SUMMARY
Insulin secretion from pancreatic β–cells is dependent on maturation and acidification of the secretory granule necessary for prohormone convertase cleavage of proinsulin. Previous studies in isolated β–cells revealed that acidification may be dependent on the granule membrane chloride channel ClC-3, in a step permissive for a regulated secretory response. In this study, immuno-electron microscopy of β–cells revealed colocalization of ClC-3 and insulin on secretory granules. Clc-3−/− mice as well as isolated islets demonstrate impaired insulin secretion; clc3−/− β–cells are defective in regulated insulin exocytosis and granular acidification. Increased amounts of proinsulin were found in the majority of secretory granules in the clc-3−/− mice while in clc3+/+ cells, proinsulin was confined to the immature secretory granules. These results demonstrate that in pancreatic β–cells chloride channels, specifically ClC-3, are localized on insulin granules and play a role in insulin processing as well as insulin secretion through regulation of granular acidification.
INTRODUCTION
Considerable progress has been made in determining many features of secretagogue-regulated insulin secretion, particularly the role of vesicle-associated ion channels in granule release (Rorsman and Renstrom, 2003). However, we are still at an early stage in defining the molecular mechanisms involved in exocytosis of the insulin granule and the important role of granule membrane ion channels in granule release. Vesicular ion channels may play several roles in secretion in addition to facilitating a specific requirement for vesicle acidification, as in the case of insulin secretion. The chloride channel ClC-3, originally identified in swelling of insulin-secreting HIT cells (Kinard et al., 2001) is expressed in the membrane of insulin-containing granules. Previous functional studies in isolated β–cells using channel specific inhibitory antibodies to elucidate ClC-3 functional expression showed that activation of ClC-3 is permissive for insulin secretion (Barg et al., 2001). This is due, at least in part, to the promotion of insulin granule acidification; various strategies to abolish acidification disrupt secretion in a similar manner. We have extended the earlier findings of Barg et al. (Barg et al., 2001) on the functional expression of ClC-3 in insulin granules to the ClC-3 knockout mouse.
A recent paper by Maritzen and colleagues (Maritzen et al., 2008) concluded ClC-3 is localized not in large dense core vesicles (LDCV) but rather in smaller, non-insulin containing vesicles – in contrast to our earlier studies (Barg et al., 2001). The Maritzen et al. studies relied heavily on subcellular fractionation using homogenates of the insulin-secreting cell line INS-1 and detected little ClC-3 in the LDCV fraction. Our current study, using primary β-cells, clearly shows colocalization of insulin and ClC-3 in LDCVs using high resolution immunoelectronmicroscopy, a method more suitable for examining channel localization. In addition, we present physiological evidence demonstrating an indisputable effect of ClC-3 expression on granule pH and insulin processing in primary β-cells.
We have obtained compelling data the analysis of which suggests that ClC-3 is gated by CaMKII in various systems (Huang et al., 2001; Mitchell et al., 2008; Robinson et al., 2004; Wang et al., 2006). Given that CaMKII is implicated in insulin secretion (Easom, 1999; Easom et al., 1997; Gromada et al., 1999; Krueger et al., 1997) and the previously published findings that ClC-3 contributes to insulin granule acidification and, therefore, granule maturation, we carried out experiments on clc-3−/− mice to determine whether the chloride channel played a significant role in the process of pancreatic β–cell granule maturation, mobilization, and finally release.
RESULTS
Impaired insulin secretion in transgenic clc-3−/− mice
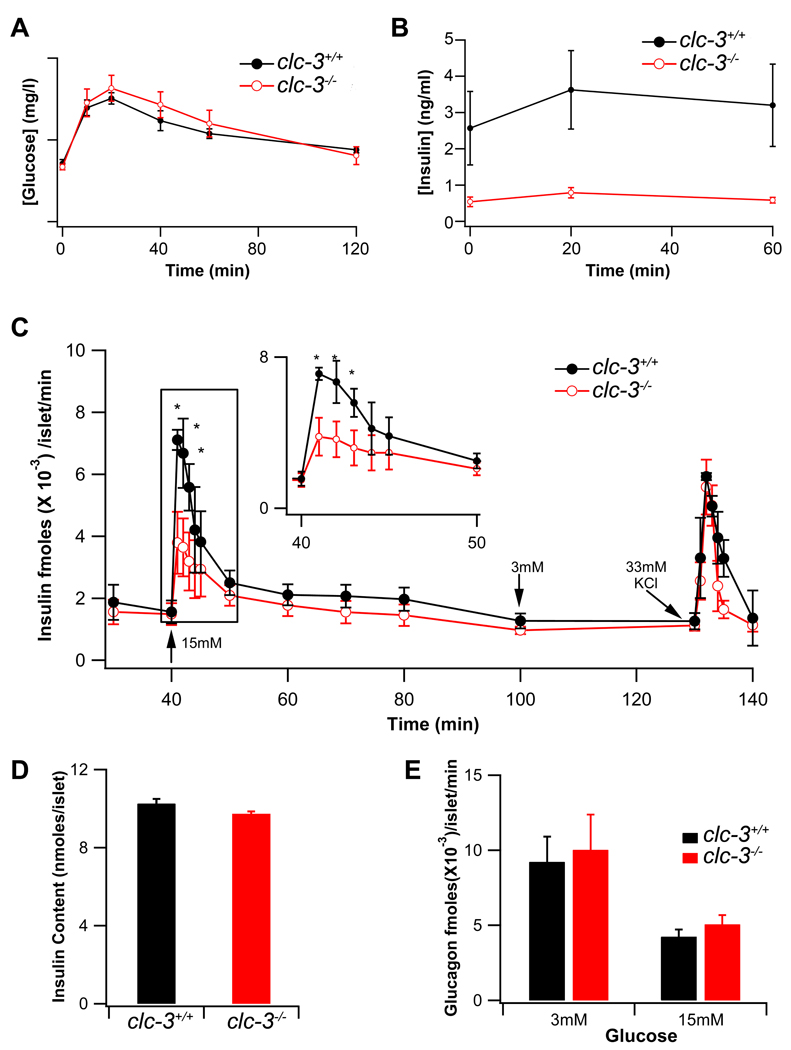
Given that ClC-3 Cl− channels localize to the β–cell granule membrane and contribute to granule acidification and mobilization (Barg et al., 2001), we anticipated that clc-3−/− mice might manifest defective insulin secretion. Data in Fig. 1 indicate that null mice exhibit a relatively normal glucose tolerance as compared to wild-type (WT) littermates (Fig. 1A). Glucose tolerance responses between subsets of clc-3−/− animals showed a high degree of variability. This could be due to an increase in insulin sensitivity in peripheral tissues and/or differences in post insulin receptor signaling in muscle and fat in subsets of clc-3−/− animals indicative of a secondary compensation resulting from the global loss of ClC-3 channels. In contrast, serum insulin levels were significantly reduced in the clc-3−/− animals over the clc-3+/+ littermate controls (Fig. 1B) consistent with a β–cell secretory defect.
Figure 1. Serum glucose and insulin levels in clc-3−/− mice compared to wild type (clc-3+/+) littermate controls, blunted first-phase insulin secretion in clc-3−/− islets.
(A) Blood glucose levels were measured in whole blood following glucose challenge, nclc-3+/+ = 6, nclc3−/− = 10. Data are plotted as the mean ± S.E.M. (B) Insulin concentration was measured in sera of clc3−/− (n=7) and clc-3+/+ (n=3) mice by ELISA; nclc-3+/+ = 6, nclc3−/− = 10. Data are plotted as the mean ± S.E.M. Note time scale differences in A and B. (C) Insulin release from isolated perifused islets from WT (closed black circles) and KO (open red circles) mice before and after elevating glucose from 3 mM to 15 mM and back to 3 mM. Data are presented in fmoles × 10−3 of insulin/islet/min as means ± S.E.M. (n=3). Asterisks mark significance at the P < 0.05 level. (D) Total insulin content in the lysed islets, measured by ELISA as in (B) and (C). Data are presented in nanomoles of insulin/islet. (E) Glucagon concentration was determined in the same fractions by ELISA. Data are presented in fmoles ×10−3 of glucagon/islet/min as means ± S.E.M. (n = 3).
Having observed a significant difference in serum insulin levels between the ClC-3 WT and null mice, we examined whether a similar secretory impairment could be observed in islets isolated from clc-3−/− animals. Perifusion assays of isolated islets revealed that first phase glucose-stimulated insulin release was significantly attenuated in the clc-3−/− islets as compared to islets from clc-3+/+ animals (Fig. 1C). Insulin release in response to maximal K+-induced depolarization, total islet insulin content, and glucagon release in response to glucose did not vary between the two genotypes (Fig. 1C, D and E). It is intriguing to note that insulin secretion to glucose is impaired, but not to high K+, as both stimuli produce cellular depolarization and stimulate insulin release by a mechanism upstream of acidification. This apparent discrepancy suggests that ClC-3 may also play a role in the pathway between glucose metabolism and cellular depolarization.
Reduced secretion in β–cell cells from clc-3−/− mice
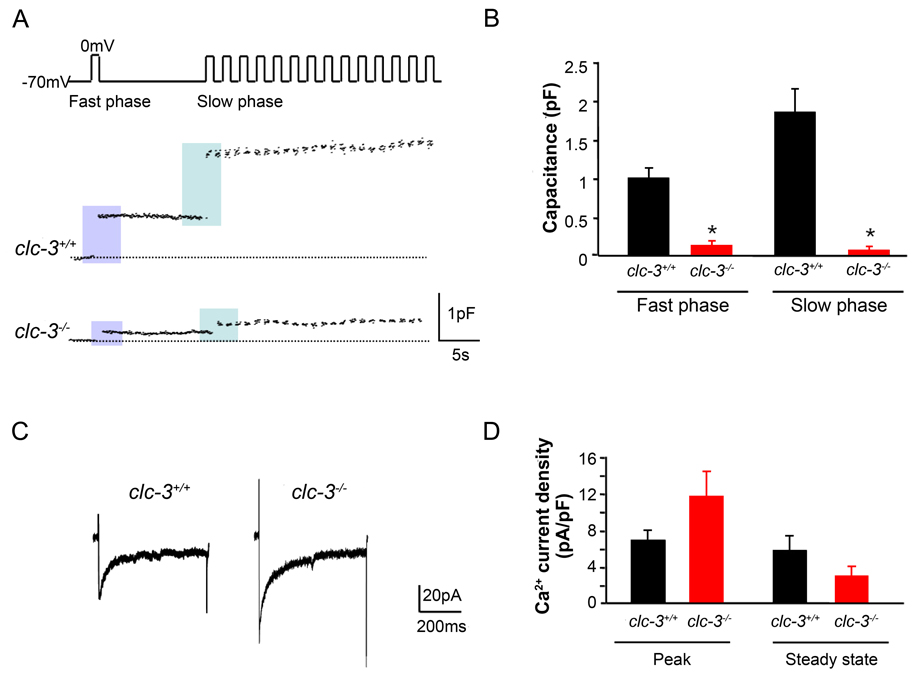
The characteristic pattern of insulin secretion in response to glucose seen at the whole animal or organ level is reflected electrophysiologically in isolated β-cells by transient increases in membrane capacitance when cells are stimulated with square-wave depolarizing pulses that open plasma membrane L-type voltage-dependent Ca2+ channels (Ammala et al., 1993). In order to confirm the role of ClC-3 in the regulation of depolarization-induced granular release, we carried out studies on β-cells isolated from both clc-3+/+ and clc-3−/− mice. Data in Fig. 2 A,B demonstrate that a fast phase (Ready Releasable Pool - RRP) of secretion, as measured by a rapid increase in membrane capacitance elicited by a single depolarizing voltage pulse, is reduced in clc-3−/− cells. Refilling of the RRP in clc-3−/− mice is inhibited as shown by a decreased membrane capacitance in response to the first depolarization in a train of depolarizing stimuli in a slow phase (Fig.2 A,B).The rate of capacitance increase in β-cells isolated from clc-3−/− mice was lower than in clc-3+/+ mice with the average rate of capacitance change (ΔCm(pF/s)) of 9.5 × 10−3±1.5 × 10−3 for clc-3+/+ and −4.3 × 10-3±3.1 × 10−3 for clc-3−/−. (Fig.S1). The decrease in depolarization-induced secretion seen in the clc-3−/− β-cells was not due to a decrease in the density of voltage-dependent channels. The peak and steady-state Ca2+ channel current density in response to step depolarizations from a holding potential of −70 mV to 0 mV was not significantly different between the clc-3+/+ and clc-3−/− cells (Fig. 2C,D).
Figure 2. Reduction in depolarization-induced exocytosis in pancreatic β–cells isolated from clc-3−/− mice compared to clc-3+/+.
(A) Perforated patch recordings from representative pancreatic β–cells showing increases in cell membrane capacitance (ΔCm) evoked by voltage-clamp depolarizations from −70 to 0 mV (500 ms, 1 Hz). (B) Histograms showing average changes in capacitance during fast and slow phase of secretion. Fast phase secretion (from a docked, readily releasable granule pool) was triggered by a single pulse from −70 to 0 mV (500 ms) 10 s before the train was applied to the cells to elicit mobilization of granules from a reserve pool of granules (slow phase). Data are mean ± S.E.M. of 4 experiments for each cell type. * P < 0.05. (C) Representative whole-cell Ca2+ currents from perforated patch capacitance experiments as in (A). Currents were elicited by 500 ms voltage-clamp depolarizations from −70 to 0 mV. (D) Summary of average changes in peak and steady-state Ca2+ current in clc-3+/+ and clc-3−/− cells. Neither average peak nor steady-state currents were significantly different from one another. Data are mean ± S.E.M. of 4 experiments for each cell type.
Interestingly, we observed endocytosis following a single depolarization in a train (Fig. 2 S). During the 500 ms interval between pulses, capacitance rose and then fell prior to the succeeding pulse often leading to a negligible net increase between pulses. To our knowledge, this has not been observed in whole cell capacitance studies of β-cells to date. We observed this behaviour in approximately 50% of the clc-3+/+ and none of the clc-3 −/− cells examined.
ClC-3 localization in insulin granules of pancreatic β–cells
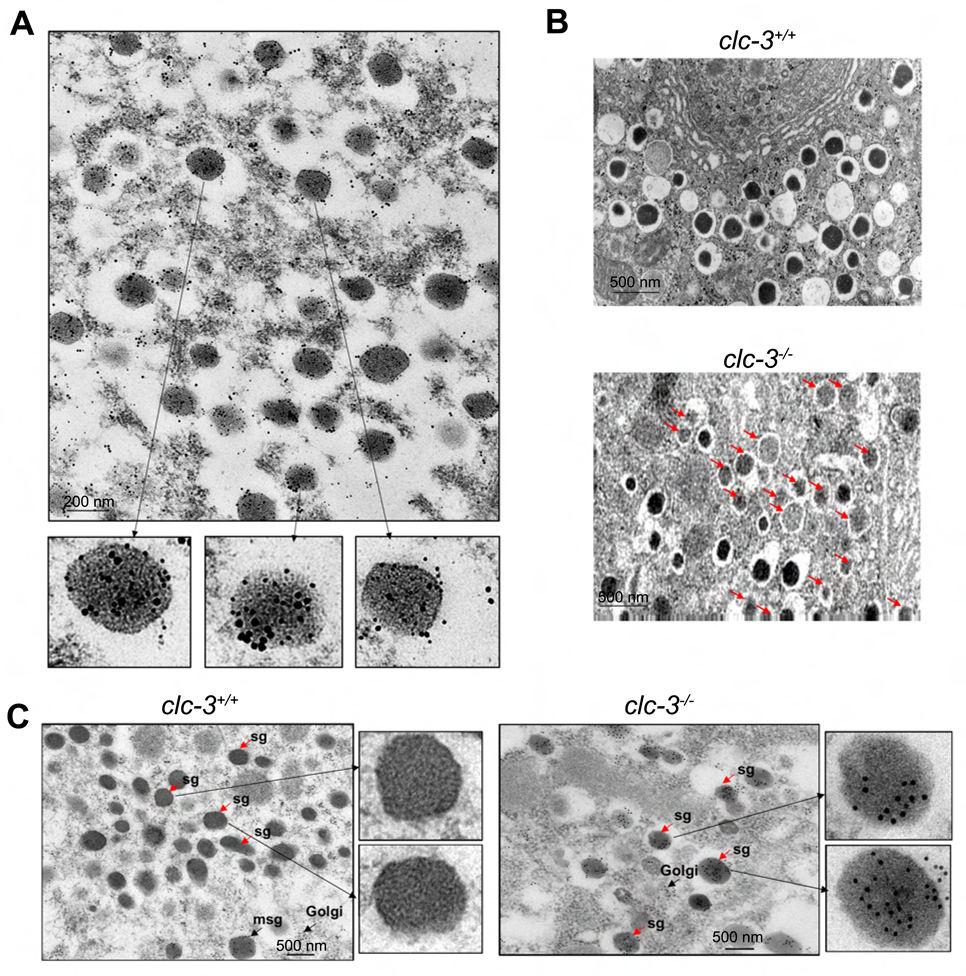
Electron microscopy on thin sections of pancreatic islets clearly demonstrates localization of ClC-3 on insulin granules (Fig. 3A). No staining with pre-immune IgG was observed (data not shown). Control experiments where anti-ClC-3 antibody was depleted with 10-fold molar concentration of soluble insulin prior to staining confirmed that the presence of ClC-3 on insulin granules is not due to non-specific recognition of insulin by anti-ClC-3 antibody or non-specific interaction of anti-ClC-3 and anti-insulin antibodies (Fig. S3).
Figure 3. Electron microscopic localization of ClC-3 on insulin granules and characterization of secretory granules in thin sections of β–cells from clc-3+/+ and clc-3−/− mice.
(A) Immunogold staining shows insulin (10 nm particles) and ClC-3 (15 nm particles) colocalized in secretory granules of β–cells. (B) Comparison of clc-3+/+ and clc-3−/− cells reveals abundant pale, immature-appearing secretory granules in the knockout cells (marked by arrows). (C) Localization of proinsulin on thin sections of pancreatic β–cells from clc-3+/+ and clc-3−/− mice. In clc-3+/+ mice proinsulin staining is observed only in the nascent (maturing) secretory granules (msg). Mature secretory granules (sg) appear free of labeling. In the β–cells from the clc-3−/− mice, proinsulin labeling is found regardless of the state of maturity.
Proinsulin subcellular localization
The foregoing observations demonstrating that insulin release in the clc-3−/− mouse is compromised at the whole animal, isolated islet, and at the single β–cell level suggested that this may be due to either a decrease in β–cell insulin granule content or alternatively impairment in insulin processing within the granules (Rouille et al., 1995).
The prohormone convertase insulin processing enzyme has an acidic pH optimum. Therefore, if granules from clc-3−/− cells are not acidified, we would expect a rise in proinsulin and a fall in insulin levels similar to that seen in granules in β-cells isolated from prohormone convertase 1/3 null (pc1/3−/−) mice (Zhu et al., 2002). We probed this possibility at the electron microscopic level. Results in Fig. 3B indicate that granules from clc-3−/− mice are less dense than wild type, a characteristic indicator of impaired granule processing from proinsulin to insulin (Furuta et al., 1998; Zhu et al., 2002). Granule number did not significantly vary between genotypes (data not shown). An examination of clc-3−/− β–cell granules showing increased levels of proinsulin as compared to granules from WT β–cells can be seen in Fig. 3C. Proinsulin labeling was carried out on thin sections of pancreatic β–cells using the proinsulin monoclonal antibody GS-9A8 and examined by immunoelectronmicroscopy. In contrast to WT β–cells, where proinsulin staining was confined to the Golgi and nascent (maturing) secretory granules, proinsulin staining in the clc-3−/− β–cells was observed in the majority of the secretory granules both maturing and at endpoint (plasmalemmal location).
Granular acidification defect in clc-3−/− β–cells
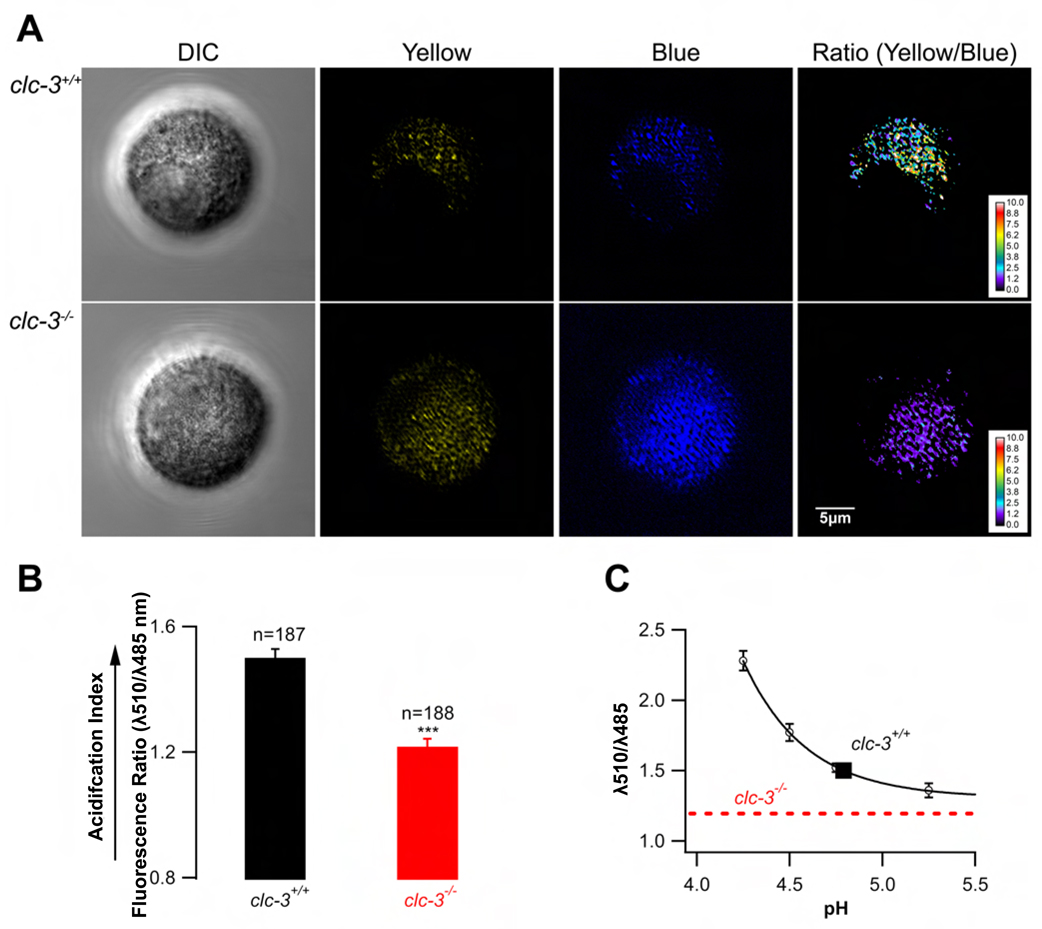
It has been shown previously that interference with granular acidification reduces exocytosis in β–cells (Barg et al., 2001). In order to compare granular acidification in clc-3−/− and clc-3+/+ cells, we carried out live cell microscopy on isolated β–cells using the ratiometric, acidotropic dye, LysoSensor Yellow/Blue DND-160 that has recently been applied to β–cell granules by Stiernet et al. (Stiernet et al., 2006). If granule acidification is dependent on ClC-3 activity, then the granule population in β–cells isolated from clc-3−/− mice should be relatively alkalotic as compared to similar populations in clc-3+/+ β–cells. Given that the acid-sensitive dye accumulates in all acidic organelles including lysosomes, we restricted our confocal imaging and analysis to regions of the cells likely to represent a high density of subplasmalemmal granules. In these experiments, we used a dual emission protocol where cells excited with 405 nm showed pH sensitive emission at 510 nm and pH insensitive emission at 485 nm (Fig. 4). The corresponding pH values for 510/485 nm ratios were obtained from an in situ calibration curve (Fig. 4 C) by interpolation. The curve was constructed using a cocktail of ionophores to clamp the intracellular pH at a known value (smooth curve through the data points in Fig. 4C). Data show that WT cells had a significantly higher acidification index than did the clc-3−/− cells (Fig.4 A, B). Their 510/485 nm ratio was 1.5 ± 0.03 corresponding to a pH of 4.79 in subplasmalemmal granules. In situ calibration of the dye in double excitation experiments (excitation at 340 and 380 nm, emission at 535 nm) also showed that WT cells exhibited a granular pH of 4.8 ± 0.1 (n = 112) (data not shown). The pH sensitivity of the dye (pKa = 4.2) precluded accurate in situ dye calibration at pH values greater than 5.2 in the fluorescence ratio range of the clc-3−/− cells. Thus, the pH of the alkalotic clc-3−/− granules can be estimated as at least one full pH unit greater than that of ClC-3 expressing cells.
Figure 4. Granule acidification in β–cells isolated from clc-3+/+ and clc-3−/− mice.
Pancreatic β–cells were loaded with the pH-sensitive, ratiometric dye LysoSensor Yellow/Blue DND-160 and visualized with confocal microscopy. (A) Dye-filled vesicles were imaged at the polar focal plane (close to the cell surface to maximize the analysis of secretory granules). The ratio of yellow (pH-sensitive) to blue (pH-insensitive) emission was used to determine organellar pH. (B) Results were analyzed using ImageJ software and presented as mean 510/485 nm fluorescence ratios ± standard errors. (C) In situ calibration curve was used to determine pH values corresponding to 510/485 nm ratios obtained experimentally. The dotted line corresponds to the experimentally obtained fluorescence ratio in clc-3−/− cells. The ratio was significantly less than that of the clc-3+/+ cells and corresponded to the asymptotic part of the pH calibration curve making an estimate of the corresponding pH ambiguous (see text).
DISCUSSION
Results described in this study establish that the Cl− channel ClC-3 is critical in the control of insulin release at the whole animal, isolated islet, and single β–cell level. Our data provide evidence that ClC-3 expressed on secretory granule membranes is involved in the maintenance of a low intragranular pH, a phenomenon increasingly recognized as important to secretion. Cl− entry across the granule membrane is thought to be required to shunt H+ influx via the V-ATPase, thus preventing the buildup of a large transgranular membrane potential.
Until recently, anion channels have received relatively little attention, as compared to their cation channel cousins, in the context of regulated insulin release. The complexity of Cl− channels became apparent with the cloning of an evolutionarily-conserved family of Cl− channels (ClCs), as well as the discovery of other channel families that principally carry Cl− (e.g. CFTR, Ca2+-activated Cl− channels) (Jentsch et al., 2002). ClC-3 is a member of the former category and is widely distributed in mammalian tissues. It can assume either a plasma membrane or intracellular membrane localization, and has been proposed as the channel mediating acidification in endosomes (Hara-Chikuma et al., 2005; Mitchell et al., 2008). Mice lacking ClC-3 exhibit a variety of defects, most prominently postnatal degeneration of the hippocampus and photoreceptors (Dickerson et al., 2002; Stobrawa et al., 2001; Yoshikawa et al., 2002). ClC-3 is present on synaptic vesicles and may play a role in glutamatergic neurotransmitter release (Stobrawa et al., 2001). Indeed, we have recently shown that ClC-3 shapes synaptic currents in developing hippocampal neurons, in part, by studying clc-3−/− mice (Wang et al., 2006).
To understand the molecular basis of secretion it is important to have assays that analyze single vesicle (granule) events and to be able to manipulate individual molecules thought to be important in the process. Capacitance measurements of cell surface area have been utilized in many systems, including β–cells (Bokvist et al., 2000; Gopel et al., 2004; Kanno et al., 2004; Rorsman and Renstrom, 2003), to investigate pools of vesicles and their role in secretion. These studies have shown that in β-cells the transient first phase of insulin secretion is mediated by a docked pool of vesicles termed the “readily releasable pool” (RRP), while the sustained second phase is due to mobilization of vesicles from a reserve pool into the RRP. Mobilization involves “priming” of the vesicles, a stage requiring ATP hydrolysis, but whether there is a single or multiple ATP-requiring processes is unclear. Exocytosis itself is almost certainly mediated by a SNARE mechanism that promotes vesicle fusion in many regulated secretory systems (Eliasson et al., 2008).
In the capacitance experiments of Barg et al. (Barg et al., 2001) conducted on isolated murine β–cells, intrapipette (intracellular) application of a functional inhibitory antibody directed against a peptide in the cytoplasmic C-terminal domain of ClC-3 significantly reduced the late slow component of exocytosis corresponding to the filling of the RRP of granules from the reserve pool (Barg et al., 2001). A comparative analysis of the size of the RRP under similar conditions was not determined in their studies. In our experiments, both fast phase (triggered by the first depolarization) and the later component of exocytosis, slow phase triggered with a train of depolarizations following a refilling period, were significantly inhibited in cells isolated from clc-3−/− mice. In contrast to data using ClC-3 inhibitory antibodies (Barg et al., 2001), our data using clc-3−/− β–cells are consistent with a model in which ClC-3 plays a role in determining both the size or release competency of the granular RRP as well as the rate of replenishment of the granular RRP from the reserve pool.
Granule acidification mechanism and potential functions
It has been known for some time that the intragranular pH (pHg) of insulin granules is acidic (pHg≈5–6, with a decrease during granule maturation) (Abrahamsson and Gylfe, 1980; Hutton, 1982; Orci et al., 1986; Pace and Sachs, 1982). Recent studies in mouse islets have shown that glucose causes an acute decrease in pHg that is dependent on the metabolism of glucose and cytoplasmic Cl− (Stiernet et al., 2006). These results suggest that there is a pathway from glucose metabolism that augments acidification, possibly by increasing Cl− movement into the granule. One important function of an acidified granular lumen is to promote the conversion of proinsulin to insulin by prohormone convertases (PCs 1–3) that have an acidic pH optimum (Rouille et al., 1995). Other granule constituents that are substrates for PCs (like islet amyloid polypeptide, IAPP) may also be dependent on acidification. The slow phase of glutamate release from synaptosomes (Zoccarato et al., 1999) shows similar pH dependence as that we have observed for β–cell granules suggesting that the model developed in β–cell may be universal for all Ca2+ regulated secretion. Moreover, our data suggest that low intragranular pH may be required not only for the priming of the granules but also for the maintenance of the granules in a releasable state. These observations are in good agreement with those made in pituitary melanotrophs (Thomas et al., 1993), glucagon-releasing pancreatic alpha-cells (Hoy et al., 2000) and chromaffin granules (Camacho et al., 2006; Pothos et al., 2002). Consequently, it may well be that development of a low pHg is required for other aspects of granule physiology that impact the priming step.
Although it is still unclear whether the regulation of granule acidification can be directly connected with the development of human type 2 diabetes, and the ClC-3 gene itself has not been so far implicated in the pathogenesis of type 2 diabetes, this study suggests that a ClC-3-mediated mechanism leading to impaired granule acidification and proinsulin processing could contribute to diabetes, especially in the context of insulin resistance.
EXPERIMENTAL PROCEDURES
Animals
All experiments on mice were performed in accordance with the University of Chicago and national guidelines and regulations, and were approved by the University of Chicago Institutional Animal Care and Use Committee. Clc-3−/− mice and wild-type control littermates were bred at the Animal Core Facility of the University of Chicago and genotyped as described previously (Dickerson et al., 2002).
Glucose homeostasis
Blood glucose and insulin concentrations were measured in 8-week-old male clc-3−/− and littermate controls after a 4-hour fast, then at the indicated times following intraperitoneal injection of glucose (2g/kg) using a Freestyle blood glucose monitor (Therasense, Inc., Alameda, CA) and mouse ultrasensitive ELISA (Alpco Diagnostics, Salem, NH).
Islet perifusion assay
Fifty islets per mouse were loaded into columns and perifused with modified Krebs-Ringer buffer containing 0.5% BSA and the indicated concentrations of glucose or KCl using a temperature-controlled automated perifusion system (Biorep Perifusion v2.0.0). Outflow was collected and hormone concentrations were measured by mouse insulin (Alpco Diagnostics, Salem, NH) and glucagon ELISA (Phoenix Pharmaceuticals, Inc., Burlingame, CA). To determine total insulin content, islets were lysed by passing 2% Triton-X-100 through the column. See Supplemental Data for detailed procedure.
Electrophysiology
Procedure for perforated patch capacitance recordings may be found in Supplemental Data.
Electron microscopy
Isolated islets were prepared for electron microscopy as described previously (Barg et al., 2001). Fixed sections were treated as indicated with anti-insulin polyclonal antibody raised in guinea pig (Millipore, Bedford, MA), custom-made anti-ClC-3 polyclonal antibody raised in rabbit (Huang et al, 2001), or anti-proinsulin monoclonal antibody GS-9A8, a generous gift of Dr. O. Madsen (BCBC Antibody Core Unit, Novo Nordisk, Malov, Denmark). Secondary antibodies conjugated to gold particles (Ted Pella, Inc., Redding, CA) were used for visualization.
Fluorescence microscopy
Isolated β-cells were loaded with LysoSensor Yellow/Blue DND-160 (Molecular Probes, Eugene, OR) and visualized using a Leica SP2 confocal microscope using 63× NA 1.5 oil objective. The preparations were excited at 405 nm, and emission was recorded at the range of 485±20 nm for blue (pH-insensitive) and 510±20 nm for yellow (pH sensitive) fluorescence. Images were taken at the polar focal plane, ROIs were drawn around fluorescent spots representing docked vesicles and intensities were measured at each emission band. Results were analyzed using ImageJ software and presented as mean 510/485 fluorescence ratios ± SEM. The pH values of the measured ratios were determined by interpolation using the in situ calibration curve.
In situ pH calibration curve
In situ pH calibration curve was performed as described in Supplemental Data.
Statistical Analyses
Statistical comparisons were performed using Student’s t-test. Unless otherwise stated, all data are expressed as a mean ± S.E.M. with the number of experiments in parentheses.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abrahamsson H, Gylfe E. Demonstration of a proton gradient across the insulin granule membrane. Acta Physiol Scand. 1980;109:113–114. doi: 10.1111/j.1748-1716.1980.tb06573.x. [DOI] [PubMed] [Google Scholar]
- Ammala C, Eliasson L, Bokvist K, Larsson O, Ashcroft FM, Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. The Journal of Physiology. 1993;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg S, Huang P, Eliasson L, Nelson DJ, Obermuller S, Rorsman P, Thevenod F, Renstrom E. Priming of insulin granules for exocytosis by granular Cl(−) uptake and acidification. Journal of Cell Science. 2001;114:2145–2154. doi: 10.1242/jcs.114.11.2145. [DOI] [PubMed] [Google Scholar]
- Bokvist K, Holmqvist M, Gromada J, Rorsman P. Compound exocytosis in voltage-clamped mouse pancreatic beta-cells revealed by carbon fibre amperometry. Pflugers Arch. 2000;439:634–645. doi: 10.1007/s004249900211. [DOI] [PubMed] [Google Scholar]
- Camacho M, Machado JD, Montesinos MS, Criado M, Borges R. Intragranular pH rapidly modulates exocytosis in adrenal chromaffin cells. J Neurochem. 2006;96:324–334. doi: 10.1111/j.1471-4159.2005.03526.x. [DOI] [PubMed] [Google Scholar]
- Dickerson LW, Bonthius DJ, Schutte BC, Yang B, Barna TJ, Bailey MC, Nehrke K, Williamson RA, Lamb FS. Altered GABAergic function accompanies hippocampal degeneration in mice lacking ClC-3 voltage-gated chloride channels. Brain Res. 2002;958:227–250. doi: 10.1016/s0006-8993(02)03519-9. [DOI] [PubMed] [Google Scholar]
- Easom RA. CaM kinase II: a protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes. 1999;48:675–684. doi: 10.2337/diabetes.48.4.675. [DOI] [PubMed] [Google Scholar]
- Easom RA, Filler NR, Ings EM, Tarpley J, Landt M. Correlation of the activation of Ca2+/calmodulin-dependent protein kinase II with the initiation of insulin secretion from perifused pancreatic islets. Endocrinology. 1997;138:2359–2364. doi: 10.1210/endo.138.6.5179. [DOI] [PubMed] [Google Scholar]
- Eliasson L, Abdulkader F, Braun M, Galvanovskis J, Hoppa MB, Rorsman P. Novel aspects of the molecular mechanisms controlling insulin secretion. The Journal of Physiology. 2008;586:3313–3324. doi: 10.1113/jphysiol.2008.155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Carroll R, Martin S, Swift HH, Ravazzola M, Orci L, Steiner DF. Incomplete processing of proinsulin to insulin accompanied by elevation of Des-31,32 proinsulin intermediates in islets of mice lacking active PC2. The Journal of Biological Chemistry. 1998;273:3431–3437. doi: 10.1074/jbc.273.6.3431. [DOI] [PubMed] [Google Scholar]
- Gopel S, Zhang Q, Eliasson L, Ma XS, Galvanovskis J, Kanno T, Salehi A, Rorsman P. Capacitance measurements of exocytosis in mouse pancreatic alpha-, beta- and delta-cells within intact islets of Langerhans. The Journal of Physiology. 2004;556:711–726. doi: 10.1113/jphysiol.2003.059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Hoy M, Renstrom E, Bokvist K, Eliasson L, Gopel S, Rorsman P. CaM kinase II-dependent mobilization of secretory granules underlies acetylcholine-induced stimulation of exocytosis in mouse pancreatic B-cells. The Journal of Physiology. 1999;518:745–759. doi: 10.1111/j.1469-7793.1999.0745p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M, Yang B, Sonawane ND, Sasaki S, Uchida S, Verkman AS. ClC-3 chloride channels facilitate endosomal acidification and chloride accumulation. The Journal of Biological Chemistry. 2005;280:1241–1247. doi: 10.1074/jbc.M407030200. [DOI] [PubMed] [Google Scholar]
- Hoy M, Olsen HL, Bokvist K, Buschard K, Barg S, Rorsman P, Gromada J. Tolbutamide stimulates exocytosis of glucagon by inhibition of a mitochondrial-like ATP-sensitive K+ (KATP) conductance in rat pancreatic A-cells. The Journal of Physiology. 2000;527(Pt 1):109–120. doi: 10.1111/j.1469-7793.2000.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Liu J, Di A, Robinson NC, Musch MW, Kaetzel MA, Nelson DJ. Regulation of human CLC-3 channels by multifunctional Ca2+/calmodulin- dependent protein kinase. The Journal of Biological Chemistry. 2001;276:20093–20100. doi: 10.1074/jbc.M009376200. [DOI] [PubMed] [Google Scholar]
- Hutton JC. The internal pH and membrane potential of the insulin-secretory granule. Biochem J. 1982;204:171–178. doi: 10.1042/bj2040171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Kanno T, Ma X, Barg S, Eliasson L, Galvanovskis J, Gopel S, Larsson M, Renstrom E, Rorsman P. Large dense-core vesicle exocytosis in pancreatic beta-cells monitored by capacitance measurements. Methods. 2004;33:302–311. doi: 10.1016/j.ymeth.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Kinard TA, Goforth PB, Tao Q, Abood ME, Teague J, Satin LS. Chloride channels regulate HIT cell volume but cannot fully account for swelling-induced insulin secretion. Diabetes. 2001;50:992–1003. doi: 10.2337/diabetes.50.5.992. [DOI] [PubMed] [Google Scholar]
- Krueger KA, Bhatt H, Landt M, Easom RA. Calcium-stimulated phosphorylation of MAP-2 in pancreatic betaTC3-cells is mediated by Ca2+/calmodulin-dependent kinase II. The Journal of Biological Chemistry. 1997;272:27464–27469. doi: 10.1074/jbc.272.43.27464. [DOI] [PubMed] [Google Scholar]
- Maritzen T, Keating DJ, Neagoe I, Zdebik AA, Jentsch TJ. Role of the vesicular chloride transporter ClC-3 in neuroendocrine tissue. J Neurosci. 2008;28:10587–10598. doi: 10.1523/JNEUROSCI.3750-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Wang X, Zhang G, Gentzsch M, Nelson DJ, Shears SB. An expanded biological repertoire for Ins(3,4,5,6)P4 through its modulation of ClC-3 function. Curr Biol. 2008;18:1600–1605. doi: 10.1016/j.cub.2008.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Madsen O, Perrelet A, Vassalli JD, Anderson RG. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol. 1986;103:2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace CS, Sachs G. Glucose-induced proton uptake in secretory granules of beta-cells in monolayer culture. Am J Physiol. 1982;242:C382–C387. doi: 10.1152/ajpcell.1982.242.5.C382. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Mosharov E, Liu KP, Setlik W, Haburcak M, Baldini G, Gershon MD, Tamir H, Sulzer D. Stimulation-dependent regulation of the pH, volume and quantal size of bovine and rodent secretory vesicles. The Journal of Physiology. 2002;542:453–476. doi: 10.1113/jphysiol.2002.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NC, Huang P, Kaetzel MA, Lamb FS, Nelson DJ. Identification of an N-terminal amino acid of the CLC-3 chloride channel critical in phosphorylation-dependent activation of a CaMKII-activated chloride current. The Journal of Physiology. 2004;556:353–368. doi: 10.1113/jphysiol.2003.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- Rouille Y, Duguay SJ, Lund K, Furuta M, Gong Q, Lipkind G, Oliva AA, Jr, Chan SJ, Steiner DF. Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Frontiers in Neuroendocrinology. 1995;16:322–361. doi: 10.1006/frne.1995.1012. [DOI] [PubMed] [Google Scholar]
- Stiernet P, Guiot Y, Gilon P, Henquin JC. Glucose acutely decreases pH of secretory granules in mouse pancreatic islets. Mechanisms and influence on insulin secretion. The Journal of Biological Chemistry. 2006;281:22142–22151. doi: 10.1074/jbc.M513224200. [DOI] [PubMed] [Google Scholar]
- Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- Thomas F, Pittman K, McFadden T, Haisch C, Peterson R, Thomas J. Reversal of type-II diabetes by pancreas islet transplant in 4 separate animal models of type-II diabetes. Transplantation Proceedings. 1993;25:992–993. [PubMed] [Google Scholar]
- Wang XQ, Deriy LV, Foss S, Huang P, Lamb FS, Kaetzel MA, Bindokas V, Marks JD, Nelson DJ. CLC-3 channels modulate excitatory synaptic transmission in hippocampal neurons. Neuron. 2006;52:321–333. doi: 10.1016/j.neuron.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Uchida S, Ezaki J, Rai T, Hayama A, Kobayashi K, Kida Y, Noda M, Koike M, Uchiyama Y, Marumo F, Kominami E, Sasaki S. CLC-3 deficiency leads to phenotypes similar to human neuronal ceroid lipofuscinosis. Genes Cells. 2002;7:597–605. doi: 10.1046/j.1365-2443.2002.00539.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Orci L, Carroll R, Norrbom C, Ravazzola M, Steiner DF. Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10299–10304. doi: 10.1073/pnas.162352799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccarato F, Cavallini L, Alexandre A. The pH-sensitive dye acridine orange as a tool to monitor exocytosis/endocytosis in synaptosomes. J Neurochem. 1999;72:625–633. doi: 10.1046/j.1471-4159.1999.0720625.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.