Abstract
There is increasing recognition that neurotrophin (NT) signaling occurs in non-neuronal tissues, including airway smooth muscle (ASM). We recently demonstrated that NTs, such as brain-derived neurotrophic factor (BDNF), enhance intracellular Ca2+ ([Ca2+]i) and force regulation in human ASM. Increased NT expression has been observed in airway diseases, such as asthma and allergy. In the present study, we tested the hypothesis that NTs contribute to inflammation-induced enhancement of ASM contractility. Using human ASM cells and real-time fluorescence [Ca2+]i imaging, we examined the contribution of the high-affinity tropomyosin-related kinase and low-affinity, pan-NT p75NTR receptors to [Ca2+]i regulation under control conditions and after exposure to the proinflammatory cytokine TNF-α (20 ng/ml). Exposure to TNF-α enhanced [Ca2+]i responses to agonist (acetylcholine, histamine). Exposure to 10 nM BDNF for even 30 minutes substantially and synergistically enhanced TNF-α effects on [Ca2+]i responses to agonist. Small interfering RNA suppression of tropomyosin-related kinase substantially blunted the effect of BDNF on [Ca2+]i responses to agonist (with greater effect on Ca2+ influx via store-operated Ca2+ entry compared with sarcoplasmic reticulum Ca2+ release) in both control and TNF-α–exposed cells. However, p75NTR suppression by small interfering RNA had no significant effect on [Ca2+]i responses in either cell group. These novel data demonstrate that NTs influence ASM contractility, and suggest a potential role for NTs in airway diseases.
Keywords: neurotrophin/tropomyosin–related kinase, lung inflammation, cytokine, sarcoplasmic reticulum, capacitative calcium entry
Airway inflammation characterizes a spectrum of diseases, including asthma, allergy and emphysema. Increased airway smooth muscle (ASM) contractility in the inflamed airway is responsible, at least in part, for the pathological airway narrowing and increased resistance to airflow in these diseases. ASM contractility is normally determined by regulation of intracellular Ca2+ ([Ca2+]i), which, in turn, involves several mechanisms, including sarcoplasmic reticulum (SR) Ca2+ release and Ca2+ influx (1–3). With airway inflammation induced by cytokines such as TNF-α, several of these regulatory mechanisms may be altered, thus contributing to increased ASM contractility (4–8).
An exciting, new investigative theme in airway physiology and pathophysiology is neurotrophins (NTs), the family of growth factors that includes brain-derived neurotrophic factor (BDNF), NT-3, and NT-4. NTs are well known to be involved in neuronal cell differentiation, morphology, and function (9–11). There is now increasing recognition that NTs and their receptors are expressed and functional in non-neuronal tissues, including ASM (12–17). Altered NT and NT receptor expression have been observed in asthma, allergy, and even in lung cancer (12, 14, 17–19). NTs may be derived from several sources within the lung, such as airway nerves, immune cells, and even ASM itself (15, 17, 20). However, the role of NTs in the lung is still under investigation. We recently reported that exogenous administration of BDNF potentiates agonist-induced [Ca2+]i and force responses in human ASM cells (16), suggesting that ASM is a potential target of NTs. Accordingly, NTs may play a role in enhanced ASM contractility with inflammation. In the present study, we examined the effect of TNF-α on NT signaling in human ASM. Using BDNF as a model NT, we determined expression and function of BDNF receptors (high-affinity tropomyosin-related kinase [TrkB] versus low-affinity p75NTR) with TNF-α, and alterations in [Ca2+]i regulation.
Some of the results in this article were presented in preliminary form at the 2007 American Thoracic Society International Meeting (San Francisco, CA).
MATERIALS AND METHODS
Human ASM Cells
The techniques for isolating human ASM cells have been previously described (21). Briefly, human bronchi were obtained from waste lung specimens incidental to patient surgery. Samples were immersed in ice-cold Hanks' balanced salt solution (HBSS) and the ASM layer dissected under a microscope. ASM tissue was finely minced in ice-cold Ca2+-free HBSS (0 Ca2+ HBSS) and cells isolated using collagenase (21). After ovomucoid/albumin separation, cell pellets were resuspended in Dulbecco's modified Eagles medium (DMEM)/F12 medium with 10% FBS (DMEM complete), centrifuged, resuspended, and seeded overnight in culture flasks or four-well coverglass-bottomed LabTek dishes (Thermo Fisher Scientific, Rochester, NY) in DMEM complete maintained at 37°C (5% CO2, 95% air). Cells were serum deprived for at least 24 hours, and experiments performed within 48 hours thereafter (necessary for TNF-α or BDNF exposure).
The procedure for procurement of waste surgical specimens was reviewed by the Mayo Clinic Institutional Review Board and determined not to be human subjects research, and exempt from institutional review board review.
Immunoprecipitation and Western Analyses
Human ASM cells were rinsed twice with HBSS, harvested, and subjected to sonication for 5 seconds in cell lysis buffer (Cell Signaling Technologies, Beverly, MA) containing 1 μM PMSF, and the resultant supernatant assayed for protein using the DC protein assay (Bio-Rad, Hercules, CA). Lysates (200 μl) were adjusted to contain 200 μg protein and 4 μg rabbit polyclonal anti-TrkB antibody (Abcam, Cambridge, MA) or 4 μg rabbit polyclonal anti-p75NTR antibody (Abcam) added overnight at 4°C with gentle rocking. The antibody–protein complex was then immunoprecipitated for 4 hours using Protein A/G Plus-Agarose (Santa Cruz Biotechnology, Santa Cruz, CA), the pellet washed four times in ice-cold PBS, and resuspended in 50 μl of cell lysis buffer and 16-μl 4× sample buffer. The supernatants were denatured at 100°C for 3 to 5 minutes and 45 μl of sample subjected to SDS-PAGE using the Criterion Gel System (Bio-Rad) and a 4–15% gradient gel. The gels were run at a current of 200 V for 60 minutes followed by transfer to PVDF membranes (Bio-Rad). Membranes were blocked for 1 hour in 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20, followed by incubation with goat polyclonal anti-TrkB (1:1,000 dilution; Neuromics, Minneapolis, MN) or mouse monoclonal anti-p75NTR (1:100 dilution) antibody overnight, and horseradish peroxidase–conjugated secondary antibody for 2 hours. Blots were then visualized by exposure to BioMax XAR film (Eastman Kodak Co., Rochester, NY) using Supersignal West Dura substrate (Pierce Biotechnology, Rockford, IL).
[Ca2+]i Imaging
The techniques for [Ca2+]i imaging of ASM cells have also been previously described (16). Briefly, ASM cells were incubated in 5 μM fura-2/AM (Invitrogen, Carlsbad, CA) and visualized using a real-time fluorescence imaging system (MetaFluor; Universal Imaging, Downingtown, PA) on a Nikon Diaphot inverted microscope. Images were acquired using a Micromax 12-bit camera system (Princeton Instruments, Trenton, NJ). Ratios of Fura 2 emissions at 510 nm when excited at 340 and 380 nm were calculated approximately every 0.75 seconds (1.33 Hz) and used to calculate [Ca2+]I, as previously described (22). Cells were perfused with HBSS and baseline [Ca2+]i level established in 25 cells per chamber using software-defined regions of interest.
Small Interfering RNA Transfection
The techniques for small interfering RNA (siRNA) transfection in human ASM cells has been previously described (21). Briefly, in 1st or 2nd passage human ASM cells grown to 40–50% confluence, negative control or protein-specific (TrkB or p75NTR) siRNAs (50 nM; Ambion) were permitted to form complexes with lipofectamine per the manufacturer's instructions. Culture media were removed and replaced with fresh serum-free DMEM/F12. siRNA and lipofectamine complexes were then added. After 24 hours, transfection medium was removed and replaced with fresh DMEM/F12 containing 10% FBS for an additional 24 hours. Finally, serum-free DMEM/F12 was used for an additional 48 hours, after which experimental protocols were followed. Specific protein suppression was confirmed by Western analysis.
TNF-α Exposure
ASM cells were exposed for 24 hours to either medium alone (control) or 20 ng/ml recombinant human TNF-α (R&D Systems. Minneapolis, MN) overnight. For cells transfected with siRNA, TNF-α was added within the last 24 hours or the serum-free period (see above). Studies on [Ca2+]i regulation were performed in the continued presence of medium alone or TNF-α.
[Ca2+]i Responses to Agonists
ASM cells exposed to medium only (control) or TNF-α were loaded with fura-2/AM and washed in HBSS for 2–3 minutes to verify cell stability. To evaluate the effect of TNF-α on agonist response, in one set of experiments, cells were exposed to 10 nM, 100 nM, or 1 μM ACh, whereas in a second set of experiments, cells were exposed to 100 nM, 1 μM, or 10 μM histamine.
To determine the effect of BDNF on [Ca2+]i responses to agonists, fura-2–loaded ASM cells exposed to medium only (control) or TNF-α were further exposed to 1 or 10 nM BDNF for 30 minutes. Initial studies examining the effect of BDNF on [Ca2+]i response to agonist was conducted with both NT concentrations. However, for additional TNF-α exposure, based on pilot studies showing minimal effects of 30-minute exposure to 1 nM BDNF in TNF-α–exposed cells, further studies examining interactions between BDNF and TNF-α were conducted using only 10 nM BDNF. This substantially simplified the combinations of drug exposures and interpretation of data. In the continued presence of BDNF (and TNF-α, if appropriate), [Ca2+]i responses to acetylcholine (ACh) or histamine were determined (concentrations as above). Baseline and peak [Ca2+]i levels were measured; amplitudes were calculated as differences between these parameters.
To determine the role of TrkB versus p75NTR receptors, ASM cells were first transfected with lipofectamine alone (transfection control) or siRNA targeting TrkB or p75NTR. After the appropriate time period (as above), cells were further exposed to serum-free medium only or to TNF-α further overnight. Finally, cells were exposed to BDNF for 30 minutes and [Ca2+]i responses to 1 μM ACh or 10 μM histamine evaluated.
Effect on SR Ca2+ Release
In separate experiments, cells were transfected with lipofectamine alone, TrkB siRNA, or p75NTR siRNA. Each group of cells was further exposed to serum-free medium or to TNF-α. All cells were then loaded with fura-2, washed with HBSS, and baseline [Ca2+]i measured. Cells were then exposed to 10 nM BDNF for 30 minutes. Extracellular Ca2+ was subsequently removed by exposure to zero-Ca2+ HBSS (5 mM EGTA). Cells were then exposed to 5 mM caffeine in zero-Ca2+ HBSS to evaluate the effect of TNF-α and interactions with BDNF effects on SR Ca2+ release.
Effect on Store-Operated Ca2+ Entry
The techniques for evaluating store-operated Ca2+ entry (SOCE) in ASM have been previously published (16, 22). ASM cells were perfused with HBSS followed by removal of extracellular Ca2+ by exposure to zero-Ca2+ HBSS for 5 minutes. Given non-SOCE Ca2+ influx in ASM (e.g., L-type Ca2+ channels) (23) cells were also exposed to 1 μM nifedipine and 10 mM KCl (clamping membrane potential) in zero-Ca2+ HBSS. Cells were then rapidly exposed to 1 μM cyclopiazonic acid (CPA; inhibitor of the sarcoendoplasmic reticulum Ca2+ ATPase) in zero-Ca2+ HBSS (and nifedipine and KCl) to induce passive SR depletion from continuing SR Ca2+ leak. When [Ca2+]i eventually reached a plateau or started trending downwards (because plasma membrane Ca2+ efflux was not inhibited), HBSS (with 2.5 mM) was rapidly reintroduced to trigger SOCE.
The above SOCE protocol was performed in fura-2–loaded ASM cells that were transfected with lipofectamine, TrkB siRNA, or p75NTR siRNA, with or without additional TNF-α exposure. BDNF was added 30 minutes before the [Ca2+]i measurements.
Statistical Analysis
Pair-wise comparisons were performed across groups using two-way ANOVA with repeated comparisons and a Bonferroni correction. At least 10 cells per group were used. Statistical significance was tested at a P value of less than 0.05. Values are reported as means (±SE).
RESULTS
Expression of TrkB and p75NTR in Human ASM Cells
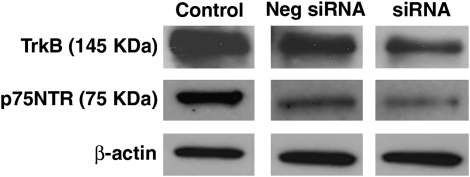
Western analyses of control ASM cells demonstrated expression of both TrkB and p75NTR (Figure 1). siRNA transfection specifically decreased TrkB or p75NTR expression, whereas negative control siRNAs (one each for TrkB and p75NTR) did not substantially change the expression of either protein (Figure 1).
Figure 1.
Expression of receptors for brain-derived neurotrophic factor (BDNF) in human airway smooth muscle (ASM) cells. Under control conditions, both tropomyosin-related kinase (TrkB) and p75NTR receptors were expressed. Transfection of ASM cells with small interfering RNA (siRNA) specifically decreased expression of TrkB and p75NTR.
Effect of BDNF on [Ca2+]i Responses to Agonist
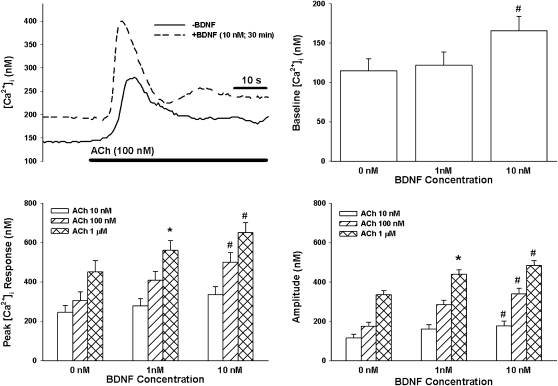
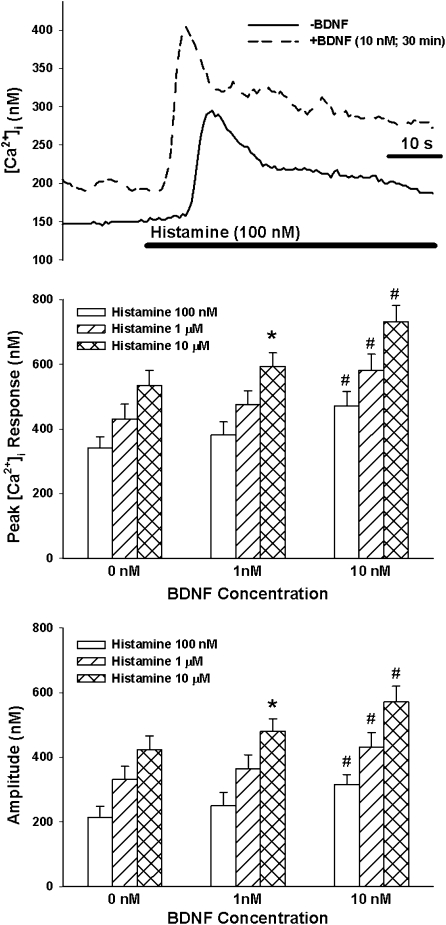
In control ASM cells (i.e., not exposed to TNF-α, BDNF, or siRNA), baseline [Ca2+]i levels ranged between 100 and 140 nM. In these cells, exposure to ACh or histamine resulted in characteristic biphasic [Ca2+]i responses, with an initial, significantly higher peak followed by a sustained elevation above baseline. Peak [Ca2+]i response to ACh (Figure 2) or histamine (Figure 3) increased progressively with increasing agonist concentration, whereas plateau [Ca2+]i stabilized at 100–150 nM above baseline. The amplitude of [Ca2+]i responses (baseline subtracted from peak) also increased with agonist exposure (Figures 2 and 3).
Figure 2.
Effect of BDNF on intracellular Ca2+ ([Ca2+]i) responses of ASM cells to acetylcholine (ACh). Under unstimulated conditions, exposure to 10 nM BDNF (but not lower concentrations) significantly increased baseline [Ca2+]i levels. In the absence of BDNF, ACh produced a concentration-dependent increase in peak [Ca2+]i responses. A 30-minute, 1 nM BDNF exposure had a significant enhancing effect only on [Ca2+]i responses to high agonist concentration. Both peak and amplitude of the [Ca2+]i responses were affected. In comparison, 30 minutes of 10 nM BDNF exposure substantially enhanced peak and amplitude responses at different agonist concentrations. Values are means (±SE). *Significant effect of 1 nM BDNF (compared with 0 nM); #significant effect of 10 nM BDNF (compared with 0 nM); P < 0.05.
Figure 3.
Effect of BDNF on [Ca2+]i responses of ASM cells to histamine. As with ACh, histamine produced concentration-dependent increases in peak [Ca2+]i responses, reflected by increases in amplitude; 1 nM BDNF significantly enhanced [Ca2+]i response to high histamine concentration. In comparison, 10 nM BDNF substantially enhanced peak and amplitude responses at different histamine concentrations. Values are means (±SE). *Significant effect of 1 nM BDNF (compared with 0 nM); #significant effect of 10 nM BDNF (compared with 0 nM); P < 0.05.
In ASM cells not exposed to TNF-α or siRNA, acute exposure (30 minutes) to 10 nM (but not 1 nM) BDNF resulted in a slow, significant increase in baseline [Ca2+]i (135–179 nM; P < 0.05 compared with no BDNF exposure; Figure 2). Subsequent exposure to 100 nM or 1 μM ACh (Figure 2), or to any histamine concentration (Figure 3), significantly increased peak [Ca2+]i and amplitude of [Ca2+]i responses compared with cells not exposed to BDNF. Plateau [Ca2+]i responses were significantly increased by 10 nM BDNF in the case of 1 μM ACh (control [142 ± 21 nM] versus BDNF [221 ± 28 nM]) and 10 μM histamine (control [128 + 19 nM] versus BDNF [179 ± 22 nM]).
Effect of TNF-α on [Ca2+]i Responses to Agonist
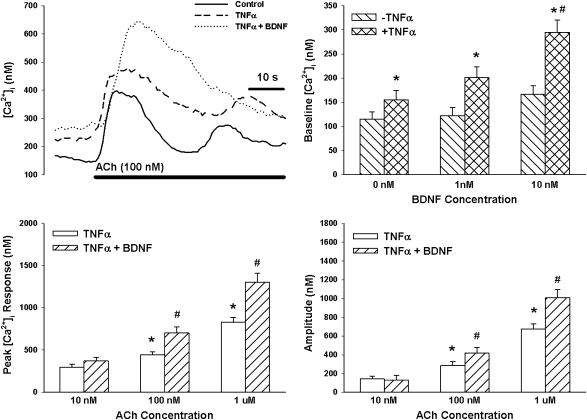
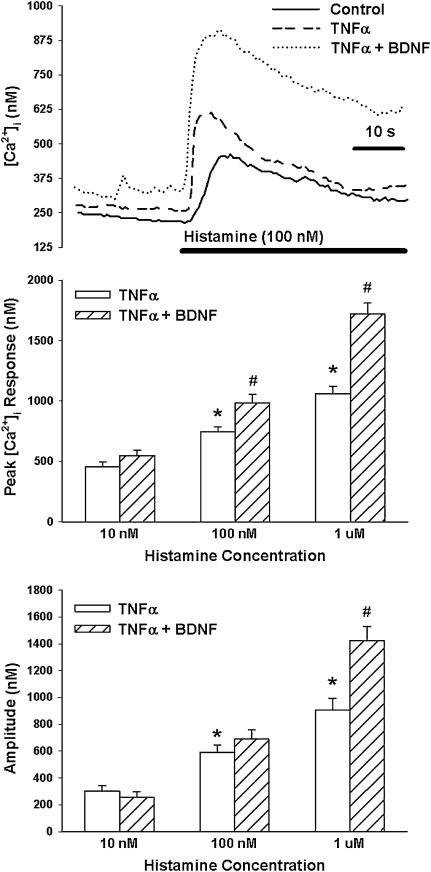
Overnight exposure to TNF-α significantly increased baseline [Ca2+]i levels (P < 0.05; Figure 4). In TNF-α–exposed cells, [Ca2+]i responses to ACh (Figure 4) as well as histamine (Figure 5) were significantly elevated, especially at higher agonist concentrations (P < 0.05 with repeated measures).
Figure 4.
Effect of TNF-α on BDNF enhancement on [Ca2+]i responses to ACh. In the absence of BDNF, overnight exposure to TNF-α alone slightly increased baseline [Ca2+]i and substantially enhanced [Ca2+]i responses to ACh, especially at higher concentrations. With the additional exposure to 30 minutes of 10 nM BDNF, there was generally further enhancement of [Ca2+]i responses, greater than that with TNF-α alone. Values are means (±SE). *Significant effect of TNF-α compared with controls; #significant effect of 10 nM BDNF compared with TNF-α alone.
Figure 5.
Effect of TNF-α on BDNF enhancement on [Ca2+]i responses to histamine. As with ACh, TNF-α alone substantially enhanced [Ca2+]i responses to histamine. Additional exposure to 10 nM BDNF further enhanced [Ca2+]i responses, greater than that with TNF-α alone. Values are means (±SE). *Significant effect of TNF-α compared with controls; #significant effect of 10 nM BDNF compared with TNF-α alone.
In ASM cells exposed overnight to TNF-α, 30-minute exposure to 10 nM BDNF substantially enhanced [Ca2+]i responses to ACh and histamine compared with TNF-α or BDNF alone. Even baseline [Ca2+]i levels were substantially enhanced, especially in the simultaneous presence of 10 nM BDNF and TNF-α. Indeed, in the simultaneous presence of TNF-α and BDNF, enhancement of baseline [Ca2+]i as well as [Ca2+]i responses (especially at higher agonist concentration) was higher than the sum of the individual enhancements by TNF-α or BDNF alone (P < 0.05; Figure 4 for ACh; Figure 5 for histamine).
Effect of TrkB versus p75NTR siRNA on [Ca2+]i Responses to Agonist
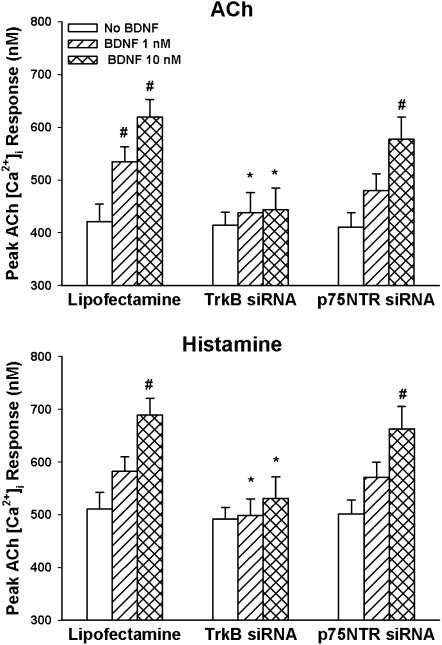
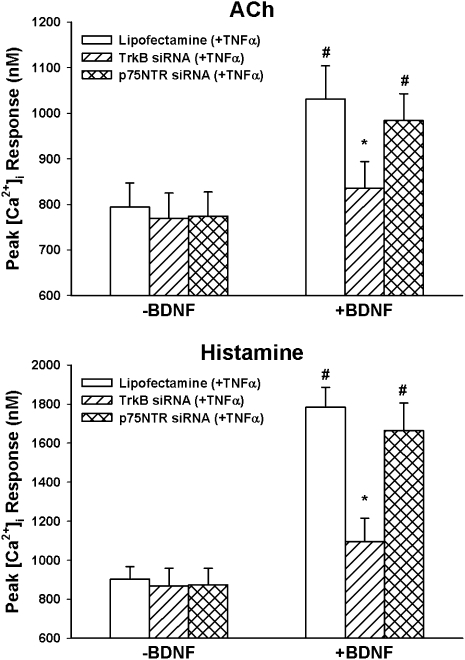
Transfection of ASM cells with siRNA targeting TrkB or p75NTR did not significantly alter baseline [Ca2+]i in ASM cells regardless of whether the cells were further exposed to TNF-α or not. In TrkB siRNA-transfected cells not exposed to TNF-α, exposure to 1 or 10 nM BDNF did not result in substantial enhancement of [Ca2+]i response to 1 μM ACh or 10 μM histamine (compared with nontransfected control cells, which showed enhancement with BDNF; Figure 6). Furthermore, TrkB siRNA blunted TNF-α–induced enhancement of BDNF effects on agonist responses (Figure 7).
Figure 6.
Effect of siRNA inhibition of expression of BDNF receptors (TrkB and p75NTR) on [Ca2+]i responses to ACh (top) and histamine (bottom). TrkB siRNA substantially abrogated the enhancing effect of BDNF on [Ca2+]i responses to agonist. In contrast, p75NTR siRNA had no substantial effect. *Significant siRNA (TrkB or p75NTR) effect compared with BDNF only; #significant BDNF effect compared with control (no BDNF). Values are means (±SE).
Figure 7.
Effect of TrkB versus p75NTR siRNA on interactions between TNF-α and BDNF in [Ca2+]i responses to ACh (top) and histamine (bottom). In the absence of BDNF, neither siRNA had substantial effects on TNF-α–induced enhancement of [Ca2+]i responses to ACh or histamine. However, TrkB siRNA substantially blunted the enhancing effect of BDNF, and p75NTR siRNA had no substantial effect on TNF-α-BDNF interactions. *Significant siRNA (TrkB or p75NTR) effect compared with BDNF only; #significant BDNF effect compared with control (no BDNF). Values are means (±SE).
In contrast to the effects of TrkB siRNA, p75NTR siRNA had no significant effects on BDNF effects on [Ca2+]i responses (Figure 6), or on TNF-α–induced enhancement of BDNF effects (Figure 7).
Effect of TrkB versus p75NTR siRNA on SR Ca2+ Release
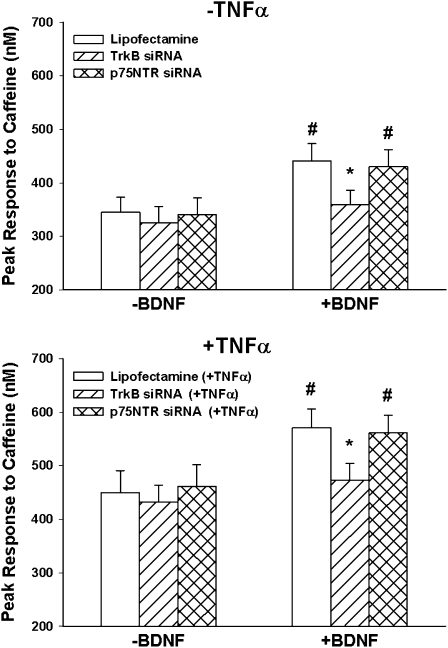
In cells not exposed to TNF-α or siRNA, exposure to 10 nM BDNF for 30 minutes substantially enhanced the peak [Ca2+]i response to 5 mM caffeine in zero-Ca2+ HBSS (Figure 8). Additional overnight exposure to TNF-α resulted in greater enhancement of these responses. With TrkB siRNA (but not p75NTR siRNA), BDNF effects were largely abrogated (Figure 8).
Figure 8.
Effect of TrkB versus p75NTR siRNA on sarcoplasmic reticulum (SR) Ca2+ release. In ASM cells not exposed to TNF-α (top), [Ca2+]i responses to 5 mM caffeine were determined in the absence of extracellular Ca2+. Under these conditions, 10 nM BDNF enhanced SR Ca2+. TrkB siRNA, but not p75NTR, blunted the enhancing effect of BDNF on the [Ca2+]i response to caffeine. In cells exposed overnight to TNF-α (bottom), [Ca2+]i responses to caffeine were increased (compared with non-exposed controls). Under these conditions, additional exposure to BDNF substantially increased caffeine responses. TrkB siRNA (but not p75NTR siRNA) blunted BDNF effects. *indicates significant siRNA (TrkB or p75NTR) effect compared with BDNF only. #Significant BDNF effect compared with control (no BDNF). Values are means ± SE. Values are means ± SE.
Effect of TrkB versus p75NTR siRNA on SOCE
Substantial SOCE was observed in all cell groups, consistent with our previous reports in human ASM (16, 22). In cells not exposed to TNF-α or siRNA, exposure to 10 nM BDNF for 30 minutes substantially increased SOCE after SR Ca2+ depletion with CPA. Enhancement of SOCE was also observed with TNF-α exposure alone (Figure 9). Combined exposure to TNF-α as well as acute BDNF resulted in substantially greater SOCE. Suppression of TrkB via siRNA blunted BDNF effects altogether, whereas p75NTR siRNA had no significant effect.
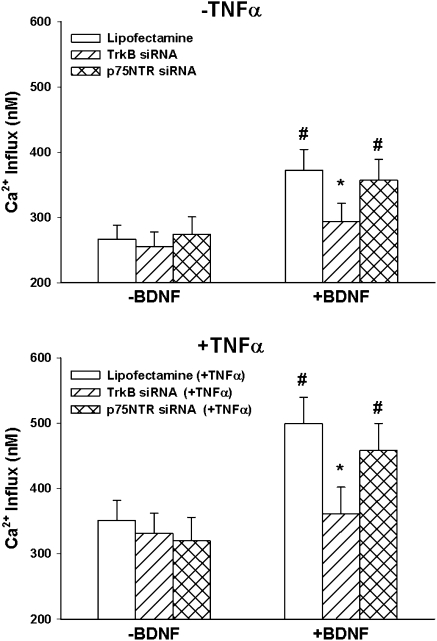
Figure 9.
Effect of TrkB versus p75NTR siRNA on store-operated Ca2+ entry (SOCE). In ASM cells exposed to zero extracellular Ca2+, sarcoplasmic reticulum Ca2+ stores were depleted using cyclopiazonic acid, as described previously (16, 22). Reintroduction of extracellular Ca2+ was used to trigger SOCE. Exposure to 10 nM BDNF before extracellular Ca2+ enhanced SOCE; in cells transfected with TrkB siRNA, but not p75NTR, this enhancing effect of BDNF on SOCE was absent. In cells exposed overnight to TNF-α (bottom), SOCE was increased. BDNF substantially increased SOCE. TrkB siRNA (but not p75NTR siRNA) blunted the BDNF effect on SOCE in TNF-α–exposed cells. *Significant siRNA (TrkB or p75NTR) effect compared with BDNF only; #significant BDNF effect compared with control (no BDNF). Values are means (±SE).
DISCUSSION
In this article, we report novel data on interactions between the proinflammatory cytokine, TNF-α, and acute BDNF exposure in terms of [Ca2+]i regulation in human ASM cells. Consistent with previous studies by several groups, TNF-α by itself enhances [Ca2+]i responses to agonists, such as ACh and histamine. Our results indicate that, in the presence of TNF-α, even brief exposure to BDNF substantially and synergistically enhances SR Ca2+ release and Ca2+ influx, thus potentiating [Ca2+]i responses to agonist. Such effects of BDNF appear to be receptor specific in that suppression of the high-affinity TrkB receptor abrogates BDNF enhancement of [Ca2+]I, whereas suppression of the low-affinity pan-NT receptor, p75NTR, does not substantially affect either TNF-α or BDNF-induced increases in [Ca2+]i.
NT in Non–Smooth Muscle Tissues
NTs, a family of highly conserved polypeptide growth factors, are well recognized to help with maintenance of different neuronal populations, axonal guidance, synaptic plasticity, and neuronal protection and repair after injury (9–11). Extensive studies have established both short- and long-term NT effects on multiple signaling cascades, generally contributing to cell survival and proliferation. In addition to relatively slow, likely genomic effects of NTs, there is increasing evidence for more rapid NT action (seconds/minutes), such as regulation of synaptic transmission (11, 24–26). In this regard, BDNF-induced transient [Ca2+]i elevation, with Ca2+ release from intracellular stores (26) and increased Ca2+ influx (27, 28) have been demonstrated in neurons.
NTs exert their effects via two structurally unrelated receptors: p75NTR, a member of the TNF receptor superfamily, and Trk receptor tyrosine kinases (9–11, 29). Although p75NTR binds to all NTs with equivalent (albeit low) affinity, BDNF and NT-4 are preferred ligands for TrkB (29, 30). NT effects may depend on the NT itself, the receptor(s) activated (p75NTR versus Trk isoforms), and duration of exposure (short term or acute versus prolonged) (26). Activation of full-length TrkB by BDNF leads to tyrosine phosphorylation, which recruits several downstream signaling enzymes and adaptor proteins (10, 11). Although p75NTR has been identified in several tissues, its role is somewhat uncertain. Initially thought to just augment Trk signaling (31), as a member of the TNF receptor family, p75NTR may modulate cell death or survival, depending on concomitant Trk activation (31, 32). However, this likely occurs with prolonged NT exposure. Overall these diverse findings in neurons emphasize the broad influence of NTs on cell signaling.
NTs in the Lung
There is increasing evidence that NTs and Trk receptors are expressed in non-neural tissues, including the lung (12–17). Ricci and colleagues (13) first demonstrated NT and receptor expression in different lung components in humans, including lung innervation and ASM itself. NTs may be derived from several other sources, including immune cells (20), suggesting a role in airway inflammation. In a recent study, we found immunocytochemical evidence for expression of BDNF, NT-3, and NT-4, as well as their receptors (i.e., TrkB, TrkC) in human ASM cells, indicating that, regardless of their source, NTs may target ASM (16). The results of the present study showing an effect of BDNF on [Ca2+]i regulation in ASM cells support this idea.
Effect of NTs on [Ca2+]i Regulation
In ASM, agonist stimulations leads to SR Ca2+ release that may occur via both IP3 receptor channels (33) as well as RyR channels (34). Typically, the plateau phase involves both sustained Ca2+ influx and continued SR Ca2+ release (34, 35). Ca2+ influx occurs via voltage-gated (23) and receptor-gated (36) channels, as well as in response to SR Ca2+ depletion (i.e., SOCE) (22).
In a recent study (16), we demonstrated that, in human ASM cells, exogenous application of BDNF (or NT-4) for even 30 minutes enhances peak [Ca2+]i response to ACh, histamine, and bradykinin, indicating NT enhancement of SR Ca2+ release. Furthermore, enhancement of ACh-induced sustained [Ca2+]i levels by BDNF was consistent with increased Ca2+ influx. Indeed, we also found that both BDNF and NT-4 enhance CPA-induced SOCE in ASM. Therefore, our previous data demonstrated direct effects of NTs (especially BDNF) on [Ca2+]i regulation. The data in the present study showing BDNF enhancement of [Ca2+]i responses to agonist are entirely consistent with those of our previous report.
Although data from other cell types demonstrate that cellular effects of NTs are mediated via Trk or p75NTR receptors, the mechanisms by which NTs influence [Ca2+]i in ASM had not been examined. In our previous study, we found that the effects of all BDNF, NT-4, and NT-3 are all significantly blunted by the tyrosine kinase inhibitor, K252a, suggesting a role for Trk receptors. The results of the present study, showing that siRNA suppression of TrkB substantially blunts the enhancing effect of BDNF on [Ca2+]i regulation, are consistent with this idea.
The role of p75NTR in ASM is currently unclear. The results of the present study suggest that p75NTR may not be involved in NT regulation of [Ca2+]i itself, as siRNA suppression of p75NTR expression had negligible effects on BDNF enhancement of [Ca2+]i responses to agonist. However, this does not rule out other potential roles for p75NTR in ASM. For example, p75NTR can modulate the activity of the GTPase RhoA (37), which then activates Rho kinase. The relevance of this pathway lies in the fact that, in addition to Ca2+-dependent regulation of force, Ca2+-independent regulation (Ca2+ sensitization) also occurs (38, 39). Such sensitization involves RhoA and its downstream target, Rho kinase (40, 41). Accordingly, it is possible that NT activation of p75NTR activates RhoA and Rho kinase, leading to alterations in force generation of ASM, in addition to NT effects on [Ca2+]i mediated via Trk receptors. This scenario remains to be established.
TNF-α, NTs, and the Airway
Although diseases, such as asthma and chronic bronchitis, are multifactorial in origin, their pathophysiology has been correlated to an inflammatory process and structural changes of both small and large airways, with infiltration by immune cells, mucus hypersecretion, and collagen deposition, critical to development of airway hyperreactivity and obstruction. In this regard, the list of cytokines implicated increases at a steady pace, only underlining the fact that airway diseases, such as asthma, involve several mechanisms. One cytokine that is produced in considerable quantities in the airway, and that has been well studied in airway inflammation, is TNF-α. Acting via its receptor, TNFR1 (p55 receptor), TNF-α has been shown to increase both agonist-induced [Ca2+]i and force in ASM of several species (6, 8, 42). TNF-α also increases second messenger expression, increasing Ca2+ release via RyR channels (4, 21). In other studies, we reported that TNF-α also enhances SOCE in human ASM (6).
There are certainly data in neuronal tissues demonstrating interactions between cytokines and NT or NT receptor expression (43, 44). However, given the relative novelty of NT findings in the lung, there are currently limited data on such interactions in ASM. In the ovalbumin-sensitized/-challenged mouse model of airway hyperresponsiveness, TrkB levels in the lung (although not specifically in ASM) have been reported to be increased (17). In ongoing studies, we have found that TrkB mRNA is increased in laser-captured microdissected samples of ASM of such mice, compared with nonhyperresponsive control mice (Y.S.P., M.A.T., and C.M.P., unpublished observations). Other cytokines, such as IL-1β, have been shown to cause a delayed increase in BDNF levels, whereas IFN-γ has been found to decrease BDNF in ASM (45). However, there are currently no published data on the effect of TNF-α on NT signaling in ASM. TNF-α may alter NT signaling by changing expression of NT receptors, or by enhancing intracellular cascades involved in NT signaling. We did not examine the latter possibility in the present study. However, we did find that exposure of human ASM cells to TNF-α increases the expression of TrkB as well as p75NTR receptor.
In the presence of TNF-α, the enhancing effect of BDNF on [Ca2+]i responses was potentiated. Indeed, in several instances, the combined effect of TNF-α and BDNF was substantially greater than that of TNF-α or BDNF alone, suggesting a synergistic effect. These data highlight the importance of both TNF-α in airway hyperresponsiveness and the role of BDNF in such changes during airway inflammation. In this regard, the mechanisms by which synergism between TNFa and BDNF may occur remain to be established. The finding that TrkB siRNA blunted the enhancing effect of TNF-α on [Ca2+]i response to agonist, with and without additional BDNF exposure, suggests that the BDNF/TrkB pathway is an integral component of the intracellular cascades by which TNF-α can enhance ASM contractility. It is interesting that, given the fact that the p75NTR is a member of the TNF receptor superfamily, we would have expected TNFa–BDNF interactions to actually involve this receptor. However, we found that, unlike TrkB, siRNA suppression of p75NTR minimally affected TNF-α enhancement of [Ca2+]i responses. Here, a caveat is that we examined only [Ca2+]i and not ASM contractility. It is already known that TNF-α can activate RhoA/Rho kinase (46), and that inhibition of Rho kinase decreases airway inflammation (47) as well as airway hyperresponsiveness (48, 49). Accordingly, it is possible that NT activation of p75NTR can modulate TNF-α effects (and vice versa) on ASM sensitivity for force generation. Furthermore, in other cell systems, BDNF can activate a number of signaling cascades (e.g., phosphatidylinositol 3 kinase, mitogen-activated protein kinases), which overlap with the signaling network activated by TNF-α (50–52). Whether synergistic activation of these common mechanisms is involved in the potentiation of [Ca2+]i responses by TNF-α and BDNF remains to be established.
In conclusion, the present study demonstrates that BDNF/TrkB signaling (but not BDNF/p75NTR) in human ASM cells regulates [Ca2+]i responses to agonist in both normal ASM and with inflammation induced by TNF-α. These effects suggest an important role of NT signaling in inflammation-induced changes in ASM contractility. Future studies should focus on the relative roles of Trk versus p75NTR signaling in terms of other changes in airway inflammation, including Ca2+ sensitivity, as well as cell proliferation and airway remodeling.
This work was supported by a Clinical Innovator Award from the Flight Attendants Medical Research Institute (Y.S.P.); National Institutes of Health grants 1 UL1 RR024150-01 (Mayo Clinic Clinical Research awards [Y.S.P. and C.M.P.]), HL74309, HL88029 (Y.S.P.), HL90595 (C.M.P.); a Mayo Clinic Early Career Development Award (Y.S.P.); and the Department of Anesthesiology, Mayo Clinic (Rochester, MN).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0151OC on February 12, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Janssen LJ. Ionic mechanisms and Ca2+ regulation in airway smooth muscle contraction: do the data contradict dogma? Am J Physiol Lung Cell Mol Physiol 2002;282:L1161–L1178. [DOI] [PubMed] [Google Scholar]
- 2.Hall IP. Second messengers, ion channels and pharmacology of airway smooth muscle. Eur Respir J 2000;15:1120–1127. [DOI] [PubMed] [Google Scholar]
- 3.Hakonarson H, Grunstein MM. Regulation of second messengers associated with airway smooth muscle contraction and relaxation. Am J Respir Crit Care Med 1998;158:S115–S122. [DOI] [PubMed] [Google Scholar]
- 4.Tirumurugaan KG, Jude JA, Kang BN, Panettieri RA, Walseth TF, Kannan MS. TNF-alpha induced CD38 expression in human airway smooth muscle cells: role of MAP kinases and transcription factors NF-kappaB and AP-1. Am J Physiol Lung Cell Mol Physiol 2007;292:L1385–L1395. [DOI] [PubMed] [Google Scholar]
- 5.Amrani Y. TNF-α and calcium signaling in airway smooth muscle cells: a never-ending story with promising therapeutic relevance. Am J Respir Cell Mol Biol 2007;36:387–388. [DOI] [PubMed] [Google Scholar]
- 6.White TA, Xue A, Chini EN, Thompson M, Sieck GC, Wylam ME. Role of transient receptor potential C3 in TNF-α–enhanced calcium influx in human airway myocytes. Am J Respir Cell Mol Biol 2006;35:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter I, Cobban HJ, Vandenabeele P, MacEwan DJ, Nixon GF. Tumor necrosis factor-alpha–induced activation of RhoA in airway smooth muscle cells: role in the Ca2+ sensitization of myosin light chain20 phosphorylation. Mol Pharmacol 2003;63:714–721. [DOI] [PubMed] [Google Scholar]
- 8.Amrani Y, Chen H, Panettieri RA Jr. Activation of tumor necrosis factor receptor 1 in airway smooth muscle: a potential pathway that modulates bronchial hyper-responsiveness in asthma? Respir Res 2000;1:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennigan A, O'Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochem Soc Trans 2007;35:424–427. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol 2000;10:381–391. [DOI] [PubMed] [Google Scholar]
- 11.Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology (Bethesda) 2005;20:70–78. [DOI] [PubMed] [Google Scholar]
- 12.Lommatzsch M, Braun A, Renz H. Neurotrophins in allergic airway dysfunction: what the mouse model is teaching us. Ann N Y Acad Sci 2003;992:241–249. [DOI] [PubMed] [Google Scholar]
- 13.Ricci A, Felici L, Mariotta S, Mannino F, Schmid G, Terzano C, Cardillo G, Amenta F, Bronzetti E. Neurotrophin and neurotrophin receptor protein expression in the human lung. Am J Respir Cell Mol Biol 2004;30:12–19. [DOI] [PubMed] [Google Scholar]
- 14.Yao Q, Haxhiu MA, Zaidi SI, Liu S, Jafri A, Martin RJ. Hyperoxia enhances brain-derived neurotrophic factor and tyrosine kinase B receptor expression in peribronchial smooth muscle of neonatal rats. Am J Physiol Lung Cell Mol Physiol 2005;289:L307–L314. [DOI] [PubMed] [Google Scholar]
- 15.Lomen-Hoerth C, Shooter EM. Widespread neurotrophin receptor expression in the immune system and other nonneuronal rat tissues. J Neurochem 1995;64:1780–1789. [DOI] [PubMed] [Google Scholar]
- 16.Prakash YS, Iyanoye A, Ay B, Mantilla CB, Pabelick CM. Neurotrophin effects on intracellular Ca2+ and force in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2006;291:L447–L456. [DOI] [PubMed] [Google Scholar]
- 17.Nassenstein C, Dawbarn D, Pollock K, Allen SJ, Erpenbeck VJ, Spies E, Krug N, Braun A. Pulmonary distribution, regulation, and functional role of Trk receptors in a murine model of asthma. J Allergy Clin Immunol 2006;118:597–605. [DOI] [PubMed] [Google Scholar]
- 18.Hoyle GW. Neurotrophins and lung disease. Cytokine Growth Factor Rev 2003;14:551–558. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhuri R, McMahon AD, McSharry CP, Macleod KJ, Fraser I, Livingston E, Thomson NC. Serum and sputum neurotrophin levels in chronic persistent cough. Clin Exp Allergy 2005;35:949–953. [DOI] [PubMed] [Google Scholar]
- 20.Ricci A, Greco S, Mariotta S, Felici L, Amenta F, Bronzetti E. Neurotrophin and neurotrophin receptor expression in alveolar macrophages: an immunocytochemical study. Growth Factors 2000;18:193–202. [DOI] [PubMed] [Google Scholar]
- 21.Sieck GC, White TA, Thompson MA, Pabelick CM, Wylam ME, Prakash YS. Regulation of store-operated Ca2+ entry by CD38 in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2008;294:L378–L385. [DOI] [PubMed] [Google Scholar]
- 22.Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2004;286:L909–L917. [DOI] [PubMed] [Google Scholar]
- 23.Worley JF, Kotlikoff MI. Dihydropyridine-sensitive single calcium channels in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 1990;259:L468–L480. [DOI] [PubMed] [Google Scholar]
- 24.McCutchen ME, Bramham CR, Pozzo-Miller LD. Modulation of neuronal calcium signaling by neurotrophic factors. Int J Dev Neurosci 2002;20:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci 2001;2:24–32. [DOI] [PubMed] [Google Scholar]
- 26.Kovalchuk Y, Holthoff K, Konnerth A. Neurotrophin action on a rapid timescale. Curr Opin Neurobiol 2004;14:558–563. [DOI] [PubMed] [Google Scholar]
- 27.Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science 2002;295:1729–1734. [DOI] [PubMed] [Google Scholar]
- 28.Li HS, Xu XZ, Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron 1999;24:261–273. [DOI] [PubMed] [Google Scholar]
- 29.Barker PA. p75NTR: a study in contrasts. Cell Death Differ 1998;5:346–356. [DOI] [PubMed] [Google Scholar]
- 30.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 2003;72:609–642. [DOI] [PubMed] [Google Scholar]
- 31.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 2002;67:203–233. [DOI] [PubMed] [Google Scholar]
- 32.Nykjaer A, Willnow TE, Petersen CM. p75NTR—live or let die. Curr Opin Neurobiol 2005;15:49–57. [DOI] [PubMed] [Google Scholar]
- 33.Coburn RF, Baron CB. Coupling mechanisms in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 1990;258:L119–L133. [DOI] [PubMed] [Google Scholar]
- 34.Kannan MS, Prakash YS, Brenner T, Mickelson JR, Sieck GC. Role of ryanodine receptor channels in Ca2+ oscillations of porcine tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol 1997;272:L659–L664. [DOI] [PubMed] [Google Scholar]
- 35.Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol Cell Physiol 1997;272:C966–C975. [DOI] [PubMed] [Google Scholar]
- 36.Murray RK, Kotlikoff MI. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol 1991;435:123–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron 1999;24:585–593. [DOI] [PubMed] [Google Scholar]
- 38.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 2003;83:1325–1358. [DOI] [PubMed] [Google Scholar]
- 39.Gerthoffer WT. Regulation of the contractile element of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 1991;261:L15–L28. [DOI] [PubMed] [Google Scholar]
- 40.Chiba Y, Sakai H, Suenaga H, Kamata K, Misawa M. Enhanced Ca2+ sensitization of the bronchial smooth muscle contraction in antigen-induced airway hyperresponsive rats. Res Commun Mol Pathol Pharmacol 1999;106:77–85. [PubMed] [Google Scholar]
- 41.Chiba Y, Takada Y, Miyamoto S, MitsuiSaito M, Karaki H, Misawa M. Augmented acetylcholine-induced, Rho-mediated Ca2+ sensitization of bronchial smooth muscle contraction in antigen-induced airway hyperresponsive rats. Br J Pharmacol 1999;127:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds AM, Holmes MD, Scicchitano R. Cytokines enhance airway smooth muscle contractility in response to acetylcholine and neurokinin A. Respirology 2000;5:153–160. [DOI] [PubMed] [Google Scholar]
- 43.Blasing H, Hendrix S, Paus R. Pro-inflammatory cytokines upregulate the skin immunoreactivity for NGF, NT-3, NT-4 and their receptor, p75NTR in vivo: a preliminary report. Arch Dermatol Res 2005;296:580–584. [DOI] [PubMed] [Google Scholar]
- 44.Tabakman R, Lecht S, Sephanova S, Arien-Zakay H, Lazarovici P. Interactions between the cells of the immune and nervous system: neurotrophins as neuroprotection mediators in CNS injury. Prog Brain Res 2004;146:387–401. [DOI] [PubMed] [Google Scholar]
- 45.Kemi C, Grunewald J, Eklund A, Hoglund CO. Differential regulation of neurotrophin expression in human bronchial smooth muscle cells. Respir Res 2006;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakai H, Otogoto S, Chiba Y, Abe K, Misawa M. TNF-alpha augments the expression of RhoA in the rat bronchus. J Smooth Muscle Res 2004;40:25–34. [DOI] [PubMed] [Google Scholar]
- 47.Henry PJ, Mann TS, Goldie RG. A rho kinase inhibitor, Y-27632 inhibits pulmonary eosinophilia, bronchoconstriction and airways hyperresponsiveness in allergic mice. Pulm Pharmacol Ther 2005;18:67–74. [DOI] [PubMed] [Google Scholar]
- 48.Gosens R, Schaafsma D, Nelemans SA, Halayko AJ. Rho-kinase as a drug target for the treatment of airway hyperresponsiveness in asthma. Mini Rev Med Chem 2006;6:339–348. [DOI] [PubMed] [Google Scholar]
- 49.Schaafsma D, Boterman M, de Jong AM, Hovens I, Penninks JM, Nelemans SA, Meurs H, Zaagsma J. Differential Rho-kinase dependency of full and partial muscarinic receptor agonists in airway smooth muscle contraction. Br J Pharmacol 2006;147:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zampieri N, Chao MV. Mechanisms of neurotrophin receptor signalling. Biochem Soc Trans 2006;34:607–611. [DOI] [PubMed] [Google Scholar]
- 51.Furuno T, Nakanishi M. Neurotrophic factors increase tumor necrosis factor-alpha–induced nuclear translocation of NF-kappaB in rat PC12 cells. Neurosci Lett 2006;392:240–244. [DOI] [PubMed] [Google Scholar]
- 52.Burke MA, Bothwell M. p75 neurotrophin receptor mediates neurotrophin activation of NF-kappa B and induction of iNOS expression in P19 neurons. J Neurobiol 2003;55:191–203. [DOI] [PubMed] [Google Scholar]