Abstract
A long-sought goal during the battle against avian influenza is to develop a new generation of vaccines capable of mass immunizing humans as well as poultry (the major source of avian influenza for human infections) in a timely manner. Although administration of the currently licensed influenza vaccine is effective in eliciting protective immunity against seasonal influenza, this approach is associated with a number of insurmountable problems for preventing an avian influenza pandemic. Many of the hurdles may be eliminated by developing new avian influenza vaccines that do not require the propagation of an influenza virus during vaccine production. Replication-competent adenovirus-free adenovirus vectors hold promise as a carrier for influenza virus-free avian influenza vaccines owing to their safety profile and rapid manufacture using cultured suspension cells in a serum-free medium. Simple and efficient mass-immunization protocols, including nasal spray for people and automated in ovo vaccination for poultry, convey another advantage for this class of vaccines. In contrast to parenteral injection of adenovirus vector, the potency of adenovirus-vectored nasal vaccine is not appreciably interfered by pre-existing immunity to adenovirus.
Keywords: adenovirus, AdHigh, avian influenza, influenza virus-free influenza vaccine, in ovo vaccine, mass immunization, nasal vaccine, RCA
A new class of avian influenza vaccine is required for mitigating a pandemic
Avian influenza (AI) not only ravages the poultry industry in multiple countries, but is also a major threat to global human health. Compelling evidence suggests that all the influenza A viruses responsible for the human pandemics of 1918 (H1N1), 1957 (H2N2) and 1968 (H3N2) were derived from AI viruses. Both the 1957 and 1968 viruses were avian/human reassortants, whereas the 1918 influenza virus (IFV) was probably an AI virus that adapted to human genetic background in toto and subsequently killed approximately 50 million people through airborne transmission [1]. In 1997, a highly pathogenic avian influenza (HPAI) H5N1 virus emerged. Evidence of sporadic bird-to-human and subsequent human-to-human transmissions, as well as its lethality in humans, is alarming [2,3]. Resurgence of the virus and its variants despite the slaughter of enormous numbers of healthy birds to create a buffer zone suggests that there may be no possibility of eradicating this fatal disease. The finding that signature mutations identified in the 1918 virus were also present in H5N1 viruses that caused human fatalities implied that some of the current H5N1 strains may be capable of inducing an infectious wave if the mutation(s) necessary for efficient human-to-human transmission shou ld appea r in a n AI virus genome [1]. Furthermore, IFV strains are capable of escaping immunologic pressure conferred by vaccination through antigenic drift and shift [4]. The consequences of denying nimble countermeasures could be dire.
Annual vaccination has been a cost-effective medical intervention in mitigating seasonal influenza [5,6] . Like the first inactivated IFV vaccines developed during the 1940s, the currently licensed trivalent (two influenza A subtypes and one influenza B virus) inactivated IFV vaccine (TIV) is still produced by propagating seed IFV strains in embryonated chicken eggs followed by collecting the allantoic fluids from which the virus is purified and inactivated. TIV formulations include whole-virus particles, disrupted particles (split vaccines) and purified antigens (subunit vaccines). The requirement for intramuscular injection of TIV is associated with a number of problems, including a limited supply of medical personnel capable of performing needle injections, reluctance to vaccination owing to pain and fear of needles (aichmophobia), transmission of blood-borne pathogens with reused needles and disposal of needles as medical waste. It is conceivable that a large proportion of the human population may not have access to adequate needle-injection services during a pandemic outbreak. In 2003, a live-attenuated (cold-adapted) IFV vaccine (LAIV) was approved for administration through nasal spray in the USA as a needle-free alternative for influenza vaccination [101].
Both TIV and LAIV are currently produced in embryonated chicken eggs based on an outmoded technology. The epidemic IFV strains and a backbone strain that grows efficiently in eggs are routinely coinoculated into the allantoic cavity of embryonated chicken eggs and incubated for approximately 12 days. It takes at least 4 months to produce the first vaccine after a candidate IFV vaccine strain is identified [7]. A high-biocontainment facility is required for propagating a lethal IFV strain (e.g., H5N1), which is a threat to production workers as well as the public if escape from the facility should occur. Propagation of human IFV strains in chicken eggs often selects variants that are not antigenically identical to the original clinical isolates owing to different growth requirements in avian and human cells [8]. Recently, a reverse-genetics system for safer and more rapid generation of a seed IFV strain using recombinant DNA technology could shorten the time required and improve success rates. This custom designer approach involves recovery of IFV particles from cells transfected with plasmids encoding each of the eight gene segments of the IFV [9].
It often takes one to two eggs to produce one dose of IFV vaccine [10]. With such a low yield, a large number of qualified eggs and huge incubator space are required for mass production. Contamination is difficult to identify in eggs since each egg is a bioreactor itself and eggs may not be internally sterile despite extensive disinfection of the egg shell. Purification of IFV particles from infected eggs during mass production often resorts to high-speed centrifugation [11]; inactivation is achieved by treating viruses with formaldehyde or β-propiolactone; and virus splitting is performed using detergents. The requirement for 100% inactivation of IFV may compromise its production in regions where the enforcement of regulation is insufficient. Trace amounts of endotoxin, egg protein, formaldehyde and preservative are often found in final TIV products [8] and their chronic impacts on health are not well understood [12]. Embryonated chicken eggs are perishable, must be ordered months in advance, and the supply could be conceivably interrupted during a pandemic when many chickens are infected or culled. Although the presence of undetectable and even unknown chicken pathogens in specific-pathogen-free eggs is not a problem for TIV owing to the inactivation process, it could be a biohazard for LAIV due to the possibility of copurification of live avian pathogens with a live vaccine. Potentially harmful reassortments generated between a LAIV vaccine strain and a wild HPAI virus within a dually infected mammalian (human or intermediate animal) host could link the high transmissibility associated with a human-adapted LAIV with the high lethality of HPAI viruses, allowing a reas-sortant to morph into a virus capable of triggering a devastating pandemic [4]. In general, inactivated H5 AI virus vaccines are only mildly immunogenic in humans even after multiple cycles of vaccination [13–17] . Overall, platforms in support of licensed influenza vaccines produced in embryonated chicken eggs are not tenable in the face of an AI pandemic. To prevent a pandemic from occurring, an audacious change in the production of AI vaccines is required.
Cell culture-based avian influenza vaccines
To break out of the traditional mold, a number of new technologies have been developed to varying stages (Table 1). IFV vaccines have been produced in cultured cells instead of embryonated chicken eggs. Advantages of using cell cultures include more rapid and streamlined production cycles, better control of the process, less chance for contamination, and increased cost– effectiveness. Unlike embryonated chicken eggs, cell lines can be kept frozen indefinitely and used to scale up manufacturing capability on a short notice. Of the various available continuous mammalian cell lines, both the African green monkey kidney (VERO) and Madin–Darby canine kidney (MDCK) cells have yielded IFV particles with higher titers usually observed in the latter because adaptation to VERO cells is required for many IFV strains [18]. However, the MDCK cell line has not been approved by the US FDA for vaccine production [101] owing to its inherent neoplastic properties of unknown mechanisms [19], even though it has been approved in Europe [8]. IFV has also been propagated in the highly characterized and standardized human PER.C6 cell line [20], as well as continuous avian cell lines [10,21] .
Table 1.
Prospects for current (licensed in the USA) and experimental human influenza vaccines.
| Influenza vaccine | Propagation of influenza virus | Egg dependence | Production speed | Replication post-vaccination | Mode of administration | Reassortment potential |
|---|---|---|---|---|---|---|
| Licensed inactivated influenza virus vaccine | Yes | Yes | Slow | No | Injection | No |
| Licensed live-attenuated influenza virus vaccine | Yes | Yes | Slow | Yes | Nasal spray | Yes |
| Inactivated influenza virus vaccine produced in MDCK, VERO and PER.C6 cells | Yes | No | Fast | No | Injection | No |
| Live attenuated influenza virus vaccine produced in MDCK cells | Yes | No | Fast | Yes | Nasal spray | Yes |
| Baculovirus-expressed influenza vaccine* | No | No | Fast | No | Injection; VLP may be administered by nasal spray | No |
| DNA-based influenza vaccine | No | No | Fast | No | Injection | No |
| Vaccinia virus-vectored influenza vaccine | No | No | Fast | Yes | Injection | No |
| Ad5-vectored influenza vaccine* | No | No | Fast | No | Nasal spray | No |
Ad5-vectored and baculovirus-expressed VLP nasal influenza vaccines can be produced rapidly without the requirement to propagate an IFV; without dependence on the supply of qualified embryonated chicken eggs; without the hazard of shedding replicating viruses; without the need to use a penetrating device; and without the potential for reassortment with wild avian influenza viruses.
Ad5: Adenovirus serotype 5; IFV: Influenza virus; MDCK: Madin–Darby canine kidney; VLP: Virus-like particle.
Since propagation of human IFV strains in chicken eggs often generates mutant strains due to difference in growth requirements between avian and human cells [8], it is conceivable that production of AI viruses in mammalian cells as an opposite may also induce undesirable mutations with antigenicity distinct from that of the original isolates. The efficacy of AI virus vaccines produced in mammalian cells, particularly at a large scale after multiple passages, remains uncertain.
IFV-free influenza vaccines
There are compelling reasons for not propagating an IFV during development and production of an influenza vaccine, since some IFV strains do not replicate well in the laboratory [22], some (e.g., H5N1) may be highly lethal to personnel [3] and all of them are prone to mutations and reassortment events [4]. LAIV is particularly unsafe as a vaccine candidate against AI owing to its capability to reassort with wild AI viruses post-administration [4], with the potential of triggering a pandemic instead of preventing one.
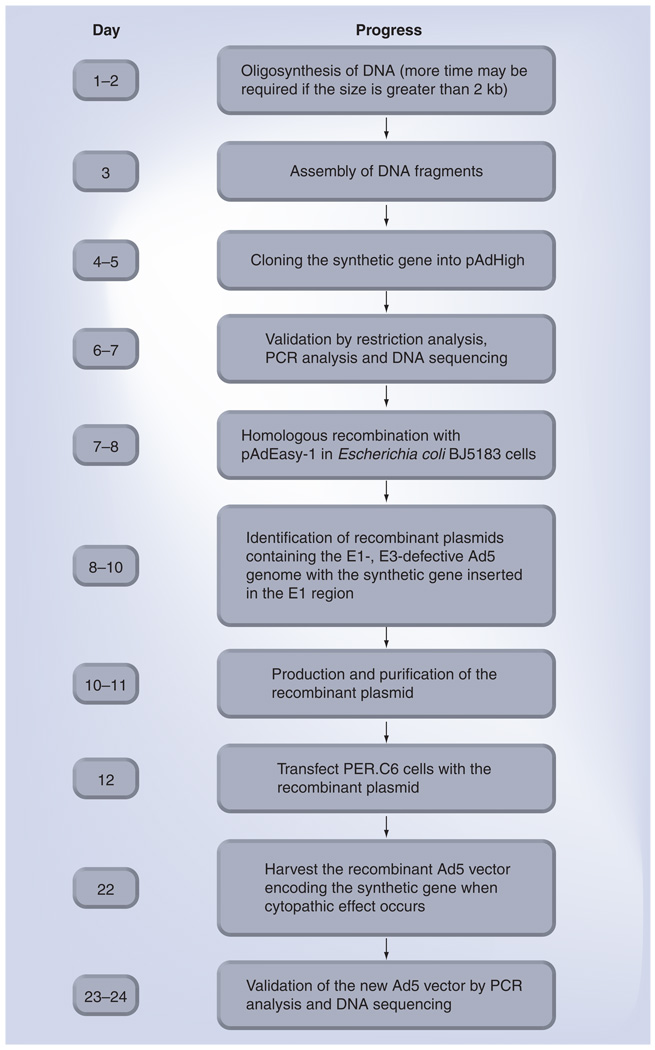
A number of IFV-free influenza vaccines have been developed (Table 1), including DNA influenza vaccines [23–25], baculovirusexpressed influenza vaccines [26–29], vaccinia virus-vectored influenza vaccines [30] and adenovirus (Ad)-vectored influenza vaccines [31–37]. The shift away from using IFV as an influenza vaccine holds advantages in cost, time, safety and production control. Since it is not possible to predict the emergence of a specific pandemic strain and stockpile an effective vaccine in advance, it is imperative to develop new AI vaccines that can be produced rapidly in response to a surge in demand with an antigenic match to the new strain soon after its identification. All of the four new classes of IFV-free influenza vaccines can be generated quickly using molecular biology techniques without the need to propagate an IFV since a defined IFV gene can be synthesized rapidly (Figure 1) once its sequence is known [35,37].
Figure 1. Rapid generation of replication-competent adenovirus (RCA)-free recombinant Ad5 vectors with the AdHigh system.
A new RCA-free Ad5 vector encoding a synthetic transgene potentially may be generated within 1 month.
Ad5: Adenovirus serotype 5.
Despite its ease of production, DNA vaccines generally suffer from low potency in nonhuman primates and humans [38,39]. Ad-vectored vaccines were significantly more immunogenic than DNA vaccines in mice [40] and humans [41]. A baculo-virus-expressed hemagglutinin (HA) vaccine was also less efficacious than its Ad-vectored counterpart in mice [33], although the former could be improved by formulation into a virus-like particle (VLP) [27]. The high potency of Ad-vectored vaccine is attributed, in part, to efficient receptor binding [42] and inter-nalization of Ad particles [43,44], the ability of Ad DNA to escape endosomal vesicles [45–47] and Ad’s competence in eliciting both humoral and cellular immune responses in mice [32,33,37] and humans [31,41,48].
Although low potency could be circumvented by adjuvantation and/or increasing the vaccine dose in conjunction with frequent booster applications, administration of DNA, baculovirus-expressed and vaccinia virus-vectored vaccines generally requires the use of penetrating devices (e.g., a syringe needle, scalpel, gene gun and in vivo electroporator) and trained medical personnel to operate these devices. Noninvasive vaccination by non-medical personnel in a single-dose regimen is crucial for mitigating a pandemic when medical resources are in short supply during a crisis. Ad-vectored nasal influenza vaccines can elicit robust and long-lasting immunity in mice [33,49] and humans [31] in a safe manner without using an invasive device. In contrast to LAIV, Ad vectors are nonreplicating once purified from packaging cells, do not require embryonated chicken eggs for production and do not reassort with wild IFV strains (the DNA genome of Ad and the RNA genome of IFV cannot exchange genetic material). Like Ad-vectored nasal influenza vaccine, the VLP-based nasal influenza vaccine was also capable of activating both arms of the immune system in animals [27]. To date, some of these IFV-free influenza vaccines have been evaluated during human clinical trials with varying degrees of success.
Adenovirus serotype 5-vectored nasal vaccine leverages gene therapy-associated hurdles to safe & effective vaccination
Among the 49 serotypes of human Ad, Ad serotype 5 (Ad5)-derived vectors have been extensively characterized, modified and employed in vaccination and gene-therapy trials [50]. Although Ad5 is highly efficient in transducing a wide variety of dividing and nondividing cells [51,52], there are limitations to its utility as a gene-therapy vector. Notably, transgene expression from an Ad5 vector is transient and the repeated administration of the vector is compromised by the inherent immunogenicity of Ad5 [53,54]. Unlike classic gene therapy, which requires persistent and high-level transgene expression in target tissues, evidence is emerging that beneficial clinical outcomes for vaccination can be achieved by inducing a transient wave of antigen expression, because short-term and low-level expression of antigens in an immunocompetent tissue could foster the longevity of memory T cells by minimizing antigen-induced apoptosis of T lymphocytes [55]. Short duration of the vaccine after mobilizing the immune repertoire also confers a high safety profile to the host owing to lack of persistent interruption of genomic homeostasis.
As Ad5 is a benign virus that frequently invades the human respiratory tract [54], the human immune system must have developed a defense mechanism against Ad5 in the airway during evolution. Ad5’s immunogenicity, although undesirable for gene therapy, provides adjuvant activity capable of boosting the immunogenicity of exo genous antigens [56]. Unlike unnatural adjuvants (manmade molecules and/or administration to an inappropriate site) that may induce unpredictable consequences [12], natural infection by Ad5 with intrinsic adjuvant activity is generally benign. Years of use as a preventative measure for adenoviral infection have demonstrated the safety of oral administration of Ad in humans [57]. A nasal spray of a nonreplicating Ad5 vector into its natural portal should, thus, represent a driver in the pursuit of safe vaccine development. Intranasal administration of soluble protein without supplemental adjuvant is generally ineffective in eliciting a potent immune response [27]; however, unnatural adjuvantation can be risky. An example of undesirable consequence when evolution is violated is the association of intranasal administration of Escherichia coli heat-labile toxin adjuvant with Bell’s palsy [58]. To date, immunization of humans by Ad5-vectored nasal influenza vaccine, which is in compliance with evolutionary medicine, has not been associated with any undesirable side effects [31].
Ad5-vectored vaccine would be of limited utility if its potency should be interfered by pre-existing Ad5 immunity since human exposure to Ad5 is common worldwide [54]. In contrast to parenteral injection of an Ad5-vectored vaccine whose potency is interfered by pre-exposure to Ad5 [41,48,59], emerging evidence shows that Ad5-vectored nasal vaccines are capable of bypassing pre-existing Ad5 immunity [31,33,40,59]. The high immunogenicity of Ad5-vectored nasal vaccine even in the presence of pre-existing Ad5 immunity could be attributed to the low threshold for vaccination in conjunction with the high efficiency of transgene delivery into the highly immunocompetent mucosal barrier. Results suggest that Ad5-vectored vaccines may broadcast a signal long enough to activate the immune system prior to elimination by pre-existing Ad5 immunity, as shown by prolonged in vivo expression of a transgene despite the presence of pre-existing Ad5 immunity in animal models [60–63], even after repetitive intranasal administration into nonhuman primates [60].
It is conceivable that a nasal vaccine may induce focused mucosal immunity against respiratory pathogens in the airway as shown by findings that LAIV was more effective than TIV in preventing influenza in humans [64,65] and nasal delivery of an Ad5-vectored tuberculosis vaccine induced more robust protective immunity against pulmonary tuberculosis than its parenteral counterpart in mice [66]. It has also been demonstrated that an Ad5-vectored nasal HIV vaccine induced greater HIV-specific IgA responses than its parenteral counterpart in mucosal secretions in mice [67]. The supremacy of nasal vaccines in conferring protection against respiratory pathogens may be attributed, in part, to IgA-mediated neutralization of virus at the mucosal surface. In contrast to nasal vaccination, intra muscular injection of TIV failed to stimulate secretory IgA and did not significantly reduce infection rates of IFV [68]. Moreover, antigens expressed from an Ad5 vector in animals’ own cells are folded into their native conformational form, correctly modified and presented by major histocompatibility complexes and, hence, they can induce more effective immune responses compared with their counterparts in biochemically purified subunit vaccines [69].
Owing to the nasal cavity’s proximity to the brain, it is crucial to determine whether Ad5 particles may induce inflammation and toxicity in the brain following nasal spray. Unlike influenza, which is associated with human neurological disorders [70], inhalation of Ad5 has not been reported to induce enc ephalitis in animals or humans. Intranasal administration of Ad5 into mice neither expressed transgenes in the brain beyond the olfactory bulb nor induced inflammatory responses in any tissue [67], whereas intracranial injection of Ad5 induced acute encephalitis [71]. It is, thus, conceivable that no significant amounts of Ad5 can enter the brain following inhalation under normal conditions. Even though a small number of Ad5 particles may infiltrate into the brain on occasion and persist in immunocompromised patients following nasal spray, administration of an E1-defective Ad5 vector will do less harm than infection by a replicating virus owing to its incompetence to amplify adverse effects through replication and late-gene expression.
Ad5-vectored vaccines can be rapidly produced & mass administered at low costs in response to a surge in demand
Generation of nonreplicating Ad5 vectors in packaging cells by trans-complementation of the defective E1 genes in the Ad5 genome is an ingenious design for construction of safe vectors. Two continuous packaging cell lines are commonly used for propagation of E1-defective human Ad5 vectors: 293, a line established by transforming human embryonic kidney cells with the left end of the Ad5 genome [72] and PER.C6, a line established by transforming human embryonic retinal cells with the Ad5 E1 region [73]. Each infected 293 packaging cell can produce up to 10,000 of Ad5 vectors [74]. However, contamination by replication-competent Ad (RCA), generated by a double-crossover event between the homologous overlapping sequences present in the Ad5 vector and the genome of the 293 packaging cell, appears to be an intrinsic problem for the 293 cell line [75,76]. Intramuscular injection of Ad5 stocks containing substantial amounts of RCA induced severe inflammation and muscular atrophy in newborn rats whereas E1-defective recombinant Ad5 was not associated with pathogenicity [76]. Since the ratio between RCA and recombinant Ad5 kept increasing in culture and a ratio of 1:40 had been detected after multiple passages owing to a replicative advantage of RCA [76], it is conceivable that large-scale production of Ad5 vectors in 293 cells may generate RCA beyond the safety margin.
Replication-competent Ad-free Ad5 vectors can be produced in PER.C6 packaging cells [73] and the yield is comparable to that of its counterpart produced in 293 cells (Tang DC, Unpublished Data). The PER.C6 cell line contains only Ad5 nucleotides 459–3510, which precludes double-crossover-type homologous recombination between the PER.C6 genome and a matched Ad5 vector with this E1 subfragment deleted. However, a helper-dependent E1-positive particle (HDEP) occurs at an extremely low frequency (not more than one HDEP in 7.5 × 1012 Ad5 particles) during production of Ad5 vectors in PER.C6 cells [77]. Unlike RCA, HDEP contains E1 derived from the PER.C6 genome but are devoid of E2, E3, E4 and late genes and, hence, cannot replicate independently [77]. In contrast to RCA that outgrows E1-defective recombinant Ad5 vector in culture [76], HDEP could be diluted out over time under low multiplicity of infection (MOI) due to its replicative dis advantage. When compared with infection by wild-type Ad5 and administration of RCA-contaminated Ad5 vectors, the number of E1 genes in RCA-free Ad5 stocks containing HDEP is vanishingly small and poses a negligible risk.
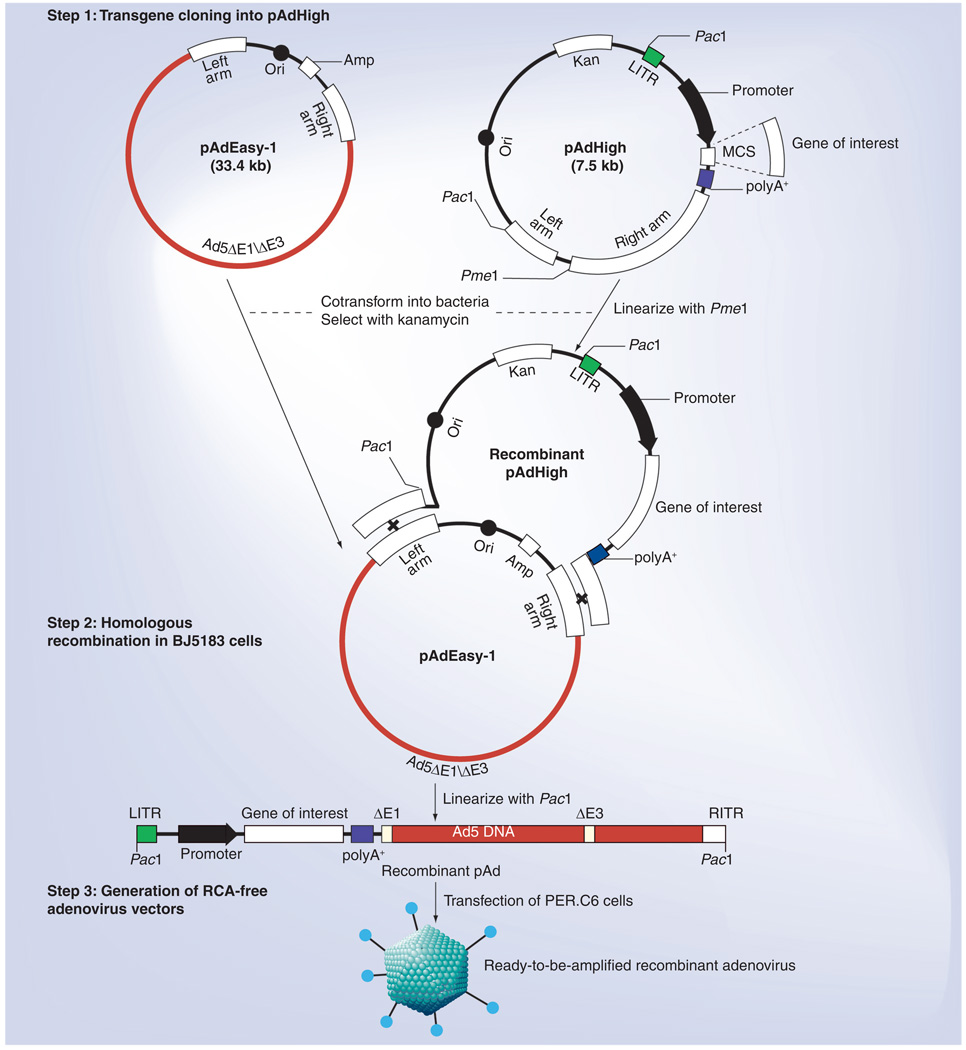
Although generation of RCA-free Ad5 vectors in PER.C6 cells foregoes many of the potential safety concerns, the conventional process through homologous recombination between shuttle and Ad5 backbone DNA in PER.C6 packaging cells is slow and the subsequent cycles of plaque purification is even more timeconsuming [73]. The finding that homologous recombination can be carried out in E. coli cells [78–80] streamlined the procedure for Ad construction by allowing recombination to occur overnight in bacterial cells and obviating the time-consuming steps of plaque purification. We have developed a new system, ‘AdHigh’, for rapid generation of RCA-free recombinant Ad5 vectors in PER.C6 cells by allowing homologous recombination to occur in E. coli cells instead of mammalian packaging cells. This new system differs from the commonly used AdEasy system [79,80] in which the defective pIX promoter found in AdEasy’s shuttle plasmids [79,81] was repaired in AdHigh’s shuttle plasmid pAdHigh. pIX plays multiple roles in Ad5’s life cycle, including stabilization of Ad5 capsids, transcriptional activation and reorganization of nuclear proteins [82,83]. Although pIX-knockout Ad5 vectors can be propagated with the same efficiency and titers as wildtype Ad5 in human 293 cells owing to trans-complementation of pIX expressed from the 293 genome [84], the PER.C6 cell is not endowed with the pIX gene [73] and hence does not support efficient propagation of Ad5 vectors with a defective pIX promoter.
To repair the defective pIX promoter in the AdEasy system, a shuttle plasmid pAdHigh was generated by shuffling DNA fragments between AdEasy’s pShuttle-CMV (provided by Johns Hopkins University, MD, USA) [79] and the PER.C6-compatible pAdApt (provided by Crucell Holland BV) [35] shuttle plasmids. pAdHigh contains Ad5 nucleotides 1–454 and 3511–5790 flanking the pAdApt-derived expression cassette under transcriptional control of the human cytomelagovirus (CMV) early promoter, Ad5 nucleotides 34931–35935, as well as bacterial sequences containing the pBR322 origin of replication and the kanamycinresistant gene (Figure 2). After cloning a transgene into the expression cassette downstream from the CMV promoter in the correct orientation, competent E. coli BJ5183 cells can be sequentially transformed with the Ad5 backbone plasmid pAdEasy-1 followed by a recombinant pAdHigh shuttle as described [80]. An RCA-free Ad5 vector encoding the transgene can be subsequently generated by transfecting PER.C6 cells with a recombinant plasmid as described (Figure 2) [79]. By performing homologous recombination between shuttle and Ad5 backbone plasmids in E. coli cells and replacing plaque purification with colony purification, a new RCA-free Ad5-vectored carrier containing a synthetic codon-optimized transgene can be generated in PER. C6 cells within 1 month using the AdHigh system (Figure 1), which fosters a more timely and less laborious process for responding quickly to the emergence of a new AI virus.
Figure 2. The AdHigh system.
The pAdHigh shuttle plasmid is a hybrid between pShuttle-CMV [79] and pAdApt [35], containing Ad5 sequences flanking an expression cassette. After cloning a transgene into the cassette, homologous recombination is allowed to occur between the shuttle plasmid pAdHigh and the Ad5 backbone plasmid pAdEasy-1 in Escherichia coli BJ5183 cells. RCA-free Ad5 vectors encoding a transgene can be generated by transfecting PER.C6 cells with the recombinant plasmid.
Ad5: Adenovirus serotype 5; Amp: Ampicillin-resistance gene; CMV: Cytomelagovirus; Kan: Kanamycin-resistance gene; Left arm: Ad5 nucleotides 34931–35935; LITR: Ad5 nucleotides 1–454; MCS: Multiple cloning site; polyA+: Simian virus 40 polyadenylation signal; Ori: PBR322 origin of replication; Promoter: CMV promoter; RCA: Replication-competent adenovirus; Right arm: Ad5 nucleotides 3511–5790 including a functional pIX promoter [81].
This schema was a modification of that for generating AdEasy-derived Ad5 vectors in 293 cells [79].
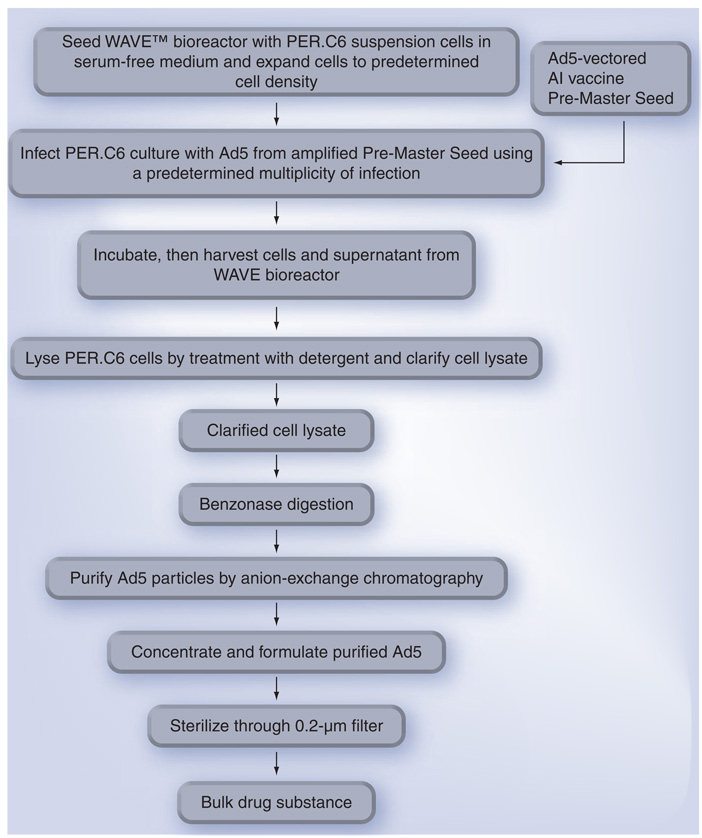
As shown in Figure 3 , RCA-free Ad5 vectors are routinely produced by infecting PER. C6 suspension cells in serum-free medium at a concentration of 1 × 106 cells per ml as described [85]. A 500-l cellbag containing Ad5-infected PER.C6 cells on a wave bioreactor can potentially produce 5 × 1015 pfu of RCA-free Ad5 vectors (equivalent to multimillion doses based on human [31] and poultry [35] trials) in a few days after infection at an appropriate MOI, although the actual yield may vary from batch to batch. Use of disposable cellbags minimizes contamination and eliminates the requirements to clean and sterilize the production system. Purification of Ad5 vectors by specific anion exchange columns allows nearly 100% recovery [86], which is easier to scale up than purification of IFV from eggs [11] or VLP from insect cells [27], which require highspeed centrifugation. Development of a stable liquid formulation enables Ad5 vectors to be stored over a year at 4°C and a few weeks at an ambient temperature without titer loss [87].
Figure 3. Schema for mass production of Ad5-vectored avian influenza (AI) vaccines.
PER.C6 suspension cells are propagated in serum-free medium in a disposable cellbag on a WAVETM bioreactor; Ad5-infected cells are lysed by treatment with a detergent; PER.C6 DNA is digested with benzonase; Ad5 particles are purified by anion exchange chromatography and formulated in A195 buffer [87]; and the Ad5-vectored AI vaccine is finally sterilized by filtration through 0.2-µm membranes. This schema was a modification of that for producing cGMP-grade Ad5-vectored AI vaccines in one of Vaxin’s Investigational New Drug applications filed with the US FDA.
Ad5: Adenovirus serotype 5.
Mass immunization of poultry with a human Ad5-vectored vaccine
As the current H5N1 AI epidemic highlights, the zoonotic origin of pandemic influenza strains suggests that mass immunization of poultry against AI should be listed as a global priority in order to safeguard public health as well as promote animal health control. Current vaccination of poultry with oil emulsion, inactivated, whole-AI-virus vaccines or fowl pox-vectored AI vaccines (Table 2) requires handling and injection of individual birds [88], which are labor intensive and time consuming. In addition, the personnel are at great risk when they have to handle a large number of live poultry during a pandemic with the possibility of accidental transmission of a field AI virus when crews travel between farms. It has been demonstrated that chickens can be effectively protected against AI by in ovo vaccination with human Ad5-vectored AI vaccines, even against mismatched strains [34 –36]. This novel AI vaccine not only protected chickens against clinical signs and death during challenge studies, but also reduced AI virus shedding [34]. The outcome is significant because this regimen can potentially minimize the hazard of uncontrolled viral circulation among flocks and prevent an AI virus from crossing the species barrier into human populations. In addition, in ovo AI vaccination can be automated with available robotic in ovo injectors for mass immunization of poultry in a time-, labor- and cost-saving manner with a superb safety profile [36]. Moreover, we have demonstrated that in ovo vaccination by an Ad5-vectored AI vaccine made from crude extract was as effective as its purified counterpart in eliciting an antibody response in chickens (Unpublished Data). By eliminating fetal bovine serum in culture medium and the requirement for Ad5 purification, the costs for mass immunization of poultry may be greatly reduced in conjunction with automated mass administration. In addition to in ovo injection, chickens can also be immunized by intraocular administration of Ad5-vectored AI vaccines [89], which may allow mass immunization of poultry by aerosol spray of Ad5 particles.
Table 2.
Prospects for current (licensed in various countries) and experimental avian influenza vaccines for the mass immunization of poultry.
| AI vaccine | Propagation of AI virus | Replication postvaccination | Mode of administration | Interference by pre-existing immunity to avian virus | Mass administration (simultaneous immunization of multiple birds by one administration) |
|---|---|---|---|---|---|
| Licensed oil emulsion inactivated whole AI virus vaccine | Yes | No | Subcutaneous or intramuscular injection after 10 days of age | No | No |
| Licensed fowl pox-vectored AI vaccine | No | Yes | Subcutaneous or intramuscular injection at or after 1 day of age | Yes | No |
| Licensed Newcastle disease virus-vectored AI vaccine | No | Yes | Aerosol spray | Yes | Yes |
| Baculovirus-expressed, oil emulsion AI vaccine | No | No | Subcutaneous injection | No | No |
| DNA-based AI vaccine* | No | No | Subcutaneous, intramuscular or in ovo injection | No | Yes |
| Ad5-vectored AI vaccine* | No | No | Subcutaneous, intramuscular or in ovo injection | No | Yes |
Ad5-vectored and DNA-based AI vaccines can be produced rapidly without the requirement to propagate an influenza virus; without dependence on the supply of qualified embryonated chicken eggs; without the hazard of shedding replicating viruses; without immunologic interference from pre-existing immunity to avian viruses; and with the potential for mass administration. However, DNA-based Al vaccine has not been licensed for commercial application owing to its low potency [88]. Although the Ad5-vectored AI vaccine is more potent than its DNA-based counterpart in immunizing chickens (Toro H, Unpublished Data), whether the former will be licensed for mass immunization of poultry remains to be seen. Low-pathogenic AI virus vaccines are not recommended for poultry immunization owing to the potential for generating harmful reassortants, as well as inducing respiratory disease and egg-production drop [88].
Ad: Adenovirus; Ad5: Adenovirus serotype 5; AI: Avian influenza.
The use of inactivated AI virus vaccines derived from the circulating or closely related AI viruses precludes differentiation between infected and vaccinated poultry by routinely used serological tests. This intrinsic problem with an AI virus vaccine has limited its use because it is difficult to prove that flocks are AI-free after vaccination. In contrast to an AI virus vaccine, the use of an Ad5-vectored AI vaccine is compatible with a differentiation between infected and vaccinated animals (DIVA) strategy because the vector encodes a limited number of AI virus-derived antigens (e.g., HA). Thus, host antibody responses to marker antigens (e.g., nuclear protein) expressed by the AI virus but not the vector can be used to discriminate natural infection from vaccination.
In contrast to avian virus (e.g., fowl pox and Newcastle disease virus)-vectored AI vaccines whose potency is often interfered by pre-existing immunity to the avian virus [36,88], we have shown that chickens immunized in ovo by the human Ad5 vector encoding a H5 HA could still be immunized post-hatch by intramuscular injection of another Ad5 vector encoding a H7 HA [35]. Evidence suggests that it is unlikely that pre-existing Ad5 immunity in chickens may significantly interfere with the potency of Ad5-vectored vaccines. This potential problem may be further minimized by injection of in ovo vaccines on E18 (day 18 of embryonic incubation in the egg), 3 days before hatch, as described [34,36], for avoiding the steep rise in yolk maternal antibody transfer to the embryonic circulation between E19 and E21 [90]. Embryonic pathogen-specific serum antibody levels are much lower on E17–E19 than from E20 to immediately post-hatch [91]. Thus, E18 appears to offer a unique window for in ovo vaccination when the level of circulating maternally derived antibody is relatively low, allowing Ad5 to transduce embryonic cells and activate the chicken immune system with minimal interference from maternal Ad5 immunity.
Conclusion
Overall, rapid and cost-effective production of Ad5-vectored AI vaccines in conjunction with existing mass administration techniques may foster the development of a powerful tool for mass immunization of humans as well as poultry in order to arrest an AI pandemic in a simple, effective, economic and safe manner without the requirement for propagating an IFV during vaccine production.
Expert commentary
Avian influenza is a major threat to global poultry industry as well as public health worldwide. It is urgent to develop a new generation of IFV-free AI vaccine that can be produced rapidly, at low costs, and easily administered by non-medical personnel, to prevent or mitigate an AI pandemic. Ad5-vectored AI vaccines hold great promise as a critical tool against AI since they comply with those criteria. Ad5-vectored AI vaccines induce robust immune responses in humans as well as poultry, and they can be applied in mass-vaccination programs (nasal spray for people and automated in ovo administration for poultry). The disadvantages identified with Ad5 vectors during classic gene therapy trials have been shown to be advantages when Ad5 vectors are used as nasal vaccine carriers. Nasal spray of a nonreplicating Ad5 vector is in compliance with evolutionary medicine and thus should be less risky than artificial vaccines, manmade adjuvants and/or un natural routes that are unfamiliar to the human immune system.
Five-year view
A human Phase I clinical trial on a RCA-free Ad5-vectored nasal AI vaccine against a H5 HA was initiated in the fourth quarter of 2008 at the University of Alabama at Birmingham, AL, USA. Human Phase II clinical trials on two different Ad5-vectored nasal AI vaccines are expected to be completed within 5 years. An US NIH Small Business Innovation Research Program Phase II grant (#2-R44-AI-068285-02) has been awarded to the Vaxin Inc.–Auburn University–Southeast Poultry Research Laboratory team for refining Ad5-vectored in ovo AI vaccines and developing an Ad5-vectored in ovo Newcastle disease vaccine during 2008–2011. Ad5-vectored in ovo vaccines are expected to enter the commercial market within 5 years.
Key issues.
It is urgent to develop an influenza virus-free avian influenza (AI) vaccine that does not require the propagation of influenza viruses and the supply of embryonated chicken eggs.
Replication-competent Ad (RCA)-free recombinant Ad5 vectors can be generated rapidly by the AdHigh system in PER.C6 cells.
RCA-free Ad5-vectored AI vaccines can be rapidly produced at low costs from PER.C6 suspension cells in serum-free medium in response to an escalation in demand.
RCA-free Ad5-vectored AI vaccines can be mass administered into people by nasal spray, as well as into poultry by automated in ovo administration.
Ad5-vectored vaccines can induce highly specific immune interventions based on well-defined antigens that are the focus of specific immune reactivity.
There should not be any safety concerns for nasal administration of a RCA-free Ad5 vector into people since the vector is nonreplicating and the procedure is in compliance with evolutionary medicine.
There should not be any safety concerns for in ovo administration of an Ad5 vector since chicken cells do not support the replication of human Ad5. It is conceivable that chickens will rapidly eliminate Ad5 after the immune repertoire is mobilized toward a beneficial immune protection following vaccination.
Acknowledgements
The authors thank the University of Alabama at Birmingham for providing the animal facility and performing a human clinical trial on an adenovirus-vectored avian influenza (H5N1) vaccine; Southern Research Institute for performing some of the assays and animal challenge studies in their BSL-3+ facility; Southeast Poultry Research Laboratory for challenging chickens with highly pathogenic avian influenza viruses in their BSL-3+ facility; and Southern Drug Research for performing a human clinical trial on an adenovirus-vectored influenza (H1N1) vaccine.
Footnotes
Financial & competing interests disclosure
The authors at Vaxin Inc. and Auburn University are shareholders of Vaxin Inc. and inventors on patents pertaining to adenovirus-vectored vaccines. The National Institutes of Health and the US Navy provided grant support. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Information resources
• Van Kampen KR, Shi Z, Gao P et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine 23, 1029–1036 (2005).
• Toro H, Tang DC, Suarez DL, Sylte MJ, Pfeiffer J, Van Kampen KR. Protective avian influenza in ovo vaccination with non-replicating human adenovirus vector. Vaccine 25, 2886–2891 (2007).
Contributor Information
De-chu C Tang, Vice President of Research, and Chief Technical Officer, Vaxin Inc., 1500 First Avenue North, Birmingham, AL 35203, USA, Tel.: +1 205 909 3738, Fax: +1 205 307 6503, tang@vaxin.com.
Jianfeng Zhang, Vaxin Inc., 1500 First Avenue North, Birmingham, AL 35203, USA, Tel.: +1 205 909 3740, Fax: +1 205 307 6503, zhang@vaxin.com.
Haroldo Toro, Department of Pathobiology, Auburn University, 264 Greene Hall, Auburn, AL 36849, USA, Tel.: +1 334 844 2662, Fax: +1 334 844 2652, torohar@vetmed.auburn.edu.
Zhongkai Shi, Vaxin Inc., 1500 First Avenue North, Birmingham, AL 35203, USA, Tel.: +1 205 909 3751, Fax: +1 205 307 6503, shi@vaxin.com.
Kent R Van Kampen, Vaxin Inc., 1500 First Avenue North, Birmingham, AL 35203, USA, Tel.: +1 205 909 3737, Fax: +1 205 307 6503, vankampen@vaxin.com.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Taubenberger JK, Reid AH, Lourens RM, et al. Characterization of the 1918 influenza virus polymerase genes. Nature. 2005;437:889–893. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]
- 2.Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1) N. Engl. J. Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 3.Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371:1464–1475. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- 4.Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine. 2002;20:3068–3087. doi: 10.1016/s0264-410x(02)00254-2. [DOI] [PubMed] [Google Scholar]
- 5.Nichol KL, Lind A, Margolis KL, et al. The effectiveness of vaccination against influenza in healthy, working adults. N. Engl. J. Med. 1995;333:889–893. doi: 10.1056/NEJM199510053331401. [DOI] [PubMed] [Google Scholar]
- 6.Nichol KL, Nordin J, Mullooly J, et al. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N. Engl. J. Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 7.Emanuel EJ, Wertheimer A. Public health. Who should get influenza vaccine when not all can? Science. 2006;312:854–855. doi: 10.1126/science.1125347. [DOI] [PubMed] [Google Scholar]
- 8.Audsley JM, Tannock GA. Cell-based influenza vaccines: progress to date. Drugs. 2008;68:1483–1491. doi: 10.2165/00003495-200868110-00002. [DOI] [PubMed] [Google Scholar]
- 9.Horimoto T, Kawaoka Y. Strategies for developing vaccines against H5N1 influenza A viruses. Trends Mol. Med. 2006;12:506–514. doi: 10.1016/j.molmed.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Smith KA, Colvin CJ, Weber PS, Spatz SJ, Coussens PM. High titer growth of human and avian influenza viruses in an immortalized chick embryo cell line without the need for exogenous proteases. Vaccine. 2008;26:3778–3782. doi: 10.1016/j.vaccine.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Morenweiser R. Downstream processing of viral vectors and vaccines. Gene Ther. 2005;12:S103–S110. doi: 10.1038/sj.gt.3302624. [DOI] [PubMed] [Google Scholar]
- 12.Tang DC, Van Kampen KR. Toward the development of vectored vaccines in compliance with evolutionary medicine. Expert Rev. Vaccines. 2008;7:399–402. doi: 10.1586/14760584.7.4.399. [DOI] [PubMed] [Google Scholar]
- 13.Subbarao K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat. Rev. Immunol. 2007;7:267–278. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenson I, Bugarini R, Nicholson KG, et al. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/ Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J. Infect. Dis. 2005;191:1210–1215. doi: 10.1086/428948. [DOI] [PubMed] [Google Scholar]
- 15.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 16.Bresson JL, Perronne C, Launay O, et al. Safety and immunogenicity of an inactivated split-virion influenza A/ Vietnam/1194/2004 (H5N1) vaccine: Phase I randomised trial. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 17.Keitel WA, Campbell JD, Treanor JJ, et al. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a Phase I-II randomized clinical trial. J. Infect. Dis. 2008;198:1309–1316. doi: 10.1086/592172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozaki H, Govorkova EA, Li C, et al. Generation of high-yielding influenza A viruses in African green monkey kidney (Vero) cells by reverse genetics. J. Virol. 2004;78:1851–1857. doi: 10.1128/JVI.78.4.1851-1857.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palese P. Making better influenza virus vaccines? Emerg. Infect. Dis. 2006;12:61–65. doi: 10.3201/eid1201.051043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pau MG, Ophorst C, Koldijk MH, et al. The human cell line PER.C6 provides a new manufacturing system for the production of influenza vaccines. Vaccine. 2001;19:2716–2721. doi: 10.1016/s0264-410x(00)00508-9. [DOI] [PubMed] [Google Scholar]
- 21.Lee CW, Jung K, Jadhao SJ, Suarez DL. Evaluation of chicken-origin (DF-1) and quail-origin (QT-6) fibroblast cell lines for replication of avian influenza viruses. J. Virol. Methods. 2008;153:22–28. doi: 10.1016/j.jviromet.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Horimoto T, Takada A, Fujii K, et al. The development and characterization of H5 influenza virus vaccines derived from a 2003 human isolate. Vaccine. 2006;24:3669–3676. doi: 10.1016/j.vaccine.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1748. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 24.Fynan EF, Webster RG, Fuller DH, et al. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc. Natl Acad. Sci. USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen MW, Cheng TJ, Huang Y, et al. A consensus-hemagglutinin-based DNA vaccine that protects mice against divergent H5N1 influenza viruses. Proc. Natl Acad. Sci. USA. 2008;105:13538–13543. doi: 10.1073/pnas.0806901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox MM. Progress on baculovirus-derived influenza vaccines. Curr. Opin. Mol. Ther. 2008;10:56–61. [PubMed] [Google Scholar]
- 27.Bright RA, Carter DM, Crevar CJ, et al. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS ONE. 2008;3:e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmood K, Bright RA, Mytle N, et al. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008;26:5393–5399. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 29.Treanor JJ, Schiff GM, Hayden FG, et al. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA. 2007;297:1577–1582. doi: 10.1001/jama.297.14.1577. [DOI] [PubMed] [Google Scholar]
- 30.Kreijtz JH, Suezer Y, de Mutsert G, et al. Recombinant modified vaccinia virus Ankara expressing the hemagglutinin gene confers protection against homologous and heterologous H5N1 influenza virus infections in macaques. J. Infect. Dis. 2009;199:405–413. doi: 10.1086/595984. [DOI] [PubMed] [Google Scholar]
- 31. Van Kampen KR, Shi Z, Gao P, et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005;23:1029–1036. doi: 10.1016/j.vaccine.2004.07.043. Demonstrates safety and immunogenicity of an adenovirus serotype 5 (Ad5)-vectored influenza vaccine in humans.
- 32.Gao W, Soloff AC, Lu X, et al. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 2006;80:1959–1964. doi: 10.1128/JVI.80.4.1959-1964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoelscher MA, Garg S, Bangari DS, et al. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet. 2006;367:475–481. doi: 10.1016/S0140-6736(06)68076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toro H, Tang DC, Suarez DL. Protective avian influenza in ovo vaccination with non-replicating human adenovirus vector. Vaccine. 2007;25:2886–2891. doi: 10.1016/j.vaccine.2006.09.047.Demonstrates that chickens can be immunized by in ovo administration of human Ad5-vectored vaccines, which not only can confer protection against avian influenza but also opens the possibility for consolidating different poultry vaccines into a single package that is amenable to automated mass immunization in a wide variety of disease settings.
- 35.Toro H, Tang DC, Suarez DL, Zhang J, Shi Z. Protection of chickens against avian influenza with non-replicating adenovirus-vectored vaccine. Vaccine. 2008;26:2640–2646. doi: 10.1016/j.vaccine.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avakian AP, Poston RM, Kong FK, Van Kampen KR, Tang DC. Automated mass immunization of poultry: the prospect for nonreplicating human adenovirus-vectored in ovo vaccines. Expert Rev. Vaccines. 2007;6:457–465. doi: 10.1586/14760584.6.3.457. [DOI] [PubMed] [Google Scholar]
- 37.Holman DH, Wang D, Raja NU, et al. Multi-antigen vaccines based on complex adenovirus vectors induce protective immune responses against H5N1 avian influenza viruses. Vaccine. 2008;26:2627–2639. doi: 10.1016/j.vaccine.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 38.Sykes K. Progress in the development of genetic immunization. Expert Rev. Vaccines. 2008;7(9):1395–1404. doi: 10.1586/14760584.7.9.1395. [DOI] [PubMed] [Google Scholar]
- 39.Lu S, Wang S, Grimes-Serrano JM. Current progress of DNA vaccine studies in humans. Expert Rev. Vaccines. 2008;7(2):175–191. doi: 10.1586/14760584.7.2.175. [DOI] [PubMed] [Google Scholar]
- 40.Shi Z, Zeng M, Yang G, et al. Protection against tetanus by needle-free inoculation of adenovirus-vectored nasal and epicutaneous vaccines. J. Virol. 2001;75:11474–11482. doi: 10.1128/JVI.75.23.11474-11482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the step study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 43.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins α v β 3 and α v β 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 44.Shayakhmetov DM, Eberly AM, Li ZY, Lieber A. Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. J. Virol. 2005;79:1053–1061. doi: 10.1128/JVI.79.2.1053-1061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. J. Virol. 2005;79:1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 47.Leopold PL, Crystal RG. Intracellular trafficking of adenovirus: many means to many ends. Adv. Drug Deliv. Rev. 2007;59:810–821. doi: 10.1016/j.addr.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 48.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoelscher MA, Jayashankar L, Garg S, et al. New pre-pandemic influenza vaccines: an egg-and adjuvant-independent human adenoviral vector strategy induces long-lasting protective immune responses in mice. Clin. Pharmacol. Ther. 2007;82:665–671. doi: 10.1038/sj.clpt.6100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang DC, Johnston SA, Carbone DP. Butyrate-inducible and tumor-restricted gene expression by adenovirus vectors. Cancer Gene Ther. 1994;1:15–20. [PubMed] [Google Scholar]
- 52.Tang DC, Jennelle RS, Shi Z, et al. Overexpression of adenovirus-encoded transgenes from the cytomegalovirus immediate early promoter in irradiated tumor cells. Hum. Gene Ther. 1997;8:2117–2124. doi: 10.1089/hum.1997.8.17-2117. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Nunes FA, Berencsi K, et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl Acad. Sci. USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nwanegbo E, Vardas E, Gao W, et al. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 2004;11:351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 56.Molinier-Frenkel V, Lengagne R, Gaden F, et al. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J. Virol. 2002;76:127–135. doi: 10.1128/JVI.76.1.127-135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tucker SN, Tingley DW, Scallan CD. Oral adenoviral-based vaccines: historical perspective and future opportunity Expert Rev. Vaccines. 2008;7:25–31. doi: 10.1586/14760584.7.1.25. [DOI] [PubMed] [Google Scholar]
- 58.Couch RB. Nasal vaccination, Escherichia coli enterotoxin, and Bell’s palsy. N. Engl. J. Med. 2004;350:860–861. doi: 10.1056/NEJMp048006. [DOI] [PubMed] [Google Scholar]
- 59. Croyle MA, Patel A, Tran KN, et al. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS ONE 3, e3548. 2008;3:e3548. doi: 10.1371/journal.pone.0003548. Demonstrates that Ad5-vectored nasal vaccines are not interfered by pre-existing Ad5 immunity.
- 60.Zabner J, Petersen DM, Puga AP, et al. Safety and efficacy of repetitive adenovirus-mediated transfer of CFTR cDNA to airway epithelia of primates and cotton rats. Nat. Genet. 1994;6:75–83. doi: 10.1038/ng0194-75. [DOI] [PubMed] [Google Scholar]
- 61.Wadsworth SC, Zhou H, Smith AE, Kaplan JM. Adenovirus vector-infected cells can escape adenovirus antigen-specific cytotoxic T-lymphocyte killing in vivo. J. Virol. 1997;71:5189–5196. doi: 10.1128/jvi.71.7.5189-5196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scaria A, St George JA, Jiang C, et al. Adenovirus-mediated persistent cystic fibrosis transmembrane conductance regulator expression in mouse airway epithelium. J. Virol. 1998;72:7302–7309. doi: 10.1128/jvi.72.9.7302-7309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen P, Kovesdi I, Bruder JT. Effective repeat administration with adenovirus vectors to the muscle. Gene Ther. 2000;7:587–595. doi: 10.1038/sj.gt.3301137. [DOI] [PubMed] [Google Scholar]
- 64.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 2007;356:685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 65.Treanor JJ, Kotloff K, Betts RF, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine. 1999;18:899–906. doi: 10.1016/s0264-410x(99)00334-5. [DOI] [PubMed] [Google Scholar]
- 66. Wang J, Thorson L, Stokes RW, et al. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 2004;173:6357–6365. doi: 10.4049/jimmunol.173.10.6357.Demonstrates that a nasal vaccine can induce a more protective immune response against respiratory pathogens than its parenteral counterpart.
- 67. Lemiale F, Kong WP, Akyurek LM, et al. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J. Virol. 2003;77:10078–10087. doi: 10.1128/JVI.77.18.10078-10087.2003.Demonstrates that an Ad5 vector does not infiltrate into the brain beyond the olfactory bulb following intranasal administration.
- 68.Betts RF, Treanor JJ. Approaches to improved influenza vaccination. Vaccine. 2000;18:1690–1695. doi: 10.1016/s0264-410x(99)00508-3. [DOI] [PubMed] [Google Scholar]
- 69.Bright RA, Carter DM, Daniluk S, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 70.Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med. Infect. Dis. 2008;6:114–124. doi: 10.1016/j.tmaid.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Thomas CE, Edwards P, Wickham TJ, Castro MG, Lowenstein PR. Adenovirus binding to the coxsackievirus and adenovirus receptor or integrins is not required to elicit brain inflammation but is necessary to transduce specific neural cell types. J. Virol. 2002;76:3452–3460. doi: 10.1128/JVI.76.7.3452-3460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59.Demonstrates that a nonreplicating Ad5 vector can be generated in bioengineered packaging cells, although replication-competent adenovirus (RCA) contamination was found many years later.
- 73. Fallaux FJ, Bout A, van der Velde I, et al. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum. Gene Ther. 1998;9:1909–1917. doi: 10.1089/hum.1998.9.13-1909.Demonstrates that a RCA-free Ad5 vector can be generated in bioengineered packaging cells.
- 74.Graham FL, Prevec L. Methods for construction of adenovirus vectors. Mol. Biotechnol. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- 75.Zhu J, Grace M, Casale J, et al. Characterization of replication-competent adenovirus isolates from large-scale production of a recombinant adenoviral vector. Hum. Gene Ther. 1999;10:113–121. doi: 10.1089/10430349950019246. [DOI] [PubMed] [Google Scholar]
- 76. Lochmuller H, Jani A, Huard J, et al. Emergence of early region 1-containing replication-competent adenovirus in stocks of replication-defective adenovirus recombinants (δ E1 + δ E3) during multiple passages in 293 cells. Hum. Gene Ther. 1994;5:1485–1491. doi: 10.1089/hum.1994.5.12-1485.Demonstrates that contamination of an El-defective Ad5 stock by RCA is an intrinsic property of 293 cells.
- 77.Murakami P, Havenga M, Fawaz F, et al. Common structure of rare replication-deficient E1-positive particles in adenoviral vector batches. J. Virol. 2004;78:6200–6208. doi: 10.1128/JVI.78.12.6200-6208.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chartier C, Degryse E, Gantzer M, et al. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J. Virol. 1996;70:4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996.Demonstrates that recombinant Ad5 vectors can be generated rapidly by allowing homologous recombination between shuttle and Ad5 backbone plasmids to occur in Escherichia coli cells.
- 79. He TC, Zhou S, da Costa LT, et al. A simplified system for generating recombinant adenoviruses. Proc. Natl Acad. Sci. USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509.Develops the AdEasy system for rapid generation of recombinant Ad5 vectors in 293 cells.
- 80.Zeng M, Smith SK, Siegel F, et al. AdEasy system made easier by selecting the viral backbone plasmid preceding homologous recombination. Biotechniques. 2001;31:260–262. doi: 10.2144/01312bm04. [DOI] [PubMed] [Google Scholar]
- 81.Babiss LE, Vales LD. Promoter of the adenovirus polypeptide IX gene: similarity to E1B and inactivation by substitution of the simian virus 40 TATA element. J. Virol. 1991;65:598–605. doi: 10.1128/jvi.65.2.598-605.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parks RJ. Adenovirus protein IX: a new look at an old protein. Mol. Ther. 2005;11:19–25. doi: 10.1016/j.ymthe.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 83.Rosa-Calatrava M, Grave L, Puvion-Dutilleul F, Chatton B, Kedinger C. Functional analysis of adenovirus protein IX identifies domains involved in capsid stability, transcriptional activity, and nuclear reorganization. J. Virol. 2001;75:7131–7141. doi: 10.1128/JVI.75.15.7131-7141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vellinga J, Van der Heijdt S, Hoeben RC. The adenovirus capsid: major progress in minor proteins. J. Gen. Virol. 2005;86:1581–1588. doi: 10.1099/vir.0.80877-0. [DOI] [PubMed] [Google Scholar]
- 85.Maranga L, Aunins JG, Zhou W. Characterization of changes in PER.C6 cellular metabolism during growth and propagation of a replication-deficient adenovirus vector. Biotechnol. Bioeng. 2005;90:645–655. doi: 10.1002/bit.20455. [DOI] [PubMed] [Google Scholar]
- 86.Perkins S. Selection and optimization of anion exchange resins for purification of type 5 adenovirus. Presented at: Viral Vectors and Vaccines Conference. Southampton, Bermuda. 2006 November 6–8; [Google Scholar]
- 87. Evans RK, Nawrocki DK, Isopi LA, et al. Development of stable liquid formulations for adenovirus-based vaccines. J. Pharm. Sci. 2004;93:2458–2475. doi: 10.1002/jps.20157.Develops a specific formulation that allows Ad5 stocks to be stored without freezing for long periods.
- 88.Swayne DE, Kapczynski D. Strategies and challenges for eliciting immunity against avian influenza virus in birds. Immunol. Rev. 2008;225:314–331. doi: 10.1111/j.1600-065X.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- 89.van Ginkel FW, Tang DC, Gulley SL, Toro H. Induction of mucosal immunity in the avian Harderian gland with a replication-deficient Ad5 vector expressing avian influenza H5 hemagglutinin. Dev. Comp. Immunol. 2009;33:28–34. doi: 10.1016/j.dci.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kowalczyk K, Daiss J, Halpern J, Roth TF. Quantitation of maternal-fetal IgG transport in the chicken. Immunology. 1985;54:755–762. [PMC free article] [PubMed] [Google Scholar]
- 91.Mast J, Gilson D, Morales D, Meulemans G, van den Berg TP. Transfer of material antibodies from the yolk sac to the chicken. In: van den Berg TP, editor. Immunosuppressive Viral Diseases in Poultry, Annual Report and Proceedings 2001. Luxembourg: Office for Official Publications of the European Communities; 2003. pp. 180–183. [Google Scholar]
Website
- 101.US FDA. www.fda.gov.