Abstract
The objective of this study was to determine if levels of repressors to myogenic regulatory factors (MRFs) differ between muscles from young adult and aged animals. Total RNA from plan-taris, gastrocnemius, and soleus muscles of Fischer 344 × Brown Norway rats aged 9 mo (young adult, n = 10) and 37 mo (aged, n = 10) was reverse transcribed and then amplified by PCR. To obtain a semiquantitative measure of the mRNA levels, PCR signals were normalized to cyclophilin or 18S signals from the corresponding reverse transcription product. Normalization to cyclophilin and 18S gave similar results. The mRNA levels of MyoD and myogenin were ~275–650% (P < 0.001) and ~500–1,100% (P < 0.001) greater, respectively, in muscles from aged compared with young adults. In contrast, the protein levels were lower in plantaris and gastrocnemius muscles and similar in the soleus muscle of aged vs. young adult rats. Id repressor mRNA levels were ~300–900% greater in fast and slow muscles of aged animals (P ≤ 0.02), and Mist 1 mRNA was ~50% greater in the plantaris and gastrocnemius muscles (P < 0.01). The mRNA level of Twist mRNA was not significantly affected by aging. Id-1, Id-2, and Id-3 protein levels were ~17–740% greater (P < 0.05) in hindlimb muscles of aged rats compared with young adult rats. The elevated levels of Id mRNA and protein suggest that MRF repressors may play a role in gene regulation of fast and slow muscles in aged rats.
Keywords: sarcopenia, muscle atrophy, transcription factors, E proteins, repressors of transcription
Skeletal Muscle from aged animals or humans has a lower basal rate of protein synthesis than muscles from younger hosts. This contributes to sarcopenia by reducing the amount of protein accumulation available for normal protein turnover or muscle repair (32, 37). It is not known which step(s) in the regulation of muscle protein synthesis fails to respond properly with aging. It is, for example, possible that specific genes are not activated properly in muscles from aged hosts. This would occur if there were transcriptional limitations in aged muscle caused, for example, by lower levels or reduced activity of muscle regulatory factors (MRFs).
MRFs are muscle-specific transcriptional proteins that belong to the basic helix-loop-helix (b-HLH) class of DNA-binding proteins (for reviews see Refs. 8 and 40). MRFs heterodimerize with E12 or E47, which are products of the E2A gene. The basic region of the HLH protein is required for binding of MRF:E protein heterodimers to a CANNTG or “E-box” domain and trans-activation of downstream muscle genes such as myosin light chain (MLC) and creatine kinase (22, 40, 41).
Although basal MRF mRNA levels are elevated during senescence (31, 33, 36), the loss of contractile tissue appears unavoidable in control sedentary conditions. The mechanisms accounting for the apparent paradoxical elevation in MRF mRNA levels and loss of muscle mass in control conditions of aged animals and the diminished accumulation of muscle mass under conditions of increased muscle loading in aged animals (7, 10, 12, 29, 33) are not known.
Repressors are HLH proteins that act as negative regulators of MRFs. For example, Id proteins bind strongly to E proteins (21) and repress MRF activity by titration of E proteins (11, 28, 39). This is related to the fact that Id lacks the basic domain, so that MRF:Id heterodimers are unable to bind to the E boxes and initiate transcription (5, 6, 14, 21). Repressors such as Mist 1 compete with MRF:E heterodimers for E boxes (e.g., as Mist 1 homodimers or Mist 1:E47 heterodimers), or they can form MyoD-Mist 1 and MRF4-Mist 1 heterodimers that do not bind DNA (26). Other repressors, such as Twist, can bind to the basic region of HLH proteins (19), thereby inhibiting the DNA binding by MRF complexes (20, 25).
We rationalized that repressor mRNA levels may be elevated in muscles from aged animals and override or prevent an increase in MRF transcriptional control, thereby contributing to sarcopenia. Therefore, we investigated whether aging is associated with increased mRNA levels of repressors Id-1, Id-2, Id-3, Mist 1, and Twist. Because Ids are known to sequester E proteins, we also hypothesized that E protein levels might become limiting with aging. Therefore, we also examined if the expression of E12 was increased with aging.
METHODS
Animals
Young adult (9 mo; n = 10) and aged (37 mo; n = 10) Fischer 344 × Brown Norway (FBN) F1 hybrid male rats (Harlan, Indianapolis, IN) were housed separately in pathogen-free conditions at 20–22°C with a 12:12-h light-dark cycle.
Plantaris, soleus, and gastrocnemius muscles were removed from animals after anesthesia (ketamine hydrochloride, 9 mg/100 g body wt and xylazine hydrochloride, 1 mg/100 g body wt). The muscles were quickly weighed, frozen in liquid nitrogen, and then stored at −80°C. Animals were euthanized with on overdose of pentobarbital sodium (60 mg/kg ip). All experiments carried approval from the Institutional Animal Use and Care Committee from West Virginia University School of Medicine. The animal care standards were followed by adhering to the recommendations for the care of laboratory animals as advocated by the American Association for Accreditation of Laboratory Animal Care and following the policies and procedures detailed in the Guide for the Care and Use of Laboratory Animals as published by the U.S. Dept. of Health and Human Services and proclaimed in the Animal Welfare Act (PL89–544, PL91–979, and PL94–279).
RNase protection assay mRNA analyses
Rat-specific RNA probes were made from RT-PCR products, and RNase protection assays (RPA) were conducted for MRFs as described in detail previously (2, 31). MRF4 primer sequences and annealing temperatures were those used by Smith et al. (38), except that EcoRI restriction sequences were included in the primers. RT-PCR using primers with EcoRI restriction sites amplified myogenin. Within each sample, resulting MRF signals were normalized by the corresponding cyclophilin signal because cyclophilin does not change with aging in muscles from FBN rats (30).
Semiquantitative RT-PCR analyses of mRNA for repressor and MRF genes
Semiquantitative RT-PCR analysis was conducted as described in detail elsewhere (2). Briefly, total RNA was extracted from muscles, treated with DNase I (Ambion, Austin, TX) to remove any contamination by DNA, and reverse transcribed with oligo(dT) primers according to standard methods (Life Technologies, Bethesda, MD). Control RT reactions were done in which the RT enzyme was omitted. One microliter of cDNA was then amplified by PCR using 100 ng of each primer, 250 μM dNTPs, 1× PCR buffer, and 2 U Taq polymerase (Sigma Chemical, St. Louis, MO) in a final volume of 50 μl. Primer pairs were designed from sequences in GenBank (Table 1).
Table 1.
Primers used for PCR amplification of cDNA
| Product | Accession No. |
Sequence | Position | TA, °C |
Cycles | PCR Length, bp |
Restriction Enzyme |
Restriction Products, bp |
|---|---|---|---|---|---|---|---|---|
| MyoD | M84176 | 5′-CCCGACGGCTCTCTCTGCTCCTT | 2636 | 61.6 | 35 | 178 | DpnI | 50; 128 |
| 3′-CGCCTGGGCCTGGGTGCA | 2813 | |||||||
| Myogenin | M24393 | 5′-TGCCACAAGCCAGACTACCCACC | 827 | 57.1 | 34 | 246 | AluI | 166; 80 |
| 3′-CGGGGCACTCACTGTCTCTCAA | 1072 | |||||||
| MRF4 | M84685 | 5′-CCGGGAGCGACAGCAGTGG | 199 | 59.3 | 35 | 298 | AluI | 138; 160 |
| 3′-TCCTGGACGACGTGGCCGA | 496 | BgI | 186; 112 | |||||
| Id-1 | D10862 | 5′-CGGCGGGCGAAGTGGTGCG | 109 | 57.3 | 38 | 355 | PstI | 189; 30; 133 |
| 3′-CACCAAGGTCGGCTGCTGGCGT | 453 | |||||||
| Id-2 | D10863 | 5′-GCCCAGCATCCCCCAGAA | 213 | 52.7 | 38 | 264 | PstI | 47; 30; 187 |
| 3′-CCATTTATTTAGCCACAG | 459 | |||||||
| Id-3 | D10864 | 5′-CTGCTACGAGGCGGTGTG | 41 | 58.2 | 38 | 472 | PstI | 186; 286 |
| 3′-AAATGAAGAGGGCTGTGG | 495 | SacII | 152; 320 | |||||
| SacI | 287; 185 | |||||||
| Mist 1 | AF049874 | 5′-CGCCCCGCTCCTCTGCTCTACC | 100 | AluI | 87; 188 | |||
| 3′-TGCTCGGTCCGTCTGGGCC | 357 | 64.1 | 38 | 275 | BgII | 142; 133 | ||
| Twist | M63649 | 5′-CACGCTGCCCTCGGACAA | 1201 | |||||
| 3′-GGGACGCGGACATGGACC | 1380 | 59.9 | 36 | 197 | DdeI | 20; 120; 57 | ||
| E12 | AF049874 | 5′-GCCCGAGAGCGCCTGCGTGTC | 378 | 63.5 | 37 | 436 | BamI | 288; 148 |
| 3′-GGACCCCCTCAACCGTGCCTC | 798 | PvuII | 66; 237 | |||||
| Desmin | X73524 | 5′-GGGCGGCGAGGGGTGATGTC | 53 | 62.1 | 34 | 551 | KpnI | 267; 284 |
| 3′-TCTTGCGGCGCAGCGGC | 603 | SmaI | 207; 344 | |||||
| MLC | D14688 | 5′-CGCCGGTCTGGTCCTCTTGTG | 18 | 59.8 | 35 | 289 | SmaI | 286; 3 |
| 3′-GTACTACTTGCTCCGGGGCCC | 306 | DdeI | 97; 192 | |||||
| GAPDH | X02231 | 5′-AAATGGGGTGATGCTGGTG | 321 | |||||
| 3′-GAGATCATCACCCTTTTGG | 411 | 54.2 | 28 | 110 | ||||
| Cyclophilin | M19533 | 5′-ACGCCGCTGTCTCTTTTC | 9 | DdeI | 150; 250; 40 | |||
| 3′-TGCCTTCTTTCACCTTCC | 431 | 54.6 | 32 | 440 | HindII | 40; 400 |
TA, annealing temperature; GAPDH, glyeraldehyde-3-phosphate dehydrogenase; MLC, essential myosin light chain. Accession No., GenBank accession number.
In preliminary experiments we verified each PCR product by DNA sequencing (see Table 1), and the number of PCR cycles was determined for each gene from RNA isolated from both young and aged animals so that analyses were done in the linear range of amplification. Amplifications of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and cyclophilin were used as positive and negative controls, respectively, because GAPDH mRNA levels are lower in muscles of aged rats compared with young adult rats and cyclophilin levels do not differ in muscles of aged and young adult rats (30).
As a second approach to evaluate the semiquantitative mRNA results, we repeated the RT-PCR analyses using 18S as an internal control. For these experiments, we made cDNA from total RNA using random primers and amplified 18S primer pairs and competimers to the primers in the PCR reaction along with the gene of interest according to the manufacture's protocols (Ambion). The competimers attenuated the 18S signal, which was consistent with the manufacturer's protocol (Ambion), but the relative extent of attenuation was similar in total RNA samples isolated from muscles of old and young adult rats (data not shown). The gene of interest was expressed as a ratio to the 18S signal in the same PCR product. Although the signal ratios of a gene normalized to cyclophilin differed from the same gene normalized to 18S under these conditions (largely due to the effect of the competimer function to attenuate the 18S signal), the relative differences between muscles of aged and young adult rats were similar when the RT-PCR signals were normalized to either cyclophilin or 18S. Therefore, we have reported the PCR signals normalized to cyclophilin.
For a particular gene, the cDNA from all muscle samples was amplified simultaneously. After amplification, 10 μl of each reaction was electrophoresed on 1.5% agarose gels. Gels were stained with ethidium bromide. Then images were captured and the signals were quantified in arbitrary units as optical density × band area, using Kodak one-dimensional (1-D) image analysis system (Eastman Kodak, Rochester, NY). PCR signals were normalized to the cyclophilin signal of the corresponding RT product to provide a semiquantitative estimate of gene expression. The PCR products were run in duplicate on different gels for each gene, and the results were averaged.
Western blot analyses
The relative level of MRF and repressor protein expression was determined in the plantaris, soleus, and gastrocnemius muscles of young adult and aged rats. Muscle samples were minced on ice and homogenized in ice-cold T-PER tissue protein extraction buffer (Pierce, Rockford, IL) containing protease inhibitors aprotinin, leupeptin, and phenylmethylsulfonyl fluoride. Solublilized protein extracts were quantified in duplicate by using bicinchoninic acid reagents (Pierce, Rockford, IL) and BSA standards. Forty micrograms of soluble protein was loaded on each lane of a 10% polyacrylamide gel and separated by routine SDS-PAGE for 1.5 h at 20°C (1). The gels were blotted to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules CA) and stained with Ponceau S red (Sigma) to confirm equal loading and transfer of proteins to the membrane. The membranes were blocked in 5% milk and probed with either anti-myogenin clone FD5 (Hybridoma Bank, Iowa University, IA) at a concentration of 3 μg/ml in Tris-buffered saline (TBS) or anti-MyoD, Id-1, Id-2, Id-3, and E12 (BD PharMingen, San Diego, CA) at a concentration of 2 μg/ml in TBS in 2% milk. Secondary antibodies were conjugated to alkaline phosphatase (Sigma), and the signals were developed by chemiluminescence (Bio-Rad). The signals were visualized by exposing the membranes to X-ray film (BioMax MS-1, Eastman Kodak), and digital records of the films were captured with a Kodak 290 camera. The resulting bands were quantified as optical density × band area, by a 1-D image software package (Eastman Kodak). Protein samples were run on SDS-PAGE gels and stained with Coomassie blue to confirm that protein loads were similar in each lane.
Statistical analyses
A one-way ANOVA was used to examine aging differences across normalized signals for each muscle using SPSS software. Significance level was set at P < 0.05.
RESULTS
Body mass and muscle characteristics
The body weight of the old rats (522 ± 10 g) was 23% higher (P < 0.02) than that of the young adult rats (427 ± 23 g). The extent of aging-associated sarcopenia was similar in each of the skeletal muscles studied from aged rats compared with the young adult rats as shown by an ~30% reduction (P < 0.05) in muscle wet weights with age (Table 2). The degree of muscle loss was even more striking when muscle mass was normalized to body weight (mg/g body wt), which was ~50–58% lower (P < 0.05) in aged rats compared with young adult rats.
Table 2.
Muscle characteristics
| Plantaris |
Soleus |
Gastrocnemius |
||||
|---|---|---|---|---|---|---|
| Young adult | Aged | Young adult | Aged | Young adult | Aged | |
| Muscle wet wt/body wt, mg/g | 1.10±0.05 | 0.59±0.02* | 0.50±0.02 | 0.25±0.02* | 5.06±0.41 | 2.27±0.23* |
| Wet wt, mg | 408±20 | 277±13* | 193±12 | 127±12* | 1,540±153 | 1,080±104* |
Values are means ± SE; n = 10/group;
Significantly different from young adult (P < 0.05).
RT-PCR and RPA analysis of MRF genes
As previously reported (2, 31), we detected mRNA bands for MyoD, myogenin, and MRF4. We observed that cyclophilin did not differ (P = 0.53) between muscles of old and young adult rats. This confirms our previous observations (30) and ensures that we were able to detect differences that were the result of age and not of experimental manipulation. Therefore, we normalized each RPA signal to the corresponding cyclophilin signal. Myogenin and MyoD signals were higher in aged than in young adult muscles (P < 0.004). Although MRF4 levels were similar in the plantaris of old and young adult rats (P = 0.49), MRF4 levels also were greater in soleus and gastrocnemius muscles of old vs. young adult rats (P < 0.04). Consistent with the RPA data and our previous observations (29), we confirmed by semiquantitative RT-PCR that cyclophilin did not change with aging in any muscle studied (P > 0.41) and that GAPDH was lower (P < 0.021) in muscles from old animals. Subsequently, for all RT-PCR analyses the PCR signal was normalized to the cyclophilin signal from the same RT product.
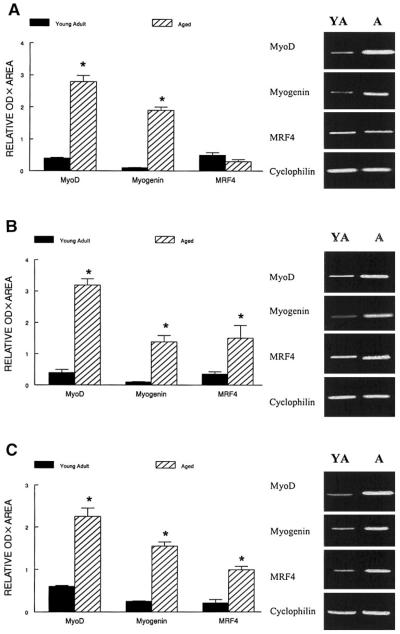
Because RPAs require a relatively large amount of RNA, we chose to examine the expression of repressor genes by RT-PCR, which requires much less RNA. However, to make sure that the semiquantitative data obtained by RT-PCR were comparable to those obtained by RPA, we analyzed the MRF signals also by RT-PCR. Representative ethidium bromide-stained gels for the MRFs after RT-PCR are shown in Fig. 1. The normalized signals obtained with semiquantitative RT-PCR and RPAs for MRFs in each muscle were similar. The relative estimates of mRNA signals by RT-PCR for MyoD and myogenin were ~650 and 1,150% greater in the plantaris muscle from aged compared with young adults (P < 0.001). In contrast, plantaris MRF4 mRNA levels were not affected by aging (P > 0. 64) as assessed by RT-PCR or RPA. In the soleus, MyoD, myogenin, and MRF4 mRNA levels were estimated to be elevated by ~700%, 1,100%, and 357% in aged compared with young adult rats (Fig. 1), and in the gastrocnemius the mRNA levels for MyoD, myogenin, and MRF4 were estimated to be ~275, 500, and 354% greater in aged rats compared with young adult animals (P < 0.002) (Fig. 1).
Fig. 1.
Representative results of RT-PCR MyoD, myogenin, MRF4, and cyclophilin from plantaris (A), soleus (B), and gastrocnemius muscles (C) isolated from young adult (YA) and aged (A) rats. PCR products were visualized with ethidium bromide. Semiquantification of PCR products was obtained by densitometric analysis of the signal product area × optical density (OD). The relative expression of each gene is presented normalized to the cyclophilin signal from the same RT product. The estimated normalized data are presented as means ± SE (n = 10/group). Data were run in duplicate on different gels for each gene. *Data from aged rats were significantly different from young adult rats in the same muscle at P < 0.01.
Myogenin and MyoD protein levels
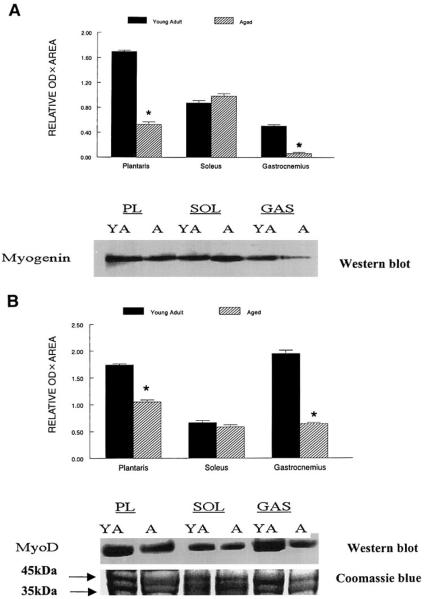
An immunoreactive band of ~35 kDa corresponded to the predicted molecular mass of the rat MyoD protein, and an ~34-kDa myogenin immunoreactive band corresponded to the myogenin protein, respectively. They were expressed in all skeletal muscles examined in the rat hindlimb, although the relative levels of these MRFs differed between muscles and with aging. Myogenin protein levels estimated from Western blots were reduced to ~32 and 20% of the levels (P < 0.01) found in muscle samples isolated from the plantaris and gastrocnemius muscles from young adult rats (Fig. 2A). A similar aging-associated pattern was observed for MyoD protein levels (P < 0.02), which were reduced to 66 and 33% of the levels for the plantaris and gastrocnemius muscles from old rats compared with young adult rats (Fig. 2B). In contrast, aging had no significant effect on myogenin and MyoD in the soleus (P > 0.27).
Fig. 2.
Representative Western blots of relative myogenin (A) and MyoD protein levels (B) in plantaris (PL), soleus (SOL), and gastrocnemius (GAS) muscles of young adult and aged rats. Forty micrograms of soluble protein extracts was loaded on each lane, separated by SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes, and incubated with either anti-myogenin or anti-MyoD primary antibodies. Proteins were run in duplicate and stained with Coomassie blue. The area of the Coomassie-stained gel corresponding to the myogenin and MyoD immunoreactive areas are shown below the Western blot. The immunoreactive signals were developed by chemiluminescence, and the resulting bands were quantified as band OD × band area as determined by Kodak one-dimensional (1-D) image software. Data are expressed in arbitrary units as means ± SEs. *Data from aged rats were significantly different from young adult rats in the same muscle at P < 0.01.
Expression of desmin and MLC mRNA
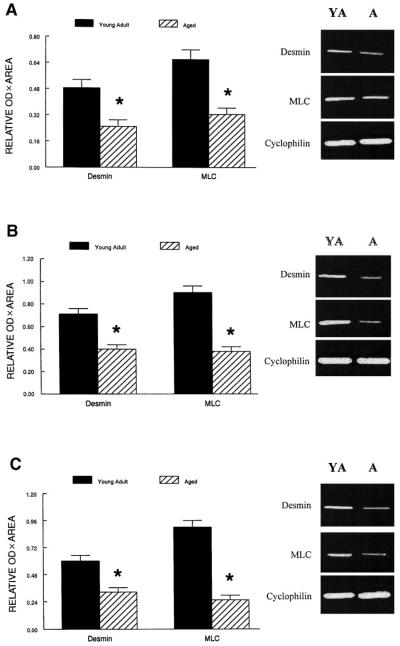
The mRNA levels of desmin and the essential MLC were evaluated because transcription of these genes is controlled by the MRF proteins (27, 42). The normalized mRNA levels for both desmin and MLC in plantaris, soleus, and gastrocnemius muscles of aged rats were ~50% (P < 0.01) of those levels in young adult rats (Fig. 3). This is similar in magnitude to the decrease in muscle mass due to aging in the rat hindlimb (i.e., muscle weight/body wt; Table 2).
Fig. 3.
Relative expression of desmin and myosin light chain (MLC) mRNAs in plantaris (A), soleus (B), and gastrocnemius muscles (C) from young adult and aged rats after RT-PCR amplification. The expression of each PCR product was normalized to the cyclophilin signal from the same RT product. Values are means and error bars represent SEs (n = 10/group). *Data from aged rats were significantly different from young adult rats in the same muscle at P < 0.01.
Estimates of repressor mRNA level normalized to 18S or cyclophilin
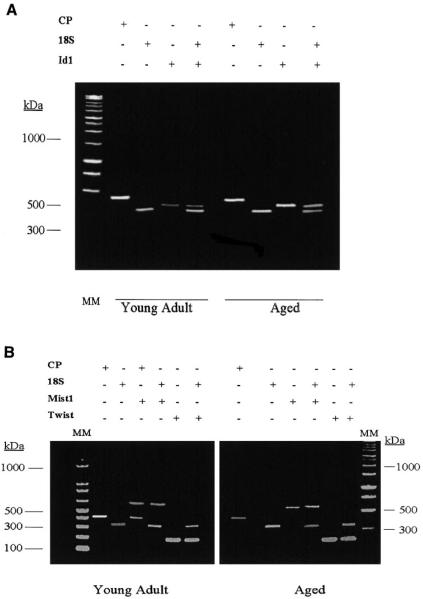
We recognize that reliable quantification of RT PCR can be difficult. However, semiquantitative estimates can be obtained if the gene of interest is normalized to an appropriate control gene. In our studies, the PCR products were normalized to cyclophilin, which was amplified from the same RT product as the gene of interest but in a different tube. To control for the possibility that normalization by cyclophilin amplified by PCR in different tubes might be an unreliable internal control, we repeated the experiments with 18S as an internal control. We generated cDNA with random primers and amplified each gene concurrent with 18S primer pairs. For PCR amplification, we used conditions where both 18S competimers to attenuate the 18S signal and the gene of interest were amplified in the same tube. We used the annealing temperature and the cycling number that were in the linear range for the gene of interest. Representative data for 18S either amplified in the presence or absence of Id-1 in young adult and aged muscles are shown in Fig. 4A. Examples of 18S and cyclophilin as internal controls are shown for Mist 1 and Twist genes in samples isolated from aged and young adult rat muscles in Fig. 4B. Although the absolute signal ratios for the gene of interest to 18S differed from the signal ratio to cyclophilin, semiquantitative estimates of aging-related differences in gene products by RT-PCR were similar whether the gene was normalized to cyclophilin [using oligo(dT) RT-based primers] or to 18S (using random primers).
Fig. 4.
A: representative RT-PCR results comparing cyclophilin (CP) and 18S internal controls for PCR amplification of repressor genes. Primer pairs are described in Table 1. CP was run at the conditions described in Table 1. 18S was run either with or without Id-1 in the same tube during PCR amplification (other genes were amplified with and without 18S under conditions described in Table 1; data not shown). Amplification of 18S alone was taken as the annealing temperature for Id-1. Although the absolute ratios of Id-1 to CP (with the genes run in different tubes but from the same RT product) differed from the absolute ratio obtained when 18S and Id-1 were run in the same tube, the relative comparisons of the Id-1-to-CP ratio and the Id-1-to-18S ratio were similar when muscles from young adult and aged rats were compared. Id-1 is illustrated in the plantaris, but data were similar for soleus and gastrocnemius muscles and for Id-2 and Id-3. These findings indicate that semiquantitative PCR analysis produces similar aging-comparative data when 18S and CP are used as internal controls. Molecular mass (MM) estimates are shown in kDa. B: representative RT-PCR results comparing CP and 18S internal controls for PCR amplification of Mist 1 and Twist genes. The conditions for PCR are given in Table 1. All other conditions are similar to those described for A.
Estimates of Id mRNA levels
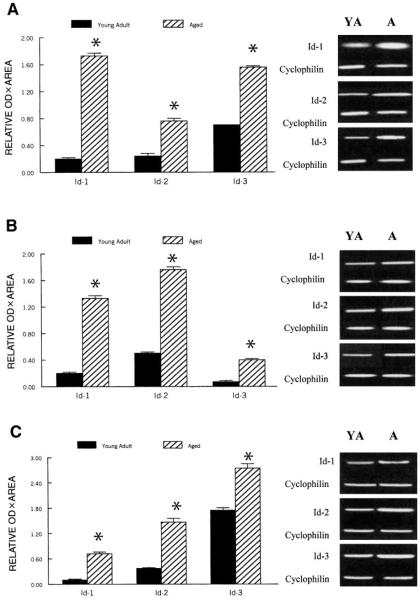
Semiquantitative RT-PCR analyses of gene signals normalized to cyclophilin indicated that mRNA levels for all three Id repressors were significantly greater in muscles of old rats compared with those of young adult rats (P < 0.01) (Fig. 5). The Id-1 repressor mRNA expression was estimated to be ~750, 560, and 810% greater in plantaris, soleus, and gastrocnemius muscles, respectively, of aged compared with young adult rats (Fig. 5). Similarly, Id-3 was ~970, 470, and 390% greater in the plantaris, soleus, and gastrocnemius muscles, respectively, of aged compared with young adult rats (Fig. 5). The aging associated increases in mRNA for Id-2 were less striking than for Id-1 and Id-3 but still amounted to ~300, 260, and 350% greater in the plantaris, soleus, and gastrocnemius muscles of aged compared with young adult rats (P < 0.01) (Fig. 5).
Fig. 5.
Relative semiquantitative expression of Id-1, Id-2, and Id-3 mRNAs in plantaris (A), soleus (B), and gastrocnemius muscles (C) from young adult and aged rats after RT-PCR amplification. The expression of each PCR product was normalized to the CP signal of the corresponding RT product. Representative gels are shown adjacent to each graph. Values are expressed in arbitrary units as means, and error bars represent SEs (n = 10/group). *Significant difference (P < 0.01) from corresponding young adult value.
Estimates of Mist 1 and Twist repressor mRNA levels
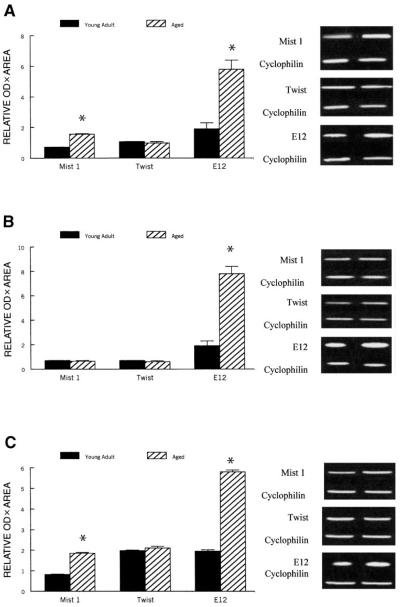
Semiquantitative analysis of PCR signals normalized to cyclophilin showed that Mist 1 was ~120% greater (P < 0.03) in plantaris and gastrocnemius muscles of aged rats compared with young adult rats, whereas Mist 1 expression was similar in the soleus of aged and young adult rats (P = 0.87). In contrast, semiquantitative data suggest that Twist mRNA levels did not differ (P = 0.69) significantly between any of the three muscles of young adult and aged animals (Fig. 6).
Fig. 6.
Relative semiquantitative expression of Mist 1, Twist, and E12 mRNAs in plantaris (A), soleus (B), and gastrocnemius muscles (C) from young adult and aged rats after RT-PCR amplification. The expression of each PCR product was normalized to the cyclophilin signal. Representative gels are shown adjacent to each graph. Values are expressed in arbitrary units as means, and error bars represent SEs (n = 10/group). *Significantly different (P < 0.01) from corresponding young adult value.
Id protein levels
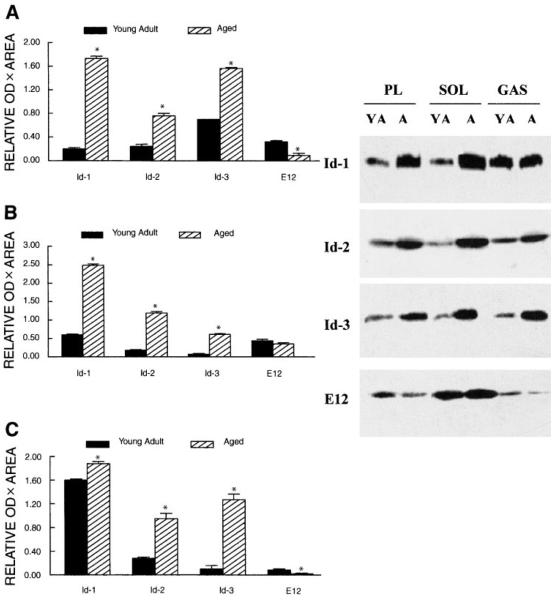
Because Id mRNA levels differed markedly in muscles of young adult and aged rats, we examined the protein levels of Id repressors in these muscles. Similar to the mRNA data, there were substantial age-related differences in repressor protein levels in muscles isolated in hindlimb muscles as determined by Western blots. Id-1 was estimated to be 200–300% greater in aged plantaris and soleus muscles, but Id-1 levels were only 14% greater in gastrocnemius muscles from aged rats compared with young adult rats (P≤ 0.04). Id-2 was 345, 237, and 558% greater in plantaris, soleus, and gastrocnemius muscles, respectively, of aged rats compared with muscles from young adult rats. Id-3 was 157, 481, and 742% greater in aged plantaris, soleus, and gastrocnemius muscles, respectively, compared with muscles in young adult rats (Fig. 7).
Fig. 7.
Representative Western blots of relative Id-1, Id-2, Id-3, and E12 protein levels in plantaris (A), soleus (B), and gastrocnemius muscles (C) of young adult and aged rats. Forty micrograms of solubilized protein extracts was loaded on each lane, separated by SDS-PAGE, transferred to PVDF membranes, and reacted to the appropriate primary antibody. Incubation was conducted with secondary antibodies conjugated to alkaline phosphatase, and the resulting signals were developed by chemiluminescence and exposed to X-ray film. Proteins were run in duplicate and stained with Coomassie blue (data not shown). Immunoreactive bands were quantified by image analysis software. Data are expressed in arbitrary units as means, and error bars represent SEs (n = 10/group) of band area × band OD. *Significantly different from young adult at P < 0.05.
E12 mRNA levels
Because both Id mRNA and protein levels were greater in aged muscles relative to young adult muscles, we examined whether E12 mRNA levels also were greater in muscles of aged animals. As estimated by the semiquantitative measures of the RT-PCR, E12 was ~210, 290, and 190% greater (P < 0.01) in plantaris, soleus, and gastrocnemius muscles, respectively, for old rats compared with young adult rats (Fig. 6).
E12 protein levels
E12 signals appeared to be greater in the slow soleus muscle compared with the faster contracting plantaris and gastrocnemius muscles in both young adult and aged rats. E12 protein levels were only ~30% of the levels measured in plan-taris and gastrocnemius muscles of aged rats compared with young adult rats. In the soleus muscle, however, no significant age-related change in the E12 protein was observed (Fig. 7).
DISCUSSION
MRF expression and aging
Sarcopenia is a loss of muscle mass and strength (16, 17), and this poses a significant problem in the elderly (18). However, the underlying mechanism of sarcopenia is not well understood. It is possible that MRFs may play a role in sarcopenia if MRF transcription and/or posttranscriptional modification occur or if their transcriptional activity is modulated with aging. The important novel findings of this study are that 1) compared with muscles from young adult rats, aging suppresses myogenin and MyoD protein levels while the mRNA levels for these MRFs are high, and 2) aging elevates mRNA and protein levels of Id in fast muscles.
Most previous studies of MRFs in aging muscle have examined only MRF mRNA levels. Our mRNA data are generally consistent with these reports (29, 31, 33, 36) showing an aging-induced increase in MRF mRNA levels in skeletal muscles of experimental animals. Consequently, mRNA levels of MyoD and myogenin do not appear to be limiting under basal conditions in sarcopenia. The age-related decrease (fast muscles) or maintenance (slow soleus muscle) of myogenin and MyoD protein levels despite elevated transcripts for both genes indicates that posttranscriptional events may limit MRF levels.
Sarcopenia was evident in the fast and slow hind-limb muscles as reflected by a 50% decline in muscle weight-to-body weight ratio during aging. This decline in muscle mass was strikingly similar to the decline in mRNA levels of the muscle-specific genes such as desmin and MLC, which are, in part, regulated by MRFs. Further work is needed to determine if the related reduction in MRF protein levels, especially in fast muscles, limits the availability of MRFs to bind to E-boxes and thereby the transcription of contractile protein genes such as desmin and MLCs. Additional studies are required to determine if the reduced MRF levels may be a consequence of reduced stability or increased degradation of MRFs during aging.
Pathways independent from MRFs may regulate experimental atrophy in young adult animals. This is not surprising because aging-associated muscle loss occurs in both fast and slow muscles, and it is much more gradual than experimental atrophy. For example, there is a 40–50% loss in slow muscle mass after ~14 days of hindlimb suspension (12, 13, 35) and an ~40% decrease in both fast and slow muscles 10 days after spinal cord transection (15) with either transient or no detectable changes in MRF mRNA levels in the atrophic muscles of young adult rodents.
Repressor expression and aging
This is the first study that we are aware of that has reported that hindlimb muscles from aged rats had higher mRNA and protein levels of Id repressors, whereas MyoD and myogenin protein levels did not increase with aging. We do not know if the chronic increase in Id repressor levels would be sufficient to reduce MRF transcriptional control of muscle-specific genes and decrease muscle protein synthesis. Additional work is needed to determine if elevated levels of Id repressor proteins and reduced levels of MRF proteins in skeletal muscles of old or young rats would result in muscle atrophy under basal conditions, without the requirement for acceleration of catabolic pathways. If this is the case, this would contrast sharply with hypertrophy, where an increase in MRF mRNA expression is accompanied by an increase in the genes they regulate (28, 30, 34). We are, however, unable to conclude from the current data if the mechanisms regulating the transcriptional activity of MRFs during muscle growth differ from those involved in their regulation during sarcopenia.
Id proteins bind strongly to E proteins and more weakly to MRFs (23) and are therefore considered a dominant negative regulator of MRFs (21) by titration of the E proteins (11, 28, 39). Although Mist 1 was not elevated in the soleus and Twist was not elevated in any muscle examined, additional studies are required to determine if repressors occupying the E-boxes and dimerizing with MRFs are less important in sarcopenia than sequestering E proteins. Nevertheless, even though Id-2 binds more strongly to MyoD than Id-3 or Id-1, and all Ids bind weakly to myogenin (11, 14, 21, 23, 25, 26, 39), future studies are needed to evaluate the possibility that high levels of Id repressors might be adequate to negatively regulate transcription of muscle genes apart from sequestering E proteins via either heterodimerizing with the MRFs to prevent DNA binding (19, 26) or forming homodimers that bind DNA and prevent MRF-E protein binding to E-boxes (23).
E proteins and aging
An increase in Id repressor protein results in increased sequestration of E proteins and, consequently, a mismatch between MRF proteins and E proteins. Therefore, we examined whether E protein levels might be elevated in muscles of aged animals in an attempt to offset the negative effect of the repressor proteins. Although E12 mRNA levels increased by ~200–300% with aging, E12 protein levels as estimated by Western blots were only ~30% of young adult rats in fast muscles and ~14% in the soleus muscle samples of senescence rats. We are currently studying if elevated Id repressor levels prevent the increase in transcription of MRF-regulated muscle-specific genes desmin and MLC and if the Id repressors may play a role in sarcopenia in part because aging alters the posttranslational control of E proteins.
Because MRF protein levels were low despite high transcript levels in muscles of aged rodents compared with young adult rats, the current study cannot establish if Id repressor sequestering of E protein levels was required to reduce MRF transcriptional control of muscle contractile genes. Nonetheless, the data suggest that Id repressors may play a role in which normal gene transcription is disrupted in aging. While common mechanisms may induce increased transcription of MRF, Id, and E genes in aging, they appear to have different posttranscriptional controls.
Perspectives
The results from this study indicate that elevated transcription of Id generally correlates to aging-associated muscle loss (i.e., sarcopenia) in hindlimb muscles of aged rodents. Increased Id levels would be expected to attenuate the transcriptional regulation of muscle genes by MRFs. However, the interpretation of the transcriptional data is limited by the semiquantification methods used in this study to analyze the gene signals, and MRF and Id mRNA levels should also be examined in follow-up studies by quantitative methods. Nevertheless, the aging-associated differences in Id and MRF protein levels as determined from Western blot data provide a sound rationale for conducting other studies that focus on posttranscriptional control of MRFs in sarcopenia. Because both E protein and Id-1 repressor levels increase with denervation (9), we propose that future studies should evaluate whether Id transcription might be influenced by the denervationreinnervation process that occurs in both fast and slow muscles with aging (3, 4, 24). Additional research will be required to determine if Id plays an important role in controlling muscle loss or impeding muscle hyper-trophy in aging.
Acknowledgments
This study was supported by National Institute on Aging Grant AG-17143.
Footnotes
The monoclonal antibody FD5 (anti-myogenin) developed by W. Wright was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Dept. of Biological Sciences, The University of Iowa, Iowa City, IA 52242.
REFERENCES
- 1.Alway SE. Overload-induced C-Myc oncoprotein is reduced in aged skeletal muscle. J Gerontol A Biol Sci Med Sci. 1997;52:B203–B211. doi: 10.1093/gerona/52a.4.b203. [DOI] [PubMed] [Google Scholar]
- 2.Alway SE, Lowe DA, Chen KD. The effects of age and hindlimb suspension on the levels of expression of the myogenic regulatory factors MyoD and myogenin in rat fast and slow skeletal muscles. Exp Physiol. 2001;86:509–517. doi: 10.1113/eph8602235. [DOI] [PubMed] [Google Scholar]
- 3.Ansved T, Larsson L. Quantitative and qualitative morphological properties of the soleus motor nerve and the L5 ventral root in young and old rats. Relation to the number of soleus muscle fibers. J Neurol Sci. 1990;96:269–282. doi: 10.1016/0022-510x(90)90138-d. [DOI] [PubMed] [Google Scholar]
- 4.Ansved T, Wallner P, Larsson L. Spatial distribution of motor unit fibres in fast- and slow-twitch rat muscles with special reference to age. Acta Physiol Scand. 1991;143:345–354. doi: 10.1111/j.1748-1716.1991.tb09242.x. [DOI] [PubMed] [Google Scholar]
- 5.Benezra R, Davis RL, Lassar A, Tapscott S, Thayer M, Lockshon D, Weintraub H. Id: a negative regulator of helix-loop-helix DNA binding proteins. Control of terminal myogenic differentiation. Ann NY Acad Sci. 1990;599:1–11. doi: 10.1111/j.1749-6632.1990.tb42359.x. [DOI] [PubMed] [Google Scholar]
- 6.Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 7.Blough ER, Linderman JK. Lack of skeletal muscle hypertrophy in very aged male Fischer 344 × Brown Norway rats. J Appl Physiol. 2000;88:1265–1270. doi: 10.1152/jappl.2000.88.4.1265. [DOI] [PubMed] [Google Scholar]
- 8.Borycki AG, Emerson CP. Muscle determination: another key player in myogenesis? Curr Biol. 1997;7:R620–R623. doi: 10.1016/s0960-9822(06)00317-4. [DOI] [PubMed] [Google Scholar]
- 9.Carlsen H, Gundersen K. Helix-loop-helix transcription factors in electrically active and inactive skeletal muscles. Muscle Nerve. 2000;23:1374–1380. doi: 10.1002/1097-4598(200009)23:9<1374::aid-mus8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Carson JA, Booth FW. Myogenin mRNA is elevated during rapid, slow, and maintenance phases of stretch-induced hypertrophy in chicken slow-tonic muscle. Pflügers Arch. 1998;435:850–858. doi: 10.1007/s004240050593. [DOI] [PubMed] [Google Scholar]
- 11.Chen B, Han BH, Sun XH, Lim RW. Inhibition of muscle-specific gene expression by Id3: requirement of the C-terminal region of the protein for stable expression and function. Nucleic Acids Res. 1997;25:423–430. doi: 10.1093/nar/25.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen KD, Alway SE. A physiological level of clenbuterol does not prevent atrophy or loss of force in skeletal muscle of old rats. J Appl Physiol. 2000;89:606–612. doi: 10.1152/jappl.2000.89.2.606. [DOI] [PubMed] [Google Scholar]
- 13.Chen KD, Alway SE. Clenbuterol reduces soleus muscle fatigue during disuse in aged rats. Muscle Nerve. 2001;24:211–222. doi: 10.1002/1097-4598(200102)24:2<211::aid-mus60>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Christy BA, Sanders LK, Lau LF, Copeland NG, Jenkins NA, Nathans D. An Id-related helix-loop-helix protein encoded by a growth factor-inducible gene. Proc Natl Acad Sci USA. 1991;88:1815–1819. doi: 10.1073/pnas.88.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont-Versteegden EE, Houle JD, Gurley CM, Peterson CA. Early changes in muscle fiber size and gene expression in response to spinal cord transection and exercise. Am J Physiol Cell Physiol. 1998;275:C1124–C1133. doi: 10.1152/ajpcell.1998.275.4.C1124. [DOI] [PubMed] [Google Scholar]
- 16.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 17.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50:5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- 18.Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123:465–468. doi: 10.1093/jn/123.suppl_2.465. [DOI] [PubMed] [Google Scholar]
- 19.Hamamori Y, Wu HY, Sartorelli V, Kedes L. The basic domain of myogenic basic helix-loop-helix (bHLH) proteins is the novel target for direct inhibition by another bHLH protein, Twist. Mol Cell Biol. 1997;17:6563–6573. doi: 10.1128/mcb.17.11.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hebrok M, Fuchtbauer A, Fuchtbauer EM. Repression of muscle-specific gene activation by the murine Twist protein. Exp Cell Res. 1997;232:295–303. doi: 10.1006/excr.1997.3541. [DOI] [PubMed] [Google Scholar]
- 21.Jen Y, Weintraub H, Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev. 1992;6:1466–1479. doi: 10.1101/gad.6.8.1466. [DOI] [PubMed] [Google Scholar]
- 22.Kadesch T. Helix-loop-helix proteins in the regulation of immunoglobulin gene transcription. Immunol Today. 1992;13:31–36. doi: 10.1016/0167-5699(92)90201-h. [DOI] [PubMed] [Google Scholar]
- 23.Langlands K, Yin X, Anand G, Prochownik EV. Differential interactions of Id proteins with basic-helix-loop-helix transcription factors. J Biol Chem. 1997;272:19785–19793. doi: 10.1074/jbc.272.32.19785. [DOI] [PubMed] [Google Scholar]
- 24.Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci. 1995;50:91–95. doi: 10.1093/gerona/50a.special_issue.91. [DOI] [PubMed] [Google Scholar]
- 25.Lemercier C, Brown A, Mamani M, Ripoche J, Reiffers J. The rat Mist1 gene: structure and promoter characterization. Gene. 2000;242:209–218. doi: 10.1016/s0378-1119(99)00523-5. [DOI] [PubMed] [Google Scholar]
- 26.Lemercier C, To RQ, Carrasco RA, Konieczny SF. The basic helix-loop-helix transcription factor Mist1 functions as a transcriptional repressor of myoD. EMBO J. 1998;17:1412–1422. doi: 10.1093/emboj/17.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Capetanaki Y. Regulation of the mouse desmin gene: transactivated by MyoD, myogenin, MRF4 and Myf5. Nucleic Acids Res. 1993;21:335–343. doi: 10.1093/nar/21.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loveys DA, Streiff MB, Kato GJ. E2A basic-helix-loop-helix transcription factors are negatively regulated by serum growth factors and by the Id3 protein. Nucleic Acids Res. 1996;24:2813–2820. doi: 10.1093/nar/24.14.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowe DA, Alway SE. Stretch-induced myogenin, MyoD, and MRF4 expression and acute hypertrophy in quail slow-tonic muscle are not dependent upon satellite cell proliferation. Cell Tissue Res. 1999;296:531–539. doi: 10.1007/s004410051314. [DOI] [PubMed] [Google Scholar]
- 30.Lowe DA, Degens H, Chen KD, Alway SE. Glyceraldehyde-3-phosphate dehydrogenase varies with age in glycolytic muscles of rats. J Gerontol A Biol Sci Med Sci. 2000;55:B160–B164. doi: 10.1093/gerona/55.3.b160. [DOI] [PubMed] [Google Scholar]
- 31.Lowe DA, Lund T, Alway SE. Hypertrophy-stimulated myogenic regulatory factor mRNA increases are attenuated in fast muscle of aged quails. Am J Physiol Cell Physiol. 1998;275:C155–C162. doi: 10.1152/ajpcell.1998.275.1.C155. [DOI] [PubMed] [Google Scholar]
- 32.Makrides SC. Protein synthesis and degradation during aging and senescence. Biol Rev Camb Philos Soc. 1983;58:343–422. doi: 10.1111/j.1469-185x.1983.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 33.Marsh DR, Criswell DS, Carson JA, Booth FW. Myogenic regulatory factors during regeneration of skeletal muscle in young, adult, and old rats. J Appl Physiol. 1997;83:1270–1275. doi: 10.1152/jappl.1997.83.4.1270. [DOI] [PubMed] [Google Scholar]
- 34.Mozdziak PE, Greaser ML, Schultz E. Myogenin, MyoD, and myosin expression after pharmacologically and surgically induced hypertrophy. J Appl Physiol. 1998;84:1359–1364. doi: 10.1152/jappl.1998.84.4.1359. [DOI] [PubMed] [Google Scholar]
- 35.Mozdziak PE, Greaser ML, Schultz E. Myogenin, MyoD, and myosin heavy chain isoform expression following hindlimb suspension. Aviat Space Environ Med. 1999;70:511–516. [PubMed] [Google Scholar]
- 36.Musaro A, Cusella De Angelis MG, Germani A, Ciccarelli C, Molinaro M, Zani BM. Enhanced expression of myogenic regulatory genes in aging skeletal muscle. Exp Cell Res. 1995;221:241–248. doi: 10.1006/excr.1995.1372. [DOI] [PubMed] [Google Scholar]
- 37.Short KR, Nair KS. The effect of age on protein metabolism. Curr Opin Clin Nutr Metab Care. 2000;3:39–44. doi: 10.1097/00075197-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Smith CK, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J Cell Physiol. 1994;159:379–385. doi: 10.1002/jcp.1041590222. [DOI] [PubMed] [Google Scholar]
- 39.Sun XH, Copeland NG, Jenkins NA, Baltimore D. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol. 1991;11:5603–5611. doi: 10.1128/mcb.11.11.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 41.Weintraub H, Dwarki VJ, Verma I, Davis R, Hollenberg S, Snider L, Lassar A, Tapscott SJ. Muscle-specific transcriptional activation by MyoD. Genes Dev. 1991;5:1377–1386. doi: 10.1101/gad.5.8.1377. [DOI] [PubMed] [Google Scholar]
- 42.Wentworth BM, Donoghue M, Engert JC, Berglund EB, Rosenthal N. Paired MyoD-binding sites regulate myosin light chain gene expression. Proc Natl Acad Sci USA. 1991;88:1242–1246. doi: 10.1073/pnas.88.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]