Abstract
Alveolar hypoxia produces widespread systemic inflammation in rats. The inflammation appears to be triggered by activation of mast cells by a mediator released from alveolar macrophages, not by the reduced systemic partial pressure of oxygen (PO2). If this is correct, the following should apply: (1) neither mast cells nor tissue macrophages should be directly activated by hypoxia; and (2) mast cells should be activated when in contact with hypoxic alveolar macrophages, but not with hypoxic tissue macrophages. We sought here to determine whether hypoxia activates isolated alveolar macrophages, peritoneal macrophages, and peritoneal mast cells, and to study the response of the microcirculation to supernatants of these cultures. Rat mesenteric microcirculation intravital microscopy was combined with primary cultures of alveolar macrophages, peritoneal macrophages, and peritoneal mast cells. Supernatant of hypoxic alveolar macrophages, but not of hypoxic peritoneal macrophages, produced inflammation in mesentery. Hypoxia induced a respiratory burst in alveolar, but not peritoneal macrophages. Cultured peritoneal mast cells did not degranulate with hypoxia. Immersion of mast cells in supernatant of hypoxic alveolar macrophages, but not in supernatant of hypoxic peritoneal macrophages, induced mast cell degranulation. Hypoxia induced release of monocyte chemoattractant protein-1, a mast cell secretagogue, from alveolar, but not peritoneal macrophages or mast cells. We conclude that a mediator released by hypoxic alveolar macrophages activates mast cells and triggers systemic inflammation. Reduced systemic PO2 and activation of tissue macrophages do not play a role in this phenomenon. The inflammation could contribute to systemic effects of diseases featuring alveolar hypoxia.
Keywords: hypoxia, systemic inflammation, alveolar macrophages, mast cells, monocyte chemoattractant protein
CLINICAL RELEVANCE
This study demonstrates a direct link, via a circulating mediator, between activation of alveolar macrophages by low alveolar PO2, and degranulation of systemic mast cells, the first step in the systemic inflammatory cascade. A candidate mediator, monocyte chemoattractant protein-1, was identified. Roles of low tissue PO2 and activation of tissue macrophages were ruled out. The phenomenon described could participate in the inflammation underlying the systemic effects seen in conditions associated with low alveolar PO2.
Alveolar hypoxia, induced by reduction of inspired PO2, initiates a rapid and widespread inflammatory response in mesentery (1), skeletal muscle (2, 3), and brain (4) of rats. The inflammation is characterized by increased microvascular levels of reactive oxygen species (ROS) (5), perivascular mast cell degranulation (2, 3, 6), increased leukocyte–endothelial adhesive interactions (1), and extravasation of albumin (7).
Studies in the cremaster microcirculation suggest that the inflammation elicited by alveolar hypoxia is not triggered by the reduction of cremaster PO2, but rather by a mediator released from a distant site and transported by the circulation. This idea is supported by two lines of evidence: first, selective reduction of cremaster PO2 does not produce mast cell degranulation and inflammation in the cremaster microcirculation unless alveolar PO2 is also reduced (2, 3); second, plasma obtained from hypoxic rats applied to the normoxic cremaster produces an inflammatory response similar to that elicited by alveolar hypoxia (8). The response to hypoxic rat plasma is not due to inflammatory mediators released into plasma by activated mast cells or adherent leukocytes of the donor rat; furthermore, the agent(s) responsible for the inflammation is not generated by blood cells (8). Further investigation has shown that the putative mediator activates mast cells and initiates an inflammatory cascade that includes activation of the renin–angiotensin (Ang) system (RAS) (9).
A role for alveolar macrophages as a source of the putative mediator is supported by findings that alveolar macrophage depletion in vivo attenuates the inflammatory response to alveolar hypoxia, and that supernatant of alveolar macrophages cultured in hypoxia induces mast cell degranulation and inflammation in normoxic cremaster muscle (10). Similar to the inflammation elicited by alveolar hypoxia (9), the response to alveolar macrophage supernatant was abrogated by pretreatment with cromolyn, a mast cell stabilizer, and with AngII receptor antagonists (10).
The present experiments were designed to provide evidence of a direct link between the activation of alveolar macrophages by the reduced alveolar PO2 and the degranulation of tissue mast cells, which initiates the inflammation of hypoxia. We reasoned that, if alveolar macrophage–borne mediator(s) triggers the inflammation by activating mast cells, reduced PO2 would not directly activate primary mast cell cultures; on the other hand, these cells would degranulate when placed in contact with supernatant of hypoxic alveolar macrophages. Furthermore, we hypothesized that hypoxia would not directly activate isolated resident tissue macrophages, and that mast cells exposed to supernatant of resident tissue macrophages cultured in hypoxia would not undergo degranulation. The results confirm our hypothesis that the inflammation of alveolar hypoxia is triggered by mediator(s) released by activated alveolar macrophages, and rule out possible contributions of local hypoxia and of resident tissue macrophages in the initiation of the inflammation. A mast cell secretagogue, monocyte chemoattractant protein (MCP)-1, was identified as a possible candidate for the putative mediator of hypoxia-induced systemic inflammation.
The phenomenon described here highlights a systemic effect of alveolar macrophage activation, and could provide a possible pathogenic mechanism to explain the systemic consequences of conditions associated with reduced alveolar PO2.
MATERIALS AND METHODS
All procedures were approved by the Animal Care and Use Committee of the University of Kansas Medical Center, an institution accredited by the American Association for Accreditation of Laboratory Animal Care.
The experiments described here included in vivo studies in which supernatant of primary cell cultures, as well as pharmacological agents, were applied topically to the mesentery of normoxic rats, and in vitro studies performed in primary cell cultures.
Alveolar macrophages in an intact animal are normally exposed to a higher PO2 than are peritoneal macrophages or peritoneal mast cells, and this difference subsists in conditions of hypoxia. Accordingly, humidified gas mixtures for the cell culture experiments were used with nominal concentrations of 21, 10, and 0% O2. All gas mixtures contained 5% CO2, with the balance made up with N2. These gas mixtures provided PO2 values that encompass the range observed in vivo, from normoxic to hypoxic conditions, in the cell types studied here. The PO2 attained in the liquid phase during gaseous equilibration in an open system depends on the efficacy of the equilibration system. In the present experiments, the cell cultures, placed in an incubator at 37°C, were gassed via a needle inserted in the cap of the culture dish and connected to the gas source. Care was taken to place the tip of the needle a few millimeters above the surface of the culture medium so as to not disturb the culture. To directly determine the efficacy of the equilibrating system, PO2 of the medium was measured directly in some of the experiments using a phosphorescence decay method (11). This technique is currently employed in our laboratory to measure microvascular PO2 of intact animals (2, 3). The actual supernatant PO2 values were (mean ± SEM): 0% O2: 4.8 ± 0.8 mm Hg; and 10% O2: 65.3 ± 0.9 mm Hg. These values were attained within 2–3 minutes of equilibration. Exposure to 21% O2 produced PO2 values outside the range of the method (>100 mm Hg). Alveolar macrophages may be exposed in vivo to PO2 of approximately 65 mm Hg in moderate cases of hypoxia. In contrast, peritoneal macrophages and mast cells exposed to 10% O2 will be in an environment which is at the high end of the PO2 values observed in vivo. On the other hand, PO2 of approximately 5 mm Hg is the value to which peritoneal macrophages, mast cells, and other systemic tissue cells would be exposed in a rat breathing 10% O2 (2, 3).
Intravital Microscopy
The procedures for intravital microscopy of the mesentery have been described in detail before (1). Briefly, male Sprague-Dawley rats (220–300 g) were anesthetized with urethane (1.5 g/kg body weight intramuscularly). PE-50 catheters (Becton-Dickinson, Sparks, MD) were placed in the jugular vein and carotid artery for injection of solutions and measurement of arterial blood pressure. The abdomen was opened via a midline incision, and the ileo-cecal portion of the intestine was gently drawn out, exteriorized, and mounted on a transparent plastic stage. Single, unbranched, postcapillary venules, with a diameter of 20–40 μm, were selected for microscopic observation. Adherent leukocytes were defined as those remaining stationary for at least 30 seconds. Leukocyte–endothelial adherence (LEA) was expressed as number of adherent leukocytes per 100 μm of venule length. Ruthenium red (5 mg/100 ml) (12) was used to estimate in vivo mast cell degranulation intensity with the AnaliSYS Software System (Soft Imaging Systems Corp., Lakewood, CO). At least five mast cells were analyzed in each field of observation. At the end of the experiment, the rats were killed with an intravenous overdose of 150 mg/kg sodium pentobarbital.
Culture of Isolated Alveolar Macrophages
Male Sprague-Dawley rats (300–350 g) were anesthetized with pentobarbital sodium (35 mg/kg, intraperitoneal). After placement of a PE-50 catheter in the jugular vein, a tracheotomy was performed, and a PE-240 catheter placed in the trachea. An overdose of pentobarbital sodium (150 mg/kg) was injected intravenously and bronchoalveolar lavage was performed, as previously described in detail (10). The cells were plated in a T-25 sterile flask at 37°C and equilibrated with 5% CO2 in air for 45 minutes until the macrophages were firmly adhered to the flask. The supernatant was replaced and, depending on the experimental protocol, the cell culture was equilibrated with humidified gas mixtures with nominal concentrations of 0, 10, or 21% O2 for 30 or 60 minutes. The supernatant was then removed and frozen at −70°C until use, or the cultures were used on the same day.
Culture of Peritoneal Mast Cells and Peritoneal Macrophages
Peritoneal macrophages and peritoneal mast cells were harvested by lavage of the peritoneal cavity in rats anesthetized with urethane (1.5 g/kg, intramuscularly). The macrophages were separated from mast cells by differential centrifugation using a Percoll solution (13). Mast cells isolated by this procedure exceed 95% purity. Macrophages isolated had essentially no contamination with mast cells.
The separated mast cells and peritoneal macrophages were resuspended in 2 ml of Dulbecco's modified Eagles medium with 10% serum and plated in a T-25 sterile flask at 37°C in 10% O2–5% CO2–85% N2 for 45 minutes. The supernatant was discarded with a pipette and replaced with 2 ml of serum-free Dulbecco's modified Eagles medium. The cell cultures were equilibrated with humidified mixtures with nominal O2 concentrations of 10% (normoxia) or 0% (hypoxia). Depending on the experimental protocol, the supernatant was removed and frozen at −70°C until use, or the cultures were used on the same day.
Measurement of H2O2 in Supernatant of Alveolar and Peritoneal Macrophages
An electrochemical detection system (Apollo 4000; World Precision Instruments, Sarasota, FL) was used to determine H2O2 concentration in 0.3 ml aliquots of supernatant of alveolar and peritoneal macrophages isolated and cultured on the day of measurement. Measurements were performed in samples removed at 15-minute intervals using an H2O2-sensitive electrode at 37°C. After measurement, the sample was returned to the culture dish. When the experiments were finished, 2 ml of a solution of 0.4% Trypan blue was added to the culture and mixed for 2 minutes. Photographs of five different areas of the culture, containing approximately 200 cells each, were obtained within 30 minutes of Trypan blue addition. Cell viability was expressed as the percentage of cells excluding Trypan blue.
Measurement of Inflammatory Mediators
The effect of hypoxia on supernatant levels of several cytokines and chemokines was investigated by initially screening cytokine and chemokine levels with a multianalyte ELISA Array (SABiosciences Corp., Frederick, MD). Agents investigated in the initial screen included IL-1β, -4, -6, -10, -12, and -17α, INF-r, TNF-α, transforming growth factor-B1, MCP-1, and macrophage inflammatory protein (MIP)-1α and -1β. The initial screen was followed by individual assay (Single Analyte ELISA; SABiosciences Corp.) to determine levels of MCP-1 in supernatant of alveolar macrophages, peritoneal macrophages, and peritoneal mast cells.
Statistical Analysis
Data are presented as means (±SEM). In both the in vivo and in vitro experiments, each preparation served as its own control, with the data obtained after a given experimental treatment compared with the average of the values obtained during the control period in the same experiment. Significance was established using a t test for paired values. Intergroup comparisons were made with a one-way ANOVA with the Bonferroni correction for multiple comparisons.
RESULTS
Experiments in the Mesenteric Microcirculation
All experiments of this series had the same format: after a 30-minute control period, approximately 0.5 ml of supernatant or of a pharmacological agent was distributed evenly over the mesentery. The microcirculation was observed for an additional 30 minutes. The supernatants used had been frozen, and were thawed at 37°C immediately before use.
Effect of alveolar or peritoneal macrophage supernatant applied on the mesentery of normoxic rats.
Supernatant of alveolar macrophages, which had been equilibrated with 10% O2, produced mast cell degranulation and LEA (Figure 1A) (n = 5 rats studied). In contrast, neither supernatant from alveolar macrophages equilibrated with 21% O2 (Figure 1B, n = 5) or from peritoneal macrophages equilibrated with 0% O2, (Figure 1C, n = 5) produced mast cell degranulation or LEA.
Figure 1.
Effect of topical application of alveolar macrophages and peritoneal macrophages supernatant on the mesentery. Top: representative microphotographs of the mesenteric microcirculation after topical application of supernatant hypoxic alveolar macrophages (A), normoxic alveolar macrophages (B), and hypoxic peritoneal macrophages (C). The large dots are used to align the optical Doppler velocimeter, and, occasionally, they are moved to obtain a better image of the leukocyte–endothelial interface for photographs. The red arrows point to the mast cells; the blue arrows identify adherent leukocytes. Bottom: average values of leukocyte–endothelial adherence (LEA), leukocytes/100 μm, and intensity of mast cell degranulation (MCD) in arbitrary units. Presented are mean (±SEM) values at the end of the control (C) and experimental (E) periods, respectively; n = 5 rats studied in each group. **P < 0.01; ***P < 0.001.
Role of the RAS on the response of the microcirculation to hypoxic alveolar macrophage supernatant.
Topical application of AngII (100 nM) produced an increase in LEA without producing mast cell degranulation (Figure 2A, n = 5). This indicates that the effect of AngII occurs at the leukocyte–endothelial interface, and is not mediated by mast cell activation.
Figure 2.
Role of the renin–angiotensin (Ang) system (RAS) on the response of the mesentery to hypoxic alveolar macrophage supernatant. Top: representative microphotographs of the mesenteric microcirculation after topical application of 10 nM AngII (A), hypoxic alveolar macrophage supernatant in rats pretreated with AngII receptor blocker (B), and hypoxic alveolar macrophage supernatant in rats treated with apocynin (C). The red arrows point to the mast cells; the blue arrows identify adherent leukocytes. Bottom: average values of LEA, leukocytes/100 μm, and intensity of MCD in arbitrary units. Presented are means (±SEM) of values at the end of the control (C) and the experimental (E) period, respectively; n = 5 in all cases. *P < 0.05; ***P < 0.001.
Application of supernatant of alveolar macrophages equilibrated with 10% O2 to the mesentery of rats pretreated with the nonspecific angiotensin II (ANGII) receptor antagonist Sar1,Thr8, AngII, continuously infused intravenously at a rate of 30 μg−1 · kg−1 · min−1 (Figure 2B, n = 5) or with the nicotinamide adenine dinucleotide phosphate reduced (NADPH) oxidase inhibitor, apocynin (1 mM applied topically; Figure 2C, n = 5), essentially produced the same results: although mast cell degranulation occurred in response to the application of supernatant, no increase in LEA was observed.
Figure 3 shows the effects of AngII receptor blockade (Figure 3A, n = 5) and of apocynin (Figure 3B, n = 5) on the response of the microcirculation to the basic mast cell secretagogue C4880 (14). When applied to the mesentery or cremaster microcirculation, C4880 induces mast cell degranulation and LEA (2, 6). In the present experiments, pretreatment with AngII receptor blocker (Figure 3A) or with apocynin (Figure 3B) did not prevent the mast cell degranulation, but inhibited the LEA induced by C4880.
Figure 3.
Role of the RAS on the microvascular response to mast cell secretagogue C4880. Top: representative microphotographs of mesenteric microcirculation illustrating the effect of topical application of C4880 after pretreatment with AngII receptor blocker (A), or with apocynin (B). Bottom: average values of LEA, leukocytes/100 μm, and intensity of MCD in arbitrary units. Presented are means (±SEM) of values at the end of the control (C) and the experimental (E) period, respectively; n = 5 in all cases. *P < 0.05; **P < 0.01.
These results indicate that, as seen in the cremaster (9, 10), the increased LEA produced in the mesentery by hypoxic alveolar macrophage supernatant is mediated by activation of the RAS; the RAS is activated by mast cell degranulation induced, in turn, by an agent released into the supernatant by hypoxic alveolar macrophages. The effectiveness of the NADPH oxidase antagonist, apocynin, on the inflammatory response further suggests that the effects of RAS activation are mediated by NADPH oxidase assembly.
Experiments in Primary Cell Cultures
H2O2 release by isolated alveolar and peritoneal macrophages.
All the experiments in this series had the same format: after a 45-minute period of equilibration with 21% O2 for alveolar and 10% O2 for peritoneal macrophages, the cultures were equilibrated for 60 minutes with the experimental gas mixture. Samples for supernatant H2O2 concentration measurement were obtained at the end of the normoxic control period and every 15 minutes thereafter.
Table 1 shows the changes in supernatant H2O2 concentration with respect to their respective control samples in the different groups. Under control conditions (21% O2), alveolar macrophage supernatant H2O2 concentration averaged 0.23 (±0.05) nM/liter/106 cells, without significant differences among groups. Continuation of exposure to 21% O2 for 1 additional hour resulted in a gradual decrease in H2O2 concentration of the supernatant. Hypoxia (nominal 10% O2 and 0% O2) produced a significant net increase in alveolar macrophage supernatant H2O2 concentration. The increase was significantly higher when the nominal O2 concentration of the equilibrating gas mixture was 0% (actual PO2, ∼5 mm Hg) than when equilibration was performed with 10% O2 (actual PO2, ∼65 mm Hg). The increase was transitory, reaching a peak at 15 minutes of equilibration, and gradually returning toward control. The increase in supernatant H2O2 produced by equilibration with 10% O2 was blocked by pretreatment with polyethylene glycol catalase. Viability of alveolar macrophages, assessed by Trypan blue exclusion at the end of the experiments, was higher than 95%, and was not influenced by the PO2, even when it reached values as low as approximately 5 mm Hg.
TABLE 1.
EFFECTS OF HYPOXIA ON H2O2 RELEASE BY ALVEOLAR AND PERITONEAL MACROPHAGES
| Supernatant Δ [H2O2]* (nM/106 cells) |
No. of Cultures | ||||||
|---|---|---|---|---|---|---|---|
| Cell Type | % O2 | 15 min | 30 min | 45 min | 60 min | % Viability | |
| Alveolar macrophages | 21 | −0.03 ± 0.02 | −0.11 ± 0.03† | −0.12 ± 0.03† | −0.09 ± 0.04‡ | 95.8 ± 0.4 | 5 |
| 10 | 0.28 ± 0.07† | 0.09 ± 0.03‡ | −0.01 ± 0.03 | −0.06 ± 0.04 | 96.9 ± 0.3 | 5 | |
| 0 | 1.05 ± 0.13§ | −0.03 ± −0.02 | −0.02 ± 0.02 | −0.02 ± 0.02 | 98.6 ± 0.6 | 5 | |
| 10 + CAT | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.00 ± 0.02 | −0.01 ± 0.02 | 96.9 ± 0.5 | 5 | |
| Peritoneal macrophages | 10 | −0.02 ± 0.01 | −0.02 ± 0.02 | −0.01 ± 0.02 | −0.02 ± 0.02 | 95.9 ± 0.9 | 5 |
| 0 |
0.01 ± 0.01 |
0.01 ± 0.01 |
−0.01 ± 0.01 |
0.01 ± 0.02 |
95.2 ± 0.5 |
5 |
|
Definition of abbreviation: CAT, catalase.
Changes in the concentration of H2O2 with respect to the control; each value (n = 5) is the average of the value at a given time minus the corresponding control.
P < 0.01.
P < 0.05.
P < 0.001.
Average peritoneal macrophage supernatant H2O2 concentration under control conditions (10% O2) was 0.31 (±0.05) nM/liter/106 cells, which was not significantly different from that observed under control conditions (21% O2) in alveolar macrophages. However, in contrast with alveolar macrophages, equilibration of peritoneal macrophages with 0% O2 was not accompanied by H2O2 release into the supernatant. Cell viability at the end of 1 hour of equilibration was also approximately 95%, and was not influenced by the supernatant PO2.
Because H2O2 activates mast cells and induces inflammation, additional experiments in the mesenteric microcirculation were performed to determine whether the small amount of H2O2 present in the alveolar macrophage supernatant after 30 minutes of hypoxia could be responsible for the inflammatory response illustrated in Figure 1A. In those experiments, supernatant of alveolar macrophages equilibrated with 10% O2 was applied topically to the mesentery. Topical application of a solution of 10 nM H2O2, a concentration over fivefold higher than that observed in the alveolar macrophage supernatant at 30 minutes of 10% O2 equilibration, did not increase LEA or mast cell degranulation above that of the untreated control samples (compare Figures 4A and 4B). Only when a solution with a concentration of 10 μM was applied did LEA and mast cell degranulation become evident (Figure 4C, n = 5).
Figure 4.
Effect of topical H2O2 on the normoxic mesentery. Top: representative microphotographs of the mesenteric microcirculation illustrating the effect of topical application of H2O2. (A) Untreated, (B) after application of 10 nM H2O2, and (C) after application of 10 μM H2O2. Because H2O2 bleaches ruthenium red, the photographs were taken after the H2O2 was washed out from the surface, after which ruthenium red was applied. Bottom: average values of LEA, leukocytes /100 μm, and intensity of MCD in arbitrary units. n = 5 in all cases. **P < 0.01; ***P < 0.001.
Effect of hypoxia on peritoneal mast cell degranulation.
Fresh peritoneal mast cell cultures (Figure 5, n = 5 cell cultures studied) were successively exposed to gas mixtures with 10 and 0% O2, and 0% O2 plus C4880. After 30 minutes of exposure to each condition, a small sample (5–10 μl, ∼20,000 cells/μl) was removed and mixed with an equal volume of a solution of ruthenium red to assess mast cell degranulation. Exposure of peritoneal mast cells to 0% O2 did not significantly increase uptake of ruthenium red from the levels observed during normoxic equilibration (compare Figures 5A and 5B). Addition of C4880 during hypoxia to cells obtained from the same cultures induced complete mast cell degranulation (Figure 5C), indicating that the hypoxic mast cells could respond normally to a general secretagogue.
Figure 5.
Effect of hypoxia on MCD. Representative photomicrographs of primary mast cell cultures. The cells represented in A–C were obtained from the same primary culture. The data on top of each photograph is the mean (±SEM) of the percent MCD in five separate experiments. (A) Normoxia (MCD = 10.0 ± 3.5%; n = 5); (B) hypoxia (MCD = 8.2 ± 1.6%; n = 5); (C) hypoxia plus C48/80 (MCD = 100 ± 0%; n = 5).
Interactions between isolated mast cells, alveolar macrophages, and peritoneal macrophages.
In these series, mast cells cultured in 10% O2 were centrifuged, and the pellet suspended in supernatant removed from fresh primary cell cultures of alveolar or peritoneal macrophages. The supernatants contained no macrophages. The mast cells resuspended in macrophage supernatant were equilibrated with 10% O2 in all cases.
Suspension of isolated mast cells in supernatant of alveolar macrophages that had been cultured in normoxia did not induce mast cell degranulation (Figure 6A, n = 5 cell cultures studied); on the other hand, suspension in supernatant of alveolar macrophages cultured in hypoxia (10% O2) significantly increased mast cell degranulation (Figure 6B, n = 5). In contrast, suspension of mast cells in supernatant of peritoneal macrophages that had been incubated with 0% O2 failed to produce mast cell degranulation (Figure 6C, n = 5).
Figure 6.
Effect of immersion of mast cells in supernatant of alveolar or peritoneal macrophages. Mast cells obtained for the same culture were immersed in supernatant of alveolar macrophages that had been equilibrated with 21% O2 (A), 10% O2 (B), or peritoneal macrophages equilibrated with 0% O2 (C). The supernatants contained no macrophages, and, after mast cell immersion, were equilibrated with 10% O2. The data above each photograph is the mean (±SEM) of the percent of MCD in five separate experiments.
Effects of hypoxia on selected cytokine levels.
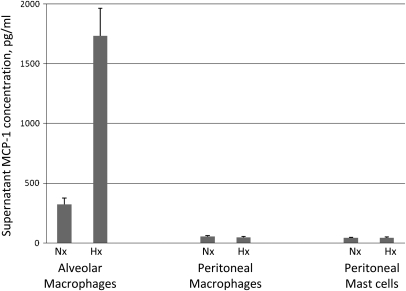
Of all the agents screened in the supernatant of alveolar macrophages using a multianalyte ELISA array (IL-1β, -4, -6, -10, -12, and -17α, IFN-γ, MCP-1, TNF-α, and MIP-1α and -1β), only MCP-1 showed a marked increase with hypoxia. Subsequent individual ELISA assays using a specific antibody showed that MCP-1 increased almost fourfold in the supernatant of alveolar macrophages exposed to hypoxia compared with the normoxic control (Figure 7). In contrast, equilibration with nominal 0% O2 does not elicit release of MCP-1 from peritoneal macrophages or mast cells (Figure 7).
Figure 7.
Effect of hypoxia on the release of monocyte chemoattractant protein (MCP)-1 by alveolar macrophages. Supernatant MCP-1 concentration of primary cultures of alveolar macrophages, peritoneal macrophages, and peritoneal mast cells exposed to 30 minutes of hypoxia (Hx) or normoxia (Nx) (n = 5 in each case).
DISCUSSION
The central findings of these experiments are the following: (1) supernatant of hypoxic alveolar macrophages elicited inflammation in the normoxic mesentery, whereas supernatant of peritoneal macrophages equilibrated with even lower PO2 values had no inflammatory effect; (2) hypoxia activated primary cultures of alveolar, but not of peritoneal macrophages; (3) mast cells in primary cultures did not degranulate when exposed to hypoxia, although degranulation occurred when mast cells were placed in contact with supernatant of hypoxic alveolar macrophages, but not of hypoxic peritoneal macrophages; (4) hypoxia induced release of MCP-1 from alveolar, but not peritoneal macrophages or mast cells.
These results demonstrate a direct link between alveolar macrophage activation by hypoxia and mast cell degranulation, and rule out possible roles of low tissue PO2 and of resident tissue macrophages in the early phase of the inflammation of alveolar hypoxia. The results add a key piece of evidence that confirms our hypothesis that the inflammation produced by alveolar hypoxia is triggered by alveolar macrophage–borne mediator(s) carried by the circulation. The release by alveolar macrophages exposed to hypoxia of a mast cell secretagogue, MCP-1, provides evidence concerning the nature of a possible mediator of inflammation.
Studies in the Mesenteric Microcirculation
There were two objectives of the in vivo studies in the mesenteric microcirculation. First, as the mast cells and resident tissue macrophages used in the present experiments were collected from the peritoneum, it was important to determine whether the responses of the primary cell cultures to the various interventions were similar to those observed in vivo. Peritoneal mast cells and macrophages were selected because they can be harvested with minimal manipulation, and, therefore, their responses are least affected by the isolation procedures. The second objective of the in vivo studies was to demonstrate whether the response of the mesenteric microcirculation to hypoxic alveolar macrophage supernatant is similar to that observed previously in the cremaster. A difference in the responses of these two vascular beds would argue against our hypothesis that the widespread inflammation of alveolar hypoxia in the intact animal is triggered by a mediator released into the circulation by activated alveolar macrophages.
Topical application of supernatant of alveolar macrophages equilibrated with 10% O2 produced essentially the same response in the mesentery as that observed previously in the cremaster microcirculation (9): mast cell degranulation, increased LEA (Figure 1A), and activation of the local RAS (Figures 2 and 3). As observed in the cremaster, activation of the RAS in the mesentery is a result of mast cell degranulation (9, 10). The mechanism by which mast cell degranulation activates the RAS is not clear. Possible pathways of activation include mast cell AngI-converting chymase (15–17), or release of renin, which has been demonstrated in myocardial mast cells (18, 19).
In addition to demonstrating the similarity of responses in the cremaster and the mesentery, the present experiments expand our previous findings in the cremaster in two important respects. First, apocynin, a blocker of the assembly of NADPH oxidase, had essentially the same effects as the AngII receptor blocker in preventing the inflammation. This provides further insight into the mechanism of action of AngII by showing that its inflammatory effects are secondary to the assembly of NADPH oxidase. A role for NADPH oxidase–generated ROS has been documented in several conditions characterized by increased RAS activity, including diabetes (20), hypertension (21, 22), and ischemia–reperfusion (17). A second important new finding of these experiments was that supernatant of peritoneal macrophages exposed to a more severe level of hypoxia than alveolar macrophages did not initiate an inflammatory response in the mesentery (Figure 1C). This observation is consistent with the failure of hypoxia to induce a respiratory burst in cultures of peritoneal macrophages (Table 1). These findings, together with the absence of in vivo cremaster inflammation during selective cremaster hypoxia in the presence of normal alveolar PO2 (2, 3), indicate that resident tissue macrophages do not contribute to the initiation of the systemic inflammation of alveolar hypoxia.
The results show that the inflammatory response to supernatant of hypoxic alveolar macrophages has similar characteristics in the mesenteric and in the skeletal muscle microcirculations, and supports the hypothesis that the widespread inflammation of alveolar hypoxia is the result of mast cell activation by an agent released by alveolar macrophages.
Alveolar macrophages, but not peritoneal macrophages, responded with a transitory release of H2O2 when exposed to hypoxia. The H2O2 release is a manifestation of the respiratory burst that occurs during macrophage activation (23), and is characterized by superoxide generation, followed by dismutation to H2O2. In addition to an important role in inactivation of phagocyted pathogens, ROS generated during the respiratory burst are thought to play a role in intracellular signal transduction (24, 25). In the present experiments, the amount of H2O2 released by alveolar macrophages was largest at the lowest PO2. The correlation between the magnitude of the respiratory burst and the severity of hypoxia in the presence of maintained cell viability indicates that this is a biological response of alveolar macrophages to reduced PO2. On the other hand, peritoneal macrophages exposed to the same PO2 did not become activated. The dissimilar effects of hypoxia on activation of the two types of macrophages are paralleled by the different effects of their supernatants on mast cells: whereas supernatant of hypoxic alveolar macrophages elicited degranulation of mast cells both in vivo (Figure 1A) and in vitro (Figure 6B), supernatant of hypoxic peritoneal macrophages exposed to much lower PO2 had none of these effects (Figures 1C and 6C). Thus, evidence obtained both in vivo and in vitro points to a specific effect of reduced PO2 on alveolar macrophages.
The discrepancy between alveolar and peritoneal macrophages in the response to hypoxia is a manifestation of the different characteristics of these two cell types. Although both originate from a common precursor in the bone marrow, alveolar macrophages in vivo are normally exposed to a higher PO2 than peritoneal macrophages. These different environments may, in part, determine their different metabolic patterns (26), and perhaps explain the dissimilar effects of hypoxia.
The present experiments clearly demonstrate a link between activation of alveolar macrophages by hypoxia and mast cell degranulation: degranulation did not occur when mast cells were directly exposed to hypoxia, but was observed when the mast cells were immersed in supernatant of alveolar macrophages that had been equilibrated in hypoxia. This shows that hypoxic alveolar macrophages release an agent that produces mast cell degranulation. This agent is not released by normoxic alveolar macrophages or by hypoxic peritoneal macrophages, which show no evidence of activation. Our earlier in vivo finding, that selective cremaster hypoxia does not induce mast cell degranulation in rats with normal alveolar PO2 (3), agrees with these in vitro observations, and demonstrates that neither reduced local PO2 nor activation of resident tissue macrophages participate in the systemic inflammation of hypoxia.
A key issue in this phenomenon is the nature of the mediator released by alveolar macrophages in response to hypoxia. There is evidence that alveolar hypoxia leads to pulmonary inflammation in rats (27), and alveolar macrophages play an important role in this phenomenon. Reduction of alveolar PO2 in rats leads to increased expression of mRNA for TNF-α, MIP-1β, and intercellular adhesion molecule-1 in the lung, all of which are reduced by depletion of alveolar macrophages (27); hypoxia also increases expression of TNF-α and MIP-1β in isolated alveolar macrophages (28). In addition, hypoxia acts as a synergist in the interaction of several pathogens with alveolar macrophages (29–31). However, although these responses may influence the development of pulmonary and systemic inflammation later on in the course of hypoxia, it is unlikely that the phenomenon described in the present experiments is a consequence of changes in gene expression. Given the rapid onset of the response to hypoxia, and the fact that the targets are the mast cells, the putative mediator of systemic inflammation is likely to be a mast cell secretagogue stored in alveolar macrophages. To provide additional information on this subject, several possible candidates were screened. MCP-1 was observed to increase significantly in the supernatant of alveolar macrophages exposed to hypoxia in the time frame used in these studies. Peritoneal macrophages or mast cells did not release MCP-1 when exposed to even lower PO2. MCP-1, a chemokine of the CC family, fits the criteria for a putative mediator of hypoxia-induced inflammation: MCP-1 induces chemotaxis of alveolar macrophages, mast cells, and human T lymphocytes (32). MCP-1 is released from alveolar macrophages in vitro in response to hypoxia and hypoxia/reoxygenation (33–35), influences distal organ damage in hemorrhagic shock (36), and activates mast cells to elicit microvascular inflammation (37, 38). Further studies are necessary to determine the mechanism underlying the release of MCP-1 by alveolar macrophages, the interaction of MCP-1 with mast cells, and whether other alveolar macrophage–borne agents participate in the activation of mast cells. Nevertheless, the demonstration of increased release of a mast cell secretagogue provides confirmation of our hypothesis that the systemic inflammation of hypoxia is initiated by an alveolar macrophage–borne mediator.
The present results highlight the role of alveolar macrophages in initiating an inflammatory response in the systemic microcirculation. Although most known functions of these cells take place within the lung, mounting evidence indicates that alveolar macrophage activation leads to systemic inflammation and microvascular function impairment. For example, after activation by phagocytosis of particulate matter, cytokines released by alveolar macrophages act on the bone marrow to mobilize platelets and leukocytes, which stimulate the release of acute-phase proteins, and lead to systemic inflammation (39, 40). The phenomenon described in the present study represents an example of systemic effects initiated by activation of alveolar macrophages by another type of stimulus; in this case, reduction of alveolar PO2.
Several conditions featuring alveolar hypoxia are accompanied by systemic effects, and inflammation has been implicated as a causative or contributory factor in these systemic effects. These include the cachexia and muscle wasting (41, 42) and the cardiovascular abnormalities (43) of chronic obstructive pulmonary disease, the suboptimal erythropoiesis of pulmonary fibrosis (44), and the cardiovascular complications of sleep apnea (45, 46). Conditions in which hypoxia develops rapidly are also accompanied by a systemic inflammatory component. For instance, pneumonia is accompanied by systemic inflammation, and elevated circulating inflammatory markers are predictors of subsequent mortality (47, 48). Although these markers may, in part, reflect the response to bacterial infection, it is conceivable that the hypoxic environment in areas of lung consolidation may lead to activation of alveolar macrophages that contribute to the systemic inflammation. Acute altitude illnesses represent another example of systemic inflammatory response to alveolar hypoxia. Rapid ascent to high altitude is increasingly frequent, and may result in acute mountain sickness or high-altitude cerebral edema (49). Although the role of inflammation in these cases is still unclear, the presence of elevated levels of circulating and tissue inflammatory markers (50–52), and the effectiveness of dexamethasone, an anti-inflammatory steroid, in the treatment of acute mountain sickness (49, 53), suggest the contribution of an inflammatory component. Whether activation of alveolar macrophages plays a role in the pathogenesis of these and other conditions associated with alveolar hypoxia should be the subject of further research.
It is important to keep in mind that reduction of alveolar PO2 in an intact organism is a complex stimulus, which sets in motion responses with different time courses; accordingly, a number of mechanisms may participate at different moments in the course of hypoxia. For example, increased leukocyte–endothelial adhesive interactions in response to hypoxia have been demonstrated in isolated human umbilical veins, in which the phenomenon described here clearly does not occur (54). However, the time course of the in vitro responses (hours vs. minutes) is quite different from that described in the present article, suggesting that the underlying mechanisms are different from those described here.
In summary, the present study provides key evidence in support of our hypothesis that the inflammation of alveolar hypoxia is initiated by the release of a mediator from alveolar macrophages. This evidence includes the identification of a possible mediator of this phenomenon, a mast cell secretagogue produced by alveolar macrophages. Further research is necessary to determine whether other agents participate, and to develop tools to antagonize the mediator's effects. This should help in understanding the possible contribution of this phenomenon to the pathogenesis of illnesses associated with reduced alveolar PO2, as well as its role in the overall strategies of adaptation of organisms to alveolar hypoxia.
Supplementary Material
This work was supported by National Heart, Lung, and Blood Institute grant HL-39443 (N.C.G.). Jie Chao is a Pre-Doctoral Fellow of the American Heart Association, Midwest Affiliate.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2008-0417OC on February 24, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Wood JG, Mattioli LF, Gonzalez NC. Hypoxia causes leukocyte adherence to mesenteric venules in non-acclimatized rats but not after acclimatization. J Appl Physiol 1999;86:873–881. [DOI] [PubMed] [Google Scholar]
- 2.Shah S, Allen J, Wood JG, Gonzalez NC. Dissociation between microvascular PO2 and hypoxia-induced microvascular inflammation. J Appl Physiol 2003;94:2323–2329. [DOI] [PubMed] [Google Scholar]
- 3.Dix R, Orth T, Allen J, Wood JG, Gonzalez NC. Activation of mast cells by systemic hypoxia, but not by local hypoxia, mediates increased leukocyte-endothelial adherence in cremaster venules. J Appl Physiol 2003;95:2495–2502. [DOI] [PubMed] [Google Scholar]
- 4.Mc Donald JT, Gonzalez NC, Wood JG. Mast cell degranulation promotes the cerebral microvascular inflammatory response to hypoxia [abstract]. FASEB J 2003;17:A1282. [Google Scholar]
- 5.Wood JG, Johnson JS, Mattioli LF, Gonzalez NC. Systemic hypoxia promotes leukocyte–endothelial adherence via reactive oxidant generation. J Appl Physiol 1999;87:1734–1740. [DOI] [PubMed] [Google Scholar]
- 6.Steiner DR, Gonzalez NC, Wood JG. Mast cells mediate the microvascular inflammatory response to hypoxia. J Appl Physiol 2003;94:325–334. [DOI] [PubMed] [Google Scholar]
- 7.Wood JG, Johnson JS, Mattioli LF, Gonzalez NC. Systemic hypoxia increases leukocyte emigration and vascular permeability in conscious rats. J Appl Physiol 2000;89:1561–1568. [DOI] [PubMed] [Google Scholar]
- 8.Orth T, Allen J, Wood JG, Gonzalez NC. Plasma from conscious hypoxic rats stimulates leukocyte–endothelial interactions in normoxic cremaster venules. J Appl Physiol 2005;99:290–297. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez NC, Allen J, Schmidt EJ, Casillan AJ, Orth T, Wood JG. Role of the renin–angiotensin system in the systemic microvascular inflammation of alveolar hypoxia. Am J Physiol Heart Circ Physiol 2007;292:H2285–H2294. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez NC, Allen JA, Blanco VG, Schmidt EJ, van Rooijen N, Wood JW. Alveolar macrophages are necessary for the systemic inflammation of acute alveolar hypoxia. J Appl Physiol 2007;103:1386–1394. [DOI] [PubMed] [Google Scholar]
- 11.Lo LW, Koch CJ, Wilson DF. Calibration of oxygen-dependent quenching of the phosphorescence of Pd-meso-tetra (4-carboxyphenyl) porphyrin: a phosphor with general application for measuring oxygen concentration in biological systems. Anal Biochem 1996;236:153–160. [DOI] [PubMed] [Google Scholar]
- 12.Shepherd RK, Duling BR. Use of ruthenium red staining to detect mast cell degranulation in vivo. Microcirculation 1995;2:363–370. [DOI] [PubMed] [Google Scholar]
- 13.Poole TJ, Zetter BR. Stimulation of rat peritoneal mast cell migration by tumor-derived peptides. Cancer Res 1983;43:5857–5861. [PubMed] [Google Scholar]
- 14.Ferry X, Brehin S, Kamel R, Kandry Y. G protein–dependent activation of mast cells by peptides and basic secretagogues. Peptides 2002;23:1507–1515. [DOI] [PubMed] [Google Scholar]
- 15.Miyakazi M, Takai S. Tissue angiotensin II generating system by angiotensin-converting enzyme and chymase. J Pharmacol Sci 2006;100:391–397. [DOI] [PubMed] [Google Scholar]
- 16.Balcells E, Meng QC, Johnson WH Jr, Oparil S, Dell'Italia LJ. Angiotensin II formation form ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol Heart Circ Physiol 1997;273:H1769–H1774. [DOI] [PubMed] [Google Scholar]
- 17.Yusof M, Kamada K, Gaskinf FS, Korthuis RJ. Angiotensin II mediates post-ischemic leukocyte–endothelial interactions: role of calcitonin gene–related peptide. Am J Physiol Heart Circ Physiol 2007;292:H3032–H3037. [DOI] [PubMed] [Google Scholar]
- 18.Silver RB, Reid AC, Mackins CJ, Askwith T, Schaefer U, Herzlinger D, Levi R. Mast cells, a unique source of renin. Proc Natl Acad Sci USA 2004;101:13607–13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackins C, Kano S, Seyedi N, Schaefer U, Reid AC, Machida T, Silver AB, Levi R. Cardiac mast cell–derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest 2006;116:1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol 2004;287:R1014–R1030. [DOI] [PubMed] [Google Scholar]
- 21.Ferrario CM, Strawn WB. Role of the renin-angiotensin system and pro-inflammatory mediators in cardiovascular disease. Am J Cardiol 2006;98:121–126. [DOI] [PubMed] [Google Scholar]
- 22.Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling LL. NADPH oxidase–derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal 2002;4:899–914. [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen N, Ma GF, Konttinen YT. Regulation of macrophage activation. Cell Mol Life Sci 2003;60:2334–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwinn MR, Vallyathan V Respiratory burst: role in signal transduction in alveolar macrophages. J Toxicol Environ Health B Crit Rev 2006;9:27–39. [DOI] [PubMed] [Google Scholar]
- 25.Iles KE, Forman HJ. Macrophage signaling and respiratory burst. Immunol Res 2002;26:95–105. [DOI] [PubMed] [Google Scholar]
- 26.Simon LM, Robin ED, Phillips JR, Acevedo J, Axline SG, Theodore J. Enzymatic basis for bioenergetic differences of alveolar versus peritoneal macrophages and enzyme regulation by molecular O2. J Clin Invest 1977;59:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madjpour C, Jewell UR, Kneller S, Ziegler U, Schwenderer R, Booy C, Kläusli L, Pasch T, Schimmer RC, Beck-Schimmer B. Decreased alveolar oxygen induces lung inflammation. Am J Physiol Lung Cell Mol Physiol 2003;284:L360–L367. [DOI] [PubMed] [Google Scholar]
- 28.Van Otteren GM, Standiford TJ, Kunkel SL, Danforth JM, Strieter RM. Alterations of ambient hypoxia modulate the expression of tumor necrosis factor and macrophage inflammatory protein-1α from murine alveolar macrophages. Am J Respir Cell Mol Biol 1995;13:399–409. [DOI] [PubMed] [Google Scholar]
- 29.Agorreta J, Zulueta JJ, Montuenga LM, Garayola M. Adrenomedullin expression in a rat model of acute lung injury induced by hypoxia and LPS. Am J Physiol Lung Cell Mol Physiol 2005;288:L536–L545. [DOI] [PubMed] [Google Scholar]
- 30.Leeper-Woodford SK, Detmer K. Acute hypoxia increases alveolar macrophage tumor necrosis factor activity and alters NF-κB expression. Am J Physiol Lung Cell Mol Physiol 1999;276:L909–L913. [DOI] [PubMed] [Google Scholar]
- 31.Vuichard D, Ganter MT, Schimmer RC, Suter D, Booy C, Reyes L, Pasch T, Beck-Schimmer B. Hypoxia aggravates lipopolysaccharide-induced injury. Clin Exp Immunol 2005;141:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.deBoer WI, Sont JK, van Schadewijk A, Stolk J, van Krieken H, Hiemstra P. Monocyte chemoattractant protein-1, interleukin-8 and chronic airways inflammation in COPD. J Pathol 2000;190:619–626. [DOI] [PubMed] [Google Scholar]
- 33.Mc Courtei AS, Farivar AS, Woolley SM, Merry HE, Wolf PS, Szabo C, Mulligan MS. Poly (ADP) ribose synthetase inhibition in alveolar macrophages undergoing hypoxia and reoxygenation. Exp Mol Pathol 2008;84:141–144. [DOI] [PubMed] [Google Scholar]
- 34.Krishnadasan B, Naidu BV, Byrne K, Fraga C, Verrier ED, Mulligan MS. The role of inflammatory cytokines in lung ischemia–reperfusion injury. J Thorac Cardiovasc Surg 2003;2003:261–272. [DOI] [PubMed] [Google Scholar]
- 35.Zhao M, Fernandez LG, Doctor A, Sharma AK, Zarbock A, Tribble CG, Kron IL, Laubach VE. Alveolar macrophage activation is a key initiation signal for acute ischemia–reperfusion injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L1018–L1026. [DOI] [PubMed] [Google Scholar]
- 36.Frink M, Lu A, Thobe BM, Hsieh Y-C, Choudhry MA, Schwacha MG, Kunkel SL, Chaudry IH. Monocyte chemoattractant protein-1 influences trauma-hemorrhage–induced distal organ damage via regulation of keratinocyte-derived chemokine production. Am J Physiol Regul Integr Comp Physiol 2007;292:R1110–R1116. [DOI] [PubMed] [Google Scholar]
- 37.Wan MX, Wang Y, Liu Q, Schramm R, Thorlacius H. CC chemokines induce P-selectin–dependent neutrophil rolling and recruitment in vivo: intermediary role of mast cells. Br J Pharmacol 2003;138:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conti P, Boucher W, Letourneau R, Feliciani C, Reale M, Barbacane RC, Vlagopoulos P, Bruneau G, Thibault J, Theoharides TC. Monocyte chemoattractant protein-1 provokes mast cell aggregation an 3[H] 5HT release. Immunology 1995;86:434–440. [PMC free article] [PubMed] [Google Scholar]
- 39.Ishii H, Hayashi S, Hogg JC, Fujii T, Goto Y, Sakamoto N, Mukae H, Vincent R, van Eeden SF. Alveolar macrophage–epithelial cell interaction following exposure to atmospheric particles induces the release of mediators involved in monocyte mobilization and recruitment. Resp Res 2005;5:87. Available from: http://respiratory-research.com/content/6/1/87 [DOI] [PMC free article] [PubMed]
- 40.Van Eeden DF, Tan WC, Suwa T, Mukae H, Terashima T, Fuji T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by particulate matter air pollutants. Am J Respir Crit Care Med 2001;164:826–830. [DOI] [PubMed] [Google Scholar]
- 41.Agusti A, Soriano B. COPD as a systemic disease. COPD 2008;5:133–138. [DOI] [PubMed] [Google Scholar]
- 42.Wüst RC, Degens H. Factors contributing to muscle wasting and dysfunction in COPD patients. Int J Chron Obstruct Pulmon Dis 2007;2:289–300. [PMC free article] [PubMed] [Google Scholar]
- 43.Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, Geyer K, Kohansal R, Burghuber OC. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;178:1211–1218. [DOI] [PubMed] [Google Scholar]
- 44.Tsantes A, Tassiopoulos S, Papadhimitriou SI, Bonovas S, Kavalierou L, Vaiapoulos G, Meletis I. Suboptimal erythropoietic response to hypoxemia in idiopathic pulmonary fibrosis. Chest 2003;124:548–553. [DOI] [PubMed] [Google Scholar]
- 45.Morgan BJ. Vascular consequences of intermittent hypoxia. Adv Exp Med Biol 2007;618:69–84. [DOI] [PubMed] [Google Scholar]
- 46.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflamation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 2008;117:2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yende S, D'Angelo J, Kellum JA, Weissfeld L, Fine J, Welch RD, Kong L, Carter M, Angus DC. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med 2008;177:1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez-Serrano S, Dorca J, Coromines M, Carratala J, Gudiol F, Manresa F. Molecular inflammatory responses measured in blood of patients with severe community-acquired pneumonia. Clin Diagn Lab Immunol 2003;10:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basnyat B, Murdoch BR. High altitude illnesses. Lancet 2003;361:1967–1974. [DOI] [PubMed] [Google Scholar]
- 50.Hartmann C, Tschopp M, Fischer R, Bidlingmaier C, Riepl R, Tschopp K, Hartmann H, Endres S, Toepfer M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 2000;12:246–252. [DOI] [PubMed] [Google Scholar]
- 51.Beidleman BA, Muza SR, Fulco CS, Cymerman A, Staab JE, Sawka MN, Lewis SF, Skrinar GS. White blood cell and hormonal responses to 4300 m altitude before and after intermittent altitude exposure. Clin Sci (Lond) 2006;111:163–169. [DOI] [PubMed] [Google Scholar]
- 52.Klausen T, Olsen NV, Poulsen TD, Richalet JP, Pedersen BK. Hypoxemia increases serum interleukin-6 in humans. Eur J Appl Physiol Occup Physiol 1997;76:480–482. [DOI] [PubMed] [Google Scholar]
- 53.Wright AD. Medicine at high altitude. Clin Med 2006;6:604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michiels C, Arnaud T, Remacle J. Endothelial cell responses to hypoxia: initiation of a cascade of cellular interactions. Biochim Biophys Acta 2000;1497:1–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.