Abstract
Signaling by Wnt/β-catenin regulates self-renewal of tissue stem cells in the gut and, when activated in the embryonic bronchiolar epithelium, leads to stem cell expansion. We have used transgenic and cell type–specific knockout strategies to determine roles for β-catenin–regulated gene expression in normal maintenance and repair of the bronchiolar epithelium. Analysis of TOPGal transgene activity detected β-catenin signaling in the steady-state and repairing bronchiolar epithelium. However, the broad distribution and phenotype of signaling cells precluded establishment of a clear role for β-catenin in the normal or repairing state. Necessity of β-catenin signaling was tested through Cre-mediated deletion of Catnb exons 2–6 in airway epithelial cells. Functional knockout of β-catenin had no impact on expression of Clara cell differentiation markers, mitotic index, or sensitivity of these cells to the Clara cell–specific toxicant, naphthalene. Repair of the naphthalene-injured airway proceeded with establishment of focal regions of β-catenin–null epithelium. The size of regenerative epithelial units, mitotic index, and restoration of the ciliated cell population did not vary between wild-type and genetically modified mice. Thus, β-catenin was not necessary for maintenance or efficient repair of the bronchiolar epithelium.
Keywords: injury, repair, β-catenin, progenitor
CLINICAL RELEVANCE
These studies help define signaling pathways that regulate airway repair and might be exploited to manipulate epithelial repair capacity.
The epithelium lining conducting airways is maintained by progenitor cells that can be hierarchically organized based upon their sensitivity or resistance to toxicants (1). Clara cells represent an abundant population of facultative progenitor cells that contribute to epithelial maintenance and repair after injury to ciliated cells (2, 3). In contrast, bronchiolar stem cells repair airways after naphthalene-induced ablation of Clara cells. Bronchiolar stem cells are located adjacent to neuroepithelial bodies and at the bronchoalveolar duct junction, have the capacity to generate Clara and ciliated cells, and proliferate infrequently in the steady state (4–6). In this regard, airway stem cells share properties of adult tissue stem cells that have been defined within other organs, such as the skin, intestine, liver, and cornea (7–11). However, even though stem cells of the airway epithelium have been spatially localized and some insight gained regarding their molecular phenotype (4–6, 12), relatively little is known of mechanisms regulating their maintenance, activation, and differentiation. Our recent demonstration that potentiation of canonical Wnt signaling in the airway epithelium leads to niche-independent expansion of undifferentiated cells with characteristics of the bronchiolar stem cell raises questions regarding roles for Wnt signaling in regulation of airway repair and stem cell maintenance in the adult airway (13).
Phenotypic analysis of transgenic and knockout mouse models have revealed a central role for β-catenin signaling in the regulation of tissue regeneration and stem cell fate within a number of organs (14–21). Nuclear translocation of β-catenin and downstream target gene activation has been shown to contribute to self-renewal of hematopoietic stem cells (18). Furthermore, lymphoid enhancing factor 1 (Lef1) and T cell factor (TCF) 4, both of which function as heterodimerization partners for β-catenin, have been shown to play central roles in the regulation of stem and transit-amplifying (TA) cell populations within the intestinal crypt (15, 17, 21). Similar observations have been made within the epidermal stem cell niche, where Wnt/β-catenin signaling regulates appropriate follicle-associated proliferation and TA cell migration (14, 22–25). Activation of downstream target genes through generation of a functional β-catenin/TCF heterodimeric transcription factor has been visualized in vivo using transgenic reporter lines in which reporter genes have been placed downstream from promoter elements harboring canonical TCF cis elements. Results of these studies demonstrated increases in TCF-optimized promoter-LacZ (TOPGal) activity in cells with either natural or ectopic elevation of β-catenin signaling (22). Activation of β-catenin reporter transgenes during the process of lung development and in response to injury suggests that the Wnt/β-catenin signaling pathway may regulate epithelial cell behavior in mature airways (13, 26, 27). In addition to roles for β-catenin signaling in normal tissue maintenance, its dysregulation through mutation of key regulatory components of the pathway has been demonstrated in a range of cancers, including numerous epidermal carcinomas and the majority of colon cancers (21, 24, 28–31). Together, these data highlight the widespread involvement of β-catenin signaling in regulating aspects of stem cell–associated proliferation, differentiation, and migration in tissue maintenance and repair.
The purpose of this study was to characterize the temporal and spatial pattern of β-catenin signaling in normal and progenitor cell–depleted airways, and to determine whether β-catenin signaling was necessary for either normal maintenance or repair of the naphthalene-injured epithelium. Our results indicate that β-catenin is activated in the steady-state and repairing bronchiolar epithelium, but that β-catenin is not necessary for either of these processes. Overall, our data support the conclusion that proliferation and differentiation of adult bronchiolar stem/progenitor cells is regulated by a β-catenin–independent signaling mechanism.
MATERIALS AND METHODS
Animal Husbandry
Colonies of either wild-type or genetically modified mice were maintained as in-house breeding colonies under specific pathogen–free conditions in an Association for Assessment and Accreditation of Laboratory Animal Care–accredited facility either at the University of Pittsburgh or Duke University. Health status was monitored quarterly using comprehensive serology screens performed on cohoused sentinel mice. All experimental and breeding animals were maintained on a 12-hour light/dark cycle and given access to food and water ad libitum. TOPGal(B6)-congenic mice were generated by back-crossing transgene-positive mice to C57Bl/6 wild-type mice through 10 generations. Mice used in naphthalene experiments were between 8 and 12 weeks of age, and weighed between 25 and 35 g. All procedures involving animal models were approved either by the University of Pittsburgh or Duke University institutional animal care and use committees.
Genotyping
Genotype was determined by PCR amplification of genomic DNA prepared from a 1-cm tail biopsy. TOPGal transgenic mice were evaluated using primers specific for the Escherichia coli LacZ coding sequence: forward primer, 5′-GTGGCAGCATCAGGGGAAAACCTT-3′ and reverse primer, 5′-GAATTCCGCCGATACTGACGGGCT-3′, and resulted in an amplicon of 476 bp. Mice harboring the CCSP-Cre transgene were identified using primers specific for bacteriophage P1 Cre recombinase: forward primer, 5′-GGACATGTTCAGGGATCGCCAGGCG-3′ and reverse primer, 5′-GCATAACCAGTGAAACAGCATTGCTG-3′, and gave a band of 267 bp. Homozygous Catnbflox(E2–6) mice were identified by PCR amplification of the wild-type and the floxed exon 2–6 alleles using primer pairs that detected the loxP modification. Primers for this analysis were: forward primer, 5′-AAGGTAGAGTGATGAAAGTTGTT-3′ and reverse primer, 5′-CACCATGTCCTCTGTCTATTC-3′. The wild-type allele resulted in a 221-bp amplicon, and the floxed exon 2–6 allele gave a 324-bp amplicon. Mice heterozygous and homozygous for the Catnbflox(E3) allele were distinguished by PCR amplification of the wild-type and modified alleles. Primers for the wild-type allele were: forward primer, 5′-GGT AGG TGA AGC TCA GCG CAG AGC-3′ and reverse primer, 5′-ACG TGT GGC AAG TTC CGC GTC ATC C-3′, and resulted in a 900-bp amplicon; the modified in allele did not yield a product (predicted length, 2.1 kb) with this primer pair. The Catnbflox(E3) allele was detected through analysis of the knocked-in neo resistance cassette. Primers were: forward primer, 5′-GAA CAA GAT GGA TTG CAC GC-3′ and reverse primer, 5′-AGG AGC AAG GTG AGA TGA CA-3′, and resulted in a 321-bp amplicon.
Naphthalene Administration
Naphthalene administration was performed as previously described (32). Naphthalene doses for wild-type littermates and genetically modified mice were: TOPGal(B6) congenic, 275 mg/kg body weight; and CCSP-Cre/Catnbflox(E2–6) (referred to as DE2–6 mice), 250 mg/kg body weight.
Bromodeoxyurindine Administration
Proliferating cells were continuously labeled with bromodeoxyuridine (BrdU; Sigma, St. Louis, MO) using a 14-d mini-osmotic pump and a delivery rate of 0.5 μl/hour (Alzet 2002; Durect, Cupertino, CA). Pumps were charged with sterile 20 mg/ml BrdU in normal saline, and primed for 2 hours at 37°C in sterile 1× PBS. Pumps were implanted subcutaneously 24 hours after naphthalene exposure. In some experiments, proliferating cells were continuously labeled by repeated intraperitoneal injection of BrdU (50 mg/kg body weight) at 12-hour intervals, starting immediately after naphthalene treatment and continuing through the 10-day recovery time point. The method of BrdU delivery did not result in appreciable differences in labeling index.
Tissue Recovery
Animals were killed by injection of 2.5% avertin to achieve a surgical plane of anesthesia, followed by exsanguination. For adult mice, tracheas were canulated and left lobes were removed and homogenized in 2.5 ml 4 M guanidinium isothiocyanate containing 0.7% 2-mercaptoethanol for RNA analysis. For standard light microscopy, right lung lobes were inflation fixed in situ for 10 minutes with 10% neutral buffered formalin (NBF) at 10 cm water pressure, removed, and immersion fixed in 10% NBF for an additional 20 minutes (for detection of β-gal activity) or 2 hours (for immunostaining). Formalin-fixed tissue was extensively washed in PBS, embedded in paraffin, and processed as indicated below for specific staining procedures. Total lung RNA was isolated from neonatal and juvenile mice (i.e., before 2 months of age) using methods detailed above.
β-gal Histochemical Detection
Accessory lobes were equilibrated to β-gal staining solution: 5 mM K3Fe(CN)3, 5 mM K4Fe(CN)6, 2 mM MgCl2, 0.02% Nonidet P-40, 0.01% sodium deoxycholate, 1× PBS, and 1 mg/ml X-gal. β-gal detection was performed in the dark at 37°C for 6 hours. These methods were optimized for uniform X-gal staining of lung tissue from ROSA26-LacZ mice to ensure complete penetration of staining solutions. After β-gal detection, tissues were washed in PBS to remove residual X-gal staining solution and postfixed for 16 hours in 10% NBF at 4°C. X-gal–stained accessory lobes were microdissected to expose the lumen of intrapulmonary conducting airways and minor daughters. The surface of the airway epithelium was examined for the presence of X-gal–stained (blue) cells using a dissecting microscope, and representative images were obtained. X-gal–stained cardiac lobes were cryoprotected, embedded in paraffin, and sectioned at 5 μm to reveal the major axial pathway as well as numerous airway terminal bronchioles.
Immunohistochemistry
Adjacent serial sections were rapidly cleared with three changes of xylenes, hydrated through graded ethanol solutions (100–70%), equilibrated to water, and quenched with 3% peroxide for 20 minutes. Sections were blocked with PBS/0.5% BSA (blocking solution) for 20 minutes and incubated with rabbit anti-CCSP (1:32,000), mouse IgG2b–anti-acetylated tubulin (1:10,000), or blocking solution overnight at 4°C. Sections were washed extensively in 1× PBS and incubated with biotinylated goat anti-rabbit Ig (1:6,000 in blocking solution) or goat anti-mouse IgG2b in blocking buffer for 1 hour at room temperature. Sections were washed and further incubated with streptavidin–horseradish peroxidase (1:4,000) in PBS. Antigen–antibody complexes were detected using a diaminobenzidine substrate detection kit (Vector Laboratories, Burlingame, CA). Images were obtained using an Olympus Provis AX70 microscope (Center Valley, PA) equipped with a digital camera and processed using Image-Pro Plus (Media Cybernetics, Silver Spring, MD) and Adobe Photoshop (San Jose, CA).
Immunofluorescence
Sections were prepared as indicated above. Antigens were retrieved through equilibration of sections to 10 mM citrate buffer (pH 6.0), microwaving for 20 minutes, and passive cooling to 4°C. Sections were blocked with 5% BSA/PBS before application of primary antibodies. Primary antibodies were diluted in 5% BSA/PBS and used at the concentrations listed in Table 1. Slides were incubated overnight at 4°C, washed, and secondary antibodies applied for 0.5 hour at room temperature. Forkhead box J1 (FoxJ1) signal was enhanced by application of 0.5% Sudan black in 70% ethanol (SU121; Spectrum, Gardena, CA) for 5 minutes, followed by extensive washing in PBS. Slides were coverslipped using Fluoromount-G containing 2 μg/ml 4′,6′-diamidino-2-phenylindole (Sigma). Antigen–antibody complexes were visualized as single optical planes using an Olympus AX70 microscope. Images were obtained as indicated above.
TABLE 1.
ANTIBODIES
| Antigen | Host | Source | Dilution | Antigen Retrieval |
|---|---|---|---|---|
| CCSP | Rabbit | In-house | 1:10,000 | No |
| Goat | In-house | 1:10,000 | No | |
| FoxJ1 | Mouse IgG1 | Dr. Steven Brody, Washington University | 1:2,000 | Yes |
| BrdU | Rat | Accurate Chemical, Inc. | 1:500 | Yes |
| β-catenin |
Mouse IgG1 |
BD Transduction Labs |
1:400 |
No |
Definition of abbreviations: BrdU, bromodeoxyuridine; CCSP, Clara cell secretory protein; FoxJ1, forkhead box J1.
Morphometric Analysis
Repair index.
Epithelial repair was quantified at the 45-day recovery time point by determining the length of basement membrane underlying CCSP-immunoreactive regions of the epithelium and dividing this value by the total length of the basement membrane. Repairing regions were defined as those bounded by two adjacent CCSP-positive cells. Lengths were determined using the measurement function of Image-Pro Plus. Values are presented as the average (±SEM) for four or five animals.
Cumulative mitotic index.
The cumulative mitotic index was defined as the number of BrdU-labeled nuclei divided by the total number of nuclei, and is presented as a percentage.
FoxJ1 index.
Ciliated cells were identified by immunofluorescence staining for FoxJ1. Ciliated cell differentiation was assessed by determining the number of FoxJ1-positive nuclei within nascent regions of epithelium and expressing this value as a percent of the total number of nuclei. Data are displayed as average percent FoxJ1-positive nuclei within nascent epithelium (±SEM).
RNA Abundance
Quantitative RT-PCR was used to assess mRNA abundance. RNA was isolated from either the left lung lobe (adult mice) or total lung (neonatal and juvenile mice) (33). Briefly, tissue was homogenized in 4 M guanidinium isothiocyanate containing 0.7% 2-mercaptoethanol, and RNA purified by organic extraction (4, 32). Either 1 μg lung RNA (test) or 500 ng commercially prepared lung RNA (calibrator; Ambion, Austin, TX) was used as template for complementary DNA synthesis. Complementary DNA was synthesized using a Superscript II Kit (Invitrogen, Carlsbad, CA) according to the manufacturer's directions (for reactions containing reverse transcriptase [RT]) or in the absence of reverse transcriptase (no reverse transcriptase [NRT]). Triplicate aliquots of RT reactions and duplicate aliquots of NRT reactions served as PCR templates. Assays-on-Demand gene expression probes (Applied Biosystems, Foster City, CA) included: secretoglobin (Scgb1a1/CCSP, Mm00442046_m1); cytochrome P450–2F2 (CyP450–2F2, Mm00484087_m1); surfactant-associated protein (Sftpc, Mm00488144_m1); FoxJ1 (Mm00807215_m1); and β glucuronidase (Gusβ, Mm00446953_m1). Cycle conditions were: 95°C for 12 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Quantitative RT-PCR for the target and control genes was performed in separate tubes to avoid possible competition and/or interference. Differential gene expression was determined using either an ABI PRISM 7,000 Sequence Detection System or Eppendorf Realplex4 and values calculated by the ΔΔCT method (34).
RESULTS
Sporadic Activation of TOPGal Transgene Activity in the Steady-State Adult Airway
To determine whether β-catenin–mediated signaling contributed to airway homeostasis, lung tissue from wild-type and TOPGal(B6)-congenic mice was analyzed by whole-mount β-gal staining with microdissection to reveal transgene activation in airways. Endogenous (eukaryotic) β-gal activity was not detected at pH 7.0 in transgene-negative tissues (data not shown). TOPGal transgene activity was weak relative to levels observed in the developing lung (Ref. 13 and data not shown), with no clear association between transgene activity and defined anatomical features of the airway (Figures 1A–1C). X-Gal histochemical staining was observed in subsets of CCSP-immunoreactive secretory cells and FoxJ1-immunorective ciliated cells of the steady-state airway (Figures 1G and 1J). No activation of the TOPGal transgene was observed in the adult steady-state alveolar epithelium. These data suggest that β-catenin–mediated signaling contributes to airway homeostasis at the level of individual cells or small cell clusters, and that it is not operative in the adjacent alveolar compartment.
Figure 1.
TOPGal transgene activity in the steady-state and repairing airway. (A–C) The spatial context of cells expressing the β-galactosidase reporter (blue) was determined in accessory lobes of untreated control mice (A) and those treated with naphthalene and allowed to recover for 3 (B) or 10 (C) days. The proximal end of the main axial pathway (arrows) is located at the top of each panel. Original magnification = 12.5×. (D–L) The identity of cells expressing the β-galactosidase reporter was determined by immunohistochemical detection (brown) of the Clara cell marker, CCSP (G–I), and the ciliated cell marker, acetylated tubulin (J–L). No primary antibody controls (D–F). Representative lung tissue sections from control mice (D, G, and J) and those treated with naphthalene and allowed to recover for 3 (E, H, and K) or 10 (F, I, and L) days are shown. Scale bars = 50 μm. (M) Abundance of CCSP mRNA was determined by quantitative RT-PCR in control mice (0) or those treated with naphthalene and allowed to recover for 3, 5, 7, or 10 days. Data are presented as mean ΔΔCT (±SEM) (n = 4). The relative distribution of β-galactosidase– and CCSP-positive cells was determined in lungs of animals treated with naphthalene and allowed to recover for 10 days (N). Examples of reparative units in bronchiolar (B) and terminal bronchiolar (TB) regions are indicated by brackets. Scale bar = 100 μm.
TOPGal Transgene Activity in the Repairing Airway Epithelium
To determine whether signaling by β-catenin was modulated during repair after extensive progenitor cell depletion, wild-type and TOPGal mice were exposed to the Clara cell toxicant, naphthalene, and allowed to recover for 2, 3, 5, or 10 days. Analysis of Clara cell gene expression demonstrated an 80% depletion of CCSP on Day 3 (Figure 1M). These data were similar to the kinetics of epithelial marker gene expression reported previously for naphthalene-exposed FVB/n mice (32), and indicated that the injury and repair process was similar in TOPGal-transgenic mice. Endogenous β-gal activity was not detected in transgene-negative tissue at any recovery time point (data not shown). Limited or no transgene activation was observed in individuals exposed to naphthalene and allowed to recover for either 2 days (data not shown) or 3 days (Figures 1B, 1E, 1H, and 1K). Transgene activation, if detected at these time points, was observed preferentially within terminal bronchioles. This period of relative transgene inactivity correlated with the period of Clara cell necrosis and initiation of the proliferative phase of the repair response (4, 6). β-gal activity, indicative of TOPGal transgene activation, was noted on Recovery Day 5 (data not shown), and was maintained at this level through Recovery Day 10 (Figures 1C, 1F, 1I, and 1L). At these time points, β-gal–positive cells were detected throughout the main axial pathway. As observed for the steady-state lung, transgene-expressing cells tended to be present as isolated cells or as small clusters within the epithelium (Figure 1N). Within repairing regions, β-gal–positive/CCSP-expressing cells were identified. However, the majority of acetylated tubulin-expressing cells within repairing regions were β-gal negative. β-gal–positive cells were also identified within nonrepairing epithelial zones. Although the pattern of TOPGal transgene activity during epithelial injury and repair further supported a role for β-catenin in airway homeostasis, the absence of transgene expression during the period of peak proliferation on Day 3, expression in terminally differentiated ciliated cells, and the lack of restriction to regenerative zones suggested that this pathway was not a proximal regulator of the repair response.
Cell Type–Specific Modification of Clara Cell Gene Expression
To probe the relationship between β-catenin–regulated gene expression and Clara cell differentiation, a null allele of the β-catenin gene was generated in CCSP-expressing cells through Cre-mediated recombination. This approach made use of a transgene in which Cre recombinase was expressed under regulation of the rat CCSP promoter (CCSP-Cre) (35, 36). Cellular specificity and kinetics of Cre recombinase activity have been described previously through analysis of mice hemizygous for the CCSP-Cre transgene and heterozygous for the ROSA-RS (14, 37). Both CCSP-positive cells and ciliated cells of adult airways expressed β-gal, a finding consistent with those of previous reports of a precursor–progeny relationship between airway CCSP-expressing cells and ciliated cells (3).
To assess the necessity of β-catenin and its signaling activity for appropriate epithelial regeneration, β-catenin–null epithelium was generated through Cre-mediated deletion of Catnb exons 2–6 (38). Necessity of β-catenin for airway epithelial maturation and regeneration was assessed in mice that were hemizygous for the CCSP-Cre transgene and homozygous for the Catnbflox(E2–6) allele (CatnbΔ(E2–6) mice). Recombination efficiency was determined by immunofluorescence localization of β-catenin within the airway epithelium (Figures 2A and 2B). Analysis of the adult wild-type lung detected lateral plasma membrane β-catenin staining in all airway epithelial cells (Figure 2A). Similar analysis of CatnbΔ(E2–6) demonstrated extensive recombination in the steady state, with overall recombination varying from a minimum of 90% (Figure 2B) to a maximum of 95%. Secretory and ciliated cells were identified within the recombined cell population (data not shown), and suggested that β-catenin deficiency did not impact the differentiation potential of CCSP-expressing cells. Occasional cells showing β-catenin immunoreactivity were usually found as isolated cells within a field of recombined cells, with the less frequent observation of small clusters (average of four cells) of β-catenin–positive cells. These cells, which presumably resulted from a lack of recombination of at least one CatnbfloxE2–6 allele, were randomly distributed in the airway, with no spatial association with any specific anatomic features, such as branchpoints or terminal bronchioles. The nonrecombined fraction of epithelial cells included pulmonary neuroendocrine cells, within which no recombination was observed (data not shown), occasional isolated ciliated cells, and rare Clara cells. These data indicate that the CCSP-Cre transgene mediated efficient generation of homozygous β-catenin–null airway epithelial secretory and ciliated cells.
Figure 2.
Cell type–specific deletion of Catnb. Efficiency of Cre recombinase–mediated modification of the CatnbfloxE2–6 allele was determined by immunostaining lung tissue from wild-type (A) and CCSP-Cre/CatnbfloxE2–6/floxE2–6 (B) mice for β-catenin (green). Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI; blue). Asterisks mark residual β-catenin immunoreactivity indicative of unrecombined airway epithelial cells. Scale bars = 50 μm.
Steady-State Phenotype of the β-Catenin–Null Airway Epithelium
Previous studies have demonstrated that loss of β-catenin in the early embryonic lung endoderm results in defects in proximal–distal lineage specification that favored proximal epithelial cell fate over distal (39). However, loss of β-catenin late in embryonic development failed to yield obvious defects in epithelial maturation at adulthood (39). Because loss of β-catenin has been shown to promote the differentiation of epithelial cells in the intestine, leading to loss of progenitor cell pools, we first determined how loss of β-catenin in the late embryonic period impacted the kinetics of epithelial differentiation and maturation in airways. Expression of a broad panel of differentiation markers for airway secretory cells (CCSP, Scgb3a2, Cyp2f2, and Cldn10), ciliated cells (FoxJ1), and alveolar type 2 cells (SP-C) were measured at the level of mRNA abundance during the course of postnatal lung maturation (Figure 3). We have previously shown that these markers are differentially regulated during postnatal maturation of airways, allowing staging of this process (40). However, no differences were observed in the expression pattern of differentiation markers between wild-type and CatnbΔ(E2–6) mice, suggesting that developmental maturation of Clara, ciliated, and alveolar epithelial cells were not impacted by loss of β-catenin. Furthermore, analysis of aged wild-type and CatnbΔE2–6 (6–12 months; n > 60) mice did not detect genotype-associated alterations in any of the above parameters or in the development of lung tumors (data not shown). These results indicate that the airway and alveolar secretory cell lineages were correctly specified and differentiated in the absence of β-catenin. A caveat with these data is that changes in the abundance or function of rare cell types would not be revealed through analysis of genes expressed by abundant cell types.
Figure 3.
Postnatal maturation of airway epithelial cells. Total RNA was isolated from either wild-type (open bars) or CCSP-Cre/CatnbfloxE2–6/floxE2–6 mice (closed bars) at the indicated postnatal stages. Abundance of the indicated mRNAs was quantified by real-time RT-PCR with normalization to β-glucuronidase. Experimental samples were compared using a standard total lung RNA sample, and data presented as average ΔΔCT (±SEM) (n = 4).
β-Catenin Is Dispensable for Repair of the Airway Epithelium
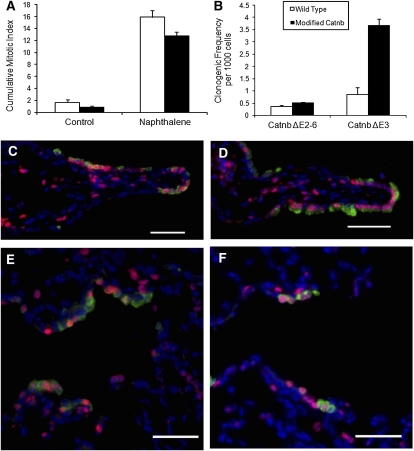
We have previously shown that bronchiolar stem cells can be revealed as a rare naphthalene-resistant population, the abundance of which is increased after potentiation of β-catenin signaling (4, 5, 13). Because loss of β-catenin signaling leads to loss of intestinal stem cells, ostensibly through differentiation into mature cell types (41), we sought to determine the impact of β-catenin deficiency on maintenance of naphthalene-resistant bronchiolar stem cells. The absence of molecular markers that specifically define this population of airway cells required the use of functional assays to investigate their behavior in normal versus β-catenin–deficient airways. Necessity of β-catenin for regeneration of the conducting airway epithelium was assessed through exposure of wild-type and CatnbΔE2–6 mice to naphthalene and recovery for 3 to 45 days (Figure 4). At the 10-day recovery time point, repairing bronchioles of wild-type mice were composed of nascent CCSP-immunoreactive Clara and ciliated cells, all of which displayed strong lateral membrane staining for β-catenin (Figures 4A and 4C). Regenerating bronchiolar epithelial cells of CatnbΔE2–6 mice were largely devoid of lateral membrane β-catenin staining, and regenerative zones were of similar size and cellular composition to those of similarly exposed and recovered wild-type mice (Figures 4B and 4D). After 45 days of recovery, regenerating CCSP-immunoreactive cells covered a similar fraction of basement membrane in airways of both wild-type and CatnbΔE2–6 mice (Figure 4E). Furthermore, analysis of CCSP mRNA abundance within total lung RNA of recovering wild-type and CatnbΔE2–6 mice demonstrated remarkably similar patterns of injury and repair (Figure 4F). Continuous BrdU labeling and histomorphometric methods were used to compare the mitotic index for naphthalene exposed wild-type and CatnbΔE2–6 mice. Dual immunofluorescence analysis of BrdU and CCSP detected similarly sized reparative units located at branch points and in terminal bronchioles of wild-type and CatnbΔE2–6 airways (Figures 5C–5F). No genotype-dependent differences in the cumulative mitotic index of wild-type and CatnbΔE2–6 tissues were detected (Figure 5A).
Figure 4.
Naphthalene susceptibility and repair of the β-catenin–null epithelium. Dual immunofluorescence detection of CCSP (red) and β-catenin (green) in wild-type (A and C) and or CatnbΔE2–6 (B and D) mice exposed to naphthalene and allowed to recover for 10 days. DAPI counterstain appears as blue. Representative images of CCSP immunoreactive regions at branch points (A and B) and terminal bronchioles (C and D) are presented. Scale bars = 50 μm. No differences were observed in the spatial pattern of bronchiolar epithelium observed between wild-type and CatnbΔE2–6 mice. The impact of genotype on epithelial repair was further determined 45 days after naphthalene exposure through analysis of secretory cell repopulation (E). Histomorphometric methods were used to determine the length of basement membrane subtended by CCSP immunoreactive epithelium in wild-type (open bar) or CatnbΔE2–6 (closed bar) mice. Results are presented as average percentages (±SEM) (n = 3). Genotype-dependent differences were not detected. The kinetics of epithelial injury and repair was compared in wild-type and CatnbΔE2–6 mice through analysis of CCSP mRNA abundance by real-time RT-PCR of total RNA isolated from lungs of control mice (0) or those treated with naphthalene and allowed to recover for 3, 10, or 45 days (F). Open bars, wild-type mice; closed bars, CatnbΔE2–6 mice. Data are presented as average ΔΔCT (±SEM) (n = 3). No genotype-dependent differences were detected.
Figure 5.
Normal proliferation of the β-catenin null epithelium. (A) The impact of β-catenin deficiency on proliferation of control and naphthalene-treated wild-type (open bars) and CatnbΔE2–6 mice (closed bars) was determined in mice that were injected with bromodeoxyuridine (BrdU) daily until Recovery Day 10. Results are reported as the average mitotic index (±SEM) (n = 3). Mitotic index did not vary significantly by genotype in steady state or during recovery from naphthalene. (B) The frequency of clonogenic cells in airway epithelial cell preparations from wild-type and CatnbΔE2–6 littermates (left bars) was determined. Cells from wild-type and CatnbΔ3 littermates served as positive control cells (right bars). Data are presented as means (±SEM) (n = 4). Significant differences in clonogenic potential were only noted between wild-type and CatnbΔE3 mice. (C–F) Tissue from mice that were continuously labeled with BrdU was dual immunostained for CCSP (green) and BrdU (red), and counterstained with DAPI (blue). Representative images of regenerating areas are shown for wild-type (C and E) and CatnbΔE2–6 (D and F) mice. Regenerating zones located at branch points (C and D) and terminal bronchioles (E and F) are presented. Scale bars = 50 μm.
Analysis of injury and repair in mice expressing a stabilized form of β-catenin (CatnbΔE3 mice [13]) suggested that β-catenin truncation resulted in amplification of bronchiolar stem cells and distribution of these cells throughout the bronchiolar epithelium. This observation led to the suggestion that stabilization of β-catenin replaced niche-derived signals that regulate stem cell maintenance and participation in epithelial repair. To determine whether deletion of β-catenin in bronchiolar stem or transit-amplifying cells has a subtle effect on the number of clonogenic cells, we compared the clonogenic frequency of wild-type and CatnbΔE2–6 epithelial cells in vitro. As previously reported, this analysis detected an increase in the number of clongenic cells in airways of CatnbΔE3 mice (Figure 5B). However, no genotype-dependent differences in clonogenic frequency were detected in CatnbΔE2–6 airway cells, and colony size and morphology did not vary by genotype (data not shown). These results indicate that deletion of β-catenin in CCSP-expressing cells does not impact the number of colony-forming cells in vitro. Overall, these results demonstrate that a β-catenin–null progenitor cell pool could reconstitute the conducting airway epithelium, and suggest that β-catenin was not necessary for repair of the conducting airway epithelium.
Differentiation of β-Catenin–Null Progenitor Cells
The ability of β-catenin–null progenitor cells to regenerate the ciliated cell population was assessed in wild-type and CatnbΔE2–6 animals 45 days after naphthalene exposure. As ciliated cell representation varies by anatomical location (42), this analysis was limited to the last 200-μm basement membrane ending in a well defined bronchoalveolar duct junction. In both wild-type and CatnbΔE2–6 tissue, dual immunofluorescent analysis of CCSP and FoxJ1 detected zones of columnar epithelium (Figures 6B and 6C), separated by regions that were deficient in CCSP-expressing cells. Quantitative analysis demonstrated that representation of FoxJ1 immunopositive cells in repairing regions did not vary by genotype (Figure 6A). These data indicate that β-catenin deficiency did not impact the differentiation potential of the bronchiolar progenitor cell pool.
Figure 6.
Normal pattern of ciliated cell differentiation after repair of the β-catenin–null epithelium. Wild-type (A) and CatnbΔE2–6 (B) mice were treated with naphthalene and allowed to recover for 45 days. Dual immunofluorescence was performed for detection of CCSP (red) and forkhead box J1 (FoxJ1; green). Asterisks mark the extent of the nascent epithelium defined by repairing patches containing CCSP-immunoreactive cells. Scale bars = 50 μm. The impact of β-catenin on ciliated cell differentiation was determined in wild-type (open bar) and CatnbΔE2–6 (hatched bar) mice that had been exposed to naphthalene and allowed to recover for 45 days (C). The FoxJ1 index was determined for repairing regions of the epithelium defined by contiguous regions containing CCSP-immunoreactive cells. Data are presented as average percent FoxJ1-positive nuclei within nascent epithelium (±SEM) (n = 3). No differences were observed between genotypes.
DISCUSSION
We have used transgenic and cell type–specific knockout strategies to determine roles for β-catenin–regulated gene expression in maintenance and repair of the bronchiolar epithelium. Analysis of TOPGal transgene activity detected β-catenin–dependent signaling in the steady-state and repairing bronchiolar epithelium. However, the broad distribution and phenotype of signaling cells precluded establishment of a clear role for β-catenin in maintenance or repair of the bronchiolar epithelium. Necessity of β-catenin signaling for maintenance of epithelial reparative capacity was tested through generation of a conditional β-catenin–null epithelium by Cre-mediated recombination within bronchiolar progenitor cell populations. Functional knockout of β-catenin had no impact on developmental maturation of the airway epithelium, expression of Clara cell differentiation markers in adult airways, epithelial mitotic index, or sensitivity of the epithelium to the Clara cell–specific toxicant, naphthalene. Repair of the naphthalene-injured airway proceeded with focal proliferation of β-catenin–null epithelium in a pattern that closely resembled that of wild-type repairing epithelium. The size of the regenerative units, epithelial mitotic index, and restoration of the ciliated cell population did not vary between wild-type and CatnbΔE2–6 mice. Thus, β-catenin signaling was not necessary for maintenance or efficient repair of the bronchiolar epithelium. These data suggest that signaling through stabilized β-catenin is not necessary for Clara cell maturation, Clara-to-ciliated cell differentiation, or maintenance of the naphthalene-resistant bronchiolar stem cell.
We have used TOPGal transgenic mice to demonstrate that naphthalene-induced progenitor cell depletion results in changes in the cellular context of β-catenin–mediated signaling. In the steady state, subsets of Clara and ciliated cells express the TCF promoter–regulated β-galactosidase reporter. This expression was lost after naphthalene-induced Clara cell injury, and was regained during the late phase of the repair response. We have previously shown that cell proliferation in response to naphthalene-induced airway injury initiates as early as 36 hours after naphthalene exposure, and peaks between 72 and 96 hours after exposure (5, 6, 43). Down-regulation of the TOPGal transgene during the active proliferative phase of the repair process, coupled with its reactivation within both secretory and postmitotic ciliated cells during the latter recovery phase, is inconsistent with the view that active β-catenin signaling is a proximal regulator of cell proliferation, as has been suggested in the gut (15, 44). This notion was supported by the finding that β-catenin–null airways exhibited a similar proliferative index to those of wild-type mice, both in the steady state and after injury. Similarly, β-catenin status did not impact the in vitro colony-forming ability of dissociated airway epithelial cells. Even though we cannot formally exclude the possibility that TOPGal transgene activation within the bronchiolar epithelium occurs through a β-catenin–independent mechanism, our data, as a whole, demonstrate that β-catenin is not necessary for regulation of cell cycle entry or exit by bronchiolar epithelial cells.
Roles for the Wnt/β-catenin signaling pathway in regulation of cell proliferation were initially revealed through analysis of tumor cells of the mammary gland and colon, for which pathway components, such as Wnt, β-catenin and adenomatous polyposis coli, were identified as proto-oncogenes (45). Even though activating mutations of the Wnt/β-catenin signaling pathway are frequently found in colorectal tumors, the frequency with which these mutations are found in association with lung cancer is very low: approximately 5% (46). Moreover, there are dramatic differences in the proliferative rate of gut versus lung epithelium. The intestinal epithelium turns over every 3 to 5 days, which requires continuous activation of stem and TA cells, whereas the lung epithelium is relatively quiescent in the normal adult state (2, 32, 47, 48). These data suggest fundamental differences between organs in signaling pathways contributing to regulation of cell proliferation and growth control in the setting of neoplastic transformation, and potentially in epithelial maintenance in the steady state. Our data suggest that β-catenin signaling fulfills roles that are more analogous to those observed in the stem/TA populations of the hair follicle rather than regulation of the intestinal stem cell hierarchy. In the hair follicle, nuclear Lef1 and TOPGal transgene activity were detected in the nonmitotic compartment at the follicle base, and colocalization with hair keratins suggested that these cells had committed to the differentiation pathway (22).
In the present study, detection of TOPGal transgene activity in differentiated cells of the bronchiolar epithelium suggest that β-catenin signaling might play a role in establishment or maintenance of the differentiated state. Similarly, reexpression of the transgene late in the reparative response to naphthalene injury support the suggestion that β-catenin was involved in reestablishment of homeostasis. However, cell type–specific deletion of β-catenin had no impact on the differentiated characteristics of Clara cells, their susceptibility to naphthalene, or their ability to differentiate into ciliated cells after injury. Importantly, the repair competence of naphthalene-injured airways suggests that loss of β-catenin within developing bronchioles did not impact establishment and/or maintenance of naphthalene-resistant bronchiolar stem cells. Naphthalene-induced airway injury was repaired through proliferation of naphthalene-resistant cells that localized to the same airway microenvironments as naphthalene-resistant stem cells in airways of wild-type mice. Furthermore, nascent β-catenin–null epithelial cells were able to repopulate the epithelium after naphthalene injury as efficiently as wild-type cells. Thus, these data indicate that β-catenin signaling is unnecessary for maintenance of naphthalene-resistant bronchiolar stem cells, for expression of differentiated characteristics by Clara cells, or for differentiation of these cells into ciliated cells.
Numerous studies investigating the molecular signaling involved in maintenance and activation of skin follicular bulge stem cells have identified roles for β-catenin signaling in regulation of cell fate determination (14, 22–24, 49, 50). In the lung, gene deletion and misexpression studies demonstrated a critical role for Wnt/β-catenin signaling in the specification of lung endoderm (26, 39, 51–54) and attenuation of β-catenin signaling in the late prenatal–early postnatal period (26, 54). A reciprocal relationship between TOPGal transgene expression and airway epithelial differentiation suggested that β-catenin–dependent gene expression in airways was down-regulated as the secretory cell differentiation program was activated (13). In agreement with other studies these data suggested that β-catenin signaling maintained airway epithelial progenitor cells in a relatively undifferentiated state, and that attenuation of this pathway was necessary for expression of differentiated secretory cell characteristics. This finding was substantiated by the demonstration that stabilization of β-catenin in Clara cells blocked postnatal secretory cell maturation and secretory-to-ciliated cell differentiation (13). The seemingly contradictory findings, dispensability of β-catenin for bronchiolar stem cell maintenance in this study versus bronchiolar stem cell expansion after potentiation of β-catenin signaling, suggests that niche-dependent signals supersede developmental β-catenin signaling during the transition from progenitor cell maintenance in the developing lung (active β-catenin signaling) to stem cell maintenance in the adult lung (loss of β-catenin signaling). Overall, our studies suggest that levels of β-catenin are tightly regulated in the developing and adult lung, with low cytoplasmic/nuclear β-catenin being the norm within the bronchiolar epithelium.
Acknowledgments
The authors thank Christine Burton and Lixia Luo for assistance with animal husbandry and genotyping.
This work was supported by National Institutes of Health RO1 grants HL064888 and HL090146 (B.R.S.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0407OC on February 12, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Stripp BR. Hierarchical organization of lung progenitor cells: is there an adult lung tissue stem cell? Proc Am Thorac Soc 2008;5:695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans MJ, Johnson LV, Stephens RJ, Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest 1976;35:246–257. [PubMed] [Google Scholar]
- 3.Evans MJ, Cabral-Anderson LJ, Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest 1978;38:648–653. [PubMed] [Google Scholar]
- 4.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein–expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 2001;24:671–681. [DOI] [PubMed] [Google Scholar]
- 5.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 2002;161:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 2000;156:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays 2002;24:91–98. [DOI] [PubMed] [Google Scholar]
- 8.Vessey CJ, de la Hall PM. Hepatic stem cells: a review. Pathology 2001;33:130–141. [PubMed] [Google Scholar]
- 9.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990;61:1329–1337. [DOI] [PubMed] [Google Scholar]
- 10.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 1989;57:201–209. [DOI] [PubMed] [Google Scholar]
- 11.Lehrer MS, Sun TT, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci 1998;111:2867–2875. [DOI] [PubMed] [Google Scholar]
- 12.Giangreco A, Shen H, Reynolds SD, Stripp BR. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L624–L630. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, Mariani T, Di PY, Taketo MM, Stripp BR. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells 2008;26:1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001;105:533–545. [DOI] [PubMed] [Google Scholar]
- 15.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 1998;19:379–383. [DOI] [PubMed] [Google Scholar]
- 16.Moles JP, Watt FM. The epidermal stem cell compartment: variation in expression levels of E-cadherin and catenins within the basal layer of human epidermis. J Histochem Cytochem 1997;45:867–874. [DOI] [PubMed] [Google Scholar]
- 17.Wong MH, Huelsken J, Birchmeier W, Gordon JI. Selection of multipotent stem cells during morphogenesis of small intestinal crypts of Lieberkuhn is perturbed by stimulation of Lef-1/beta-catenin signaling. J Biol Chem 2002;277:15843–15850. [DOI] [PubMed] [Google Scholar]
- 18.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signaling in self-renewal of haematopoietic stem cells. Nature 2003;423:409–414. [DOI] [PubMed] [Google Scholar]
- 19.Eaves CJ. Manipulating hematopoietic stem cell amplification with Wnt. Nat Immunol 2003;4:511–512. [DOI] [PubMed] [Google Scholar]
- 20.Love R. Beta-catenin, brains, and beyond. Lancet Neurol 2002;1:272. [DOI] [PubMed] [Google Scholar]
- 21.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 2002;111:241–250. [DOI] [PubMed] [Google Scholar]
- 22.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 1999;126:4557–4568. [DOI] [PubMed] [Google Scholar]
- 23.Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998;95:605–614. [DOI] [PubMed] [Google Scholar]
- 24.Niemann C, Owens DM, Hulsken J, Birchmeier W, Watt FM. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 2002;129:95–109. [DOI] [PubMed] [Google Scholar]
- 25.Watt FM. The stem cell compartment in human interfollicular epidermis. J Dermatol Sci 2002;28:173–180. [DOI] [PubMed] [Google Scholar]
- 26.Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol 2004;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan D, Sehgal A, Yao J, Engelhardt JF. Lef1 transcription factor expression defines airway progenitor cell targets for in utero gene therapy of submucosal gland in cystic fibrosis. Am J Respir Cell Mol Biol 1998;18:750–758. [DOI] [PubMed] [Google Scholar]
- 28.Hatsell S, Rowlands T, Hiremath M, Cowin P. Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia 2003;8:145–158. [DOI] [PubMed] [Google Scholar]
- 29.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science 2002;296:1644–1646. [DOI] [PubMed] [Google Scholar]
- 30.Niemann C, Unden AB, Lyle S, Zouboulis Ch C, Toftgard R, Watt FM. Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci USA 2003;100:11873–11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagenaar RA, Crawford HC, Matrisian LM. Stabilized beta-catenin immortalizes colonic epithelial cells. Cancer Res 2001;61:2097–2104. [PubMed] [Google Scholar]
- 32.Stripp BR, Maxson K, Mera R, Singh G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol 1995;269:L791–L799. [DOI] [PubMed] [Google Scholar]
- 33.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987;162:156–159. [DOI] [PubMed] [Google Scholar]
- 34.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res 1996;6:986–994. [DOI] [PubMed] [Google Scholar]
- 35.Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, Tonon G, McNamara K, Marconcini LA, Hezel A, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene 2006;25:2105–2112. [DOI] [PubMed] [Google Scholar]
- 36.Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Andalcio T, Shapiro SD, Mariani TJ. Epithelial cell PPARgamma is an endogenous regulator of normal lung maturation and maintenance. Proc Am Thorac Soc 2006;3:510–511. [DOI] [PubMed] [Google Scholar]
- 37.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999;21:70–71. [DOI] [PubMed] [Google Scholar]
- 38.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre–mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 2001;128:1253–1264. [DOI] [PubMed] [Google Scholar]
- 39.Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 2003;278:40231–40238. [DOI] [PubMed] [Google Scholar]
- 40.Zemke AC, Snyder JC, Brockway BL, Drake JA, Reynolds SD, Kaminski N, Stripp BR. Molecular staging of epithlieial maturation using secretory cell–specific genes as markers. Am J Respir Cell Mol Biol 2009;40:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 2003;17:1709–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tichelaar JW, Wert SE, Costa RH, Kimura S, Whitsett JA. HNF-3/forkhead homologue-4 (HFH-4) is expressed in ciliated epithelial cells in the developing mouse lung. J Histochem Cytochem 1999;47:823–832. [DOI] [PubMed] [Google Scholar]
- 43.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 2004;164:577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 2007;27:7551–7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell 2000;103:311–320. [DOI] [PubMed] [Google Scholar]
- 46.Ohgaki H, Kros JM, Okamoto Y, Gaspert A, Huang H, Kurrer MO. APC mutations are infrequent but present in human lung cancer. Cancer Lett 2004;207:197–203. [DOI] [PubMed] [Google Scholar]
- 47.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol 2008;295:L231–L234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003–1007. [DOI] [PubMed] [Google Scholar]
- 49.DasGupta R, Rhee H, Fuchs E. A developmental conundrum: a stabilized form of beta-catenin lacking the transcriptional activation domain triggers features of hair cell fate in epidermal cells and epidermal cell fate in hair follicle cells. J Cell Biol 2002;158:331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watt FM. Epidermal stem cells: markers, patterning and the control of stem cell fate. Philos Trans R Soc Lond B Biol Sci 1998;353:831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 2002;129:4831–4842. [DOI] [PubMed] [Google Scholar]
- 52.Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, et al. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol 2005;283:226–239. [DOI] [PubMed] [Google Scholar]
- 53.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev 2000;92:55–81. [DOI] [PubMed] [Google Scholar]
- 54.Dean CH, Miller LA, Smith AN, Dufort D, Lang RA, Niswander LA. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol 2005;286:270–286. [DOI] [PubMed] [Google Scholar]