Abstract
Recently, it was discovered that sialic acid residues on group B streptococcal (GBS) capsular polysaccharides (CPS) are O-acetylated. Since GBS vaccine development has focused on de-O-acetylated CPS, it became germane to investigate the influence of de-O-acetylated GBS vaccine formulations on functional activity of sera against strains that bear the O-acetyl modification. Post-immunization sera from healthy adult recipients of de-O-acetylated GBS CPS-tetanus toxoid conjugate vaccines were evaluated in opsonophagocytosis assays using 20 GBS clinical isolates representing type Ia, Ib, II, III, or V CPS that varied in amount of O-acetylation from 2 to 40 percent. Ninety percent or greater opsonophagocytosis and killing of all strains were achieved, using CPS type-specific post-immunization sera. These data indicate that de-O-acetylated CPS-conjugate vaccines contain immunogenic epitopes that offer protection against GBS, independent of O-acetyl CPS modifications. Thus, presence of O-acetyl groups on the GBS CPS is not essential for functional antibodies to be elicited by GBS glycoconjugate vaccines.
Keywords: Group B Streptococcus, Vaccine, O-acetylation
1. Introduction
Group B Streptococcus (GBS) remains the leading cause of sepsis and meningitis in neonates and young infants despite universal antenatal screening and maternal intrapartum antibiotic prophylaxis for GBS culture-positive women [1]. GBS also causes invasive disease in pregnant women leading to fetal loss, preterm labor, chorioamnionitis, and early postpartum febrile morbidity [2]. In addition, the incidence of GBS disease is on the rise in non-pregnant adults, particularly in those with underlying medical conditions and the elderly [3]. Case-fatality rates are as high as 15% in adults ≥65 years of age and 20% in preterm infants [1-3]. GBS meningitis infant survivors often suffer from severe sequelae including metal retardation, spastic quadriplegia, and hearing or vision loss [4]. Immunization with a GBS vaccine has theoretical potential to prevent significant morbidity and mortality from perinatal infections in the mother and neonate [5]. Further, GBS immunization of adults with underlying medical conditions and those ≥65 years of age also would have a significant public health impact [6].
All GBS strains possess a capsular polysaccharide (CPS) on their surface. CPS is a major virulence factor and the primary component in GBS vaccines being developed [7, 8]. Nine antigenically distinct CPSs have been characterized: Ia, Ib, and II through VIII [9]. All CPSs are high molecular weight polymers and Ia, Ib, II, III and V are composed of repeating units of glucose, galactose, N-acetylglucosamine, and N-acetylneuraminic acid (sialic acid) [5]. Sialic acid refers to a family of related nine-carbon acidic sugars situated prominently at terminal positions of many eukaryotic surface-exposed glycoconjugates [10]. Many pathogenic bacteria including GBS display sialic acids on their cell surfaces that function to evade host immune systems. Loss of capsular sialic acid in a mutant GBS strain is associated with attenuated virulence in animal models of infection [11]. Additional studies have shown that sialic acids can inhibit alternative complement deposition and block underlying immunogenic epitopes [12, 13]. Both mechanisms of sialic acid evasion allow GBS to resist opsonophagocytosis and killing of GBS by dampening different arms of the complement cascade. Interestingly, antibodies that are protective against type III CPS depend on the presence of sialic acid residues, which dictate a conformational structure of the polysaccharide [14].
Recently, it was reported that terminal sialic acid residues of GBS CPSs are O-acetylated [15]. This finding had been overlooked in previous studies primarily because base treatment during GBS CPS purification removes O-acetyl groups on sialic acid [15]. Thus, GBS vaccine research has focused on de-O-acetylated CPS. It seemed reasonable to consider that the presence of O-acetyl groups could introduce unique epitopes to GBS CPSs or mask other antigenic regions leading to altered host recognition, depending on O-acetylation status. Therefore, we investigated the impact of CPS O-acetylation on the effectiveness of current de-O-acetylated CPS-based vaccine candidates. We evaluated for the first time the functional activity of antibodies elicited by GBS de-O-acetylated CPS-tetanus toxoid conjugate vaccines against GBS strains with varying degrees of O-acetylation.
2. Methods
2.1 Post-vaccination human sera
Healthy non-pregnant adults enrolled in GBS vaccine studies received GBS Ia, Ib, II, III, or V CPS-tetanus toxoid (TT) conjugate vaccines [16-19]. Vaccine CPS sialic acids did not contain O-acetyl groups due to base treatment during vaccine preparation [16]. Paired blood samples were collected before and 8 to 17 weeks after immunization. Pooled CPS-specific sera (standard human reference sera [SHRS]) were derived from post-immunization sera from individuals who developed high concentrations of CPS-specific IgG and/or IgM after immunization with a single dose of GBS type-specific CPS–TT conjugate vaccine. Concentrations of CPS-specific antibodies in each SHRS pool were measured as previously reported [16-19] by quantitative precipitin analysis in combination with radioactive antigen-binding assay (Table 1) using methods detailed by Guttormsen et al [20]. Briefly, quantitative precipitin analysis [21] was employed to establish the concentrations of CPS-specific antibodies in each SHRS. The concentration of each CPS-specific IgG or IgM assigned to a SHRS was based on the sum of values obtained by quantitative precipitation of antibodies [22, 23]. Serial dilutions of each SHRS allowed generation of a standard curve based on optical density values determined at 405 and 630 nm. Sera were stored at -80°C until testing.
Table 1.
Concentrations of GBS glycoconjugate vaccine-induced CPS-specific IgG and IgM in Standard Human Reference Sera (SHRS).
| CPS Type | No. of Vaccine Recipients in Serum Pool | CPS-specific IgG (μg/ml) | CPS-specific IgM (μg/ml) | Total CPS-specific antibody (μg/ml) |
|---|---|---|---|---|
| Ia | 5 | 47.2 | <0.1 | 47.2 |
| Ib | 5 | 35.4 | <0.1 | 35.4 |
| II | 7 | 30.4 | 26.1 | 56.5 |
| III | 5 | 83.5 | <0.1 | 83.5 |
| V | 5 | 11.6 | 25.1 | 36.7 |
2.2. Bacterial strains
Twenty GBS isolates (type Ia [n=5], Ib [n=2], II [n=3], III [n=7], and V [n=3]) were evaluated, including at least 1 prototype strain for each CPS type. The prototype strains were A909 (Ia), UAB (Ib [previously called UAB-II] [15]), DK23 (II), D136 (III), and NCTC 10/84 (V). These well-characterized strains were originally gifts from Drs. Rebecca Lancefield, Dennis Kasper, or Jarmila Jelinkova. COH1 was a gift from Craig Rubens. The remaining 14 GBS isolates were obtained from the Streptococcal Immunology Laboratory collection at Baylor College of Medicine. Except for A909 and D136 (initially obtained from Rebecca Lancefield), all strains were isolated from neonates with invasive early- or late-onset GBS infections of varying severity and clinical expression. Strains were isolated from blood in 15 patients and from cerebrospinal fluid in 3 patients. All strains were stored at -80°C in aliquots of Todd-Hewitt broth (THB) with 20% glycerol until testing.
2.3. O-acetylation analysis
Analysis of O-acetylation on sialic acid was performed by methods previously described [15]. Briefly, GBS strains were grown overnight in THB and washed in phosphate-buffered saline (PBS). Sialic acids were released by acid hydrolysis with 2M acetic acid and the low molecular weight material derivatized with 1,2-diamino-4,5-methylene dioxybenzene (DMB). Reverse-phase high performance liquid chromatography (HPLC) analysis was performed by Glycotechnology Core Resource Laboratory at the University of California, San Diego. Identification of O-acetylation peaks was based on known standards from bovine submaxillary mucin and was confirmed by their sensitivity to NaOH treatment. Quantitation of O-acetylation was performed by automated integration of peak areas. To be consistent with recent publications evaluating the genetic basis for O-acetyl regulation in GBS, we used O-acetylation at the 7 and 9-carbon positions (excluding 8-O acetylation) as a measure of total O-acetyl modification. The “8-OAc” peak is a minor component of GBS sialic acids and is only partially NaOH sensitive.
2.4. Opsonophagocytosis assay
SHRSs containing post-immunization antibodies against type Ia, Ib, II, III, or V CPS (Table 1) were tested for their ability to promote opsonization, phagocytosis and killing by human polymorphonuclear neutrophils (PMNs) of homologous CPS-type strains containing varying levels of O-acetylation. While SHRS Ia, Ib and III consists almost exclusively of CPS-specific IgG, a substantial amount of CPS-specific IgM is found in SHRS II and V; both antibody isotypes are opsonic in vitro. The opsonophagocytosis assay was adapted from a previously described method [24]. Reaction mixtures for opsonization consisted of 50μl of bacteria (2-3 × 106 colony-forming units [cfu]), 100μl of serial dilutions of heat-inactivated SHRS, and 150μl of PBS; these were incubated for 30 minutes at 4°C and then centrifuged at 1600 RPM at 4°C. The supernatant was decanted, and 30μl of infant rabbit complement (Serotec) with 220μl of PBS were gently vortexed, and 50μl of PMNs (1.5×106) from a healthy adult were added and incubated for 40 minutes at 37°C. Results were expressed as the mean log10 reduction in cfu of GBS before and after incubation and represented the mean of 2 to 3 experiments.
2.5. Statistical analysis
Analysis of variance was used to test for differences of sialic acid O-acetylation or opsonophagocytosis between groups. Spearman rho correlation analysis of percent O-acetylation and amount of antibody required to kill one log10 GBS were performed using STATA (version 9.0). P <0.05 was considered significant.
3. Results
3.1. O-acetylation analysis
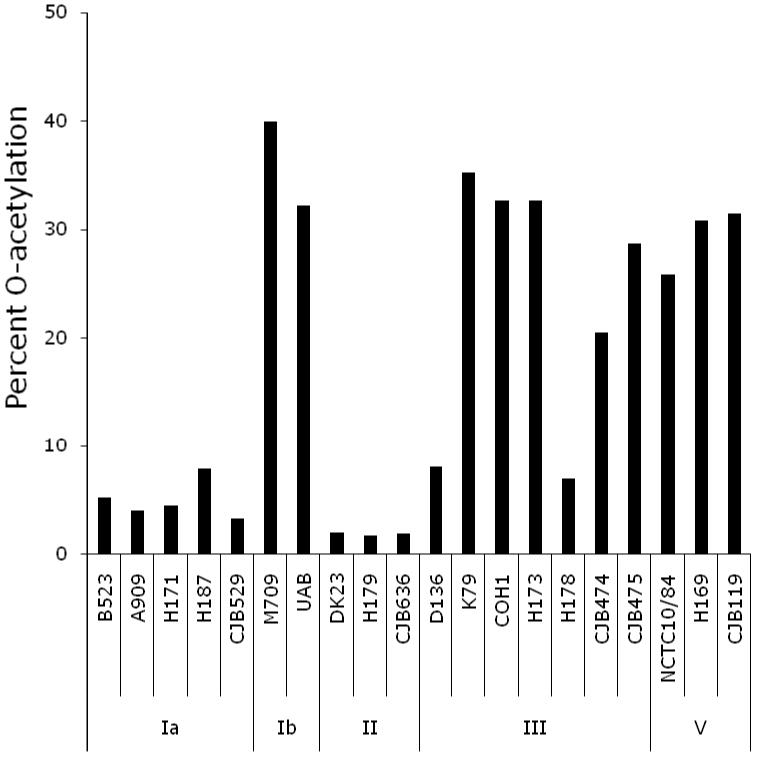
Sialic acids were hydrolyzed from 20 GBS strains containing Ia, Ib, II, III or V CPS and were analyzed by DMB-HPLC. The relative areas of peaks corresponding to retention times of DMB-derivatized standards for 7- and 9-mono-O-acetylated sialic acid were summed to calculate percent of total sialic acids that were O-acetylated. All strains exhibited O-acetylation ranging from 2 to 40 percent of total sialic acids (Figure 1). Variation was noted, but among the strains studied types Ib, III, and V demonstrated higher levels of O-acetylation than types Ia and II (ANOVA, p=<0.001). These data suggest that O-acetylation is likely not a factor for immunization studies with GBS CPS serotype Ia and II conjugate vaccines. In contrast, high levels of O-acetylation observed on CPS serotypes Ib, III, and V confirmed that analysis of the effect of native O-acetylation of the opsonophagocytic activity of sera from individuals vaccinated with these de-O-acetylated CPS conjugate vaccine preparations was warranted.
Figure 1.
The percent of total sialic acids that were O-acetylated at carbon positions 7 and 9 varied by GBS strain. High levels of O-acetylation were observed for CPS types Ib and V; low levels of O-acetylation were observed for CPS types Ia and II. CPS type III showed the most variation.
3.2. Functional activity of sera from adults immunized with de-O-acetylated GBS glycoconjugate vaccines
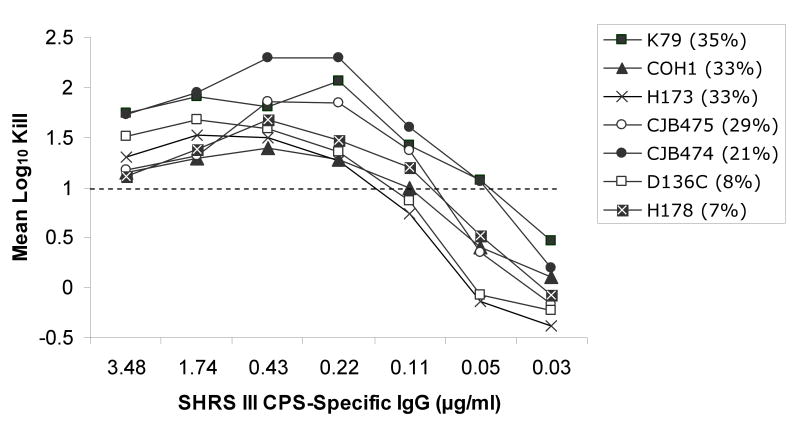
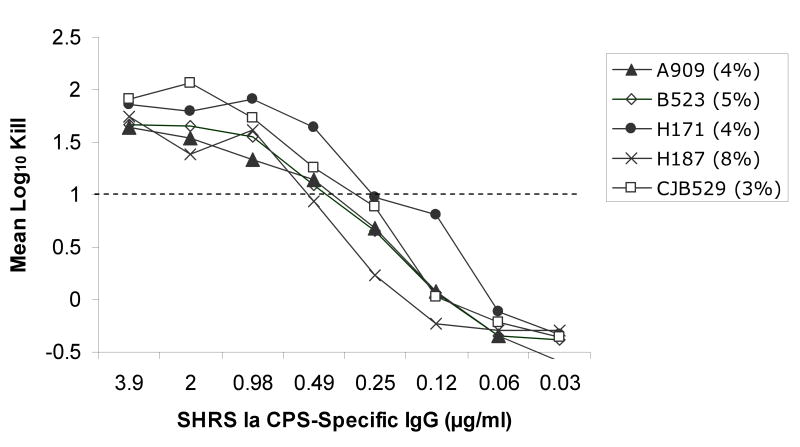
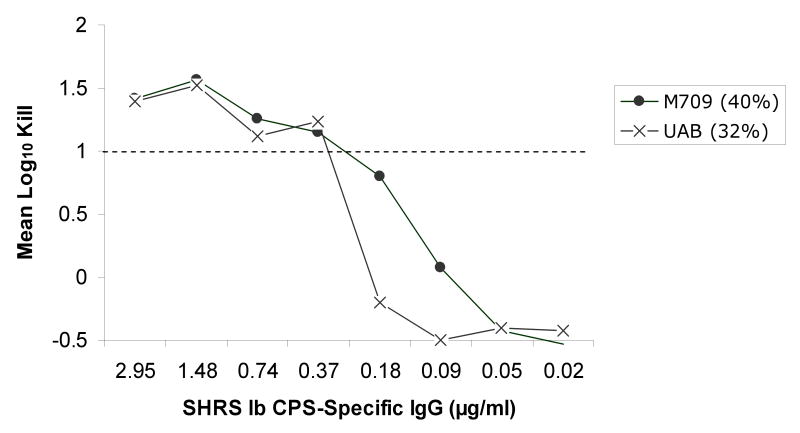
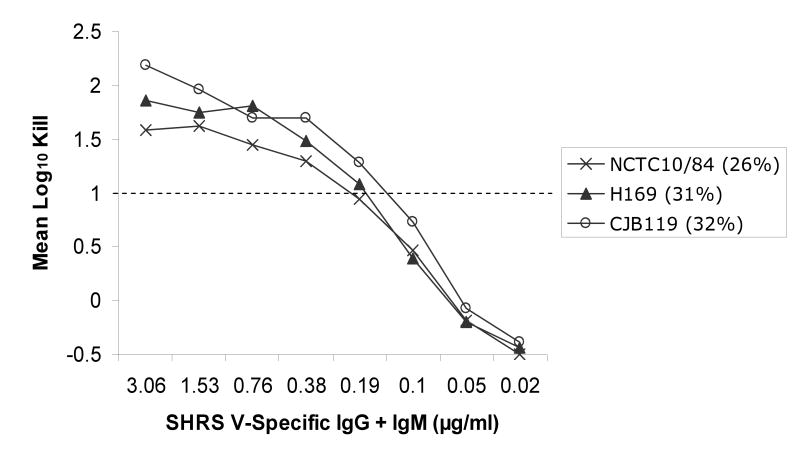
Utilizing an opsonophagocytosis assay, CPS-specific standard human reference serum pools containing CPS-specific antibodies from sera of adults after immunization with de-O-acetylated CPS glycoconjugate vaccines were used to test their ability to promote opsonization, phagocytosis and killing of GBS strains containing varying levels of sialic acid O-acetylation. Significant opsonophagocytosis and killing was evidenced by one or more log10 reduction in colony-forming units of all 20 GBS clinical isolates. This represented over 90% reduction of the initial inoculum for each strain that was dependent on the presence of vaccine-induced antibodies and in the case of type II and V, both CPS-specific IgG and IgM. The antibody concentration required to achieve at least one log10 reduction in colony-forming units ranged from ∼0.1 to 0.6 ug/ml within and among CPS types, but did not appear to correlate with the degree of strain O-acetylation (rs =-0.69, p<0.001). This finding was particularly relevant to serotype III GBS strains, which can express either high or low levels of the O-acetyl modification (Figure 1). Thorough analyses of the type III strains did not reveal evidence that O-acetylation has a substantial effect on the recognition of GBS by antibodies generated in response to de-O-acetylated vaccine preparations (Figure 2a). In fact, a lower CPS-specific antibody concentration (∼0.22 μg/ml) was required to promote opsonophagocytosis and killing of type III strains compared to other CPS types (ANOVA, p=0.004; Figure 2b-d).
Figure 2a-d.
Opsonophagocytosis and killing by human PMN of CPS GBS strains with varying levels of sialic acid O-acetylation (indicated as %) in the presence of standard human reference sera (SHRS) containing glycoconjugate vaccine-induced, CPS-specific-antibodies and infant rabbit complement. Vaccine-induced antibodies functioned effectively against all GBS strains. In particular, despite high levels of O-acetylation, CPS types Ib, III, and V GBS were killed by de-O-acetylated vaccine-induced antibodies. (Figure for opsonophagocytosis of type II CPS with low O-acetylation is not shown.)
4. Discussion
We show here for the first time that antibodies elicited by de-O-acetylated GBS CPS-tetanus toxoid conjugate vaccines function effectively in promoting opsonophagocytosis and killing of GBS independent of the extent of O-acetyl modification of CPS sialic acid residues. These findings are particularly relevant to serotype III GBS strains, which account for nearly two-thirds of late-onset infant disease cases, more than a third of which present as meningitis, for which there is no prevention strategy [3]. We show that serotype III strains can vary in capsular O-acetylation levels, but that the occurrence of O-acetyl groups is not essential for functional activity of antibodies elicited by GBS glycoconjugate vaccine and does not appear to block immunogenic epitopes present in this de-O-acetylated vaccine. Post-immunization sera achieved at least one log10 reduction (90% killing) of clinical type III GBS strains with low and high levels of O-acetylation.
Less than 0.6 μg per ml of CPS-specific antibodies in pooled post-immunization sera from healthy adults was sufficient for significant opsonophagocytosis and killing of CPS type Ia, Ib, II, III and V strains. These in vitro antibody concentrations are in concordance with previously published quantities found to be “protective” against CPS type Ia, Ib, and III strains [25-27]. Further, CPS-specific human IgG concentrations of 1.0 and 0.2 μg/ml, respectively, have been reported to protect mice against a 90% lethal dose challenge against type Ia and Ib strains [25, 26] and infants infected with type Ia and Ib GBS disease have concentrations below these in their acute sera. For infants and adults with invasive type III disease, concentrations of less than 2 μg/ml have been detected in acute sera [27]. Pre-immunization sera tested previously showed low levels (0 to <0.3 log10 reduction in cfu/ml) of opsonophagocytic activity against all CPS types [16-19].
Studies of vaccines derived from several pathogenic bacteria have shown varying effects of O-acetylation on immunogenicity of polysaccharide structures. In murine models of Neisseria meningitidis group A and Salmonella typhi Vi CPS infection, O-acetyl groups appear to be important for vaccine-induced protection [28, 29]. On the other hand, several human trials of bacterial CPS vaccines have shown very little impact of O-acetyl groups on immunogenicity and functional activity of the induced antibodies [30-32]. McNeely et al. [30] showed that antibodies raised against O-acetylated and de-O-acetylated Streptococcus pneumoniae type 9V CPS were able to opsonize 9V organisms in vitro. In humans and infant rhesus monkeys, backbone-specific antibody was sufficient for opsonic killing of type 9V. Giardina et al. [31] found that post-immunization sera from adults given an O-acetylated meningococcal group W135 CPS vaccine showed little or no discrimination between O-acetylated and non-O-acetylated strains as quantitated in serum bactericidal assays. Clinical trials of group C meningococcal CPS vaccines in O-acetylated and de-O-acetylated forms also demonstrated that O-acetylation was not required for immunogenicity of the vaccine [32].
The data presented here strongly suggest that in the case of GBS, O-acetyl modifications of the CPS do not have a significant effect on the functional activity of antibodies generated against the de-O-acetylated structure. Because these studies employed de-O-acetylated CPS conjugate vaccines, they cannot address the possibility that O-acetyl groups confer novel immunogenic epitopes. However, given the chemical lability of O-acetyl esters and the effectiveness of the de-O-acetylated CPS vaccines, such studies may not be feasible or necessary.
Theoretically, immunization with an O-acetylated GBS CPS could induce antibody against the O-acetyl groups and the backbone. In evaluations of Staphylococcus aureus type 5 and 8 CPS vaccines [33], immunization with the native CPS conjugates with 75% O-acetylation elicited a high proportion of antibodies directed against the O-acetyl moiety, but all of the vaccines constructed also produced antibodies to the backbone moieties. However, other studies have found that presence of O-acetyl groups in vaccine can actually interfere with the ability to induce antibody to the protective epitope. De-O-acetylated meningococcal group C CPS vaccine induced a twofold greater titer of antibody compared to the O-acetylated vaccine in humans [32]. Thus, antibody formed to the de-acetylated version of the vaccine will recognize the bacteria regardless of the presence or location of the O-acetyl groups.
A potential limitation of this study is that we tested opsonophagocytic activity with only a single serum pool for each CPS type. It is theoretically possible that if individual serum samples had been examined, O-aceylation-dependent differences in activity may have been found. However, previous studies of individual pre- and post-immunization sera done prior to the discovery of O-acetylation showed that opsonophagocytic activity of in excess of 90% was significantly correlated with concentration of CPS-specific antibody [16-19].
This investigation demonstrates that current de-O-acetylated GBS CPS-based conjugate vaccine formulations [16-19] induce antibodies that function effectively against major disease-causing types of GBS regardless of the presence of high or low percentage of O-acetylated sialic acid residues. Consideration should be given to further investigation with the use of isogenic strains that only differ in levels of O-acetylation to control for variation in other capsular features. However, our results using clinical strains support the presumption that protective epitopes for GBS CPS are contained on the de-O-acetylated form. We are hopeful that further trials of GBS conjugate vaccines or newly developed GBS vaccine formulations in young women, adults with defined medical conditions, and the elderly will show that the growing burden of invasive GBS disease in these groups and neonates can be substantially reduced.
Acknowledgments
Financial Support: Supported in part by Contract N01 AI-25495 from the National Institutes of Health, National Institute of Allergy and Infectious Diseases to CJB.
Footnotes
Potential Conflicts of Interest: MSE is a consultant for Novartis Vaccines and Diagnostics. No conflict of interest for PSP, KTE, ALL, MAR and CJB.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. Trends in Perinatal Group B Streptococcal Disease – United States, 2000-2006. MMWR Morb Mortal Wkly Rep. 2009;58:109–12. [PubMed] [Google Scholar]
- 2.Schrag SJ, Zywicki S, Farley MM, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 3.Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008;299:2056–65. doi: 10.1001/jama.299.17.2056. [DOI] [PubMed] [Google Scholar]
- 4.Edwards MS, Rench MA, Haffar AAM, Murphy MA, Desmond MM, Baker CJ. Long-term sequelae of group B streptococcal meningitis in infants. J Pediatr. 1985;106:717–722. doi: 10.1016/s0022-3476(85)80342-5. [DOI] [PubMed] [Google Scholar]
- 5.Baker CJ, Edwards MS. Group B streptococcal conjugate vaccines. Arch Dis Child. 2003;88:375–8. doi: 10.1136/adc.88.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards MS, Rench MA, Palazzi DL, Baker CJ. Group B streptococcal colonization and serotype-specific immunity in healthy elderly persons. Clin Infect Dis. 2005;40:352–7. doi: 10.1086/426820. [DOI] [PubMed] [Google Scholar]
- 7.Rubens CE, Wessels MR, Heggen LM, Kasper DL. Transposon mutagenesis of type III group B Streptococcus: Correlation of capsule expression with virulence. Proc Natl Acad Sci. 1987;84:7208–12. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johri AK, Paoletti LC, Glaser P, et al. Group B Streptococcus: global incidence and vaccine development. Microbiol. 2006;4:932–42. doi: 10.1038/nrmicro1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cieslewicz MJ, Chaffin D, Glusman G, et al. Structural and genetic diversity of group B Streptococcus capsular polysaccharides. Infect Immun. 2005;73:3096–103. doi: 10.1128/IAI.73.5.3096-3103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Severi E, Hood DW, Thomas GH. Sialic acid utilization by bacterial pathogens. Microbiol. 2007;153:2817–22. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 11.Wessels MR, Rubens CE, Benedi VJ, Kasper DL. Definition of bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc Natl Acad Sci. 1989;86:8983–7. doi: 10.1073/pnas.86.22.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards MS, Nicholson-Weller A, Baker CJ, Kasper DL. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980;151:1275–87. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards MS, Kasper DL, Jennings HJ, Baker CJ, Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immun. 1982;128:1278–83. [PubMed] [Google Scholar]
- 14.Jennings HJ, Lugowski C, Kasper DL. Conformational aspects critical to the immunospecificity of the type III group B streptococcal polysaccharide. Biochem. 1981;20:4511–8. doi: 10.1021/bi00519a001. [DOI] [PubMed] [Google Scholar]
- 15.Lewis AL, Nizet V, Varki A. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc Natl Acad Sci. 2004;101:11123–8. doi: 10.1073/pnas.0403010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasper DL, Paoletti LC, Wessels MR, Guttormsen HK, Carey VJ, Jennings HJ, Baker CJ. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Invest. 1996;98:2308–14. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker CJ, Paoletti LC, Wessels MR, et al. Safety and immunogenicity of capsular polysaccharide-tetanus toxoid conjugate vaccines for group B streptococcal types Ia and Ib. J Infect Dis. 1999;179:142–50. doi: 10.1086/314574. [DOI] [PubMed] [Google Scholar]
- 18.Baker CJ, Paoletti LC, Rench MA, et al. Use of capsular polysaccharide-tetanus toxoid vaccine for type II group B Streptococcus in healthy women. J Infect Dis. 2000;182:1129–38. doi: 10.1086/315839. [DOI] [PubMed] [Google Scholar]
- 19.Baker CJ, Paoletti LC, Rench MA, Guttormsen HK, Edwards MS, Kasper DL. Immune response of healthy women to 2 different group B streptococcal type V capsular polysaccharide-protein conjugate vaccines. J Infect Dis. 2004;189:1103–12. doi: 10.1086/382193. [DOI] [PubMed] [Google Scholar]
- 20.Guttormsen HK, Baker CJ, Edwards MS, Paoletti LC, Kasper DL. Quantitative determination of antibodies to type III group B Streptococcal polysaccharide. J Infect Dis. 1996;173:142–50. doi: 10.1093/infdis/173.1.142. [DOI] [PubMed] [Google Scholar]
- 21.Gotschlich EC, Rey RT, Sparks KJ. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J Clin Invest. 1972;51:89–96. doi: 10.1172/JCI106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madore DV, Strong NM, Quataert SA. Validation and standardization of serologic methods for evaluation of clinical immune responses to vaccines. In: Paoletti LC, McInnes PM, editors. Vaccines from Concept to Clinic: A guide to the development and clinical testing of vaccines for human use. CRC Press, LLC; Boca Raton, FL: 1999. pp. 43–75. [Google Scholar]
- 23.Guttormsen HK, Baker CJ, Nahm MH, et al. Type III group B streptococcal polysaccharide includes antibodies that cross-react with Streptococcus pneumonia type 14. Infect Immune. 2002;70:1724–1738. doi: 10.1128/IAI.70.4.1724-1738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards MS, Baker CJ, Kasper DL. Opsonic specificity of human antibody to the type III polysaccharide of group B Streptococcus. J Infect Dis. 1979;140:1004–8. doi: 10.1093/infdis/140.6.1004. [DOI] [PubMed] [Google Scholar]
- 25.Klegerman ME, Boyer KM, Papierniak CK, Gotoff SP. Estimation of the protective level of human IgG antibody to the type-specific polysaccharide of group B Streptococcus type Ia. J Infect Dis. 1983;148:648–55. doi: 10.1093/infdis/148.4.648. [DOI] [PubMed] [Google Scholar]
- 26.Gotoff SP, Papierniak CK, Klegerman ME, Boyer KM. Quantitation of IgG antibody to the type-specific polysaccharide of group B Streptococcus type Ib in pregnant women and infected infants. J Pediatr. 1984;105:628–30. doi: 10.1016/s0022-3476(84)80436-9. [DOI] [PubMed] [Google Scholar]
- 27.Baker CJ, Edwards MS, Kasper DL. Role of antibody to native type III polysaccharide of group B Streptococcus in infant infection. Pediatrics. 1981;68:544–9. [PubMed] [Google Scholar]
- 28.Berry DS, Lynn F, Lee CH, Frasch CE, Bash MC. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune response. Infect Immun. 2002;70:3707–13. doi: 10.1128/IAI.70.7.3707-3713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szu SC, Li XR, Stone AL, Robbins JB. Relation between structure and immunologic properties of the Vi capsular polysaccharide. 1991;59:4555–61. doi: 10.1128/iai.59.12.4555-4561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeely TB, Staub JM, Rusk CM, Blum MJ, Donnelly JJ. Antibody responses to capsular polysaccharide backbone and O-acetate side groups of Streptococcus pneumoniae type 9V in humans and rhesus macaques. Infect Immun. 1998;66:3705–10. doi: 10.1128/iai.66.8.3705-3710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giardina PC, Longworth E, Evans-Johnson RE, et al. Analysis of human serum Immunoglobulin G against O-acetyl-positive and O-acetyl-negative serogroup W135 meningococcal capsular polysaccharide. Clin Diagn Lab Immunol. 2005;12:586–92. doi: 10.1128/CDLI.12.5.586-592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinhoff MC, Lewin EB, Gotschlich EC, Robbins JB. Group C Neisseria meningitidis variant polysaccharide vaccines in children. Infect Immun. 1981;34:144–6. doi: 10.1128/iai.34.1.144-146.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fattom AI, Sarar J, Basham L, Ennifar S, Naso R. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect Immun. 1998;66:4588–92. doi: 10.1128/iai.66.10.4588-4592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]