Abstract
Transforming growth factor (TGF)-α is a ligand for the epidermal growth factor receptor (EGFR). EGFR activation is associated with fibroproliferative processes in human lung disease and animal models of pulmonary fibrosis. Overexpression of TGF-α in transgenic mice causes progressive and severe pulmonary fibrosis; however, the intracellular signaling pathways downstream of EGFR mediating this response are unknown. Using a doxycycline-regulatable transgenic mouse model of lung-specific TGF-α expression, we observed increased PCNA protein and phosphorylation of Akt and p70S6K in whole lung homogenates in association with induction of TGF-α. Induction in the lung of TGF-α caused progressive pulmonary fibrosis over a 7-week period. Daily administration of rapamycin prevented accumulation of total lung collagen, weight loss, and changes in pulmonary mechanics. Treatment of mice with rapamycin 4 weeks after the induction of TGF-α prevented additional weight loss, increases in total collagen, and changes in pulmonary mechanics. Rapamycin prevented further increases in established pulmonary fibrosis induced by EGFR activation. This study demonstrates that mammalian target of rapamycin (mTOR) is a major effector of EGFR-induced pulmonary fibrosis, providing support for further studies to determine the role of mTOR in the pathogenesis and treatment of pulmonary fibrosis.
Keywords: epidermal growth factor receptor, PI3K, Akt, mTOR
CLINICAL RELEVANCE
Expression of epidermal growth factor receptor (EGFR) and EGFR ligands have been identified in animal models of pulmonary fibrosis and human disease. Using a transgenic mouse model of pulmonary fibrosis caused by lung-specific expression of the EGFR ligand, transforming growth factor-α, the present study demonstrates that administration of rapamycin prevents both the initiation and the progression of established pulmonary fibrosis and associated alterations in lung mechanics. These findings support the need for further studies to carefully determine the role of mammalian target of rapamycin activation in the pathogenesis of pulmonary fibrosis and to assess the efficacy of therapies designed to inhibit its activity.
Pulmonary fibrosis is characterized by mesenchymal cell proliferation in the lung, expansion of the extracellular matrix (ECM), and extensive remodeling of the lung parenchyma (1). Clinical diseases causing pulmonary fibrosis are heterogeneous and include connective tissue disorders, occupational and environmental exposures, and interstitial lung diseases (ILD) (2). Currently there are no proven therapies that prevent or reverse pulmonary fibrosis, emphasizing the need to identify new molecular targets.
The molecular pathways leading to pulmonary fibrosis are complex and likely multifactorial. Animal models overexpressing the cytokines TNF-α, GM-CSF, IL-11, IL-13, and IL-1β develop varying degrees of pulmonary fibrosis associated with inflammation. In addition, several growth factors regulate matrix deposition and fibroblast proliferation in the lung, including connective tissue growth factor, platelet-derived growth factor (PDGF), basic fibroblast growth factor, insulin-like growth factor, and transforming growth factor (TGF)-β1 (3, 4). TGF-α, along with epidermal growth factor (EGF) and amphiregulin are ligands for the epidermal growth factor receptor (EGFR). We previously generated doxycycline (Dox)-regulatable transgenic mice wherein lung epithelial-specific expression of TGF-α caused progressive and extensive vascular adventitial, peribronchial, interstitial, and pleural fibrosis that was independent of inflammatory or developmental influences (5). Gene expression profiles observed after expression of TGF-α in the mouse lung were similar to those found in pulmonary fibrotic disease in humans (6).
The EGFR is a membrane-bound receptor tyrosine kinase that belongs to a subfamily of four closely related receptors: HER1/EGFR/ERBB1, HER2/NEU/ERBB2, HER3/ERBB3, and HER4/ERBB4. After ligand binding, these receptors form homo- and heterodimers leading to autophosphorylation of tyrosine residues in the cytosolic domains of the proteins. The phosphorylated tyrosine residues become docking sites for signaling molecules that activate multiple downstream effector pathways including MAPK, Src kinases, STAT, and the phosphatidylinositol 3-kinase pathway (PI3K) (7, 8). The mammalian target of rapamycin (mTOR) a is highly conserved intracellular serine/threonine kinase and a major downstream component of PI3K (9). Activation of mTOR, in complex with raptor (mTORC1), leads to phosphorylation of ribosomal P70S6 kinase (S6K) and eukaryotic initiation factor-4E–binding protein-1 (4E-BP1). Both S6K and 4E-BP1 control the translation of specific mRNAs and protein synthesis involved in cell cycle regulation (9). Inhibitors of mTOR, such as rapamycin bind to an intracellular cytoplasmic receptor, the FK506-binding protein-12 (9). This complex interacts and inhibits mTOR function leading to cell cycle arrest in the G1 phase. In addition to blocking cell proliferation, mTOR inhibitors have been identified with anti-inflammatory, anti-tumor, and anti-fibrotic properties that implicate mTOR signaling in a wide range of cellular functions.
In this study we first evaluated whether PI3K/Akt-mTOR pathway is activated by expression of TGF-α in the lungs of transgenic mice. We then determined the role of mTOR in the initiation and propagation of pulmonary fibrosis by administering rapamycin at the time of TGF-α induction. Finally, we determined the effectiveness of rapamycin as a late treatment for established and progressive fibrosis in the TGF-α model.
MATERIALS AND METHODS
Transgenic Mice
CCSP-rtTA activator mice expressing the reverse tetracycline-responsive transactivator (rtTA) under control of the 2.3-kb rat Clara cell secretory protein (CCSP) (a.k.a. secretoglobin, family 1A, member 1 [Scgb1a1]) gene promoter (10) were mated to conditional Dox-regulated transgenic mice containing the human TGF-α cDNA under the control of seven copies of the tetracycline operon ((TetO)7-cmv TGFα) plus a minimal CMV promoter (5). Single transgenic (CCSP-rtTA+/−) and bitransgenic CCSP-rtTA+/−/(TetO)7-cmv TGF-α+/− mice (herein called CCSP/TGF-α mice) were produced within the same litter by mating homozygous CCSP-rtTA+/+ mice to hemizygous (TetO)7-cmv TGFα+/− mice. Mice were genotyped as previously described (5). All mice were derived from the FVB/NJ inbred strain. Mice were maintained in virus-free containment and protocols were approved by the Institutional Animal Use and Care Committee of the Cincinnati Children's Hospital Research Foundation. To induce TGF-α expression, adult transgenic mice (8–12 wk old) were placed on Dox-containing drinking water (0.5 mg/ml) and food (62.5 mg/kg). Dox-containing water was replaced three times per week.
Administration of Erlotinib and Rapamycin
Erlotinib is a small molecule tyrosine kinase inhibitor specific for EGFR. Erlotinib powder (100 mg/kg; OSI Pharmaceuticals Melville, NY) was suspended in 0.5% methyl cellulose (0.015 mg/ml; 37°C; Colorcon, West Point, PA). Three hours before administration, food and water were removed from cages. Mice were then anesthetized (Isoflurane; Abbott Labs, Chicago, IL), and 150 to 250 μl sterile erlotinib was administered by gavage using a 20-gauge feeding catheter (Harvard Apparatus, Holliston, MA). Rapamycin (LC Laboratories, Woburn, MA) was prepared in a 30 mg/ml stock solution by dissolving 2 mg in 67 μL 100% EtOH. Rapamycin was diluted to a 0.6 mg/ml concentration in 0.25% PEG400, 0.25% Tween20. Mice were then anesthetized (Isoflurane; Abbott Labs), and intraperitoneal rapamycin (4 mg/kg) or vehicle was administered using a 1-ml syringe with a 27G1/2 needle (BD Syringe, Franklin Lakes, NJ). Drug dosage was based upon previous studies in mice using rapamycin and was not adjusted for changes in body weight during the study period (11). Mice were weighed at the beginning of the study and at weekly intervals.
Western Blots
Western blot analysis was performed on lung homogenates of CCSP/TGF-α mice treated with either 1 or 4 days of Dox. Controls were littermate single transgene CCSP/- mice treated with 1 day of Dox. Protein concentrations were assessed using a Bradford Assay and protein loaded on a 4 to 20% Tris/glycine SDS-PAGE (Invitrogen, Carlsbad, CA) and electroblotted to PVDF membranes (0.45 μm; Bio-Rad, Hercules, CA). Blots were blocked with 5% nonfat dry milk in TBST (10 mM Tris, pH 8, 150 mM NaCl, 0.1% Tween 20) and incubated with antibodies against total and phosphorylated Akt (Ser 473; Cell Signaling Technology, Beverly, MA), total and phosphorylated p70 S6 kinase (Thr 389; Cell Signaling Technology), phosphorylated EGFR (pY1086; Epitonic, Burlingame, CA), total EGFR (rabbit polyclonal; kind gift from Dr. Brad Warner, Washington University) and proliferating cell nuclear antigen (PCNA) (Cell Signaling Technology). Blots were washed in TBST and incubated with goat anti-rabbit horseradish peroxidase–conjugated (Calbiochem/EMD Biosciences, Madison, WI) secondary antibodies and developed on film by chemiluminescence using the ECL Plus system (Amersham Biosciences, Piscataway, NJ). Densitometry was performed using the volume integration function on a PhosphorImager software Imagequant 5.2 (Molecular Dynamics, Sunnyvale, CA) after scanning the films.
Rapamycin Prevention and Late Treatment Studies
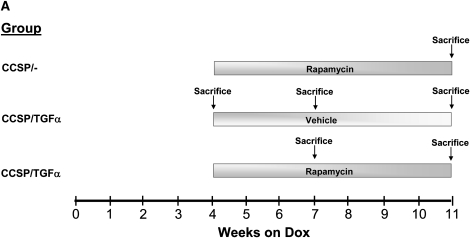
To determine if rapamycin prevented the fibrotic effects of TGF-α expression, CCSP/TGF-α mice were administered 7 weeks of Dox, and treated with either rapamycin (4 mg/kg once daily 6 d/wk) or vehicle. Controls were littermate single transgene CCSP/- mice administered Dox and treated with rapamycin.
For the late treatment studies, CCSP/TGFα mice were administered 4 wk of Dox. A subgroup of mice were killed at 4 weeks to measure endpoints as a “fibrotic baseline” to determine the effectiveness of rapamycin for reversing or preventing progression of established fibrosis. At the beginning of the fifth week, remaining mice were then treated with either vehicle or rapamycin while continuing on Dox. Mice in both groups were killed after 3 or 7 weeks of treatment (7 or 11 wk of total Dox, respectively). Control mice were littermate single transgene CCSP/- mice administered Dox for 11 weeks and treated with 7 weeks of rapamycin beginning on the fifth week of Dox. Endpoints for the prevention and late treatment studies were changes in lung histology, body weights, total lung collagen, and lung mechanics.
Lung Histology and Immunohistochemistry
Mice were killed with pentobarbital sodium (65 mg/ml) euthanasia solution (Fort Dodge Animal Health, Fort Dodge, IA), and lungs were inflation fixed using 4% paraformaldehyde at 25 cm H2O of pressure, and then allowed to fix overnight at 4°C. Fixed lungs were then washed with phosphate-buffered saline (PBS), dehydrated through a graded series of ethanols, and processed for paraffin embedding. Sections (5 μm) were loaded onto polysine slides for immunostaining, hematoxylin and eosin (H&E) staining as previously described (12). Immunostaining was performed on CCSP/TGF-α mice treated with 4 days of Dox and compared with CCSP/TGF-α mice not receiving Dox. Immunostaining for phosphorylated Akt (Ser 473; Cell Signaling) used citrate/TBST antigen unmasking with antibody diluted 1:50. Immunostaining for phosphorylated S6 (Ser 235/236; Cell Signaling) used citrate/TBST antigen unmasking with antibody diluted 1:1,000. Secondary antibodies and DAB detection were preformed as previously described (5).
Total Lung Collagen
Total lung collagen was determined by measuring total soluble collagen (Sircol Collagen Assay, Biocolor, Ireland). The left lung was homogenized in 5 ml 0.5 M acetic acid containing pepsin (1 mg/10 mg tissue; Sigma-Aldrich, St. Louis, MO) and incubated (24 h; 24°C; with 240 rpm shaking). Sircol dye was added (1 ml/100 μl; 30 min), the sample centrifuged (12,000 rpm for 12 min), and the pellet was suspended (1 ml 0.5 M NaOH). The optical density measured with a spectrophotometer (540 nm).
Pulmonary Mechanics
Lung mechanics were assessed on anesthetized mice using a computerized Flexi Vent system (SCIREQ, Montreal, PQ, Canada), as previously described (13, 14). Briefly, mice were anesthetized with ketamine and xylazine, tracheostomized, and then ventilated with a tidal volume of 8 ml/kg at a rate of 450 breaths/minute and positive end-expiratory pressure (PEEP) of 2 cm H2O computerized by the SCIREQ system, thereby permitting analysis of dynamic lung compliance. The ventilation mode was changed to forced oscillatory signal (0.5–19.6 Hz), and respiratory impedance was measured. Tissue elastance was obtained for mice at 2 cm H2O PEEP by fitting a model to each impedance spectrum. With this system, the calibration procedure removed the impedance of the equipment and tracheal tube.
Statistics
Using normal plots and tests for normality (Shapiro-Wilk and Kolmogorov-Smirnov), all response variables showed a significant departure from the normality assumption. Therefore, log transformations of the above response variables were used to compare group means in a one-way ANOVA. Differences in group means were calculated and tested using a simulation-based adjustment for multiple comparisons. For analysis of changes in body weights, a repeated measures analysis was conducted with Group, Week, Group*Week, and Baseline as the factors, to compute differences in Group*Week means. A separate ARMA (1, 1) variance/covariance structure was used for each Group. Differences in selected (a priori) Group*Week means were calculated and tested using a simulation-based adjustment for multiple comparisons.
RESULTS
TGF-α Induces Phosphorylation of the Akt/mTOR Pathway
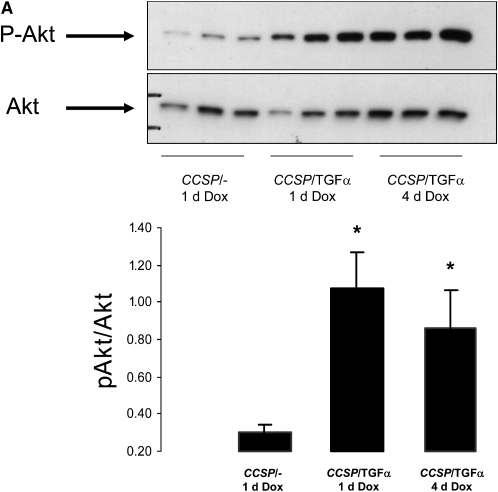
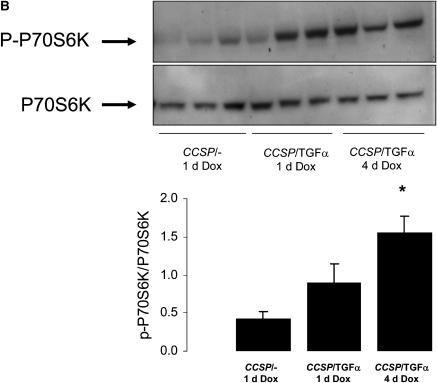
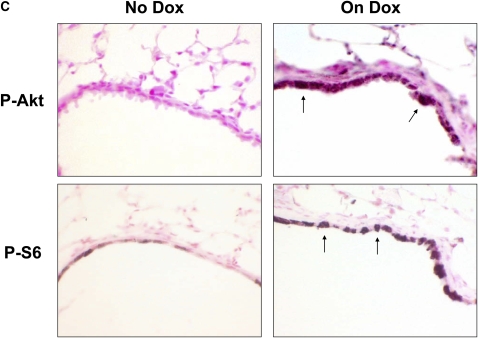
CCSP/TGF-α mice were treated with Dox to induce TGF-α expression for 1 and 4 days. Induction of TGF-α caused increased phosphorylation of Akt (p-Akt) and P70S6K (p-P70S6K) as measured by Western blot of whole lung homogenate (Figures 1A and 1B). Increased p-Akt and phosphorylated S6 were identified by immunohistochemistry primarily in epithelial cells (Figure 1C). Less prominent staining was also present in mesenchymal cells along the lung pleura and perivascular and peribronchial adventitia (not shown).
Figure 1.
Transforming growth factor (TGF)-α induces phosphorylation of the Akt, P70S6K, and S6. Epithelial cell TGF-α expression increased phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) activity as assessed by increased phosphorylation of Akt, P70S6K, and S6. CCSP/TGF-α mice were treated with doxycycline (Dox) to induce TGF-α expression for 1 or 4 days and compared with littermate control single transgene CCSP/- mice treated with 1 day of Dox. Immunoblotting of lung homogenates from CCSP/TGF-α mice demonstrated (A) increased phosphorylation of Akt at Ser473 and (B) phosphorylation of P70S6K at Thr389. Immunostaining for phosphorylated Akt and phosphorylated S6 demonstrated increased staining in the airway epithelium in CCSP/TGF-α mice after 4 days of Dox compared with CCSP/TGF-α mice not receiving Dox (C, arrows). Mean ± SE, n = 3–6 mice of each genotype; * P < 0.05.
Erlotinib and Rapamycin Inhibit TGF-α–Induced PCNA Expression
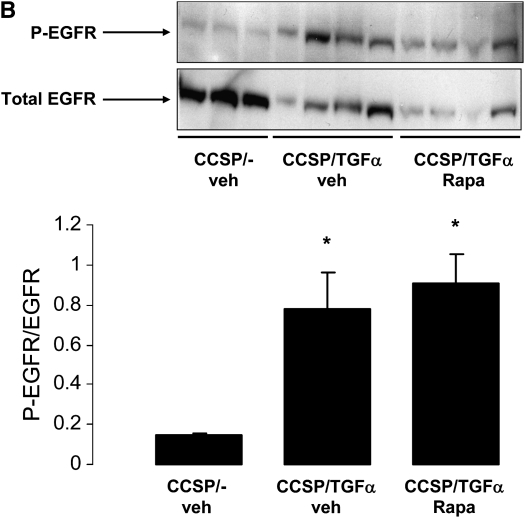
CCSP/TGF-α mice pretreated with erlotinib (100 mg/kg) then administered Dox for 24 hours demonstrated reduced p-Akt, p-P70S6K, and total PCNA in whole lung homogenates compared with vehicle-treated CCSP/TGF-α mice (Figure 2A). CCSP/TGF-α mice pretreated with rapamycin (4 mg/kg) then administered Dox for 24 hours demonstrated reduced p-P70S6K and PCNA in whole lung homogenates compared with vehicle-treated CCSP/TGF-α mice with no change in p-Akt (Figure 2A), indicating that TGF-α–induced proliferation was mediated through the mTOR pathway. Administration of Dox and rapamycin did not alter increased phosphorylation of EGFR in lung homogenates of CCSP/TGF-α mice (Figure 2B).
Figure 2.
Erlotinib and rapamycin inhibit TGF-α–induced phosphorylation of P70S6K and PCNA. (A) Pretreatment of CCSP/TGF-α mice with erlotinib (100 mg/kg) prevented increased phosphorylation of Akt and P70S6K and increased levels of PCNA after 1 day of Dox. Pretreatment of CCSP/TGF-α mice with rapamycin (Rapa, 4 mg/kg) did not alter phosphorylation of Akt, but prevented increased phosphorylation of P70S6K and increased levels of PCNA after 1 day of Dox. (B) Rapamycin did not effect phosphorylation of EGFR (Y1086) in lung homogenates after 1 day of Dox. Mean ± SE, *P < 0.05 compared with vehicle (veh)-treated (the rapamycin vehicle or 0.25% PEG400, 0.25% Tween 20) CCSP/- controls.
Rapamycin Prevents TGF-α–Induced Pulmonary Fibrosis
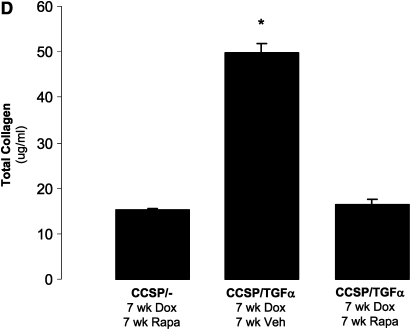
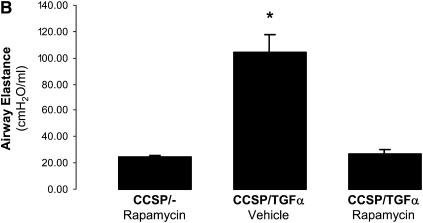
CCSP/TGF-α mice were treated with Dox to induce TGF-α expression and concomitantly treated daily with either vehicle or rapamycin (4 mg/kg 6 d/wk) for 7 weeks (Figure 3A). Control mice were CCSP/- mice administered 7 weeks of Dox and rapamycin. Body weights of CCSP/TGF-α mice treated with 7 weeks of Dox and administered vehicle decreased 14.7 ± 0.2% from baseline, while CCSP/TGF-α mice treated with 7 weeks of Dox and administered rapamycin increased 4.0 ± 0.1% from baseline, similar to that of CCSP/- mice (+4.9 ± 0.1%, P < 0.001) (Figure 3B). Induction of TGF-α caused extensive fibrosis localized to the pleural surfaces and to the perivascular and peribronchial adventitia. Rapamycin reduced pulmonary fibrosis with minimal residual disease, represented by scattered areas of perivascular pulmonary fibrosis and pleural thickening (Figure 3C). Increases in lung collagen content (Figure 3D) and altered lung mechanics (Figures 4A–4D) in CCSP/TGF-α vehicle-treated mice were all prevented in the rapamycin-treated group.
Figure 3.
Rapamycin prevents TGF-α–dependent pulmonary fibrosis. CCSP/TGF-α mice were administered 7 weeks of Dox, and treated with either rapamycin (4 mg/kg once daily 6 d/wk) or vehicle. Controls were littermate single transgene CCSP/- mice administered Dox and treated with rapamycin. The treatment protocol is represented schematically in A. (B) Rapamycin prevented TGF-α–induced decreases in progressive body weight loss. Sections of lungs were stained with hematoxylin and eosin. (C) CCSP/TGF-α mice administered rapamycin at the initiation of Dox caused a marked attenuation of pulmonary fibrosis compared with CCSP/TGF-α vehicle-treated mice. (D) Rapamycin prevented TGF-α–induced increases in total lung collagen. Photomicrographs shown are representative of lungs from seven mice in each group. All photomicrographs are taken at the same magnification, and bar is 200 μm. *P < 0.05 compared with CCSP/- control and CCSP/TGF-α vehicle-treated mice.
Figure 4.
Rapamycin prevents TGF-α–dependent changes in lung mechanics. Rapamycin administered daily to CCSP/TGF-α mice at the time of Dox treatment prevented TGF-α–mediated (A–C) increases in airway resistance, and airway and tissue elastance, and (D) decreases in compliance compared with CCSP/TGF-α receiving vehicle. *P < 0.05 compared with CCSP/- control and CCSP/TGF-α vehicle-treated mice.
Rapamycin Prevents Progression of Established TGF-α–Induced Pulmonary Fibrosis
To determine whether rapamycin influences the progression of established fibrosis, after 4 weeks of Dox treatment, CCSP/TGF-α mice were administered either daily rapamycin or vehicle while remaining on Dox (Figure 5A). Body weights of CCSP/TGF-α mice treated with vehicle decreased 25% from baseline after 8 weeks of Dox (Figure 5B). Between Weeks 8 and 11, body weight loss stabilized but was likely influenced by the deaths of three severely affected mice. Rapamycin administered at the beginning of Week 5 prevented further body weight loss compared with vehicle-treated mice, but body weights remained less than those of control mice. Lung fibrosis as assessed by histology, total lung collagen, and lung mechanics was improved compared with that of vehicle-treated mice at 7 weeks and 11 weeks, but was unchanged compared with vehicle-treated mice after 4 weeks of Dox (Figures 5C–5D and 6A–6D).
Figure 5.
Rapamycin prevents progression of TGF-α–dependent pulmonary fibrosis. After 4 weeks of Dox, CCSP/TGF-α mice were administered either daily rapamycin or vehicle. Rapamycin-treated mice were evaluated after 3 or 7 weeks of treatment. Vehicle-treated CCSP/TGF-α mice were recovered at 4, 7, and 11 weeks of Dox for comparison. Control mice were CCSP/- mice treated with rapamycin after 4 weeks of Dox. The treatment protocol is represented schematically in A. (B) Dox-induced expression of TGF-α caused progressive weight loss. Body weight stabilized in mice treated with rapamycin 4 weeks after treatment with Dox. Sections of lungs were stained with hematoxylin and eosin. (C) Expression of TGF-α caused progressive adventitial fibrosis. CCSP/TGF-α mice administered rapamycin after 4 weeks of Dox demonstrated reduced (C) adventitial fibrosis and (D) total lung collagen compared with CCSP/TGF-α vehicle-treated mice receiving 7 or 11 weeks of Dox. *P < 0.05 compared with rapamycin-treated controls. +P < 0.05 compared with 7 and 11 week vehicle-treated CCSP/TGF-α mice. Small images of dead mice in B denote mouse death during week.
Figure 6.
Rapamycin prevents progression of TGF-α–dependent changes in lung mechanics. CCSP/TGF-α transgenic mice administered rapamycin 4 weeks after treatment with Dox demonstrated (A–C) reduced increases in airway resistance, and airway and tissue elastance, and (D) decreases in compliance compared with vehicle-treated CCSP/TGF-α mice receiving 7 and 11 weeks of Dox. *P < 0.05 compared with CCSP/- control mice; + P < 0.05 compared with 7 and 11 week vehicle-treated CCSP/TGF-α transgenic mice.
DISCUSSION
Using a transgenic mouse model of pulmonary fibrosis caused by lung specific expression of the EGFR ligand, TGF-α, the present study demonstrates that administration of rapamycin prevents both the initiation and the progression of established pulmonary fibrosis and associated alterations in lung mechanics. Increased expression of EGFR ligands and activation of EGFR have been implicated in the pathogenesis of pulmonary fibrosis in a number of animal models including bleomycin, naphthalene, asbestosis, and ovalbumin-induced lung injury (15–18). Signaling pathways downstream of EGFR have not been identified in these models. Our findings demonstrate the activation of the PI3K-Akt-mTOR pathway in association with the induction of EGFR-mediated pulmonary fibrosis. The efficacy of rapamycin in preventing TGF-α–induced pulmonary fibrosis was similar to a previous study in which the EGFR inhibitors gefitinib and erlotinib also prevented fibrosis in the CCSP/TGF-α transgenic model (19). Together, these data support mTOR as a major effector of EGFR-mediated pulmonary fibrosis.
The antifibrotic effects of mTOR inhibition have recently been reported in several rat models of chronic kidney disease, including diabetic nephropathy, chronic glomerulosclerosis, and tubulointerstitial fibrosis (20–22). Likewise, rapamycin prevented extracellular matrix deposition in CCL4-induced liver fibrosis in rats (23). In rat models of established liver cirrhosis, rapamycin reduced fibrosis and attenuated disease progression (24). In pulmonary fibrosis models there are limited data on activation of mTOR and the effectiveness of rapamycin treatment. The rapamycin analog SDZ RAD prevented bleomycin-induced pulmonary fibrosis, although it was unclear whether changes in lung inflammation may have contributed to these improvements (25). CCSP/TGF-α transgenic mice develop fibrosis independent of inflammation (5, 6). Therefore, the efficacy of rapamycin in this study is unlikely to be attributed to the anti-inflammatory properties of rapamycin.
Fibrosis in the CCSP/TGF-α transgenic model is progressive, allowing assessment of rapamycin in reversing established and accumulating fibrosis. The effectiveness of mTOR inhibition in established and ongoing lung fibrosis has not been previously studied. Mice treated with 3 weeks of rapamycin after 4 weeks of Dox had significantly reduced cachexia, lung collagen, and changes in lung mechanics compared with mice receiving Dox and vehicle for 7 and 11 weeks (Figures 5 and 6). However, lung collagen and lung mechanics in these rapamycin-treated mice were not different from those of CCSP/TGF-α mice after 4 weeks of Dox. In our previous study, lung collagen accumulation and abnormalities in lung mechanics were partially reversed by removing dox treatment without any additional pharmacologic intervention, showing that TGF-α–induced lesions are at least partially reversible (6). Since the rapamycin treatment did not completely reverse fibrotic changes, which were present in mice treated with Dox for 4 weeks, we cannot determine if rapamycin is inhibiting progression or partially reversing established fibrotic changes. Mice that received an additional 4 weeks of rapamycin (7 wk total) did not show further benefit. Together, these results demonstrate that rapamycin was effective in preventing the progression of fibrosis and improvements were maintained. An extended rapamycin treatment period or increased rapamycin doses may be necessary to further reduce the extent of fibrosis. However, in CCSP/TGF-α mice treated with the EGFR inhibitor gefitinib in a late treatment study, fibrosis was not completely reversed (19). Thus, some degree of pulmonary fibrosis in the CCSP/TGF-α model may be irreversible. Alternatively, additional profibrotic pathways activated after the establishment of fibrosis may remain active after removal of EGFR or mTOR signaling.
The mTOR regulates the rate of cell growth, proliferation, and protein synthesis in a number of cell lines, including fibroblasts, vascular smooth muscle, and epithelial cells (22, 26). Pulmonary fibrosis in the TGF-α model is characterized by epithelial and mesenchymal proliferation and increased extracellular matrix deposition (6, 27), and both processes were effectively inhibited with rapamycin administration in the prevention studies. Although mTOR is generally considered a downstream substrate of Akt, there is increasing evidence that mTOR signals independently of Akt activation through the ERK-mediated pathway (26, 28). Both S6K and 4E-BP1 possess several phosphorylation sites and are activated not only by mTOR, but also by PI3K and ERK (9, 29). Additional studies using specific pathway component inhibitors, phosphorylation assays, and specific rescue experiments in vitro will be needed to precisely identify the signaling pathways leading to mTOR activation. In addition, studies of gene targets of mTOR will be needed to further identify other fibrotic components causing fibrosis in this model.
In addition to EGFR, the mTOR pathway may be regulated by multiple fibrotic activators. Although the Smad pathway is believed to be the primary conduit for signals from the TGF-β1 receptors, emerging evidence supports the importance of non-SMAD pathways. Fibroblasts stimulated with TGF-β1 demonstrate Smad-independent proliferation and matrix protein production which are regulated by MAPK and PI3K pathways (23, 30, 31). Pulmonary fibrosis caused by TGF-β1 was attenuated when mice were treated with an Akt inhibitor (32). Platelet-derived growth factors are profibrotic cytokines that have also been implicated in inflammatory models of lung fibrosis (33, 34). Platelet-derived growth factors act via two receptors which, like EGFR, are receptor tyrosine kinases and activate PI3K and MAPK (35). Future studies targeting mTOR, S6K, and 4E-BP1 will be valuable in determining whether the mTOR pathway influences diverse fibrotic processes in the lung.
Rapamycin and related analogs are currently used clinically as an immunomodulating agent for kidney transplant (9). Abnormal activation of signaling pathways of mTOR appears to occur frequently in human cancer, which led to the evaluation of the antiproliferative effects of rapamycin in malignant neoplasms. EGFR inhibitors combined with rapamycin analogs are in trials in lung cancer, with the rationale that mTOR inhibition may rescue proliferation pathways that remain activated in tumors resistant to EGFR-specific tyrosine kinase inhibitors (36). The utility of mTOR inhibition in clinical pulmonary disease is currently under investigation. Rapamycin was used to successfully treat a patient with idiopathic pulmonary fibrosis (IPF) (37). The effectiveness of rapamycin for IPF is currently under investigation in a randomized clinical trial (38). Lymphangioleiomyomatosis (LAM) is a progressive lung disease characterized by infiltration of abnormal smooth muscle–like cells and formation of parenchymal cysts. Inhibition of the mTOR pathway with Sirolimus improved spirometric measurements and gas trapping in LAM patients that persisted after cessation of treatment (39).
The predominant pathways involved in experimental fibrosis and the response to pharmacologic agents varies widely with the specific strain of mice used and with the specific pro-fibrotic challenge. Results from this study using FVB/N mice need to be tested in additional mouse strains and other models of pulmonary fibrosis before extrapolating results form this study directly to human disease.
In summary, the present study demonstrates that rapamycin prevents and inhibits progression of ongoing pulmonary fibrosis caused by expression of TGF-α and increased EGFR signaling. These findings support the need for further studies to carefully determine the role of mTOR activation in the pathogenesis of pulmonary fibrosis and to assess the efficacy of therapies designed to inhibit its activity.
Acknowledgments
The authors thank Matt Fenchel, who provided assistance in statistical analysis of data, and technical assistance in measuring lung mechanics from Angelica Falcone.
This work was supported by National Institutes of Health grants: HL086598 (W.D.H.), HL061646 (M.I.), HL058795 (T.R.K.), HL90156 (J.A.W.) and HL61646 (J.A.W.), and American Heart Association grant 0740069N (T.D.L.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0377OC on February 24, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Cook DN, Brass DM, Schwartz DA. A matrix for new ideas in pulmonary fibrosis. Am J Respir Cell Mol Biol 2002;27:122–124. [DOI] [PubMed] [Google Scholar]
- 2.Crystal RG, Gadek JE, Ferrans VJ, Fulmer JD, Line BR, Hunninghake GW. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med 1981;70:542–568. [DOI] [PubMed] [Google Scholar]
- 3.Crystal RG, Bitterman PB, Mossman B, Schwarz MI, Sheppard D, Almasy L, Chapman HA, Friedman SL, King TE Jr, Leinwand LA, et al. Future research directions in idiopathic pulmonary fibrosis: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2002;166:236–246. [DOI] [PubMed] [Google Scholar]
- 4.Kuwano K, Hagimoto N, Hara N. Molecular mechanisms of pulmonary fibrosis and current treatment. Curr Mol Med 2001;1:551–573. [DOI] [PubMed] [Google Scholar]
- 5.Hardie WD, Le Cras TD, Jiang K, Tichelaar JW, Azhar M, Korfhagen TR. Conditional expression of transforming growth factor-alpha in adult mouse lung causes pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2004;286:L741–L749. [DOI] [PubMed] [Google Scholar]
- 6.Hardie WD, Korfhagen TR, Sartor MA, Prestridge A, Medvedovic M, Le Cras TD, Ikegami M, Wesselkamper SC, Davidson C, Dietsch M, et al. Genomic profile of matrix and vasculature remodeling in tgf-{alpha} induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2007;37:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 2003;284:31–53. [DOI] [PubMed] [Google Scholar]
- 8.Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev 2006;20:1496–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther 2007;82:381–388. [DOI] [PubMed] [Google Scholar]
- 10.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 2000;275:11858–11864. [DOI] [PubMed] [Google Scholar]
- 11.Zanesi N, Aqeilan R, Drusco A, Kaou M, Sevignani C, Costinean S, Bortesi L, La Rocca G, Koldovsky P, Volinia S, et al. Effect of rapamycin on mouse chronic lymphocytic leukemia and the development of nonhematopoietic malignancies in Emu-TCL1 transgenic mice. Cancer Res 2006;66:915–920. [DOI] [PubMed] [Google Scholar]
- 12.Garvey W, Fathi A, Bigelow F, Carpenter B, Jimenez C. Improved Movat pentachrome stain. Stain Technol 1986;61:60–62. [DOI] [PubMed] [Google Scholar]
- 13.Schuessler TF, Bates JH. A computer-controlled research ventilator for small animals: design and evaluation. IEEE Trans Biomed Eng 1995;42:860–866. [DOI] [PubMed] [Google Scholar]
- 14.Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl AK, Hull WM, Wert SE, Whitsett JA. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Invest 2004;113:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu JY, Morris GF, Lei WH, Corti M, Brody AR. Up-regulated expression of transforming growth factor-alpha in the bronchiolar-alveolar duct regions of asbestos-exposed rats. Am J Pathol 1996;149:205–217. [PMC free article] [PubMed] [Google Scholar]
- 16.Madtes DK, Elston AL, Hackman RC, Dunn AR, Clark JG. Transforming growth factor-alpha deficiency reduces pulmonary fibrosis in transgenic mice. Am J Respir Cell Mol Biol 1999;20:924–934. [DOI] [PubMed] [Google Scholar]
- 17.Van Winkle LS, Isaac JM, Plopper CG. Distribution of epidermal growth factor receptor and ligands during bronchiolar epithelial repair from naphthalene-induced Clara cell injury in the mouse. Am J Pathol 1997;151:443–459. [PMC free article] [PubMed] [Google Scholar]
- 18.Vargaftig BB, Singer M. Leukotrienes mediate part of Ova-induced lung effects in mice via EGFR. Am J Physiol Lung Cell Mol Physiol 2003;285:L808–L818. [DOI] [PubMed] [Google Scholar]
- 19.Hardie WD, Davidson C, Ikegami M, Leikauf GD, Le Cras TD, Prestridge A, Whitsett JA, Korfhagen TR. EGF receptor tyrosine kinase inhibitors diminish transforming growth factor-alpha-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;294:L1217–L1225. [DOI] [PubMed] [Google Scholar]
- 20.Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, Rama I, Vidal A, Grinyo JM. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 2006;17:1395–1404. [DOI] [PubMed] [Google Scholar]
- 21.Wu MJ, Wen MC, Chiu YT, Chiou YY, Shu KH, Tang MJ. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int 2006;69:2029–2036. [DOI] [PubMed] [Google Scholar]
- 22.Kramer S, Wang-Rosenke Y, Scholl V, Binder E, Loof T, Khadzhynov D, Kawachi H, Shimizu F, Diekmann F, Budde K, et al. Low-dose mTOR inhibition by rapamycin attenuates progression in anti-thy1-induced chronic glomerulosclerosis of the rat. Am J Physiol Renal Physiol 2008;294:F440–F449. [DOI] [PubMed] [Google Scholar]
- 23.Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol 2007;22:S79–S84. [DOI] [PubMed] [Google Scholar]
- 24.Neef M, Ledermann M, Saegesser H, Schneider V, Reichen J. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J Hepatol 2006;45:786–796. [DOI] [PubMed] [Google Scholar]
- 25.Simler NR, Howell DC, Marshall RP, Goldsack NR, Hasleton PS, Laurent GJ, Chambers RC, Egan JJ. The rapamycin analogue SDZ RAD attenuates bleomycin-induced pulmonary fibrosis in rats. Eur Respir J 2002;19:1124–1127. [DOI] [PubMed] [Google Scholar]
- 26.Gerasimovskaya EV, Tucker DA, Stenmark KR. Activation of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferation. J Appl Physiol 2005;98:722–731. [DOI] [PubMed] [Google Scholar]
- 27.Hardie WD, Piljan-Gentle A, Dunlavy MR, Ikegami M, Korfhagen TR. Dose-dependent lung remodeling in transgenic mice expressing transforming growth factor-alpha. Am J Physiol Lung Cell Mol Physiol 2001;281:L1088–L1094. [DOI] [PubMed] [Google Scholar]
- 28.Kayampilly PP, Menon KM. Follicle-stimulating hormone increases tuberin phosphorylation and mammalian target of rapamycin signaling through an extracellular signal-regulated kinase-dependent pathway in rat granulosa cells. Endocrinology 2007;148:3950–3957. [DOI] [PubMed] [Google Scholar]
- 29.Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol 2005;16:29–37. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Salgado C, Fuentes-Calvo I, Garcia-Cenador B, Santos E, Lopez-Novoa JM. Involvement of H- and N-Ras isoforms in transforming growth factor-beta1-induced proliferation and in collagen and fibronectin synthesis. Exp Cell Res 2006;312:2093–2106. [DOI] [PubMed] [Google Scholar]
- 31.Rahimi RA, Leof EB. TGF-beta signaling: a tale of two responses. J Cell Biochem 2007;102:593–608. [DOI] [PubMed] [Google Scholar]
- 32.Kang HR, Lee CG, Homer RJ, Elias JA. Semaphorin 7A plays a critical role in TGF-beta1-induced pulmonary fibrosis. J Exp Med 2007;204:1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice AB, Moomaw CR, Morgan DL, Bonner JC. Specific inhibitors of platelet-derived growth factor or epidermal growth factor receptor tyrosine kinase reduce pulmonary fibrosis in rats. Am J Pathol 1999;155:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, Lee LB, McMahon G, Grone HJ, Lipson KE, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 2005;201:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008;22:1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gridelli C, Maione P, Rossi A. The potential role of mTOR inhibitors in non-small cell lung cancer. Oncologist 2008;13:139–147. [DOI] [PubMed] [Google Scholar]
- 37.Buschhausen L, Kamm M, Arns W, Schulze-Lohoff E, Weber M. Med Klin (Munich) 2005;100:161–164. (Successful treatment of a severe case of idiopathic pulmonary fibrosis with rapamycin). [DOI] [PubMed] [Google Scholar]
- 38.Walter N, Collard HR, King TE Jr. Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc 2006;3:330–338. [DOI] [PubMed] [Google Scholar]
- 39.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 2008;358:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]