Abstract
Rationale: Obesity increases the risk and severity of sleep-disordered breathing. The degree to which excess body weight contributes to blood oxygen desaturation during hypopneic and apneic events has not been comprehensively characterized.
Objectives: To quantify the association between excess body weight and oxygen desaturation during sleep-disordered breathing.
Methods: A total of 750 adult participants in the Wisconsin Sleep Cohort Study were assessed for body mass index (BMI) (kg/m2) and sleep-disordered breathing. The amount of SaO2, duration, and other characteristics of 37,473 observed breathing events were measured during polysomnography studies. A mixed-effects linear regression model estimated the association of blood oxygen desaturation with participant-level characteristics, including BMI, gender, and age, and event-level characteristics, including baseline SaO2, change in Vt, event duration, sleep state, and body position.
Measurements and Main Results: BMI was positively associated with oxygen desaturation severity independent of age, gender, sleeping position, baseline SaO2, and event duration. BMI interacted with sleep state such that BMI predicted greater desaturation in rapid eye movement (REM) sleep than in non-REM sleep. Each increment of 10 kg/m2 BMI predicted a 1.0% (SE, 0.2%) greater mean blood oxygen desaturation for persons in REM sleep experiencing hypopnea events associated with 80% Vt reductions.
Conclusions: Excess body weight is an important predictor of the severity of blood oxygen desaturation during apnea and hypopnea events, potentially exacerbating the impact of sleep-disordered breathing in obese patients.
Keywords: sleep apnea, overweight, hypoxia
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Obesity increases the risk of sleep-disordered breathing, but the extent to which excess body weight influences the amount of blood oxygen desaturation during apneic and hypopneic events has not been characterized.
What this Study Adds to the Field
We show that body mass index is a significant independent predictor of the amount of oxygen desaturation during apneas and hypopneas. The impact of sleep disordered breathing may be greater in obesity due to exacerbation of intermittent hypoxia.
Obesity is one of the most important risk factors for sleep-disordered breathing (SDB) (1). Epidemiological studies have shown that the prevalence of SDB is strongly associated with excess body weight (2) and that weight gain independently predicts the development of SDB (3). Moreover, most (4), but not all (5), interventional studies have shown that weight loss is effective in reducing apneas and hypopneas. As the prevalence of obesity increases across industrialized nations, it is predicted that obesity will cause a corresponding increase in the prevalence of SDB (1, 6).
The frequency of apneas and hypopneas increases with obesity (3, 7). The frequency of breathing events is an important predictor of the consequences of SDB, with a longitudinal dose–response relationship between the apnea-hypopnea index (AHI) and the prevalence of cardiovascular morbidity (8). The severity of oxygen desaturation during obstructive breathing events and the cumulative burden of nocturnal hypoxia are important factors in this relationship; they also predict cardiovascular morbidity in patients with SDB, independent of the frequency of breathing events (9, 10). Because excess body weight is known to predispose to more severe oxygen desaturation during voluntary apneas (11), obese patients with SDB may be disadvantaged by greater oxygen desaturation during apneas and hypopneas and by increased event frequency.
The primary aim of the present study was to measure the impact of obesity on oxygen desaturation in a large population of 30- to 62-year-old, community-dwelling men and women studied as part of the Wisconsin Sleep Cohort Study. Multiple regression analysis of over 37,000 breathing events was used to develop a predictive model to test the hypothesis that obesity was associated with greater oxygen desaturation during apneas and hypopneas independent of confounding variables and covariates.
METHODS
Participants
Participants were 750 adults from the Wisconsin Sleep Cohort Study (3) (described in the online supplement), an ongoing epidemiologic investigation of the natural history of SDB. The study was approved by the University of Wisconsin Health Sciences Institutional Review Board. Participants gave written informed consent.
Measurements
Body habitus, including height (m); weight (kg); and waist, neck, and hip circumference (cm), were measured using standard procedures (12). Body mass index (BMI) was calculated (kg/m2). Data on medical history, medication use, smoking habits, and alcohol consumption were obtained by questionnaires. Participants underwent attended polysomnography (Polygraph model 78; Grass Instruments, Quincy, MA). Measurements (described in detail in the online supplement) included electroencephalography, electrooculography, chin electromyography, SaO2, oral and nasal airflow, thoracic and abdominal excursions, and body position.
Sleep scoring was performed using conventional criteria (13). Respiratory signals were analyzed with an automated Sleep Analysis Program (SAP). This software program, developed for the Wisconsin Sleep Cohort Study and designed for the detection and characterization of hypopnea and apnea events of SDB, was previously described and validated (14) and is described in the online supplement. All breathing events that produced a change in SaO2 of 2% or greater were analyzed. The following parameters were recorded for each event: SaO2 at the start of the event (baseline SaO2), end-event SaO2, ΔSaO2 (end-event minus initial SaO2), relative change in Vt during an event (ΔVt), event duration (scored from the Vt signal), body position, sleep state (rapid eye movement [REM] or non-REM [NREM] sleep), and time of event (time since first event). Hypopneas were defined as a reduction in Vt of 20% or greater, and apneas were defined as cessation of breathing for at least 10 seconds. The AHI was calculated as the mean number of apneas and hypopneas associated with ΔSaO2 2% or greater per hour of sleep (AHI2%). Additional models that included only events associated with ΔSaO23% or greater were also examined.
Statistical Analysis
Mixed-effects linear regression models were used to examine the effect of excess body weight on ΔSaO2 during breathing events after accounting for covariates. The primary predictor variable was BMI. Alternative body habitus measures, such as neck and waist circumferences, were examined. Additional variables examined as covariates or interacting variables included: age, gender, ΔVt, time of event, sleep state, body position in which event occurred, baseline SaO2, event duration, FEV1, FVC, FEV1 % predicted, cigarette smoking, and alcohol consumption.
Using SAS PROC MIXED (SAS Institute Inc, Cary, NC), ΔSaO2 was regressed on the predictor variables, which were modeled as random (intercept and baseline SaO2) or fixed (all other predictors) effects. Interactions among predictor variables were also evaluated. Statistically significant (two-sided P value < 0.05) covariates, interactions, and quadratic terms were retained in presented models.
The primary model examined breathing events that produced 2% or greater ΔSaO2. Because most subjects had multiple breathing events, a within-subject correlation structure was fitted in the models. The best-fitting correlation structure (assessed by likelihood ratio tests and the Bayesian Information Criterion) was a “spatial” covariance matrix (spatial exponential anisotropic) with time of event (unequally spaced, within-subject) as the single spatial dimension (15).
RESULTS
A total of 1463 participants had technically adequate baseline polysomnography sleep studies. Of these subjects, 675 were not suitable for SAP analyses because the sleep studies were performed after the Wisconsin Sleep Cohort migrated to an alternative software platform that was not compatible with SAP analysis. Of the 788 remaining SAP-analyzable studies, 17 were excluded due to participant-reported, physician-diagnosed heart attack, heart failure, stroke, or emphysema, and 21 were excluded due to the use of sleep apnea treatment (continuous positive airway pressure) at the time of sleep study.
Demographic and anthropometric details of the 750 participants are presented in Table 1 and in Tables E1 through E5 in the online supplement. The mean age of the participants was 45 years, and 56% were male. Mean BMI was 29 (SD, 6) kg/m2, and mean AHI2% was 9.5 (SD, 12.0).
TABLE 1.
DESCRIPTIVE CHARACTERISTICS OF 750 PARTICIPANTS INCLUDED IN SLEEP ANALYSIS PROGRAM ANALYSIS
| Variable | Mean (SD) | Range |
|---|---|---|
| Age, yr | 45 (7) | 30–62 |
| Body mass index, kg/m2 | 29 (6) | 18–62 |
| Neck circumference, cm | 38 (4) | 27–53 |
| Waist:hip ratio (men) | 0.95 (0.06) | 0.79–1.24 |
| Waist:hip ratio (women) | 0.82 (0.07) | 0.34–1.08 |
| AHI2%, SAP-scored, events/h | 9.5 (12.0) | 0.3–91 |
| AHI4%, SAP-scored, events/h | 4.5 (8.7) | 0.1–79 |
| FEV1, % predicted | 107 (16) | 43–153 |
| FEV1/FVC | 0.82 (0.07) | 0.36–1.0 |
| Epworth Sleepiness Scale | 8.4 (4.3) | 0–22 |
| Current smoker, n (%) |
148 (20%) |
Definition of abbreviation: AHI = apnea-hypopnea index.
The 750 participants had a mean sleep time of 6.0 (SD, 1.1) hours and a total of 37,473 breathing events. Table 2 shows the number of breathing events by BMI categories. Obese participants (BMI ≥30 kg/m2) comprised 40% of the cohort but contributed 62% of breathing events.
TABLE 2.
DISTRIBUTION OF PARTICIPANTS (N = 750) AND BREATHING EVENTS (N = 37,473) BY BODY MASS INDEX (BMI) CATEGORY
| BMI Category |
|||||
|---|---|---|---|---|---|
| <25 kg/m2 | 25–29.9 kg/m2 | 30–34.9 kg/m2 | 35–39.9 kg/m2 | ≥40 kg/m2 | |
| Participants, n (%) | 199 (27) | 257 (34) | 184 (25) | 57 (8) | 53 (7) |
| Breathing events, n (%) | 3,889 (10) | 10,380 (28) | 13,210 (35) | 5,013 (13) | 4,981(13) |
Table 3 shows descriptive data for all breathing events. The mean oxygen desaturation (ΔSaO2) was 4.8% (SD, 3.1%). Due to the right-skewed distribution of ΔSaO2, the majority of events were associated with a ΔSaO2 of less than 4% (median ΔSaO2, 3.7%; range, 2–24%). Most breathing events were hypopneas (92%), and a disproportionate number of breathing events occurred in REM sleep: Mean REM sleep time was 18% of total sleep time but encompassed 26% of all breathing events.
TABLE 3.
DESCRIPTIVE DATA FOR SLEEP ANALYSIS PROGRAM–ANALYZED BREATHING EVENTS (N = 37,473)
| Variable | Mean (SD) | Median | Range |
|---|---|---|---|
| ΔSaO2, % | 4.8 (3.1) | 3.7 | 2.0–24.0 |
| SaO2 at start of event, % | 95 (3) | 95 | 79–100 |
| SaO2 at end of event, % | 90 (4) | 91 | 75–98 |
| ΔVt, % | 74 (21) | 77 | 20–100 |
| Event duration, s |
29 (13) |
26 |
10–90 |
Regression Analysis
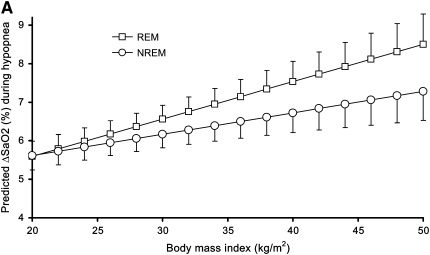
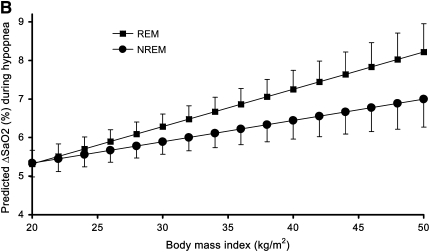
The regression model (Table 4) fitted a significant linear relationship between BMI and ΔSaO2 independent of age, gender, sleeping position, baseline SaO2, and event duration. For example, the model presented in Table 4 predicts that 50-year-old nonsmoking men with a BMI of 30 kg/m2 and a baseline SaO2 of 100% who experience a 30-second hypopnea with an 80% ΔVt while on their side in NREM sleep would be expected to have a mean ΔSaO2 of 5.9% (95% confidence interval, 5.5–6.2%). The primary variable of interest in this study is BMI, with a regression coefficient of 0.055 (SE, 0.014). This indicates that for each increment in BMI of 1 kg/m2, a mean 0.055% greater ΔSaO2 is expected. Thus, in the example, if BMI was 40 kg/m2 rather than 30 kg/m2, with all other variables unchanged, we would predict an average 0.55% greater desaturation (i.e., total ΔSaO2 of 6.4%). The model also showed statistically significant interactions between BMI and sleep state as well as BMI and ΔVt, indicating that: (1) the effect of BMI on ΔSaO2 was considerably greater during REM sleep, and (2) the impact of ΔVt on ΔSaO2 increased with increasing BMI (Table 4). Thus, for example, for hypopneas occurring during REM sleep, the coefficient would be 0.097 (= 0.055 + 0.042), and we predict an additional desaturation of approximately 1.0% for each 10 kg/m2 increment in BMI (e.g., a total estimated mean ΔSaO2 of 6.9% for the 40 kg/m2 vs. 30 kg/m2 example above). No other examined variables (e.g., age and gender) were found to significantly interact with BMI. The interaction of sleep state and BMI from the regression model is depicted in Figure 1A for men and in Figure 1B for women. The additional model that included only events associated with ΔSaO2 ≥3% yielded very similar results to those presented in Table 4. In the ΔSaO2 ≥3% model, the analogous coefficient for BMI in Table 4 (i.e., 0.055; SE, 0.014) was 0.052 (SE, 0.015).
TABLE 4.
ΔSaO2 PREDICTION MODEL AND THE IMPACT OF CHANGING INDIVIDUAL VARIABLES ON AN EVENT WITH A PREDICTED ΔSaO2 OF 5.9% (THE PREDICTED ΔSaO2 FOR AN EVENT WITH THE CHARACTERISTICS INDICATED IN THE “BASELINE VALUE” COLUMN)
| Variable* | Coefficient† (SE) | Baseline Value | Comparison Value | Expected Difference in ΔSaO2 (%) | Expected new ΔSaO2 (%) |
|---|---|---|---|---|---|
| BMI‡ | 0.055 (0.014) | 30 kg/m2 | 40 kg/m2 | +0.55 | 6.4 |
| Sleep state‡ | 0.39 (0.05) | NREM sleep | REM sleep | +0.39 | 6.3 |
| ΔVt‡ | 0.030 (0.002) | ||||
| (ΔVt)2 | 0.00054 (0.00005) | 80% | 90% | +0.35 | 6.2 |
| Baseline SaO2 | 0.52 (0.04) | ||||
| (Baseline SaO2)2 | 0.025 (0.003) | 100% | 98%§ | −0.93 | 4.9 |
| Event type | 0.25 (0.07) | Hypopnea | Apnea‖ | +0.25 | 6.1 |
| Body position | 0.54 (0.07) | Lateral | Back | +0.54 | 6.4 |
| Event duration | 0.025 (0.002) | ||||
| (Event duration)2 | 0.00016 (0.00005) | 30 s | 60 s | +0.88 | 6.8 |
| Time of event | −0.022 (0.011) | 1st Event | 1 h After 1st event | −0.02 | 5.9 |
| Gender | −0.26 (0.08) | Male | Female | −0.26 | 5.6 |
| Age | 0.028 (0.006) | 50 yr | 60 yr | +0.28 | 6.2 |
| Smoking |
0.20 (0.07) |
Nonsmoking |
1 Pack/d |
+0.20 |
6.1 |
To aid interpretation of the model intercept (5.88; SE, 0.17), select variables (BMI, ΔVt, baseline SaO2, event duration, and age) were “centered” in fitting the model by subtracting constants from their observed values as indicated by the “Starting value” column (i.e., BMI was centered at 30 kg/m2 [“BMI” = BMI − 30]; ΔVt at 80% [“ΔVt” = ΔVt − 80%]; etc.).
All coefficients (including quadratic and interaction terms) are significantly different from 0 with a P < 0.001, except for smoking (P = 0.008) and time of event (P = 0.04).
There were also significant interactions of BMI × sleep state (interaction coefficient, 0.042; SE, 0.009) and BMI × ΔVt (interaction coefficient, 0.00082; SE, 0.00018), indicating that the association of BMI and ΔSaO2 is stronger in REM compared with NREM sleep and that the effect of ΔVt on ΔSaO2 increases with increasing BMI.
The fitted quadratic relationship between baseline SaO2 and ΔSaO2 suggested that baseline SaO2 < 80% was strongly associated with greater ΔSaO2 reductions but also that there are modest decreased expected reductions in ΔSaO2 with increasing baseline SaO2 for baseline SaO2 > 80%.
Comparing a hypopnea with ΔVt = 80% to an apnea with ΔVt = 100% would yield an expected 1.1% greater ΔSaO2 (i.e., 5.9 + 1.1 = 7.0% “expected new ΔSaO2”).
Figure 1.
Predicted ΔSaO2 for a hypopnea (100% baseline SaO2, 30 seconds in duration, with an 80% ΔVt, in supine 50-year-old subjects) by body mass index and sleep state in (A) men and (B) women. Error bars indicate upper (REM) and lower (NREM) 95% confidence limit for predicted mean ΔSaO2.
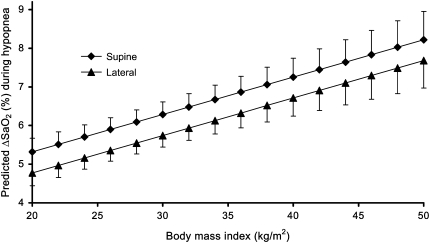
The regression model also revealed the importance of other variables (in addition to BMI) in determining the ΔSaO2 (Table 4). The supine position was associated with increased desaturation, predicting an estimated 0.54% greater ΔSaO2 compared with that expected during similar events occurring in the prone or lateral position (Figure 2). As expected, longer event duration and greater ΔVt produced greater ΔSaO2. After accounting for event-level characteristics, increasing age and smoking also independently predicted greater oxygen desaturation. Men were estimated to have an average 0.26% (SE, 0.08%) greater ΔSaO2 than women. That is, if a hypopnea with a given set of characteristics (duration, ΔVt, etc.) was expected to produce a 5.00% ΔSaO2 in a man, then a similar hypopnea in a woman would be expected to produce a 4.74% ΔSaO2.
Figure 2.
Predicted ΔSaO2 for a hypopnea (100% baseline SaO2, 30 seconds in duration, with an 80% ΔVt during REM sleep, in supine 50-year-old subjects) by body mass index and sleeping position in women. Error bars indicate upper (supine) and lower (lateral) 95% confidence limit for predicted mean ΔSaO2.
Eight percent of all events were identified by SAP as apneas. Due to the relatively few apnea events, we had insufficient power to perform apnea-only modeling. However, in the regression analysis of all respiratory events (Table 4), an indicator variable for apnea was significant (coefficient, 0.25; SE, 0.07), indicating that apneas produced greater oxygen desaturation than might be expected from extrapolating hypopnea events to ΔVt = 100%.
In addition to BMI, we examined other measures of body habitus in relation to oxygen desaturation, including neck and waist girth and the waist:hip ratio. None of these measures was a substantially better predictor of ΔSaO2 than BMI. However, we have included regression model results in the online supplement (Table E7) that describe the estimated sex-specific associations of BMI, neck girth, waist girth, and waist:hip ratio with ΔSaO2. We also examined the addition of spirometric measures of lung function, including FEV1% predicted and FEV1/FVC, to the presented models. In this population of healthy working adults, these variables had no appreciable effect on the other predictor variable coefficients, nor were they statistically significant predictors of ΔSaO2 independent of the other variables in the model.
DISCUSSION
The findings of this study show that BMI is an important predictor of SaO2during SDB. The regression model estimated a linear relationship between BMI and ΔSaO2 independent of age, gender, sleeping position, baseline SaO2, and event duration. The model also showed two interactions. First, the impact of BMI is significantly modified by sleep state; thus, ΔSaO2 during apnea or hypopnea is predicted to be greater in REM compared with NREM sleep but is especially so in persons with higher BMI. Second, during hypopnea, the (expected) positive association between ΔVt and ΔSaO2 is most marked in obese individuals.
Our model also showed that additional variables were significant predictors of the severity of the oxygen desaturation. Supine sleeping position was an independent predictor of greater oxygen desaturation, compared with the lateral or prone position. The SaO2 at the start of the breathing event (baseline SaO2), and the reduction in tidal volume occurring during hypopnea, were also predictors of oxygen desaturation.
Previous studies evaluating the effect of obesity on oxygen desaturation during SDB have not systematically examined or accounted for potential interacting or confounding factors (16, 17). In the present study, we found that body habitus predicts oxygen desaturation severity during apneic and hypopneic events independent of individual- and event-level characteristics. Sleeping position (18), sleep state (19), duration of breathing event (20), gender (21), and baseline oxygen saturation (22) have been previously reported to influence the severity of oxygen desaturation during an apneic/hypopneic event. Our model has also shown that these variables are significant independent predictors of the severity of oxygen desaturation; thus, for instance, supine sleeping position predicted greater oxygen desaturation during breathing events, compared with the lateral or prone position. Moreover, our model demonstrates that male gender, increasing age, and smoking are significant independent predictors of ΔSaO2.
Mechanisms by which Obesity Increases ΔSaO2 during Apnea and Hypopnea
The rate of desaturation during apnea is influenced by many physiological variables, including alveolar volume (23). The reservoir of oxygen in the lung is proportional to the alveolar volume, as shown in healthy individuals who experience significantly greater ΔSaO2 after voluntary apnea at residual volume compared with voluntary apnea at total lung capacity (24). The effect of excess body weight on lung volume is the principal mechanism by which BMI influences ΔSaO2 (23), an effect likely accentuated in the presence of greater whole-body oxygen demand (25). Excess body weight causes significant reduction in all lung volumes, with the greatest reduction in FRC and expiratory reserve volume (ERV) even in mildly obese and overweight individuals (26–28). In morbid obesity, FRC may approach residual volume (26). In SDB, ERV has been shown to be strongly correlated with the severity of apnea-induced desaturation (29) and mean nocturnal oxygen saturation (17, 30). Weight loss has been shown to result in improvements in overnight oxygen saturation in parallel with increase in the FRC and ERV (31).
In addition to reducing the reserve of oxygen in the lung, excess body weight affects the ventilation-perfusion ratio, another important determinant of ΔSaO2 (23). In obesity, the combined effect of a reduction in FRC and an increase in closing volume (32) results in a greater propensity for small airway closure, causing ventilation perfusion mismatching and pulmonary shunting, thereby exacerbating oxygen desaturation (33, 34).
The effect of obesity on lung volume and rate of oxygen consumption may also explain the significant interaction between BMI and sleep state predicted by our model. During REM sleep, further increase in upper airway resistance (35) may increase the work of breathing, while reduction in lung volumes and ventilation perfusion mismatching (36) increase the rate of oxygen desaturation. Moreover, chest wall excursion is reduced during the atonia of REM sleep, and there is a greater dependence upon the diaphragmatic contribution to ventilation (37, 38), which may be impinged by abdominal adiposity.
Reduction in lung volume could also explain the greater oxygen desaturation observed in the supine sleeping position. Compared with the sitting and lateral decubitus positions, supine posture causes a significant reduction in FRC and ERV in healthy individuals (39, 40).
Our primary measure of body habitus, the BMI, does not characterize the peripheral and central distribution of excess body fat (41). Therefore, we examined alternative measures of body habitus. Waist girth might be expected to show similar, if not stronger, associations with ΔSaO2 as BMI because central adiposity may have greater impact on lung function due to diaphragmatic displacement and thoracic wall impingement by visceral and chest wall fat. Although BMI is highly correlated with end expiratory lung volume (42), a recent study has reported that waist girth is strongly associated with impaired lung function independent of BMI (43). In our sample, the sex- and age-adjusted correlation of BMI and waist girth was 0.90, and only BMI was a significant predictor of ΔSaO2 when both measures were included in our model simultaneously. Thus, BMI and waist girth might be viewed as essentially interchangeable in our data. We chose to focus our results on BMI because this is the most commonly used metric of excess body weight.
Strengths and Limitations
A strength of our study is the use of data from a large community-based sample. Over 37,000 breathing events were detected in participants with a wide spectrum of body habitus and severity of SDB, allowing examination of the independent impact of individual variables and interaction between variables. Previous studies investigating the impact of obesity on ΔSaO2 have only evaluated selected populations of sleep clinic patients (16) or patients assessed for bariatric surgery (17). Because there was little racial diversity in our sample (94% white; see Table E4), we were not able to examine whether associations between obesity and ΔSaO2 varied by race.
Two limitations need to be considered when interpreting the findings of our study. First, detailed pulmonary function testing was not available in our study population. Excess body weight predominantly results in a restrictive lung defect with reduction in FRC and ERV (26), and it is possible that inclusion of these variables in our predictive model may have altered the measured impact of BMI on ΔSaO2. The lack of lung volume measurements also limits our physiological interpretation of the model; although we speculate that the impact of excess body weight on lung volume is the key mechanism by which obesity causes greater ΔSaO2, we cannot rule out other factors. For instance, right to left cardiac shunting causes increased oxygen desaturation (44), and recent reports have identified a higher prevalence of patent foramen ovale in patients with sleep apnea compared with persons without sleep apnea (45, 46). Although we cannot exclude unidentified patent foramen ovale causing greater ΔSaO2 in our study population, we are not aware of any data to suggest that patent foramen ovale becomes more prevalent as BMI increases. Second, although the SAP uses calibrated respiratory inductance plethysmography for measurement of respiratory events, the calibration may become unreliable after a change in sleeping position (47). However, because the SAP measures relative differences in Vt based on the preceding breaths, this limitation is unlikely to have strongly influenced our results.
The present study concentrated on oxygen desaturation during discrete apnea and hypopnea breathing events, but obese individuals are also at risk of nocturnal hypoventilation and hypercapnia. This issue could not be evaluated in our model but may also be important in view of the association of the obesity hypoventilation syndrome with increased mortality and morbidity (48).
Clinical Implications
Our predictive model provides clinically important information to show that obese individuals are exposed to a greater burden of oxygen desaturation during sleep-disordered breathing. Although this analysis does not confirm the long-term adverse consequences of greater oxygen desaturation in obese individuals, the detrimental effects of intermittent hypoxia are well described (49), and there is evidence to show that the severity of intermittent hypoxia is important in the pathophysiologic consequences of SDB. For example, hypopneas associated with a 4% or greater ΔSaO2 are a better predictor of prevalent cardiovascular disease compared with events associated with a 3% or less ΔSaO2 (10), and, in a recent, novel experimental model of intermittent hypoxia in humans, healthy adults exposed to intermittent hypoxia during sleep over a 2-week period developed a significant increase in waking mean SaO2 of 5 mm Hg (50).
Summary
The body mass index is an important predictor of the severity of oxygen desaturation during apneic and hypopneic events of sleep-disordered breathing, independent of age, gender, sleeping position, smoking history, baseline SaO2, and event duration. The association of BMI with oxygen desaturation is particularly strong in REM sleep. Excess body weight has been widely demonstrated to be strongly associated with a greater risk of having SDB and with an increased number of breathing events among persons with SDB. For the first time, we have comprehensively quantified the additional burden that excess body weight has on the severity of individual breathing events. These findings contribute to a fuller understanding of the impact of excess body weight on SDB.
Supplementary Material
Acknowledgments
The authors thank Terry Young and Jerry Dempsey for advice and comments and Diane Austin, Safwan Badr, Linda Evans, Laurel Finn, K. Mae Hla, Tony Jacques, Kathy Kenison, Hyon Kim, Mari Palta, Andrew Peppard, Andrea Peterson, Kathryn Pluff, Amanda Rasmuson, K.C. Seow, James Skatrud, Robin Stubbs, Mary Sundstrom, Basel Taha, Steven Weber, and Eric Young for technical expertise.
Supported by NIH grants R01HL62252, R01AG14124, RR03186, and 1 UL1RR025011; by the British Heart Foundation (N.W.); and by the Wellcome Trust (M.J.M.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200905-0773OC on July 30, 2009
Conflict of Interest Statement: P.E.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.R.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.J.M. received more than $100,001 from ResMed as a collaborator on the SERVE-HF trial, more than $100,001 from Embla, and $10,001 to $50,000 from NMT Medical as a collaborator on the SPACE study.
References
- 1.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol 2005;99:1592–1599. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Walsleben JA, Finn L, Enright P, Samet JM. Predictors of sleep-disordered breathing in community-dwelling adults: the sleep heart health study. Arch Intern Med 2002;162:893–900. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015–3021. [DOI] [PubMed] [Google Scholar]
- 4.Tuomilehto HP, Seppa JM, Partinen MM, Peltonen M, Gylling H, Tuomilehto JO, Vanninen EJ, Kokkarinen J, Sahlman JK, Martikainen T, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med 2009;179:320–327. [DOI] [PubMed] [Google Scholar]
- 5.Lettieri CJ, Eliasson AH, Greenburg DL. Persistence of obstructive sleep apnea after surgical weight loss. J Clin Sleep Med 2008;4:333–338. [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CLCM, McDowell MA, Flegal KM. Obesity among adults in the United States—no change since 2003–2004. NCHS data brief no 1. Hyattsville, MD: National Center for Health Statistics; 2007. [PubMed]
- 7.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the sleep heart health study. Arch Intern Med 2005;165:2408–2413. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 9.Baguet JP, Hammer L, Levy P, Pierre H, Launois S, Mallion JM, Pepin JL. The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence. Chest 2005;128:3407–3412. [DOI] [PubMed] [Google Scholar]
- 10.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med 2008;177:1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurewitz AN, Sampson MG. Voluntary breath holding in the obese. J Appl Physiol 1987;62:2371–2376. [DOI] [PubMed] [Google Scholar]
- 12.Measurement Descriptions and Techniques. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric standardization reference manual. Champaign, Il: Human Kinetics: 1988. pp. 1–55.
- 13.Rechtscahaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. National Institutes of Health publication no. 204. Washington, DC: US Government Printing Office; 1968.
- 14.Taha BH, Dempsey JA, Weber SM, Badr MS, Skatrud JB, Young TB, Jacques AJ, Seow KC. Automated detection and classification of sleep-disordered breathing from conventional polysomnography data. Sleep 1997;20:991–1001. [DOI] [PubMed] [Google Scholar]
- 15.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models, 2nd ed. Cary, NC: SAS Institute Inc.; 2007.
- 16.Sato M, Suzuki M, Suzuki J, Endo Y, Chiba Y, Matsuura M, Nakagawa K, Mataki S, Kurosaki N, Hasegawa M. Overweight patients with severe sleep apnea experience deeper oxygen desaturation at apneic events. J Med Dent Sci 2008;55:43–47. [PubMed] [Google Scholar]
- 17.Lehrman SG, Limann B, Koshy A, Aronow WS, Ahn C, Maguire G. Association of lung volumes with nocturnal oxygen saturation in obese persons: a possible role for therapeutic continuous positive airway pressure. Am J Ther 2008;15:221–224. [DOI] [PubMed] [Google Scholar]
- 18.Oksenberg A, Khamaysi I, Silverberg DS, Tarasiuk A. Association of body position with severity of apneic events in patients with severe nonpositional obstructive sleep apnea. Chest 2000;118:1018–1024. [DOI] [PubMed] [Google Scholar]
- 19.Series F, Cormier Y, La Forge J. Influence of apnea type and sleep stage on nocturnal postapneic desaturation. Am Rev Respir Dis 1990;141:1522–1526. [DOI] [PubMed] [Google Scholar]
- 20.Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest 1985;87:432–436. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc 2008;5:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strohl KP, Altose MD. Oxygen saturation during breath-holding and during apneas in sleep. Chest 1984;85:181–186. [DOI] [PubMed] [Google Scholar]
- 23.Farmery AD, Roe PG. A model to describe the rate of oxyhaemoglobin desaturation during apnoea. Br J Anaesth 1996;76:284–291. [DOI] [PubMed] [Google Scholar]
- 24.Findley LJ, Ries AL, Tisi GM, Wagner PD. Hypoxemia during apnea in normal subjects: mechanisms and impact of lung volume. J Appl Physiol 1983;55:1777–1783. [DOI] [PubMed] [Google Scholar]
- 25.Crummy F, Piper AJ, Naughton MT. Obesity and the lung: 2—obesity and sleep-disordered breathing. Thorax 2008;63:738–746. [DOI] [PubMed] [Google Scholar]
- 26.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest 2006;130:827–833. [DOI] [PubMed] [Google Scholar]
- 27.Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, Gattinoni L. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg 1998;87:654–660. [DOI] [PubMed] [Google Scholar]
- 28.Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol 2005;98:512–517. [DOI] [PubMed] [Google Scholar]
- 29.Series F, Cormier Y, La Forge J. Role of lung volumes in sleep apnoea-related oxygen desaturation. Eur Respir J 1989;2:26–30. [PubMed] [Google Scholar]
- 30.Bradley TD, Martinez D, Rutherford R, Lue F, Grossman RF, Moldofsky H, Zamel N, Phillipson EA. Physiological determinants of nocturnal arterial oxygenation in patients with obstructive sleep apnea. J Appl Physiol 1985;59:1364–1368. [DOI] [PubMed] [Google Scholar]
- 31.Hakala K, Maasilta P, Sovijarvi AR. Upright body position and weight loss improve respiratory mechanics and daytime oxygenation in obese patients with obstructive sleep apnoea. Clin Physiol 2000;20:50–55. [DOI] [PubMed] [Google Scholar]
- 32.Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. Effects of mass loading the respiratory system in man. J Appl Physiol 1964;19:959–966. [DOI] [PubMed] [Google Scholar]
- 33.Douglas FG, Chong PY. Influence of obesity on peripheral airways patency. J Appl Physiol 1972;33:559–563. [DOI] [PubMed] [Google Scholar]
- 34.Farebrother MJ, McHardy GJ, Munro JF. Relation between pulmonary gas exchange and closing volume before and after substantial weight loss in obese subjects. BMJ 1974;3:391–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudgel DW, Martin RJ, Johnson B, Hill P. Mechanics of the respiratory system and breathing pattern during sleep in normal humans. J Appl Physiol 1984;56:133–137. [DOI] [PubMed] [Google Scholar]
- 36.Fletcher EC, Gray BA, Levin DC. Nonapneic mechanisms of arterial oxygen desaturation during rapid-eye-movement sleep. J Appl Physiol 1983;54:632–639. [DOI] [PubMed] [Google Scholar]
- 37.Tabachnik E, Muller NL, Bryan AC, Levison H. Changes in ventilation and chest wall mechanics during sleep in normal adolescents. J Appl Physiol 1981;51:557–564. [DOI] [PubMed] [Google Scholar]
- 38.Stradling JR, Chadwick GA, Frew AJ. Changes in ventilation and its components in normal subjects during sleep. Thorax 1985;40:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Behrakis PK, Baydur A, Jaeger MJ, Milic-Emili J. Lung mechanics in sitting and horizontal body positions. Chest 1983;83:643–646. [DOI] [PubMed] [Google Scholar]
- 40.Hurewitz AN, Susskind H, Harold WH. Obesity alters regional ventilation in lateral decubitus position. J Appl Physiol 1985;59:774–783. [DOI] [PubMed] [Google Scholar]
- 41.Frankenfield DC, Rowe WA, Cooney RN, Smith JS, Becker D. Limits of body mass index to detect obesity and predict body composition. Nutrition 2001;17:26–30. [DOI] [PubMed] [Google Scholar]
- 42.Babb TG, Wyrick BL, DeLorey DS, Chase PJ, Feng MY. Fat distribution and end-expiratory lung volume in lean and obese men and women. Chest 2008;134:704–711. [DOI] [PubMed] [Google Scholar]
- 43.Leone N, Courbon D, Thomas F, Bean K, Jégo B, Leynaert B, Guize L, Zureik M. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 2009;179:509–516. [DOI] [PubMed] [Google Scholar]
- 44.Johansson MC, Eriksson P, Peker Y, Hedner J, Rastam L, Lindblad U. The influence of patent foramen ovale on oxygen desaturation in obstructive sleep apnoea. Eur Respir J 2007;29:149–155. [DOI] [PubMed] [Google Scholar]
- 45.Beelke M, Angeli S, Del Sette M, Gandolfo C, Cabano ME, Canovaro P, Nobili L, Ferrillo F. Prevalence of patent foramen ovale in subjects with obstructive sleep apnea: a transcranial Doppler ultrasound study. Sleep Med 2003;4:219–223. [DOI] [PubMed] [Google Scholar]
- 46.Shanoudy H, Soliman A, Raggi P, Liu JW, Russell DC, Jarmukli NF. Prevalence of patent foramen ovale and its contribution to hypoxemia in patients with obstructive sleep apnea. Chest 1998;113:91–96. [DOI] [PubMed] [Google Scholar]
- 47.Whyte KF, Gugger M, Gould GA, Molloy J, Wraith PK, Douglas NJ. Accuracy of respiratory inductive plethysmograph in measuring tidal volume during sleep. J Appl Physiol 1991;71:1866–1871. [DOI] [PubMed] [Google Scholar]
- 48.Nowbar S, Burkart KM, Gonzales R, Fedorowicz A, Gozansky WS, Gaudio JC, Taylor MR, Zwillich CW. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004;116:1–7. [DOI] [PubMed] [Google Scholar]
- 49.Levy P, Pepin JL, Arnaud C, Tamisier R, Borel JC, Dematteis M, Godin-Ribuot D, Ribuot C. Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives. Eur Respir J 2008;32:1082–1095. [DOI] [PubMed] [Google Scholar]
- 50.Tamisier R, Gilmartin GS, Launois SH, Pépin JL, Nespoulet H, Thomas R, Lévy P, Weiss JW. A new model of chronic intermittent hypoxia in humans: effect on ventilation, sleep and blood pressure. J Appl Physiol 2009;107:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.