Abstract
Rationale: Indices that assess the load on the respiratory muscles, such as the tension–time index (TTI), may predict extubation outcome.
Objectives: To evaluate the performance of a noninvasive assessment of TTI, the respiratory muscle tension time index (TTmus), by comparison to that of the diaphragm tension time index (TTdi) and other predictors of extubation outcome in ventilated children.
Methods: Eighty children (median [range] age 2.1 yr [0.15–16]) admitted to pediatric intensive care units at King's College and St Mary's Hospitals who required mechanical ventilation for more than 24 hours were studied.
Measurements and Main Results: TTmus, maximal inspiratory pressure, respiratory drive, respiratory system mechanics, and functional residual capacity using a helium dilution technique, the rapid shallow breathing and CROP indices (compliance, rate, oxygenation, and pressure) indexed for body weight were measured and standard clinical data recorded in all patients. TTdi was measured in 28 of the 80 children using balloon catheters. Eight children (three in the TTdi group) failed extubation. TTmus (0.199 vs. 0.09) and TTdi (0.157 vs. 0.07) were significantly higher in children who failed extubation. TTmus greater than 0.18 (n = 80) and TTdi greater than 0.15 (n = 28) had sensitivities and specificities of 100% in predicting extubation failure. The other predictors performed less well.
Conclusions: Invasive and noninvasive measurements of TTI may provide accurate prediction of extubation outcome in mechanically ventilated children.
Keywords: diaphragm, respiratory muscles, pediatric critical care, weaning
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Extubation failure is a recognized problem in pediatric intensive care and clinicians are frustrated by their inability to accurately predict extubation outcome.
What This Study Adds to the Field
Our results suggest that invasive and noninvasive measurements of tension–time index, which are assessments of the load/capacity balance of respiratory muscles, may accurately predict extubation outcomes in mechanically ventilated children.
Patients are extubated when assessed to be capable of sustaining spontaneous ventilation without respiratory support. Premature extubation leading to cardiorespiratory compromise necessitates reintubation and reinstitution of mechanical ventilation and increases mortality and morbidity (1), whereas prolonged ventilatory support exposes the child to increased risk of nosocomial infection and lung injury (2). Extubation failure has been reported to occur in 4 to 10% of children (3, 4), hence an accurate predictor of extubation outcome would be of significant clinical value (1).
Univariate indices that examine a single aspect of physiological function often have poor predictive power probably because they do not fully reflect all the pathophysiological processes affecting extubation outcome (5). Accurate prediction is more likely using multivariate indices that integrate a number of physiological functions (6). Studies of multivariate indices in children, such as the rapid-shallow breathing index (RSB) (respiratory rate [RR] divided by tidal volume [Vt]) (5) and the CROP index (compliance, RR, oxygenation, and inspiratory pressure [Pi]), however, have been limited and yielded contradictory results. We (7) and others (8, 9) have shown that the predictive power of those indices in children is poor, whereas Baumeister and colleagues (10), demonstrated that the CROP index discriminated strongly between successful and unsuccessful extubation.
Ventilation is dependent on the drive from the central nervous system, the capacity of the respiratory muscles, and the load imposed on them. Factors most likely to be predictive of extubation outcome are those assessing the effectiveness of breathing efforts and/or the load on the respiratory muscles (8). The tension–time index (TTI) of the diaphragm (TTdi) (11, 12), is a measure of the load capacity ratio of the diaphragm. It is derived by relating the mean transdiaphragmatic pressure per breath (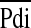 ) to the maximal inspiratory transdiaphragmatic pressure (Pdimax) and the inspiratory time (Ti) to the total respiratory cycle time (Ttot). A TTdi in excess of 0.15 is indicative of an unsustainable load on the respiratory muscles (11, 12). Measurement of the TTdi, however, is invasive, requiring the use balloon catheters. A noninvasive TTI measurement (TTmus), based on airway pressure, has been developed (13) and used in children (14, 15). A further advantage of TTmus is that it reflects the contribution of all the inspiratory muscles, rather than being specific to the diaphragm.
) to the maximal inspiratory transdiaphragmatic pressure (Pdimax) and the inspiratory time (Ti) to the total respiratory cycle time (Ttot). A TTdi in excess of 0.15 is indicative of an unsustainable load on the respiratory muscles (11, 12). Measurement of the TTdi, however, is invasive, requiring the use balloon catheters. A noninvasive TTI measurement (TTmus), based on airway pressure, has been developed (13) and used in children (14, 15). A further advantage of TTmus is that it reflects the contribution of all the inspiratory muscles, rather than being specific to the diaphragm.
We hypothesized that extubation was least likely to succeed in those children who had a high load-to-capacity ratio of the respiratory system and TTmus and TTdi would provide the most reliable predictors of extubation outcome. Our aims, therefore, were in a prospective study to evaluate TTmus measurement in children receiving mechanical ventilation by comparison to TTdi and compare their performance to that of other commonly applied “predictors” of extubation outcome.
Some of the results of these studies have been previously reported in the form of abstracts (16, 17).
METHODS
Additional detail on the methods is provided in the online data supplement.
Patients
Children who were mechanically ventilated for more than 24 hours were studied. Informed, written consent was obtained before performing the measurements. Research Ethics Committees at King's College and St Mary's Hospital NHS Trusts approved the study. Measurements were performed when the children were deemed ready for extubation by the clinical team.
Equipment
Respiratory flow was measured using a pneumotachograph connected to a three-way sliding occlusion valve (Hans Rudolph Inc., Kansas City, MO), attached to the endotracheal tube. Airway pressure was measured from a side port on the pneumotachograph. Transdiaphragmatic pressure (Pdi) was measured using balloon catheters positioned in the mid esophagus (esophageal pressure) (18) and stomach (gastric pressure) (Ackrad Laboratories, Cranford, NJ). Flow and pressure signals were recorded and displayed on a computer.
Functional residual capacity (FRC) was measured to characterize the patient's respiratory status and also evaluated as a univariate index of extubation outcome; a helium gas dilution system was used (Equilibrated Biosystems, Melville, NY) as previously described (19). FRC was expressed as the mean of paired measurements and adjusted to body weight (FRC/kg) and body length (FRC/cm). The coefficient of repeatability of FRC measurements in ventilated children is 4.4 ml/kg (20).
Protocol
Patients were studied supine with stable blood gases and blood pressure (Figure 1). Endotracheal tube suction was undertaken 10 to 15 minutes before performing each study. FRC and respiratory system compliance and resistance, using the single breath occlusion technique (21), were measured first while the children were mechanically ventilated at the settings determined by the clinical team. Dynamic compliance was calculated from the volume delivered by positive pressure inflation on breaths unaffected by spontaneous respiratory activity.
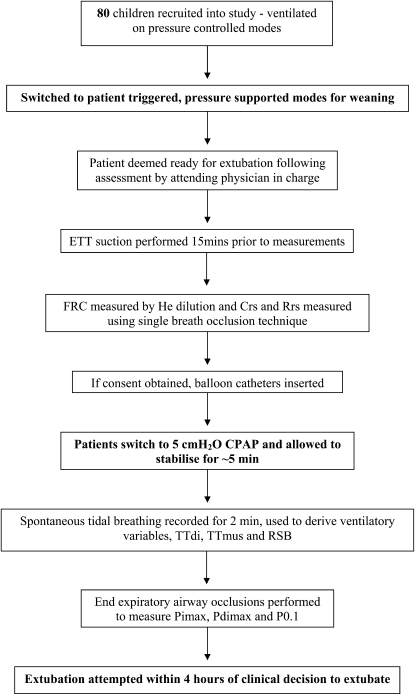
Figure 1.
Flow diagram indicating the timing and conditions under which measurements were performed. (CPAP = continuous positive air pressure; Crs = respiratory system compliance; ETT = endotracheal tube; FRC = functional residual capacity; He = helium; Pdimax = maximal transdiaphragmatic inspiratory pressure; Pimax = maximal inspiratory pressure; P0.1 = pressure generated in the first 100 milliseconds of airway occlusion; Rrs = respiratory system resistance; RSB = rapid shallow breathing; TTdi = diaphragm tension–time index; TTmus = muscle tension–time index.
Balloon catheters were then inserted and the patient switched over to 5 cm H2O continuous positive airway pressure (CPAP). After stabilization (∼ 5 min), 2 minutes of spontaneous tidal breathing was recorded and respiratory flow and pressures measured. End expiratory airway occlusions were then performed to measure respiratory drive, maximal inspiratory airway (Pimax) and, if balloon catheters were present, maximal transdiaphragmatic (Pdimax) pressures.
TTdi (11), TTmus (13), and the RSB and CROP indices modified for pediatric use (10) were calculated as previously described. Immediately before extubation a sample for blood gas analysis was taken from an indwelling arterial catheter sited for clinical purposes. All ventilator settings and standard clinical variables were recorded.
Analysis
Extubation failure was defined as reintubation and ventilation within 24 hours. The agreement between TTdi and TTmus was assessed using Bland and Altman analysis (22). Differences between patients who failed or did not fail extubation were assessed for statistical significance using the Mann Whitney U test. Spearman correlation coefficients were calculated to assess the relationship of patient age and duration of ventilation on physiologic data and extubation failure. The sensitivity and specificity for TTdi were calculated using the predefined value of 0.15 (11, 12). Regression analysis was performed between TTdi and TTmus to derive the cutoff value for TTmus. Values greater than the respective cutoffs were regarded as predictive of extubation failure. For the remaining variables, cutoff values that provided a high specificity and sensitivity were determined. Receiver operating characteristics (ROC) curves were constructed. The areas under each ROC curve were then compared to identify the most predictive index of extubation failure (23). These analyses were exploratory in nature and therefore there was no requirement to correct for multiple comparisons.
RESULTS
Eighty infants and children (median [range] age 2.1 [0.15–16] years, weight 13 [2.6–65] kg and height 85.5 [52–165] cm) with a median (range) duration of mechanical ventilation of 3 (1.2–83) days were studied. Their diagnoses were: respiratory failure (n = 23), liver transplantation (n = 23), sepsis (n = 9), neurosurgery (n = 9), liver failure (n = 6), meningitis (n = 3), gastrointestinal surgery (n = 2), neuromuscular disease (n = 2), congenital cystic adenoid malformation (n = 2), and liver tumor (n = 1). All patients, except one, were studied within 4 hours before extubation being attempted by the clinical team; the remaining patient was assessed 6 hours before successful extubation. There was no statistically significant difference between the time to extubation in those patients in whom extubation was successful or failed.
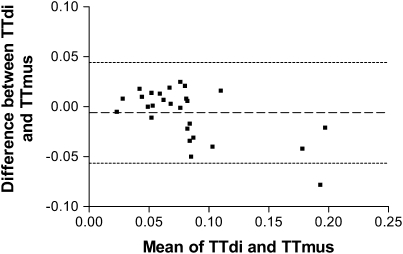
TTmus was measured in all patients and TTdi and TTmus compared in a subgroup of 28 children. There was no statistically significant difference between the median TTdi (0.071) and TTMus (0.072) (P = 0.57), but Bland-Altman analysis demonstrated a mean (SD) difference of −0.006 (0.025) between the results of the two techniques (Figure 2). Spearman correlation analysis indicated highly significant relationships between TTdi and TTmus (r = 0.75; P < 0.0001), between mean airway pressure per breath (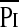 ) and
) and 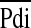 (r = 0.58; P < 0.01), Pimax and Pdimax (r = 0.85; P < 0.0001), and
(r = 0.58; P < 0.01), Pimax and Pdimax (r = 0.85; P < 0.0001), and 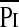 /Pimax and
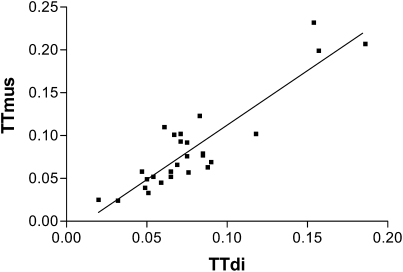
/Pimax and 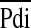 /Pdimax (r = 0.83; P < 0.0001). There was also a significant correlation between TTdi and the transdiaphragmatic pressure time product (r = 0.51; P < 0.01). Using the predefined cutoff of 0.15 (11, 12) for TTdi, regression analysis indicated a cutoff for TTmus of 0.18. (Figure 3).
/Pdimax (r = 0.83; P < 0.0001). There was also a significant correlation between TTdi and the transdiaphragmatic pressure time product (r = 0.51; P < 0.01). Using the predefined cutoff of 0.15 (11, 12) for TTdi, regression analysis indicated a cutoff for TTmus of 0.18. (Figure 3).
Figure 2.
Bland Altman analysis of diaphragm tension–time index (TTdi) and muscle tension–time index (TTmus).
Figure 3.
Regression analysis of diaphragm tension–time index (TTdi) versus muscle tension–time index (TTmus) to derive cutoff for TTmus: y = 1.2701x − 0.0149.
In the whole group, extubation failed in eight patients (10%), the median (range) time to failure after extubation was 1 (1–2) hours. Those who failed extubation did not differ significantly in their age, height, weight, duration of ventilation, or diagnosis from the children who were successfully extubated (Table 1; see Table E1 in the online supplement). There were no significant correlations between patient age duration of ventilation and extubation outcome. Similarly, the diagnoses of these patients did not differ markedly from the rest of the cohort (Table 1). There were statistically significant differences between the patients in whom extubation succeeded compared with those in whom extubation failed for TTmus (P < 0.0001), respiratory drive as assessed from the pressure generated in the first 100 milliseconds of airway occlusion (P0.1) (P < 0.047), Pimax (P < 0.012), and the fraction of Pimax developed by the respiratory muscles per breath at rest, 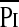 /Pimax (P < 0.001) (Table 2). There were no statistically significant differences in Ti and Ttot, although there was a trend for Ttot to be shorter in the children who failed extubation, which became statistically significant when expressed as a function of Ti; Ti/Ttot (P < 0.001). In addition, FRC/kg (P < 0.021), maximum peak inspiratory pressure used (PIPmax) (P < 0.006) and the maximum fractional concentration of inspired oxygen used (FiO2max) (P < 0.017) were also significantly different between extubation successes and extubation failures.
/Pimax (P < 0.001) (Table 2). There were no statistically significant differences in Ti and Ttot, although there was a trend for Ttot to be shorter in the children who failed extubation, which became statistically significant when expressed as a function of Ti; Ti/Ttot (P < 0.001). In addition, FRC/kg (P < 0.021), maximum peak inspiratory pressure used (PIPmax) (P < 0.006) and the maximum fractional concentration of inspired oxygen used (FiO2max) (P < 0.017) were also significantly different between extubation successes and extubation failures.
TABLE 1.
DIAGNOSIS, AGE, WEIGHT, HEIGHT, DURATION OF VENTILATION, AND REASON FOR REINTUBATION OF THE CHILDREN WHO FAILED EXTUBATION
| Diagnosis | Age (yr) | Weight (kg) | Height (cm) | Duration of Ventilation (d) | Reason for Failure |
|---|---|---|---|---|---|
| ARDS | 3.0 | 14.0 | 98 | 12 | Severe respiratory distress |
| CF, respiratory failure | 16.0 | 47.0 | 142 | 5 | Severe respiratory distress |
| Gastrointestinal surgery, myopathy | 0.2 | 3.0 | 54 | 12 | Respiratory failure with high Pco2 |
| Liver transplant | 8.0 | 30.4 | 130 | 1.2 | Respiratory failure with lung collapse |
| Sepsis, respiratory failure | 1.0 | 8.5 | 72 | 37 | Severe respiratory distress |
| Meningococcemia | 1.2 | 12.0 | 77 | 14 | Severe respiratory distress |
| CCAM | 0.8 | 6.3 | 70 | 1.8 | Respiratory distress, high Pco2 |
| Myopathy, leukemia |
14.0 |
57.0 |
160 |
43 |
Severe respiratory distress |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; CF = cystic fibrosis; CCAM = congenital cystic adenomatoid malformation.
TABLE 2.
RESULTS ACCORDING TO EXTUBATION OUTCOME
| Variable | Success, Median (Range) (n = 72) | Failure, Median (Range) (n = 8) | P Value |
|---|---|---|---|
| Age, yr | 2.1 (0.15–16) | 2.1 (0.2–16) | 0.936 |
| Weight, kg | 13 (2.6–65) | 13 (3–57) | 0.748 |
| Height, cm | 85.5 (52–165) | 87.5 (54–160) | 0.879 |
| Vt/kg body weight, ml/kg | 7.5 (4.2–21.6) | 5.6 (5.1–11.6) | 0.210 |
| Ti, s | 0.75 (0.34–1.55) | 0.69 (0.51–1.16) | 0.879 |
| Ttot, s | 2.005 (0.71–10.4) | 1.49 (0.9–2.19) | 0.056 |
| Ti/Ttot | 0.41 (0.15–0.66) | 0.52 (0.45–0.61) | 0.0009 |
| RR, bpm | 31.4 (6.1–85) | 39.7 (27.2–66.5) | 0.118 |
| P0.1, cm H2O | 2.8 (1–6.4) | 4.05 (1.5–7.1) | 0.047 |
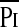 , cm H2O , cm H2O |
9.8 (2.9–37.2) | 11.6 (8.5–24.2) | 0.151 |
| Pimax, cm H2O | 46.1 (20.7–98.5) | 30.45 (21.5–58.8 | 0.012 |
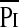 /Pimax /Pimax
|
0.23 (0.07–0.63) | 0.39 (0.3–0.57) | 0.0002 |
| TTmus | 0.09 (0.02–0.17) | 0.2 (0.183–0.26) | 0.0001 |
| Crs/kg body weight, ml/cm H2O/kg | 0.99 (0.28–2.8) | 0.94 (0.52–0.98) | 0.448 |
| Rrs, cm H2O/L/s | 25.7 (10.18–146.7) | 35.2 (10.45–51.98) | 0.974 |
| FRC, ml | 262.0 (57.5–1,585.5) | 262.0 (54.5–1,585.5) | 0.786 |
| FRC/kg body weight, ml/kg | 21.2 (17.6–27.3) | 18.9 (17.3–22.1) | 0.021 |
| FRC/cm body length, ml/cm | 3.2 (1.0–9.6) | 3.85 (1.0–7.0) | 0.910 |
| RSB index | 4.5 (0.3–17.3) | 5.4 (3.6–11.3) | 0.217 |
| CROP index | 1.0 (0.1–2.9) | 0.4 (0.2–0.5) | 0.282 |
| Duration of ventilation, d | 3 (1.2–83) | 12 (1.2–43) | 0.222 |
| PIPmax, cm H2O | 22 (16–34) | 27 (20–36) | 0.006 |
| FiO2max | 0.5 (0.3–1) | 0.7 (0.5–1) | 0.017 |
| PIP at extubation, cm H2O | 16 (10– 20) | 16.5 (15–20) | 0.084 |
| FiO2 at extubation | 0.3 (0.21–0.45) | 0.32 (0.25–0.4) | 0.289 |
| PaO2 at extubation, mm Hg | 92.25 (34.5–140.25) | 68.25 (44.25–141.0) | 0.245 |
| PaCO2 at extubation, mm Hg | 41.63 (3.38–63.45 | 41.625 (31.58–51.0) | 0.791 |
| Mean airway pressure at extubation, cm H2O | 7 (5–6) | 7.5 (7.0–9.0) | 0.103 |
| PaO2, mm Hg | 170.7 (99.5–317.5) | 185.1 (134.4–242.5) | 0.378 |
| Time from measurements to extubation, h |
1.12 (0.25–6.0) |
1.0 (0.5–2.0) |
0.497 |
Definition of abbreviations: CROP = compliance, respiratory rate, oxygenation, and inspiratory pressure; Crs = respiratory system compliance; FRC = functional residual capacity; Pi = inspiratory airway pressure; Pimax = maximum inspiratory airway pressure; PIP = peak inspiratory pressure; P0.1 = pressure generated in the first 100 milliseconds of airway occlusion; RR = respiratory rate; Rrs = respiratory system resistance; RSB = rapid shallow breathing; Ti = inspiratory time; TTmus = muscle tension–time index; Ttot = total respiratory cycle time.
In the subgroup of 28 patients who had invasive measurements using balloon catheters, 3 failed extubation and differed statistically with regard to their median TTdi, TTmus, Ti/Ttot, Pimax, and the fraction of Pimax and Pdimax developed per breath (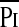 /Pimax and
/Pimax and 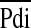 /Pdimax, respectively) (Table 3). In addition, FRC/kg (P < 0.005), PIPmax (P < 0.005), and FiO2max (P < 0.005) were also statistically significantly different between extubation successes or failures (Table E2).
/Pdimax, respectively) (Table 3). In addition, FRC/kg (P < 0.005), PIPmax (P < 0.005), and FiO2max (P < 0.005) were also statistically significantly different between extubation successes or failures (Table E2).
TABLE 3.
MAXIMUM SENSITIVITY AND SPECIFICITY OF INDIVIDUAL VARIABLES IN PREDICTING EXTUBATION FAILURE WITH AREAS UNDER THE RECEIVER OPERATING CHARACTERISTICS CURVE FOR ALL 80 SUBJECTS
| Variable | Area Under ROC Curve | Value | Sensitivity (%) | Specificity (%) | P Value |
|---|---|---|---|---|---|
| TTmus | 1.000 | >0.18 | 100 | 100 | 0.0001 |
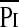 /Pimax /Pimax
|
0.911 | <0.33 | 87.5 | 87.5 | 0.0001 |
| Ti/Ttot | 0.859 | >0.45 | 69 | 87.5 | 0.001 |
| PIPmax, cm H2O | 0.794 | >27.5 | 50 | 86.1 | 0.007 |
| FRC/kg body weight, ml/kg | 0.789 | <19.15 | 50 | 86 | 0.021 |
| Pimax, cm H2O | 0.773 | <32.6 | 63 | 85 | 0.012 |
| FiO2max | 0.754 | >0.825 | 25 | 89.0 | 0.019 |
| P0.1, cm H2O | 0.715 | >4.45 | 50 | 88.0 | 0.047 |
| Ttot, s | 0.707 | >1.055 | 25 | 88 | 0.056 |
| PIP at extubation, cm H2O | 0.684 | >16.5 | 50 | 73.6 | 0.089 |
| RR, bpm | 0.669 | >41.6 | 50 | 69.4 | 0.089 |
| Mean airway pressure at extubation, cm H2O | 0.659 | >7.5 | 50 | 73.6 | 0.094 |
 , cm H2O , cm H2O |
0.656 | <10.02 | 51 | 75 | 0.149 |
| Vt/kg body weight, ml/kg | 0.645 | <5.65 | 57 | 84 | 0.21 |
| RSB index | 0.643 | >10.9 | 14.3 | 95.2 | 0.217 |
| PaO2 at extubation, mm Hg | 0.634 | <65.89 | 43 | 85 | 0.245 |
| Duration of ventilation, d | 0.631 | >11 | 62.5 | 77.8 | 0.226 |
| CROP index | 0.622 | <0.15 | 27 | 63 | 0.291 |
| FiO2 at extubation | 0.606 | >0.315 | 50 | 80.6 | 0.328 |
| Crs/kg body weight, ml/cm H2O/kg | 0.605 | <0.605 | 20 | 86 | 0.448 |
| PaO2, mm Hg | 0.595 | >193.70 | 50 | 81.9 | 0.378 |
| Crs, ml/cm H2O | 0.532 | <6.59 | 20 | 80 | 0.817 |
| Ti, s | 0.516 | >0.69 | 50 | 41.7 | 0.879 |
| Rrs, cm H2O/L/s | 0.495 | >35.09 | 60 | 72.7 | 0.164 |
| FRC/cm body length, ml/cm | 0.486 | <1.9 | 33 | 83 | 0.91 |
| PaCO2 at extubation, mm Hg | 0.470 | >46.4 | 25 | 76.4 | 0.785 |
| FRC, ml |
0.466 |
>243 |
50 |
63 |
0.786 |
For definition of abbreviations, see Table 2.
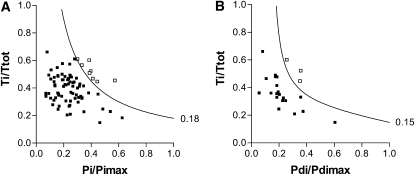
Using a cutoff of 0.18, TTmus was the most sensitive and specific predictor of extubation outcome in the 80 patients, with area under the ROC curve of 1.0 (Table 3, Figure 4A). Of the clinical data, PIPmax, and FiO2max performed best with areas under the curve of 0.794 (P = 0.007) and 0.754 (P = 0.019), respectively.
Figure 4.
Relationship between duration of respiratory muscle contraction (Ti/Ttot) and contractile capacity of (A) respiratory muscles (Pi/Pimax) and (B) diaphragm (Pdi/Pdimax) for patients in whom extubation was successful (▪) and in whom extubation failed (□). Curved lines are the isopleths indicating the cutoff for extubation outcome for muscle tension–time index (0.18) and diaphragm tension–time index (0.15). (Pdi = transdiaphragmatic inspiratory pressure; Pdimax = maximum Pdi; Pi = inspiratory airway pressure; Pimax = maximum Pi; Ti = inspiratory time; Ttot = total respiratory cycle time.
For the subgroup of 28 patients who had invasive measurements, TTdi and TTmus performed equally as well in predicting extubation outcome with areas under the ROC curves of 1.0 for both measurements (Table E3), using cutoffs of 0.18 and 0.15, respectively (Figure 4B). In addition, a Pimax less than 32.2 cm H2O had 100% sensitivity and specificity in predicting extubation failure, whereas FRC/kg, PIPmax, PaO2 at extubation, and FiO2max all had areas under the ROC curves greater than 0.9 (Table E2).
DISCUSSION
We have demonstrated that TTmus was an accurate predictor of extubation outcome in ventilated children. When compared with TTdi, TTmus provided an accurate assessment of the load capacity ratio of the inspiratory muscles in ventilated children, avoiding the requirement to perform invasive measurements. Clinical judgment and other commonly used multivariate indices, such as the RSB and CROP index, were neither sensitive nor specific in predicting extubation outcome in children. The extubation failure rate in our study (10%) was similar to that seen in previous studies (1, 4, 8). Respiratory diseases were the primary or admitting diagnosis in only 29% of subjects, but respiratory system involvement was present in the majority of our patients. Many of the remaining children with a nonrespiratory diagnosis had reduced respiratory system compliance and required significant ventilatory support, and the children who had undergone liver transplantation have potential diaphragm dysfunction as a result of surgery or underlying disease (24). TTmus provided accurate prediction of extubation outcome across a broad range of pathophysiological conditions, and hence our results emphasize that the performance of TTmus merits further investigation in another pediatric study population. The ability to sustain unsupported ventilation is dependent on the drive from the central nervous system, the capacity of the respiratory muscles, and the load imposed on them. TTmus reflects this respiratory drive modulated load/capacity balance of the diaphragm/respiratory muscles and is ideally suited for use in children as they are independent of the changes in respiratory muscle strength that occur with age.
The standard policy of the intensive care unit was followed with regard to the ventilatory mode used for weaning in our study. The decision to extubate was made by the clinical team and no trial of spontaneous breathing was performed before this decision being made, hence test referral and spectrum bias were excluded (25, 26). Patients were switched to CPAP while the measurements were performed; the total period of spontaneous breathing, including the time taken for stabilization and the measurements, was approximately 15 minutes. All the patients tolerated the brief period of CPAP required to perform the measurements without signs of cardiorespiratory decompensation or distress, but eight patients subsequently failed extubation and required reintubation within 24 hours. Post-extubation CPAP, noninvasive ventilation, or negative extrathoracic pressure was not used. All extubation indices were compared in an individual, thus the weaning and extubation policy did not influence that comparison. A standardized procedure was adopted for all measurements, which were performed in a strict order to minimize disturbance to the patient and concomitant changes to their physiological status. All patients, except one, were studied within 4 hours of extubation being attempted and there was no influence of the time to extubation on extubation outcome.
The greater TTmus in the patients who failed extubation was due to both changes in 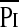 /Pimax and Ti/Ttot. Previous studies have shown that children with cystic fibrosis (14), obstructive lung diseases (27), or neuromuscular disease (15) have elevated TTmus relative to normal control subjects due primarily to an increase in the
/Pimax and Ti/Ttot. Previous studies have shown that children with cystic fibrosis (14), obstructive lung diseases (27), or neuromuscular disease (15) have elevated TTmus relative to normal control subjects due primarily to an increase in the 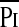 /Pimax. In the patients with obstructive lung disease, the increase in the
/Pimax. In the patients with obstructive lung disease, the increase in the 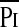 /Pimax term was due principally to an increase in
/Pimax term was due principally to an increase in 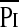 , most likely due to the added resistive load of airway obstruction (14), whereas in children with neuromuscular disease it was due to a decrease in Pimax (15). In the current study, the change in the
, most likely due to the added resistive load of airway obstruction (14), whereas in children with neuromuscular disease it was due to a decrease in Pimax (15). In the current study, the change in the 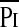 /Pimax term was due to a decrease in Pimax indicating a decrease in functional inspiratory muscle strength and a greater inspiratory demand in relation to inspiratory reserve. Atrophy and remodeling from inactivity (28); injury from overuse (29); sepsis (30); medications such as aminoglycosides, steroids, or neuromuscular blockers (31); malnutrition; and chronic illnesses (32, 33) can also detrimentally affect respiratory muscle strength. In addition the degree of consciousness, respiratory drive, and lung volume can also affect measurement of Pimax (34). Adult patients are often conscious or semiconscious when Pimax is measured and their degree of mental alertness will directly affect their cooperation and ability to perform the test (35). The test is often applied in patients who are sufficiently awake to respond to commands, but their level of consciousness may prevent them from making truly maximal efforts (36). In the current study, sedation was stopped when children were deemed ready for extubation and patients were studied once this decision was made and within 4 hours of any attempted extubation (median 1 h). At the time of study, the patients were not fully awake, hence their Pimax results reflect their respiratory muscle function and respiratory drive not fully influenced by volition and the patient's degree of alertness.
/Pimax term was due to a decrease in Pimax indicating a decrease in functional inspiratory muscle strength and a greater inspiratory demand in relation to inspiratory reserve. Atrophy and remodeling from inactivity (28); injury from overuse (29); sepsis (30); medications such as aminoglycosides, steroids, or neuromuscular blockers (31); malnutrition; and chronic illnesses (32, 33) can also detrimentally affect respiratory muscle strength. In addition the degree of consciousness, respiratory drive, and lung volume can also affect measurement of Pimax (34). Adult patients are often conscious or semiconscious when Pimax is measured and their degree of mental alertness will directly affect their cooperation and ability to perform the test (35). The test is often applied in patients who are sufficiently awake to respond to commands, but their level of consciousness may prevent them from making truly maximal efforts (36). In the current study, sedation was stopped when children were deemed ready for extubation and patients were studied once this decision was made and within 4 hours of any attempted extubation (median 1 h). At the time of study, the patients were not fully awake, hence their Pimax results reflect their respiratory muscle function and respiratory drive not fully influenced by volition and the patient's degree of alertness.
Although Pimax was significantly lower in the patients who failed extubation compared with those in whom extubation was successful, the predictive accuracy of the index, as shown by the area under the ROC curve (0.773), was relatively poor. This is consistent with previous data (8, 37), with patients able to generate large negative inspiratory pressures still failing extubation. A number of other univariate indices, P0.1, FRC/kg, PIPmax, and FiO2max, were statistically significantly different between extubation failure and success. P0.1 is a recognized index of respiratory drive and in the current study we found that it was significantly increased in the children who failed extubation. This finding is consistent with the increased respiratory drive often observed in adult patients who fail extubation (38) but is in contrast with the results of our previous study, in which a low value was predictive of extubation failure (37). In our previous study, however, only a minority of patients had a primary respiratory diagnosis. Lung volume related to body weight was significantly lower in patients who failed extubation, but there were no statistically significant differences observed for compliance or resistance results. Studies in infants have shown that a low FRC/kg measured before (39) and after extubation (40) can reliably predict subsequent respiratory failure and reintubation. Of the available clinical data, the maximum PIP and FiO2 needed at any point during mechanical ventilation were significantly higher in children who failed extubation, suggesting more severe underlying disease. The absence of statistically significant differences in ventilator settings or oxygen requirement immediately before extubation reflects that the children were extubated at similar severities of disease.
As with previous studies (7–9), we demonstrated poor sensitivity and specificity of both CROP and RSB indices. The CROP index incorporates compliance results, which poorly discriminate between patients who fail or succeed a weaning trial (41). Using 5 cm H2O CPAP rather than no pressure support during the measurements may have affected the performance of the RSB index (26) as values are higher during unassisted breathing than with pressure support (26). In children, however, when RSB was assessed without pressure support, the area under the ROC curve was only 0.57 (9). The broad age range and hence respiratory rates encountered in the pediatric population may make the RSB index inappropriate as a predictor of extubation outcome in that setting (8). It is also important to emphasize that the RSB was developed as a test to identify patients' readiness for a weaning trial and not as a predictor of extubation failure (5). Previous studies have evaluated the performance of RSB as an extubation index (7, 8, 10) and yielded contradictory results. We therefore believed it appropriate to include the RSB index as a comparator in the current study.
Based on the work by Bellemare and Grassino (11, 12), Milic-Emili (42) suggested that TTdi could predict weaning outcome. A TTdi in excess of 0.15 was suggested to be indicative of impending respiratory failure (42) and hence was used in this study as the cutoff. Our study is the first to compare in children the performance of invasive TTI measurements to those noninvasive TTmus values derived from airway pressure. A TTmus greater than 0.18 differentiated between extubation failure and success. This is markedly lower than TTmus values quoted for cystic fibrosis (>0.33) (14) and neuromuscular disease (>0.4) (15), above which respiratory failure would ultimately occur. Those studies (14, 15), however, were performed in patients who were clinically stable at the time of study and none were in respiratory failure. In the original validation study by Ramonatxo and colleagues (13) when cutoff values for TTmus were calculated for healthy subjects, a range of values from 0.16 to 0.25 were obtained. Only one study (43) has assessed the use of noninvasive tension time indices to predict extubation outcome in children. Noizet and colleagues (43) derived tension–time indices from P0.1, the mean airway pressure during mechanical ventilation, and a pressure–time index using the inspiratory pressure during unsupported, spontaneous breathing. None of these indices performed well at predicting extubation outcome; the best predictor was a combination of indices that included the P0.1, RSB, Pimax, and TTI derived from mean airway pressure. All the children studied, however, also underwent a spontaneous breathing trial before extubation and those failing this were excluded from the analysis. Excluding these patients may well have biased the results by reducing the number of extubation failures (43).
Conclusion
The results of noninvasive measurement of the load capacity ratio of the inspiratory muscles (TTmus) agreed closely with those derived from invasive measurements (TTdi) and provided an accurate predictor of extubation outcome in the mechanically ventilated infants and children studied. TTmus merits further validation in another pediatric study population.
Supplementary Material
Supported by The Wellcome Trust (grant no. 067882/Z/02/Z).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200811-1725OC on August 20, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kurachek SC, Newth CJ, Quasney MW, Rice T, Sachdeva RC, Patel NR, Takano J, Easterling L, Scanlon M, Musa N, et al. Extubation failure in pediatric intensive care: a multiple-center study of risk factors and outcomes. Crit Care Med 2003;31:2657–2664. [DOI] [PubMed] [Google Scholar]
- 2.Elward AM. Pediatric ventilator-associated pneumonia. Pediatr Infect Dis J 2003;22:445–446. [DOI] [PubMed] [Google Scholar]
- 3.Baisch SD, Wheeler WB, Kurachek SC, Cornfield DN. Extubation failure in pediatric intensive care incidence and outcomes. Pediatr Crit Care Med 2005;6:312–318. [DOI] [PubMed] [Google Scholar]
- 4.Fontela PS, Piva JP, Garcia PC, Bered PL, Zilles K. Risk factors for extubation failure in mechanically ventilated pediatric patients. Pediatr Crit Care Med 2005;6:166–170. [DOI] [PubMed] [Google Scholar]
- 5.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 1991;324:1445–1450. [DOI] [PubMed] [Google Scholar]
- 6.Tobin MJ. Predicting weaning outcome. Chest 1988;94:227–228. [DOI] [PubMed] [Google Scholar]
- 7.Manczur TI, Greenough A, Rafferty GF, Pryor D. Comparison of predictors of extubation from mechanical ventilation in children. Pediatr Crit Care Med 2000;1:28–32. [DOI] [PubMed] [Google Scholar]
- 8.Khan N, Brown A, Venkataraman ST. Predictors of extubation success and failure in mechanically ventilated infants and children. Crit Care Med 1996;24:1568–1579. [DOI] [PubMed] [Google Scholar]
- 9.Farias JA, Alia I, Esteban A, Golubicki AN, Olazarri FA. Weaning from mechanical ventilation in pediatric intensive care patients. Intensive Care Med 1998;24:1070–1075. [DOI] [PubMed] [Google Scholar]
- 10.Baumeister BL, el-Khatib M, Smith PG, Blumer JL. Evaluation of predictors of weaning from mechanical ventilation in pediatric patients. Pediatr Pulmonol 1997;24:344–352. [DOI] [PubMed] [Google Scholar]
- 11.Bellemare F, Grassino A. Effect of pressure and timing of contraction on human diaphragm fatigue. J Appl Physiol 1982;53:1190–1195. [DOI] [PubMed] [Google Scholar]
- 12.Bellemare F, Grassino A. Evaluation of human diaphragm fatigue. J Appl Physiol 1982;53:1196–1206. [DOI] [PubMed] [Google Scholar]
- 13.Ramonatxo M, Boulard P, Prefaut C. Validation of a noninvasive tension-time index of inspiratory muscles. J Appl Physiol 1995;78:646–653. [DOI] [PubMed] [Google Scholar]
- 14.Hayot M, Guillaumont S, Ramonatxo M, Voisin M, Prefaut C. Determinants of the tension-time index of inspiratory muscles in children with cystic fibrosis. Pediatr Pulmonol 1997;23:336–343. [DOI] [PubMed] [Google Scholar]
- 15.Mulreany LT, Weiner DJ, McDonough JM, Panitch HB, Allen JL. Noninvasive measurement of the tension-time index in children with neuromuscular disease. J Appl Physiol 2003;95:931–937. [DOI] [PubMed] [Google Scholar]
- 16.Harikumar G, Greenough A, Moxham J, Rafferty GF. Comparison of invasive and non-invasive measurements of tension time index in ventilated children [abstract]. Am J Respir Crit Care Med 2006;3:A256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harikumar G, Moxham J, Greenough A, Rafferty GF. Evaluation of non-invasive tension time index as a predictor of extubation outcome in ventilated children [abstract]. Am J Respir Crit Care Med 2006;3:A752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 1982;126:788–791. [DOI] [PubMed] [Google Scholar]
- 19.Rafferty GF, Greenough A, Dimitriou G, Kavadia V, Laubscher B, Polkey MI, Harris ML, Moxham J. Assessment of neonatal diaphragm function using magnetic stimulation of the phrenic nerves. Am J Respir Crit Care Med 2000;162:2337–2340. [DOI] [PubMed] [Google Scholar]
- 20.Williams O, Dimitriou G, Hannam S, Rafferty GF, Greenough A. Lung function and exhaled nitric oxide levels in infants developing chronic lung disease. Pediatr Pulmonol 2007;42:107–113. [DOI] [PubMed] [Google Scholar]
- 21.Manczur TI, Greenough A, Rafferty GF, Milner AD. Measurement of pulmonary mechanics in the paediatric intensive care unit: a comparison of techniques during pressure- and volume-limited ventilation. Pediatr Pulmonol 2000;30:265–268. [DOI] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 23.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 24.Manczur TI, Greenough A, Rafferty GF, Dimitriou G, Baker AJ, Mieli-Vergani G, Rela SM, Heaton N. Diaphragmatic dysfunction after pediatric orthotopic liver transplantation. Transplantation 2002;73:228–232. [DOI] [PubMed] [Google Scholar]
- 25.Tobin MJ, Jubran A. Variable performance of weaning-predictor tests: role of Bayes' theorem and spectrum and test-referral bias. Intensive Care Med 2006;32:2002–2012. [DOI] [PubMed] [Google Scholar]
- 26.Tobin MJ, Jubran A. Meta-analysis under the spotlight: focused on a meta-analysis of ventilator weaning. Crit Care Med 2008;36:1–7. [DOI] [PubMed] [Google Scholar]
- 27.Gaultier C, Perret L, Boule M, Baculard A, Grimfeld A, Girard F. Occlusion pressure and breathing pattern in children with chronic obstructive pulmonary disease. Bull Eur Physiopathol Respir 1982;18:851–862. [PubMed] [Google Scholar]
- 28.Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med 2004;170:626–632. [DOI] [PubMed] [Google Scholar]
- 29.Reid WD, Belcastro AN. Chronic resistive loading induces diaphragm injury and ventilatory failure in the hamster. Respir Physiol 1999;118:203–218. [DOI] [PubMed] [Google Scholar]
- 30.Lin MC, Ebihara S, El Dwairi Q, Hussain SN, Yang L, Gottfried SB, Comtois A, Petrof BJ. Diaphragm sarcolemmal injury is induced by sepsis and alleviated by nitric oxide synthase inhibition. Am J Respir Crit Care Med 1998;158:1656–1663. [DOI] [PubMed] [Google Scholar]
- 31.Polkey MI, Moxham J. Clinical aspects of respiratory muscle dysfunction in the critically ill. Chest 2001;119:926–939. [DOI] [PubMed] [Google Scholar]
- 32.Hart N, Tounian P, Clement A, Boule M, Polkey MI, Lofaso F, Fauroux B. Nutritional status is an important predictor of diaphragm strength in young patients with cystic fibrosis. Am J Clin Nutr 2004;80:1201–1206. [DOI] [PubMed] [Google Scholar]
- 33.Knook LM, de Kleer IM, van der Ent CK, van der Net JJ, Prakken BJ, Kuis W. Lung function abnormalities and respiratory muscle weakness in children with juvenile chronic arthritis. Eur Respir J 1999;14:529–533. [DOI] [PubMed] [Google Scholar]
- 34.Marini JJ, Smith TC, Lamb V. Estimation of inspiratory muscle strength in mechanically ventilated patients: the measurement of maximal inspiration pressure. J Crit Care 1986;1:32–38. [Google Scholar]
- 35.Multz AS, Aldrich TK, Prezant DJ, Karpel JP, Hendler JM. Maximal inspiratory pressure is not a reliable test of inspiratory muscle strength in mechanically ventilated patients. Am Rev Respir Dis 1990;142:529–532. [DOI] [PubMed] [Google Scholar]
- 36.Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 2003;167:120–127. [DOI] [PubMed] [Google Scholar]
- 37.Manczur TI, Greenough A, Pryor D, Rafferty GF. Assessment of respiratory drive and muscle function in the pediatric intensive care unit and prediction of extubation failure. Pediatr Crit Care Med 2000;1:124–126. [DOI] [PubMed] [Google Scholar]
- 38.Sassoon CS, Te TT, Mahutte CK, Light RW. Airway occlusion pressure. An important indicator for successful weaning in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1987;135:107–113. [DOI] [PubMed] [Google Scholar]
- 39.Kavvadia V, Greenough A, Dimitriou G. Prediction of extubation failure in preterm neonates. Eur J Pediatr 2000;159:227–231. [DOI] [PubMed] [Google Scholar]
- 40.Dimitriou G, Greenough A, Laubscher B. Lung volume measurements immediately after extubation by prediction of “extubation failure” in premature infants. Pediatr Pulmonol 1996;21:250–254. [DOI] [PubMed] [Google Scholar]
- 41.Jubran A, Tobin MJ. Passive mechanics of lung and chest wall in patients who failed or succeeded in trials of weaning. Am J Respir Crit Care Med 1997;155:916–921. [DOI] [PubMed] [Google Scholar]
- 42.Milic-Emili J. Is weaning an art or a science? Am Rev Respir Dis 1986;134:1107–1108. [DOI] [PubMed] [Google Scholar]
- 43.Noizet O, Leclerc F, Sadik A, Grandbastien B, Riou Y, Dorkenoo A, Fourier C, Cremer R, Leteurtre S. Does taking endurance into account improve the prediction of weaning outcome in mechanically ventilated children? Crit Care 2005;9:R798–R807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.