Abstract
γ-Glutamyl transpeptidase (GGT) catalyzes the transfer of the glutamyl moiety from glutathione, and glutathione S-conjugates to acceptors to form another amide or to water to produce free glutamate. Functionally, GGT plays important roles in glutathione homeostasis and mercapturic acid metabolism. The expression of GGT is increased as an adaptive response upon the exposure of oxidative stress. The underlying mechanism of this, however, is nebulous, as GGT gene structure is complex and its transcription is usually controlled by multiple promoters that generate several subtypes of GGT mRNAs. Studies reveal that signaling pathways such as Ras, ERK, p38MAPK, and PI3K are involved in the induction of GGT gene expression in response to oxidative stress. Thus, not surprisingly, induction of GGT mRNA subtypes and the involvement of multiple signaling pathways vary depending on cell type and stimuli.
Keywords: glutathione, signal transduction, oxidative stress, transcription
CLINICAL RELEVANCE
γ-Glutamyl transpeptidase is an important enzyme in the maintenance of the steady-state concentration of glutathione both inside cells and in the extracellular fluids. The enzyme activity is used diagnostically, but abnormal activity, both higher and lower than normal, is a likely contributor in the pathology of diseases in which an oxidative component is involved.
Glutathione (glutamyl-cysteinyl-glycine, GSH) is the most abundant nonprotein thiol in most cells. As a substrate for the glutathione peroxidases (GPx) and glutathione S-transferases (GSTs), GSH plays key roles in protection against oxidative stress and in detoxification/metabolism of endogenous and exogenous compounds, including carcinogens and drugs. In addition, GSH also plays roles in cell cycle regulation, cell signaling, and apoptosis (1–3).
γ-Glutamyl transpeptidase (GGT), a glycosylated protein that is partially embedded into the outer surface of the plasma membrane, catalyzes the transfer of the γ-glutamyl moiety from glutathione or glutathione-conjugates to acceptors like amino acids and dipeptides (4). By breaking down extracellular GSH into its constitutive amino acids, GGT provides cysteine, the rate-limiting amino acid, for GSH de novo synthesis. As such, GGT is critical for maintaining GSH and cysteine homeostasis (5–8), and its deficiency results in oxidative stress and cellular susceptibility to oxidant injury (8, 9). In addition, GGT also catalyzes the metabolism of endogenous compounds such as leukotriene C4 and xenobiotics after their conjugation with GSH (10). In this reaction, GSH conjugates are cleaved by GGT into γ-glutamyl group and cysteinylglycine-conjugates; the latter are further cleaved by peptidases until their final conversion to mercapturic acids and excretion into urine (11, 12). Therefore, GGT plays critical roles in antioxidant defense, detoxification, and inflammation processes. Table 1 summarizes some diseases and pathophysiologic changes associated with GGT. Although the association between increased serum GGT and some of these diseases may be causal, the symptoms and pathologic changes as a result of GGT deficiency clearly indicate that GGT is an indispensable component of the GSH antioxidant and detoxifying systems and may be potentially involved in oxidative stress and inflammation.
TABLE 1.
GGT-ASSOCIATED DISEASES AND/OR PATHOPHYSIOLOGIC CHANGES
| GGT | Associated Changes | Clinical Significance |
|---|---|---|
| Increased serum GGT | Obstructive liver diseases, pancreatitis, liver and pancreatic cancer, chronic alcoholic liver disease (24) | Clinical marker |
| Increased serum GGT | Type II diabetes (75), cardiovascular disease and stroke (76) | Epidemiologic association |
| GGT deficiency | Disrupted glutathione homeostasis, mental retardation and other central nervous system symptoms (70), disrupted LTC4 metabolism (77) | Human |
| GGT deficiency | Disrupted glutathione homeostasis, deficient LTD4 synthesis (78), DNA damage (18), oxidative stress in the lung (8), reproductive defects (79), cataract (80) | Mouse model |
| GGT deficiency |
Attenuated fibrosis in bleomycin-induced pulmonary fibrosis model (20), more airway hyperresponsiveness in asthma model (21) |
Mouse model |
Definition of abbreviation: GGT, γ-glutamyl transpeptidase.
GGT IN THE LUNG
In the lung, GGT is mainly expressed in Clara and type II epithelial cells, while other alveolar space cells, including alveolar macrophages, have undetectable GGT mRNA and protein, although the macrophage appears to adsorb the enzyme from the extracellular fluid (13–15). Epithelial lining fluid (ELF) also expresses high GGT activity, which originates from alveolar type II cells. The ELF GSH content has been reported to be as high as 400 μM in humans (16) and 2 mM in rats (17), and this alveolar GSH pool is not only an extracellular line of antioxidant defense, but also an important cysteine source for alveolar space cells after the cleavage of GSH by GGT. GGT deficiency alters the GSH/GSSG ratio and decreases the content of cysteine and GSH in alveolar space cells, which show signs of oxidative stress even under normal oxygen environment (8, 18). GGT-deficient mice are more susceptible to pulmonary oxygen toxicity than are wild-type mice (7–9, 18, 19) and display distinct inflammatory and fibrotic responses in the lung (20). In addition, mice deficient in GGT (GGT-rel) exhibit more airway hyperreactivity in experimental asthma models (21). All of this evidence strongly suggests that GGT plays important roles in oxidative stress and inflammation-associated pulmonary diseases such as chronic obstructive pulmonary disease (COPD), asthma, and pulmonary fibrosis.
GGT REGULATION
The expression of GGT level is affected by many physiologic and pathologic factors. Elevated serum GGT activity has been generally considered a clinical marker of diseases such as liver diseases, alcohol consumption, stroke, and diabetes mellitus (22–26), and an increase of cellular GGT activity is frequently found in various types of cancers, including lung, liver, prostate, and breast cancers. In the past two decades, studies have established that GGT expression could also be increased upon the exposure to oxidants. Increased GGT expression facilitates the salvage of cysteine from extracellular GSH and export of GSH S-conjugates. From this point of view, elevated GGT expression is an adaptive response to protect against oxidative and toxic stress, as first demonstrated with quinone-induced oxidative stress in epithelial cells (27, 28). Subsequently, GGT induction as an adaptive response was demonstrated in many other studies (8, 9, 29). Despite these important findings, the mechanism of GGT regulation in response to oxidative stress remains unclear. This brief review focuses on the regulation of GGT in response to oxidative/electrophile stress and the possible signaling pathways involved.
GGT GENE STRUCTURE
The gene of GGT has a complex structure, and its expression is usually controlled by several promoters. Full details of the GGT gene structures can be found in previous reviews (30–32). Here, to provide sufficient detail in the context of understanding GGT regulation, the gene structures of rat and human are briefly described.
Rat
In the rat, GGT is a single copy gene and its expression is controlled by five tandemly positioned promoters (P1–P5, numbered from the transcription start site). With alternative splicing, these promoters potentially generate seven transcripts that share the same coding region but different 5′-untranslated regions (5′-UTRs). These transcripts (namely mRNA I, II, III, IV-1, IV-2, V-1, and V-2) are expressed in a tissue-specific manner, and that is believed to be determined by the activation of specific promoters (31, 32). For instance, among the five promoters, P1 and P2 are active in kidney, while P4 was found to be active in intestine and epididymis. Different development/differentiation phases may also affect the activity of specific promoters. P3, P4, and P5, for example, are all active in fetal liver precursor cells; however, P4 and P5 become inactive when these cells differentiate into hepatocytes (33). Although the profile of GGT promoter activity and subtype mRNA expression in some specific tissues or cell types has been described, limited overall information on this is available in most rat tissues and cells.
Human
The GGT gene structure and its expression in human are only partially understood. Human GGT is a multigene family consisting of at least seven GGT genes or pseudogenes (34). It is assumed that the genomic organization of human GGT genes is similar to that of rat; that is, its transcription is also controlled by multiple tandemly positioned promoters. Several human GGT cDNAs have been cloned from hepatoma cells, placenta, lung, and pancreas. These GGT transcripts, defined as type I mRNA, exhibit different 5′-UTRs but share the same coding sequence (35). They are transcribed from a single GGT gene located on chromosome 22 (34). The promoter regulating the transcription of this type of GGT mRNA has been partially studied, and several putative cis-elements, including TRE (12-O-tetradecanoyl-phorbol-13 acetate response element, often referred to as the AP-1 or activating protein 1–binding element), binding sites for activating protein 2 (AP-2) and specificity protein 1 (SP-1), are found in its proximal region (35). The expression of four other human GGT genes has also been demonstrated, and their expression seemed to be tissue specific (34).
A recent study by Heisterkamp and coworkers systematically analyzed the gene structure and the expression of all human GGT-related genes (36). Thirteen homologs of human GGT gene were identified after searching the human genome, and at least six of them appear to be active (to code mRNAs). Among them, GGT 1 and GGT 5 have been found to encode proteins exhibiting GGT activity. The protein products of other active GGT genes, however, have not been demonstrated. Two human GGT genes were found to encode only the light chain. The best characterized GGT gene, GGT 1, is translated into a single protein that can be cleaved into a light and a heavy chain (37, 38). The light chain contains GGT activity while the heavy chain anchors the protein to the plasma membrane. GGT 1 gene was also reported to transcribe the mRNA expression of the light chain in the lung driven by a cryptic promoter present in the introns (39). The protein products of these possible light chain mRNAs, however, have not been elucidated. In general, it remains largely unknown about the expression profile, the gene structure, and the regulatory mechanism of the human GGT genes.
GGT INDUCTION IN RESPONSE TO OXIDATIVE STRESS
The expression of many enzymes in the GSH antioxidant/detoxifying system is increased upon the exposure to oxidative stress, including GCL (rate-limiting enzyme in de novo GSH synthesis), MRPs (transporting GSH/GSSG and GSH S-conjugates out of cells), and GSTs (40–42). As a key enzyme involved in GSH homeostasis and GSH conjugates metabolism, it is unsurprising that GGT is also induced by oxidative stress. Many substances, especially those that generate reactive oxygen/nitrogen species (ROS/RNS) and/or perturb the redox homeostasis, such as aflatoxin B1, NO2, redox cycling quinones, and alcohol, increase GGT expression in various cells and tissues (27, 43–45).
Kugelman and colleagues first demonstrated the induction or increased expression of GGT in response to oxidative stress (27). Menadione, a redox cycling quinone, significantly increased GGT activity and the total mRNA level in rat alveolar L2 cells in a time- and dose-dependent manner. This finding has been confirmed by many studies in rodent models with the use of various agents that are themselves oxidants or produce ROS and electrophiles, such as NO2 (44), hyperoxia (46), ethoxyquin, aflatoxin B1 (43), and hypoxanthine and xanthine oxidase (45). These studies also indicated that the redox regulation of GGT expression occurs at the transcription level.
Compared with that in rodent models, limited studies on the regulation of GGT expression by oxidative stress have been reported in humans. Although it has been known for a long time that human GGT level is increased in oxidative stress–associated pathologic conditions such as alcohol consumption and inflammation (47), or by agents that generate ROS in vivo, such as TNF-α (48) and phorbol 12-myristate 13-acetate (49), little direct evidence has appeared in the literature concerning whether the up-regulation of GGT is via a redox mechanism under these conditions. Ripple and coworkers described the only available evidence that directly links human GGT induction to oxidative stress (50). In their study, GGT activity was increased by H2O2 and menadione in a human prostate carcinoma cell line (LNCap cells). Unfortunately, no other evidence concerning human GGT induction by oxidants has appeared, and so the mechanism remains obscure and therefore it is an area open to future investigation.
DIFFERENTIAL REGULATION OF GGT mRNAS BY OXIDATIVE STRESS
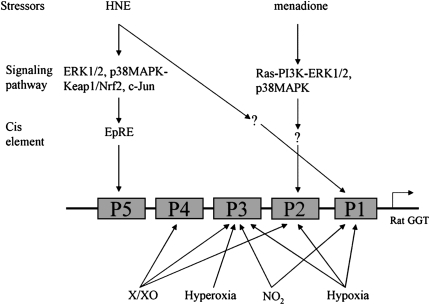
As discussed above, rat GGT usually has several subtypes of mRNAs generated from multiple promoters. Studies have found that the regulation of these different GGT mRNAs (or the response of GGT promoters) by oxidants may be different. Joyce-Brady and colleagues studied the activation of different GGT promoters in response to hypoxia and hyperoxia in rat alveolar type II cells. It was found that upon the exposure of hypoxia (4% O2), GGT P1 and P2 became activated, although normally only P3 was active (51). However, upon the exposure to hyperoxia (40% O2), P3 but not P1 or P2 was activated. Both hypoxia and hyperoxia produce ROS, and their bioeffects are assumed to be associated with ROS. On the other hand, another study from the same group demonstrated that both mRNA I and III (or activation of P1 and P3) were induced upon the exposure to NO2 in rat lung (44). These results suggest that GGT promoters may be regulated via different mechanisms.
The differential response of rat GGT promoters to oxidants was also observed by Markey and colleagues (45). In this study, GGT mRNAs II, III, and IV, but not I, were induced by oxidants generated from hypoxanthine oxidation by xanthine oxidase in rat initial segment of epididymis. The degree of up-regulation of these three mRNAs, however, was different, depending on the ROS concentration and exposure time. For instance, at 6.5 hours of exposure, mRNA II was increased by19% with low ROS exposure versus 74% with high ROS exposure; for mRNA III and IV, the corresponding increase was 70% versus 43%, and 74% versus 74%, respectively. The authors proposed that mRNA II or P2 was the most sensitive to oxidative stress. This study also noticed that only mRNA III could be up-regulated by a system that generated hydroxyl radical (·OH), but other subtypes of mRNAs were not. These findings indicate that the differential regulation of rat GGT promoters is not only associated with the feature of inducers, but also with the oxidant concentration and cell types. This point is further supported by recent studies from our lab (52–54). These studies demonstrated that 4-hydroxynonenal (HNE), a lipid peroxidation product, significantly increased the total GGT mRNA at transcription level in rat alveolar L2 cells. Using primers against each subtype of GGT mRNA, it was shown that HNE only increased the transcription of mRNA I and V-2, but not others (52). GGT mRNA V was widely distributed in rat tissues, and thus it may be involved in the GGT induction in the aforementioned studies, in which the regulation of mRNA V was not investigated.
The differential regulation of the expression of subtypes of GGT mRNA is also observed in humans. Daubeuf and colleagues investigated the regulation of GGT mRNA subtypes by TNF-α, TPA, and sodium butyrate in four human cell lines (HepG2, LNCap, HeLa, and U937), and found the three subtypes of GGT mRNAs investigated (type A, B, and C) were differentially regulated in both a cell- and agent-dependent manner (55). For example, TNF-α and TPA increased but butyrate reduced the expression of type A and C mRNA in HepG2 cells, while in LNCap cells all agents reduced the expression of three subtypes. On the other hand, in HeLa cells, TNF-α reduced type A level, while butyrate increased it. Although redox changes may not be involved in the regulation mechanism, this study reveals the potential of differential regulation of the expression of subtypes of GGT mRNAs (or the activation of corresponding promoters) in oxidative stress–mediated GGT induction in humans.
In summary, available studies suggest that each subtype of GGT mRNAs, which is regulated through a distinct promoter, responds differentially to oxidative stress. The promoter activation depends not only on tissues/cell types, but also on inducing agents and exposure factors. It remains unclear why GGT expression is regulated in such a complex and precise mechanism when one common mechanism would work, further study on this may advance our understanding on the gene regulation under various stress conditions.
SIGNALING PATHWAYS INVOLVED IN OXIDATIVE STRESS–MEDIATED GGT INDUCTION
The activity of many signaling molecules or pathways could be directly or indirectly altered upon oxidative stress. These pathways include but are not limited to phospholipases, isoforms of protein kinase C (PKC), protein tyrosine kinases, protein tyrosine phosphatases, phosphatidylinositol 3-kinase (PI3K), and all three major mitogen-activated protein kinase (MAPK) pathways (56–63). According to the extensive studies in the past decade, the induction of antioxidant/detoxifying genes by oxidative stress is mediated through redox-sensitive pathways, though the mechanism by which these pathways are initially activated remains incompletely understood. The induction of heme oxygenase 1 (HO-1) by oxidative stress, for example, is mediated through MAPKs, PKC-ζ, PI3K, Akt, PKC-δ, and/or PKA, depending on the cell types and inducers (64, 65).
Although it has been known for a long time that GGT is induced by oxidative stress, few studies have investigated the signaling pathways involved (Figure 1). Since total GGT mRNA is usually composed of several subtypes and the regulation of these transcripts by oxidative stress is different, the signaling pathways involved should be specified to a specific subtype of mRNA. Our lab investigated this by determining the signaling pathways involved in HNE-mediated GGT induction. It was found that pretreating cells with either ERK1/2 pathway inhibitor (PD98059) or p38MAPK inhibitor (SB203580) partially decreased GGT mRNA V-2 level induced by HNE in rat alveolar type II (L2) cells. When cells were pretreated with a mixture of both inhibitors, the induction was completely abrogated (54). In contrast, PKC isoforms and PI3K were not involved. This study clearly shows that ERK1/2 and p38MAPK were involved in the HNE-mediated activation of the rat GGT P5 promoter. A recent study by Pandur and coworkers investigated the signaling pathway involved in rat GGT P2 promoter activation by menadione in rat colorectal carcinoma cell line (CC531) (66). They demonstrated that Ras and its several downstream pathways, including PI3K/AKT, ERK1/2, and p38MAPK, were involved in menadione-caused P2 activation. It is hard to draw a conclusion based only on the two studies, but it seems that the signaling pathways involved in the activation of individual GGT promoter by oxidative stress may be different. To define the factors (cell types, inducers, etc.) involved in these differences, however, further research is clearly required.
Figure 1.
Signaling pathways involved in rat γ-glutamyl transpeptidase (GGT) regulation by oxidative stressors X/XO, xanthine/xanthine oxidase. Bottom panel lists only the induction of subtypes of GGT mRNA by oxidative stressors, the signaling pathways involved have not been demonstrated.
cis- AND trans-ELEMENTS INVOLVED IN GGT REGULATION BY OXIDATIVE STRESS
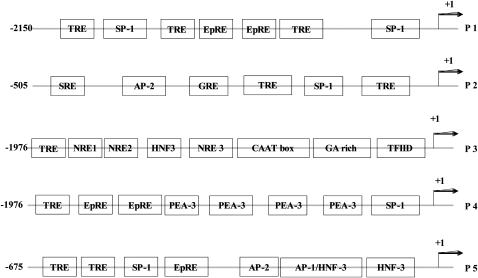
In the past decade, studies have identified many transcription factors (trans-elements) that are redox sensitive, such as c-Jun, Nrf2 (Nuclear erythroid 2 p45-related factor 2), and NF-κB. After activation by oxidants/electrophiles, these transcription factors bind to specific sequences or cis-element in the promoters and increase the expression of target genes. Based on the sequence of known redox-sensitive cis-elements, some putative redox-sensitive cis-elements are located in rat GGT promoters (Figure 2), such as TRE (12-O-tetradecanoyl-phorbol-13 acetate response element) and the electrophile response element (EpRE, often referred to as the ARE or antioxidant response element). Among them, EpRE has gained extensive attention due to its involvement in the regulation of many antioxidant/detoxifying genes. The most well-established EpRE-binding protein (transcription factor) is Nrf2, a protein sequestered in the cytosol under resting conditions mediated by association with Keap1, which assists in the rapid ubiquitination and short half-life of Nrf2 (67). Upon exposure to oxidants/electrophiles, Nrf2 disassociates from Keap1 via redox modification of Keap1 and/or phosphorylation of Nrf2. Nrf2 then translocates to the nucleus, where it forms heterodimers with other proteins and binds to EpRE to enhance gene transcription. Phosphorylation of Nrf2 is also required for translocation, and the protein kinases involved may be activated through redox signaling (68, 69).
Figure 2.
Some putative redox-sensitive DNA cis-elements in the 5′-UTR of rat GGT promoters. AP-2, activating protein 2; GRE, glucocorticoid response element; HNF-3, hepatocyte nuclear factor-3; NRE, nuclear response element; PEA-3, polymavirus enhancer activator-3; SP-1, specificity protein 1; SRE, serum response element; TFIID, transcription initiation factor IID; TRE (AP1 binding site), 12-O-tetradecanoyl-phorbol-13 acetate response element; EpRE, electrophile response element.
In studies on the cis- and trans-elements involved in GGT induction by oxidative stress, Joyce-Brady and colleagues reported that an EpRE motif might be responsible for the rat GGT P3 activation by hyperoxia, since its deletion led to the abrogation of the induction of mRNA III (51). Studies from our lab determined the cis-and trans-elements involved in rat GGT P5 activation by HNE (53, 54). It was found that the EpRE motif located at −109–117 nt upstream of the transcription start site was involved in P5 activation by HNE. Using an in vitro method (supershift or immunodepletion modifications of the electrophoretic mobility shift assay), several transcription factors were identified in the EpRE–protein complex, including Nrf1, Nrf2, Jun-B, c-Jun, Fos-B, c-Fos, Fra1, and Fra2. Among them, the binding of Nrf2 and c-Jun to EpRE was confirmed with chromatin immunoprecipitation (ChIP) assay (54). This clearly shows the critical role of Nrf2/EpRE in HNE-mediated activation of rat GGT P5. However, in contrast with the finding of Joyce-Brady and coworkers that EpRE was possibly involved in GGT P3 activation by hyperoxia (51), P3 was not activated in our study. This apparent inconsistency likely reflects the complexity of GGT regulation mechanism and suggests that its promoter activity may be regulated in both cell- and inducing agent–dependent manners. There have been no reports concerning the cis-elements or transcription factors involved in the oxidative stress–mediated activation of rat GGT P1, P2, and P4. Considering the potential difference in the regulation of rat GGT promoters as discussed above, it is expected that cis-elements and transcription factors other than EpRE and Nrf2 might be involved in the redox induction of P1, P2, and P4.
There is also little direct evidence available on the cis- and trans-elements involved in the redox regulation of human GGT genes. However, some reports provided clues of the potential involvement of some cis- and trans-elements in human GGT regulation. Daubeuf and colleagues reported that human GGT (GGT 1) was up-regulated by phorbol 12-myristate 13-acetate (PMA) in Hela cells through the AP-1–binding site at −214/−225 nt upstream of the transcription start site, and c-Jun was part of the AP-1 complex (49). Reuter and coworkers found that NF-κB–binding site located at −110/−119 on the 5′-UTR was responsible for TNF-α–induced GGT induction in a human leukemia cell line (48). Studying whether human GGT is induced in a redox-sensitive manner and determining the underlying mechanism are future challenges in GGT regulation research.
FUTURE PERSPECTIVE
It would be of great interest to know how GGT is involved in diseases associated with oxidative stress and inflammation, such as pulmonary fibrosis, asthma, and Alzheimer's disease. GGT knockout mice and the human subjects with GGT deficiency or single nucleotide polymorphism (SNP) would be good models for such studies. Fortunately, the human GGT deficiency, which is associated with mental retardation, is apparently very rare and only several cases have been reported (70–74), while there is no report of SNPs for human GGT so far. Another important aspect for future study is the regulation of GGT genes, especially in humans. This would include the tissue-/cell-specific expression of each GGT gene and its regulation under various conditions such as oxidative stress. Although it has been well established in rat models, direct evidence of GGT induction in humans in response to oxidative stress is still lacking. Knowledge on which human GGT genes are regulated, what signaling pathways are involved, and what is the expression profile of different GGT transcripts in responsive to oxidative stress would help in understanding how GGT is involved in normal physiology and diseases and potentially facilitate developing novel therapeutic or preventive strategies against these diseases.
Acknowledgments
The authors thank current and former members of the laboratory, Ewa Rajpert-DeMeyts, Min Chang, Rui-Ming Liu, Amir Kugelman, Dale Dickinson, Karen Iles, Mike Shi, Tim Robison, Jinah Choi, and Evelyn Gozal and collaborators, Victor Darley-Usmar, Julie Andersen, Yannick Laperche, Cecelia Giulivi, Nora Heisterkamp, and John Groffen, for their contributions to our investigations into the regulation of γ-glutamyl transpeptidase.
The work cited from our laboratory in this review was supported by ES05511 from the National Institute of Environmental Health Sciences, National Institutes of Health.
Originally Published in Press as DOI: 10.1165/rcmb.2009-0169TR on August 14, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med 2007;43:883–898. [DOI] [PubMed] [Google Scholar]
- 2.Circu ML, Aw TY. Glutathione and apoptosis. Free Radic Res 2008;42:689–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pallardo FV, Markovic J, Garcia JL, Vina J. Role of nuclear glutathione as a key regulator of cell proliferation. Mol Aspects Med 2009;30:77–85. [DOI] [PubMed] [Google Scholar]
- 4.Tate SS, Meister A. Gamma-glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem 1981;39:357–368. [DOI] [PubMed] [Google Scholar]
- 5.Meister A. On the enzymology of amino acid transport. Science 1973;180:33–39. [DOI] [PubMed] [Google Scholar]
- 6.Hanigan MH, Ricketts WA. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry 1993;32:6302–6306. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman MW, Wiseman AL, Shi ZZ, Carter BZ, Barrios R, Ou CN, Chevez-Barrios P, Wang Y, Habib GM, Goodman JC, et al. Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci USA 1996;93:7923–7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jean JC, Liu Y, Brown LA, Marc RE, Klings E, Joyce-Brady M. Gamma-glutamyl transferase deficiency results in lung oxidant stress in normoxia. Am J Physiol Lung Cell Mol Physiol 2002;283:L766–L776. [DOI] [PubMed] [Google Scholar]
- 9.Barrios R, Shi ZZ, Kala SV, Wiseman AL, Welty SE, Kala G, Bahler AA, Ou CN, Lieberman MW. Oxygen-induced pulmonary injury in gamma-glutamyl transpeptidase-deficient mice. Lung 2001;179:319–330. [DOI] [PubMed] [Google Scholar]
- 10.Paolicchi A, Sotiropuolou M, Perego P, Daubeuf S, Visvikis A, Lorenzini E, Franzini M, Romiti N, Chieli E, Leone R, et al. Gamma-glutamyl transpeptidase catalyses the extracellular detoxification of cisplatin in a human cell line derived from the proximal convoluted tubule of the kidney. Eur J Cancer 2003;39:996–1003. [DOI] [PubMed] [Google Scholar]
- 11.Jakoby WB. Enzymatic basis of detoxication. New York: Academic Press; 1980.
- 12.Soleo L, Strzelczyk R. [Xenobiotics and glutathione.] G Ital Med Lav Ergon 1999;21:302–308. [Italian] [PubMed] [Google Scholar]
- 13.Oakes SM, Takahashi Y, Williams MC, Joyce-Brady M. Ontogeny of gamma-glutamyltransferase in the rat lung. Am J Physiol 1997;272:L739–L744. [DOI] [PubMed] [Google Scholar]
- 14.Joyce-Brady M, Takahashi Y, Oakes SM, Rishi AK, Levine RA, Kinlough CL, Hughey RP. Synthesis and release of amphipathic gamma-glutamyl transferase by the pulmonary alveolar type 2 cell. Its redistribution throughout the gas exchange portion of the lung indicates a new role for surfactant. J Biol Chem 1994;269:14219–14226. [PubMed] [Google Scholar]
- 15.Forman HJ, Skelton DC. Protection of alveolar macrophages from hyperoxia by gamma-glutamyl transpeptidase. Am J Physiol 1990;259:L102–L107. [DOI] [PubMed] [Google Scholar]
- 16.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol 1987;63:152–157. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland MWGM, Nelson J, Lyen Y, Forman HJ. Oxygen toxicity: Loss of lung macrophage function without metabolite depletion. J Free Radic Biol Med 1985;1:209–214. [DOI] [PubMed] [Google Scholar]
- 18.Rojas E, Valverde M, Kala SV, Kala G, Lieberman MW. Accumulation of DNA damage in the organs of mice deficient in gamma- glutamyltranspeptidase. Mutat Res 2000;447:305–316. [DOI] [PubMed] [Google Scholar]
- 19.Jean JC, Harding CO, Oakes SM, Yu Q, Held PK, Joyce-Brady M. Gamma-glutamyl transferase (GGT) deficiency in the GGTenu1 mouse results from a single point mutation that leads to a stop codon in the first coding exon of GGT mRNA. Mutagenesis 1999;14:31–36. [DOI] [PubMed] [Google Scholar]
- 20.Pardo A, Ruiz V, Arreola JL, Ramirez R, Cisneros-Lira J, Gaxiola M, Barrios R, Kala SV, Lieberman MW, Selman M. Bleomycin-induced pulmonary fibrosis is attenuated in gamma-glutamyl transpeptidase-deficient mice. Am J Respir Crit Care Med 2003;167:925–932. [DOI] [PubMed] [Google Scholar]
- 21.Han B, Luo G, Shi ZZ, Barrios R, Atwood D, Liu W, Habib GM, Sifers RN, Corry DB, Lieberman MW. Gamma-glutamyl leukotrienase, a novel endothelial membrane protein, is specifically responsible for leukotriene D(4) formation in vivo. Am J Pathol 2002;161:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wannamethee G, Ebrahim S, Shaper AG. Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol 1995;142:699–708. [DOI] [PubMed] [Google Scholar]
- 23.Hanigan MH. Gamma-glutamyl transpeptidase, a glutathionase: its expression and function in carcinogenesis. Chem Biol Interact 1998;111–112:333–342. [DOI] [PubMed]
- 24.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci 2001;38:263–355. [DOI] [PubMed] [Google Scholar]
- 25.Bots ML, Salonen JT, Elwood PC, Nikitin Y, Freire de Concalves A, Inzitari D, Sivenius J, Trichopoulou A, Tuomilehto J, Koudstaal PJ, et al. Gamma-glutamyltransferase and risk of stroke: the EUROSTROKE project. J Epidemiol Community Health 2002;56:i25–i29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emdin M, Passino C, Donato L, Paolicchi A, Pompella A. Serum gamma-glutamyltransferase as a risk factor of ischemic stroke might be independent of alcohol consumption. Stroke 2002;33:1163–1164. [DOI] [PubMed] [Google Scholar]
- 27.Kugelman A, Choy HA, Liu R, Shi MM, Gozal E, Forman HJ. Gamma-glutamyl transpeptidase is increased by oxidative stress in rat alveolar l2 epithelial cells. Am J Respir Cell Mol Biol 1994;11:586–592. [DOI] [PubMed] [Google Scholar]
- 28.Liu RM, Hu H, Robison TW, Forman HJ. Differential enhancement of gamma-glutamyl transpeptidase and gamma- glutamylcysteine synthetase by tert-butylhydroquinone in rat lung epithelial l2 cells. Am J Respir Cell Mol Biol 1996;14:186–191. [DOI] [PubMed] [Google Scholar]
- 29.Liu RM, Hu H, Robison TW, Forman HJ. Increased gamma-glutamylcysteine synthetase and gamma-glutamyl transpeptidase activities enhance resistance of rat lung epithelial l2 cells to quinone toxicity. Am J Respir Cell Mol Biol 1996;14:192–197. [DOI] [PubMed] [Google Scholar]
- 30.Pawlak A, Lahuna O, Bulle F, Suzuki A, Ferry N, Siegrist S, Chikhi N, Chobert MN, Guellaen G, Laperche Y. Gamma-glutamyl transpeptidase: a single copy gene in the rat and a multigene family in the human genome. J Biol Chem 1988;263:9913–9916. [PubMed] [Google Scholar]
- 31.Taniguchi N, Ikeda Y. Gamma-glutamyl transpeptidase: catalytic mechanism and gene expression. Adv Enzymol Relat Areas Mol Biol 1998;72:239–278. [DOI] [PubMed] [Google Scholar]
- 32.Chikhi N, Holic N, Guellaen G, Laperche Y. Gamma-glutamyl transpeptidase gene organization and expression: a comparative analysis in rat, mouse, pig and human species. Comp Biochem Physiol B Biochem Mol Biol 1999;122:367–380. [DOI] [PubMed] [Google Scholar]
- 33.Holic N, Suzuki T, Corlu A, Couchie D, Chobert MN, Guguen-Guillouzo C, Laperche Y. Differential expression of the rat gamma-glutamyl transpeptidase gene promoters along with differentiation of hepatoblasts into biliary or hepatocytic lineage. Am J Pathol 2000;157:537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Courtay C, Heisterkamp N, Siest G, Groffen J. Expression of multiple gamma-glutamyltransferase genes in man. Biochem J 1994;297:503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visvikis A, Pawlak A, Accaoui MJ, Ichino K, Leh H, Guellaen G, Wellman M. Structure of the 5′ sequences of the human gamma-glutamyltransferase gene. Eur J Biochem 2001;268:317–325. [DOI] [PubMed] [Google Scholar]
- 36.Heisterkamp N, Groffen J, Warburton D, Sneddon TP. The human gamma-glutamyltransferase gene family. Hum Genet 2008;123:321–332. [DOI] [PubMed] [Google Scholar]
- 37.Nash B, Tate SS. Biosynthesis of rat renal gamma-glutamyl transpeptidase: evidence for a common precursor of the two subunits. J Biol Chem 1982;257:585–588. [PubMed] [Google Scholar]
- 38.Barouki R, Finidori J, Chobert MN, Aggerbeck M, Laperche Y, Hanoune J. Biosynthesis and processing of gamma-glutamyl transpeptidase in hepatoma tissue culture cells. J Biol Chem 1984;259:7970–7974. [PubMed] [Google Scholar]
- 39.Leh H, Chikhi N, Ichino K, Guellaen G, Wellman M, Siest G, Visvikis A. An intronic promoter controls the expression of truncated human gamma-glutamyltransferase mrnas. FEBS Lett 1998;434:51–56. [DOI] [PubMed] [Google Scholar]
- 40.Yamane Y, Furuichi M, Song R, Van NT, Mulcahy RT, Ishikawa T, Kuo MT. Expression of multidrug resistance protein/gs-x pump and gamma-glutamylcysteine synthetase genes is regulated by oxidative stress. J Biol Chem 1998;273:31075–31085. [DOI] [PubMed] [Google Scholar]
- 41.Borud O, Mortensen B, Mikkelsen IM, Leroy P, Wellman M, Huseby NE. Regulation of gamma-glutamyltransferase in cisplatin-resistant and -sensitive colon carcinoma cells after acute cisplatin and oxidative stress exposures. Int J Cancer 2000;88:464–468. [PubMed] [Google Scholar]
- 42.Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic Biol Med 2002;33:974. [DOI] [PubMed] [Google Scholar]
- 43.Griffiths SA, Good VM, Gordon LA, Hudson EA, Barrett MC, Munks RJ, Manson MM. Characterization of a promoter for gamma-glutamyl transpeptidase activated in rat liver in response to aflatoxin b1 and ethoxyquin. Mol Carcinog 1995;14:251–262. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi Y, Oakes SM, Williams MC, Takahashi S, Miura T, Joyce-Brady M. Nitrogen dioxide exposure activates gamma-glutamyl transferase gene expression in rat lung. Toxicol Appl Pharmacol 1997;143:388–396. [DOI] [PubMed] [Google Scholar]
- 45.Markey CM, Rudolph DB, Labus JC, Hinton BT. Oxidative stress differentially regulates the expression of gamma-glutamyl transpeptidase mrnas in the initial segment of the rat epididymis. J Androl 1998;19:92–99. [PubMed] [Google Scholar]
- 46.Knickelbein RG, Ingbar DH, Seres T, Snow K, Johnston RB Jr, Fayemi O, Gumkowski F, Jamieson JD, Warshaw JB. Hyperoxia enhances expression of gamma-glutamyl transpeptidase and increases protein S-glutathiolation in rat lung. Am J Physiol 1996;270:L115–L122. [DOI] [PubMed] [Google Scholar]
- 47.Emdin M, Passino C, Pompella A, Paolicchi A. Serum gamma-glutamyl transpeptidase: a prognostic marker in cardiovascular diseases. Biofactors 2003;17:199–205. [DOI] [PubMed] [Google Scholar]
- 48.Reuter S, Schnekenburger M, Cristofanon S, Buck I, Teiten MH, Daubeuf S, Eifes S, Dicato M, Aggarwal BB, Visvikis A, et al. Tumor necrosis factor alpha induces gamma-glutamyltransferase expression via nuclear factor-kappab in cooperation with SP1. Biochem Pharmacol 2009;77:397–411. [DOI] [PubMed] [Google Scholar]
- 49.Daubeuf S, Duvoix A, Wellman-Rousseau M, Diederich M, Visvikis A. Phorbol ester regulation of the human gamma-glutamyltransferase gene promoter. Biochem Biophys Res Commun 2004;313:300–307. [DOI] [PubMed] [Google Scholar]
- 50.Ripple MO, Henry WF, Rago RP, Wilding G. Prooxidant-antioxidant shift induced by androgen treatment of human prostate carcinoma cells. J Natl Cancer Inst 1997;89:40–48. [DOI] [PubMed] [Google Scholar]
- 51.Joyce-Brady M, Oakes SM, Wuthrich D, Laperche Y. Three alternative promoters of the rat gamma-glutamyl transferase gene are active in developing lung and are differentially regulated by oxygen after birth. J Clin Invest 1996;97:1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H, Dickinson DA, Liu RM, Forman HJ. 4-hydroxynonenal increases gamma-glutamyl transpeptidase gene expression through mitogen-activated protein kinase pathways. Free Radic Biol Med 2005;38:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Liu H, Dickinson DA, Liu RM, Postlethwait EM, Laperche Y, Forman HJ. Gamma-glutamyl transpeptidase is induced by 4-hydroxynonenal via epre/nrf2 signaling in rat epithelial type ii cells. Free Radic Biol Med 2006;40:1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang H, Liu H, Iles KE, Liu RM, Postlethwait EM, Laperche Y, Forman HJ. 4-hydroxynonenal induces rat gamma-glutamyl transpeptidase through mitogen-activated protein kinase-mediated electrophile response element/nuclear factor erythroid 2-related factor 2 signaling. Am J Respir Cell Mol Biol 2006;34:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daubeuf S, Accaoui MJ, Pettersen I, Huseby NE, Visvikis A, Galteau MM. Differential regulation of gamma-glutamyltransferase mRNAs in four human tumour cell lines. Biochim Biophys Acta 2001;1568:67–73. [DOI] [PubMed] [Google Scholar]
- 56.Burdon RH. Oxyradicals as signal transducers. In: Forman HJ, Cadenas E, editors. Oxydative stress and signal transduction. New York: Chapman & Hall; 1997. pp. 289–322.
- 57.Claiborne A, Yeh JI, Mallett TC, Luba J, Crane EJ III, Charrier V, Parsonage D. Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry 1999;38:15407–15416. [DOI] [PubMed] [Google Scholar]
- 58.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med 2000;28:1349–1361. [DOI] [PubMed] [Google Scholar]
- 59.Nakashima I, Kato M, Akhand AA, Suzuki H, Takeda K, Hossain K, Kawamoto Y. Redox-linked signal transduction pathways for protein tyrosine kinase activation. Antioxid Redox Signal 2002;4:517–531. [DOI] [PubMed] [Google Scholar]
- 60.Aslan M, Ozben T. Oxidants in receptor tyrosine kinase signal transduction pathways. Antioxid Redox Signal 2003;5:781–788. [DOI] [PubMed] [Google Scholar]
- 61.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors 2003;17:287–296. [DOI] [PubMed] [Google Scholar]
- 62.Barford D. The role of cysteine residues as redox-sensitive regulatory switches. Curr Opin Struct Biol 2004;14:679–686. [DOI] [PubMed] [Google Scholar]
- 63.Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys 2008;477:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 2006;86:583–650. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Forman HJ. Acrolein induces heme oxygenase-1 through PKC-delta and PI3K in human bronchial epithelial cells. Am J Respir Cell Mol Biol 2008;38:483–490. [DOI] [PubMed] [Google Scholar]
- 66.Pandur S, Pankiv S, Johannessen M, Moens U, Huseby NE. Gamma-glutamyltransferase is upregulated after oxidative stress through the Ras signal transduction pathway in rat colon carcinoma cells. Free Radic Res 2007;41:1376–1384. [DOI] [PubMed] [Google Scholar]
- 67.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor NRF2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem 2003;278:21592–21600. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating nrf2 activation in response to chemical stress. Free Radic Biol Med 2004;37:433–441. [DOI] [PubMed] [Google Scholar]
- 69.Giudice A, Montella M. Activation of the NRF2-are signaling pathway: a promising strategy in cancer prevention. Bioessays 2006;28:169–181. [DOI] [PubMed] [Google Scholar]
- 70.Iida M, Yasuhara T, Mochizuki H, Takakura H, Yanagisawa T, Kubo H. Two Japanese brothers with hereditary gamma-glutamyl transpeptidase deficiency. J Inherit Metab Dis 2005;28:49–55. [DOI] [PubMed] [Google Scholar]
- 71.Schulman JD, Goodman SI, Mace JW, Patrick AD, Tietze F, Butler EJ. Glutathionuria: inborn error of metabolism due to tissue deficiency of gamma-glutamyl transpeptidase. Biochem Biophys Res Commun 1975;65:68–74. [DOI] [PubMed] [Google Scholar]
- 72.Wright EC, Stern J, Ersser R, Patrick AD. Glutathionuria: gamma-glutamyl transpeptidase deficiency. J Inherit Metab Dis 1980;2:3–7. [DOI] [PubMed] [Google Scholar]
- 73.Goodman SI, Mace JW, Pollack S. Serum gamma-glutamyl transpeptidase deficiency. Lancet 1971;1:234–235. [DOI] [PubMed] [Google Scholar]
- 74.Hammond JW, Potter M, Wilcken B, Truscott R. Siblings with gamma-glutamyltransferase deficiency. J Inherit Metab Dis 1995;18:82–83. [DOI] [PubMed] [Google Scholar]
- 75.Lee DH, Silventoinen K, Jacobs DR Jr, Jousilahti P, Tuomileto J. Gamma-glutamyltransferase, obesity, and the risk of type 2 diabetes: Observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab 2004;89:5410–5414. [DOI] [PubMed] [Google Scholar]
- 76.Whitfield JB, Zhu G, Nestler JE, Heath AC, Martin NG. Genetic covariation between serum gamma-glutamyltransferase activity and cardiovascular risk factors. Clin Chem 2002;48:1426–1431. [PubMed] [Google Scholar]
- 77.Mayatepek E, Okun JG, Meissner T, Assmann B, Hammond J, Zschocke J, Lehmann WD. Synthesis and metabolism of leukotrienes in gamma-glutamyl transpeptidase deficiency. J Lipid Res 2004;45:900–904. [DOI] [PubMed] [Google Scholar]
- 78.Shi ZZ, Han B, Habib GM, Matzuk MM, Lieberman MW. Disruption of gamma-glutamyl leukotrienase results in disruption of leukotriene D(4) synthesis in vivo and attenuation of the acute inflammatory response. Mol Cell Biol 2001;21:5389–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar TR, Wiseman AL, Kala G, Kala SV, Matzuk MM, Lieberman MW. Reproductive defects in gamma-glutamyl transpeptidase-deficient mice. Endocrinology 2000;141:4270–4277. [DOI] [PubMed] [Google Scholar]
- 80.Chevez-Barrios P, Wiseman AL, Rojas E, Ou CN, Lieberman MW. Cataract development in gamma-glutamyl transpeptidase-deficient mice. Exp Eye Res 2000;71:575–582. [DOI] [PubMed] [Google Scholar]