Abstract
Background
Little is known about the serotonin1A receptor (5-HT1A) in bipolar depression despite altered 5-HT1A binding in major depressive disorder (MDD). Utilizing PET and the radioligand [C-11]WAY100635, regional 5-HT1A binding was compared between depressed bipolar disorder (BD) and control subjects.
Methods
Brain 5-HT1A binding potential (BPF = Bmax/KD, where Bmax is the total number of available receptors and 1/KD is the receptor affinity of the ligand) was measured in 32 currently depressed, medication-free BD subjects and 47 healthy controls. Participants were also genotyped for the 5-HT1A promoter polymorphism C(-1019)G.
Results
The bipolar depressed group demonstrated higher 5-HT1A BPF across all regions of interest (ROIs; p=0.022). Post-hoc analyses indicated that male BD patients had higher 5-HT1A BPF than male controls (p=0.025), with higher 5-HT1A BPF found in every region (by 102% in raphe nuclei and 31-54% in the 12 forebrain ROIs). Whereas, female subgroups did not differ in 5-HT1A BPF (p=0.32). 5-HT1A BPF did not correlate with depression severity. The GG genotype was overrepresented at a trend level in the BD group (p=0.057). Number of copies of the G-allele was associated with higher 5-HT1A BPF in raphe (p=0.0050), amygdala (p=0.022), and hippocampus (p=0.041).
Conclusion
Higher 5-HT1A BPF in depressed males with BD suggests higher raphe autoreceptor binding, potentially causing less serotonin release and compensatory upregulation of postsynaptic 5-HT1A receptors in forebrain regions. The raphe effect may be partly genetic. Lack of difference in 5-HT1A BPF between BD and control females may reflect greater effect of prior antidepressant exposure in BD females.
Keywords: Bipolar, depression, serotonin, 5-HT1A receptor, positron emission tomography (PET)
INTRODUCTION
Bipolar disorder has high prevalence (1) and substantial morbidity (2) and mortality (3, 4). Bipolar disorder is characterized by mania and major depressive episodes (MDEs) that are clinically indistinguishable from those of major depressive disorder (MDD). Most of the disease burden and almost all suicides in bipolar disorder are attributed to the occurrence of depressive episodes, the longest duration illness phase in this condition (5-7).
Impaired serotonergic (5-HT) function is implicated in the pathophysiology of depression and manic phases. Brainstem serotonin-1A autoreceptors (5-HT1A) regulate 5-HT neuronal firing, and other 5-HT auto- and heteroreceptors mediate serotonin effects in many forebrain regions. The downregulation or desensitization of the brainstem 5-HT1A autoreceptors in raphe nuclei that occurs over weeks with serotonin selective reuptake inhibitor (SSRI) treatment mediates greater 5-HT neurotransmission to forebrain regions and is thought to underlie the lag time to antidepressant action (8).
To date, in vivo positron emission tomography (PET) imaging studies report altered 5-HT1A binding in MDD although both the direction of the effect and the brain regions involved differ among studies (9-11). We now report on in vivo 5-HT1A binding using PET and [C-11]WAY100635, comparing 32 medication-free bipolar disorder patients in an MDE with 47 controls. Participants were also genotyped for the functional promoter C(-1019)G polymorphism in the 5-HT1A gene to explore effects on 5-HT1A binding in bipolar disorder. We hypothesized higher 5-HT1A binding in bipolar depression, similar to the pattern we found in MDD, which would be partly dependent on the G allele promoter variant.
METHODS AND MATERIALS
PARTICIPANTS
Thirty-two patients who presented in a MDE and met DSM-IV criteria for bipolar I or bipolar II disorder on Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P) (12), psychiatric interview, and chart review were enrolled. Forty-seven healthy volunteer control participants were also enrolled if free of Axis I diagnosis by SCID-I-NP (12) and without history of mood or psychotic disorder or suicide in any first-degree relative. MA- or PhD-level psychologists performed all SCID interviews with independent diagnostic assessment by a research psychiatrist. Consensus diagnoses were determined by at least 3 senior clinicians. Controls were recruited concurrently with bipolar disorder patients. Data from 43 of the 47 controls have previously been reported (10). Control participants had their respective PET scans between 8/17/1999 and 6/6/2007. Bipolar participants had their respective PET scans between 6/14/1999 and 6/27/2007. All participants were 18-65 years old. Depressed bipolar patients scored ≥ 16 on the 17-item Hamilton Depression Rating Scale (HDRS-17) upon screening. They were free of antidepressant and mood stabilizer medication for at least 14 days (42 days for fluoxetine) and oral antipsychotics for at least 21 days before PET scan. No patients were on depot antipsychotics. Small doses of shorter-acting benzodiazepines were the only psychotropic permitted, used only as needed until 72 hours before PET. For ethical reasons, no patients were tapered off of medications effective for their mood disorder for the purpose of this research. Exclusion criteria for all participants were: significant medical condition as determined by history, physical examination, routine blood and urine tests; positive urine toxicology; positive pregnancy test; history of alcohol or substance use disorder in lifetime for controls and within the previous 6 months for bipolar disorder patients; lifetime exposure to 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”); current or planned pregnancy; or lack of capacity to provide informed consent.
Only three of the 32 bipolar disorder participants were naïve to treatment with an antidepressant medication (12/32 had the last antidepressant discontinued > 120 days before PET; 17/32 had the last antidepressant discontinued 15-120 days before PET, mean ± SD, 41.0 ± 30.2 days). Comorbid Axis I disorders in the bipolar disorder group included panic disorder (n=7), posttraumatic stress disorder (n=6), obsessive compulsive disorder (n=5), simple phobia (n=2), social anxiety disorder (n=1), and generalized anxiety disorder (n=1). Twelve of the 32 met DSM-IV criteria for a past history (> 6 months before screening) of alcohol or substance use disorder (9 alcohol; 5 cannabis; 3 cocaine; 1 stimulant; 1 opiate).
All participants provided written informed consent after description of the study protocol as approved by the Institutional Review Boards of the New York State Psychiatric Institute and Columbia University Medical Center.
CLINICAL ASSESSMENTS
The HDRS-17 (13), the Beck Depression Inventory (BDI) (14), and the Global Assessment Scale (15) were utilized to assess depression severity and functional impairment. Lifetime aggression was rated using the Brown-Goodwin Aggression Inventory (16) and manic/hypomanic symptoms using the Young Mania Rating Scale (17).
RADIOCHEMISTRY AND INPUT FUNCTION MEASUREMENT
[Carbonyl-C-11]WAY100635 [N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl) cyclohexane carboxamide] was prepared as previously described (18). Measurements of arterial input function, metabolites, and free plasma fraction (fP) were made as described previously (18, 19). No group differences between controls and bipolar disorder were present in: specific activity of [C-11]WAY100635 (1.56 ± 0.73 mCi/nmol versus1.88 ± 1.04 mCi/nmol; t=-1.50, df=51.3, p=.14); injected mass (2.86 ± 1.88 μg versus 2.31 ± 1.97 μg; t=1.27, df=77, p=.21; or injected dose (8.17±3.52 mCi versus 6.70±3.58 mCi; t=1.81, df=77, p=.08).
IMAGE ACQUISITION AND ANALYSIS
Acquisitions of MRI and PET data were performed as described previously (19). Image analysis was performed using MATLAB 2006b (The Mathworks, Natick, MA) with extensions to the following open source packages: Functional Magnetic Resonance Imaging of the Brain’s Linear Image Registration Tool (FLIRT) v5. (20), Brain Extraction Tool (BET) v1.2 (21), and University College of London’s Statistical Parametric Mapping (SPM5) normalization (22) and segmentation routines (23). To correct for subject motion during the PET scan, de-noising filter techniques were applied to all PET images starting at frame five. The eighth frame was used as a reference to which all other frames were aligned using rigid body FLIRT. For registration, a mean of the motion corrected frames eight through 18 was registered using FLIRT.
Region of interests (ROI) were drawn on each subject’s MRI as previously described (18) and included: the ventral prefrontal cortex (VPFC), medial prefrontal cortex (MPFC), dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), cingulate (posterior) cortex (CIN), amygdala (AMY), hippocampus (HIP), parahippocampal gyrus (PHG), insular cortex (INS), temporal cortex (TEM), parietal cortex (PAR), and occipital cortex (OCC). Because the boundaries of the median and dorsal raphe nuclei are not identifiable on MRI, a 2 cm3 ellipsoid was manually placed on the raphe nuclei of each individual’s mean PET image, completely encompassing the high [C-11]WAY100635 binding region of the posterior midbrain. A cylindrical reference region was drawn in cerebellar white matter, as this region of cerebellum is virtually devoid of 5-HT1A (19). For cortical regions, the ROIs were modified to include only gray matter voxels using SPM5-generated gray matter probability (GMp) maps. SPM5 segments the structural MRI image of each subject using the default tissue probability maps as priors. It produces GMp maps for each subject using a single generative model that combines the registration and tissue classification components of segmentation, and includes parameters that account for image intensity nonuniformity (23). PET image voxels within a cortical ROI at each frame were multiplied by the corresponding gray matter probability map value. The average gray matter corrected intensities were obtained by taking the sum of the PET activity within each cortical ROI and dividing by the sum of the GMp map voxel values within that ROI. Data analysts were blind to subject identity and diagnostic grouping.
DERIVATION OF OUTCOME MEASURES
Regional distribution volumes (V) of [C-11]WAY100635 were derived as previously described (18). Consensus nomenclature for distribution and binding parameters, which we now employ, was recently established by an international group of experts in pharmacokinetic modeling (24). For comparison purposes with outcome data from other investigators, BPND was also derived by the simplified reference tissue model (SRTM) (25) as previously described (18), using the first 90 minutes of PET emission data and the cerebellar gray matter as the reference region.
GENOTYPING
The functional 5-HT1A gene promoter region single nucleotide polymorphism (SNP) known as 5-HT1A C(-1019)G (a.k.a. rs6295) was genotyped for a biallelic classification (CC, CG, or GG) for each participant as previously described (26, 27).
STATISTICS
The log transform of BPF was used to stabilize the variance across regions. Also, the necessity to address the non-normal distribution of binding of [C-11]WAY100635 for statistical comparisons has been recognized by our group and others (28-30). Linear mixed-effects models were fit to the ROI-level BPF estimates with region, diagnostic group, sex, and lifetime aggression score as fixed effects and subject as the random effect. Standard errors (SE) were computed for each estimated BPF value using a bootstrap algorithm that takes into account errors in metabolite, plasma, and brain data (31) and observations were weighted accordingly in the linear mixed-effects models.
RESULTS
The healthy volunteer and bipolar disorder groups were of comparable age (t=-0.14; p=0.89) and sex distribution (χ2, p=0.87). Table 1 provides socio-demographic and clinical data for the two groups. The ROI analysis of the [C-11]WAY100635 PET data revealed expected differences in the binding to 5-HT1A, expressed as BPF, across brain regions (F12,900=976.1, p<.0001).
Table 1. Sociodemographic and Clinical Data of the Study Groups.
| Controls (n = 47) | Bipolar Depressed (n = 32) | Statistic (df) | P value | |
|---|---|---|---|---|
| Sex, M/F, No. | 20/27 | 13/19 | χ2(1) = 0.029 | NS (0.87) |
| Age, mean ± SD, y | 38.1 ± 14.7 | 38.4 ± 9.7 | t(77.0) = -0.143 | NS (0.89) |
| Bipolar I/bipolar II, No. | NA | 18/14 | ||
| Inpatient/Outpatient, No. | NA | 16/16 | ||
| Age at 1st major depressive episode, mean ± SD, y | NA | 20.3 ± 9.8 | ||
| Age at 1st manic or hypomanic episode, mean ± SD, y | NA | 28.0 ± 10.6 | ||
| Length of current major depressive episode, wk | NA | 35.3 ± 64.0 | ||
| Antidepressant history, exposed/naïve, No. | NA | 29/3 | ||
| Previous suicide attempts, No. (%) | NA | 19 (59%) | ||
| Number of suicide attempts in attempters (n = 19), No. | NA | 1.6 ± 2.1 | ||
| HDRS (17-item) score, mean ± SD | 0.6 ± 0.9 | 18.0 ± 4.9 | t(31.7) = -17.9 | <0.001 |
| Beck Depression Inventory score, mean ± SD | 1.7 ± 2.6 | 25.7 ± 11.0 | t(33.4)= -12.1 | <0.001 |
| Young Mania Scale score | 0.2 ± 0.6 | 6.4 ± 7.1 | t(30.3) = -4.8 | <0.001 |
| Global Assessment Scale score, mean ± SD† | 90.1 ± 4.5 | 47.6 ± 10.5 | t(35.8) = 21.0 | <0.001 |
| Aggression Inventory Score (Brown-Goodwin)† | 14.0 ± 3.7 | 20.0 ± 6.5 | t(43.4) = -4.7 | <0.001 |
| 5-HT1APR C(-1019)G biallelic genotype, No. (%)† | ||||
| CC | 15 (32.6) | 8 (25.8) | ||
| CG | 27 (58.7) | 15 (48.4) | ||
| GG | 4 (8.7) | 8 (25.8) | FET* | NS (0.057)** |
Abbreviations: HDRS, Hamilton Depression Rating Scale; NA, not applicable; NS, not significant
Based on 46 healthy volunteers and 31 depressed bipolar patients
Fisher’s exact test (two-sided) comparing count data for homozygotes with GG v. non-GG homozygotes (i.e. CC and CG) between groups
Fisher’s exact test (one-sided) p=0.045
Controls vs. Bipolar Depressed
The depressed bipolar group had higher 5-HT1A BPF than the control group (F1,74=5.45, p=0.022). There was also an effect of sex on 5-HT1A BPF that depended on brain region (F12,900=2.20, p=.010); and a significant 3-way interaction indicated this pattern of influence also differed between bipolar disorder patients and controls (F12,900=2.60, p=.0021). Based on a model that constrains equal effect size for all regions and both sexes, we estimate 25.1% higher 5-HT1A BPF for bipolar depressed subjects relative to controls.
Post hoc testing by brain region demonstrated higher 5-HT1A BPF in the bipolar group in all ROIs (see Table 2). Figure 1 shows the weighted mean ± standard error of 5-HT1A BPF for all ROIs in controls and bipolar disorder patients. For HIP, there was also a sex by diagnosis interaction (F1,75=4.32, p=0.041). And for RN there was also an effect of sex (F1,75=5.49, p=0.022) and a sex by diagnosis interaction (F1,75=12.51, p=0.00070), indicating the binding pattern in RN at least contributed to the 3-way interaction in the main analysis.
Table 2. Linear Mixed Effects Model Results for Each Region of Interest (ROI).
| Effect of Diagnosis | ||
|---|---|---|
| ROI | F(1,75) | P |
| Anterior cingulate cortex (ACC) | 6.64 | 0.012 |
| Amygdala (AMY) | 6.52 | 0.013 |
| Cingulate body (CIN) | 8.55 | 0.005 |
| Dorsolateral prefrontal cortex (DLPFC) | 5.86 | 0.018 |
| Hippocampus (HIP) | 5.57 | 0.021 |
| Insular cortex (INS) | 5.68 | 0.020 |
| Medial prefrontal cortex (MPFC) | 8.91 | 0.004 |
| Occipital cortex (OCC) | 9.11 | 0.004 |
| Orbital prefrontal cortex (OPFC) | 6.56 | 0.012 |
| Parietal cortex (PAR) | 6.60 | 0.012 |
| Parahippocampal gyrus (PHG) | 9.24 | 0.003 |
| Temporal cortex (TEM) | 6.08 | 0.016 |
| Raphe nuclei (RN) | 18.32 | 0.00005 |
Figure 1.
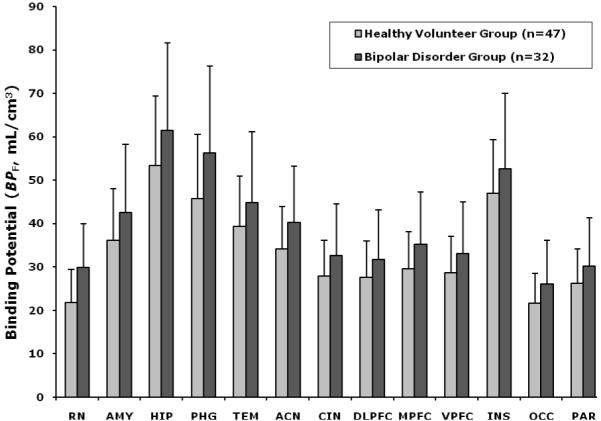
Radiolabeled [C-11]WAY-100635 binding potential (BPF) estimates for the 5-HT1A receptor in nonmedicated depressed bipolar patients and controls in 13 regions of interest. Comparison of 5-HT1A BPF between the bipolar group and control group demonstrates higher 5-HT1A BPF in bipolar patients (p=.022). Also, there was a region x sex interaction (p=0.010) and a 3-way interaction, region x sex x diagnosis (p=0.0021). Post-hoc comparisons demonstrate higher 5-HT1A BPF in the bipolar group in every ROI (see Table 2). ACN, anterior cingulate cortex; AMY, amygdala; CIN, cingulate body; DLPFC, dorsolateral prefrontal cortex; HIP, hippocampus; INS, insula; MPFC, medial prefrontal cortex; OCC, occipital cortex; PAR, parietal cortex; PHG, parahippocampal gyrus; TEM, temporal cortex; VPFC, ventral prefrontal cortex; RN, raphe nuclei. The height of each vertical bar represents the weighed means of 5-HT1A BPF for the region, and the error bar represent the standard error of the weighted estimate.
Effects of Sex
As we reported previously (19, 32), females controls have higher BPF than male controls in all ROIs. To explore the sex differences in 5-HT1A BPF between diagnostic groups, post hoc testing for each sex was carried out across all ROIs. Among the males, both the main effect of diagnosis (F1,31=5.52, p=0.025) and the region by diagnosis interaction (F12,372=2.78, p=0.0012) terms were statistically significant. In contrast, 5-HT1A BPF in female bipolar patients did not differ from female controls (F1,44=1.03, p=0.32), nor was the interaction term significant (F12,528=1.08, p=0.38). In male bipolar patients, weighted means of 5-HT1A BPF were 102% higher in RN and 29-50% higher throughout the 12 forebrain ROIs compared to male controls.
Figure 2A and 2B show scatter plots for raphe nuclei and hippocampus of raw 5-HT1A BPF values for individual male subjects, also displaying the associated standard error (31) for each BPF estimate. To demonstrate visually regions of higher binding in male bipolar patients, Figure 3 provides voxel maps of mean 5-HT1A BPF for the male bipolar patient subgroup and the male control subgroup. This illustrates how binding in bipolar males is elevated across the brain, including elevated binding in the brainstem raphe autoreceptors.
Figure 2.
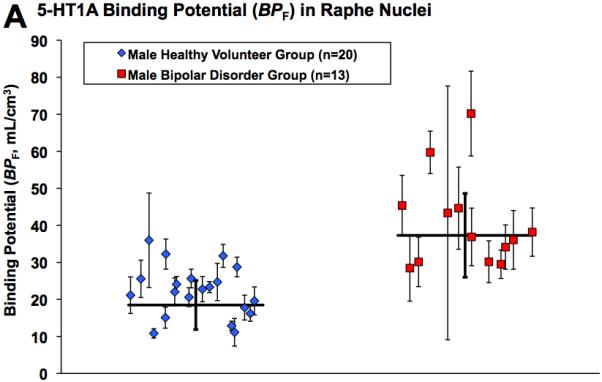
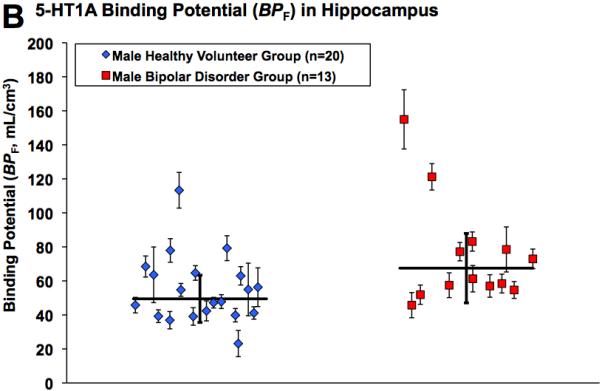
Radiolabeled [C-11]WAY-100635 binding potential (BPF) estimates for the 5-HT1A receptor in male bipolar patients and male controls in A) raphe nuclei and B) hippocampus. Diamonds and squares represent single measurements of BPF in controls and bipolar patients, respectively. Thin capped vertical error bars represent standard errors computed using a bootstrap algorithm that takes into account errors in metabolite, plasma, and brain data (31). Weighted group mean and standard error of the weighted mean of BPF are represented by thick horizontal lines and thick capped vertical lines, respectively.
Figure 3.
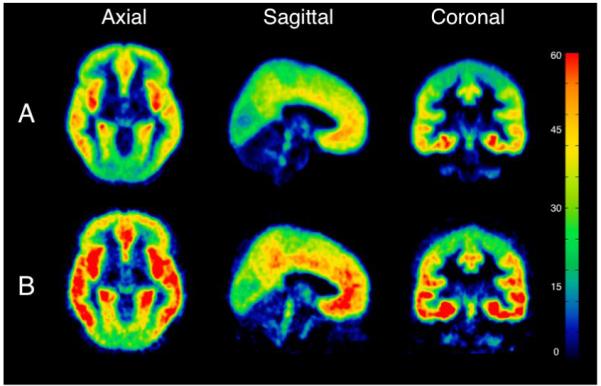
Mean binding potential (BPF) voxel maps for A) the male healthy volunteer group and B) the male bipolar depressed group. Each voxel intensity is the mean of the 5-HT1A BPF measurements for that voxel among each group. The color bar represents 5-HT1A BPF level in mL/cm3.
Clinical Correlates
No correlations were identified between depression or mania severity scores on the HDRS-17, YMRS or BDI total scores and the natural log of BPF in any of the 13 ROIs. This included no significant correlations for the controls, bipolar disorder patients, bipolar disorder males, and bipolar disorder females.
Age did not correlate with the natural log of 5-HT1A BPF in any ROI for the total sample: ACC (r=-.04, p=.74), AMY (r<.01, p=.98), CIN (r=-.12, p=.28), DLPFC (r=-.09, p=.45), HIP (r=-.02, p=.88), INS (r=-.10, p=.36), MPFC (r=-.05, p=.66), OCC (r=-.11, p=.33), OPFC (r=-.06, p=.62), PAR (r=-.12, p=.28), PHG (r=.01, p=0.92), TEM (r=-.05, p=.64), and RN (r=-.08, p=.51). Nor were there any significant partial correlations between the natural log of 5-HT1A BPF and age when controlling for sex and diagnosis: ACC (r=-.02, p=.89), AMY (r=.03, p=.80), CIN (r=-.11, p=.33), DLPFC (r=-.07, p=.54), HIP (r=.01, p=0.95), INS (r=-.09, p=.45), MPFC (r=-.03, p=.79), OCC (r=-.10, p=.40), OPFC (r=-.04, p=.74), PAR (r=-.11, p=.36), PHG (r=.01, p=.95), TEM (r=-.04, p=.77), and RN (r=-.06, p=.63).
Other clinical variables did not affect results. There were no differences in 5-HT1A BPF between bipolar subtypes I versus II or related to suicide attempter status within the bipolar depressed group. Controlling for lifetime aggression history score, previously shown to correlate inversely with 5-HT1A BPF in healthy volunteers (32), in the model did not alter findings related to bipolar disorder.
Plasma and Nonspecific Binding
Free fraction in plasma or fP of [C-11]WAY100635 was higher in controls than in patients with bipolar disorder (8.1 ± 2.5% v. 5.9 ± 2.0%, respectively; t77=4.22; p<0.000067). Cerebellar distribution volume or VND of [C-11]WAY100635 did not differ between controls and patients with bipolar depression (0.29 ± 0.11 v. 0.26 ± 0.10 mL/g respectively; t77=1.40; p=0.17).
C(-1019)G Promoter Polymorphism
Genotyping for the 5-HT1A C(-1019)G promoter polymorphism demonstrated that 8/31 (25.8%) of the bipolar patients and 4/46 (8.7%) of controls were homozygous for the G allele variant (Fisher’s exact test, 2-tailed, p=0.057). Frequency of the G allele presence did not differ between males (66%) and females (73%; Fisher’s exact test, p=.47). There was a relationship between 5-HT1A BPF and number of G alleles that was dependent on brain region (F12,900=2.39, p=0.0049). Post hoc testing revealed that number of G alleles was associated with higher 5-HT1A BPF in AMY (t=-2.34, p=0.022), HIP (t=-2.08, p=0.041), and RN (t=-2.90, p=0.0050); and in RN there was also a sex by G-allele interaction (t=2.07, p=0.042), indicating number of G-alleles in male depressed bipolars was associated with higher 5-HT1A BPF in RN.
Alternative Reference Region and Outcome Measure
Several PET studies using [C-11]WAY100635 have not acquired an arterial input function, used shorter duration data acquisition, and employed the SRTM method of image analysis and used cerebellar gray (rather than white) matter as reference region (9, 11, 33). For SRTM, BPND is the only possible binding outcome measure, as total and free arterial plasma concentrations are necessary for calculation of BPP and BPF, respectively (see Innis et al (24) for nomenclature). To allow comparison of our findings in bipolar disorder with data from other groups using SRTM rather than the 2-tissue compartment model, we also performed an SRTM analysis (using only the first 90 min of PET emission data and employing cerebellar gray matter as reference region). By this method, we found no significant differences in BPND across all ROIs in the bipolar disorder group compared to controls (F1,74=3.32; p=0.073), no effect of sex (F1,74=0.19; p=0.66), no sex by diagnosis interaction (F1,74=1.04; p=0.31), and no 3-way interaction of region, sex, and diagnosis (F12,900=0.92; p=0.53). BPND was nonsignificantly lower by 5.1-13.9% across the ROIs in the bipolar group.
DISCUSSION
To our knowledge, this is the first published report employing PET to assess 5-HT1A binding in vivo in a homogeneous, non-medicated, patient sample with bipolar depression. Using the 5-HT1A antagonist radioligand [C-11]WAY100635, we identified in bipolar depression higher 5-HT1A BPF, in particular in the raphe autoreceptors and to a lesser degree across all terminal field ROIs. Post-hoc analyses indicated the higher 5-HT1A BPF was specific to male bipolar depressed patients compared with male controls. In contrast, female bipolar depressed patients did not differ in 5-HT1A BPF from female controls throughout the brain. Healthy females had higher binding than healthy males, as we previously reported (18, 19).
It has been demonstrated in vitro that the C(-1019) allele (rs6295) of the polymorphic region of the 5-HT1A gene promoter region is part of a 26 base pair imperfect palindrome that binds a transcriptional repressor, nuclear deformed epidermal autoregulatory factor (NUDR), that suppresses expression in raphe but not hippocampal neuron cultures (34). It would be predicted that the G allele would be associated with higher raphe autoreceptor binding and not with higher terminal field binding. Previously, we reported among MDD patients and controls that carriers of at least one G allele have higher 5-HT1A BPF in raphe nuclei (10). In the present sample, there was a trend for an overrepresentation of the GG genotype the bipolar group (8.7%, controls; 25.8%, bipolar disorder). In raphe nuclei there was a relationship between number of copies of the G allele and higher raphe 5-HT1A BPF. Similar relationships identified in hippocampus and amygdala do not withstand correction for multiple comparisons. Yet the sample sizes in this study are not sufficient to differentiate effect of bipolar disorder diagnosis from effect of genotype on raphe nuclei 5-HT1A expression; however, it is possible that the G allele is part of the reason for higher raphe autoreceptor binding in males with bipolar disorder. We did not find a sex difference in G allele frequency so that effect is explained by some other mechanism. Thus, the present results are at least consistent with a genetic mechanism by which G allele carriers have higher expression of 5-HT1A autoreceptors in the raphe nuclei, potentially producing lower serotonin neuron firing and less serotonin release at nerve terminals. That in turn may result in some post-synaptic receptor upregulation, although preclinical data supporting upregulation of terminal field 5-HT1A in serotonin depleted states is lacking.
We previously reported higher 5-HT1A BPF in antidepressant naïve patients with MDD (10). The present finding of higher 5-HT1A BPF in bipolar depression is similar to our prior finding in MDD. Although, in bipolar depression the estimated 25% difference relative to controls is slightly larger than that found in the prior antidepressant naïve MDD cohort, which was 19%. Also, the effect in bipolar depression is apparently not substantially diminished by prior history of antidepressant medication exposure. Unfortunately, only 3 of 32 in the present bipolar disorder sample were antidepressant naïve, precluding meaningful analysis by antidepressant exposure history. If indeed prior antidepressant exposure has a similar lasting reduction in 5-HT1A density and function as revealed in preclinical models (35, 36), an antidepressant-naïve sample of bipolar depressed males may demonstrate even higher 5-HT1A BPF.
Alternatively, prior antidepressant exposure in bipolar disorder may result in a sex-specific reduction in 5-HT1A in the females and not males so that, among antidepressant-exposed patients, higher binding is only demonstrable in the males. In support of this hypothesis, female bipolars with prior antidepressant exposure greater than 120 days from PET (n=5) had higher mean 5-HT1A BPF than those with exposure between 15 and 120 days from PET (n=13; data not shown). Also, there was a high rate of 5-HT1A C(-1019)G polymorphism GG genotype in our bipolar male subsample, 33%, compared with 21% in the bipolar female subsample. It may thus be speculated that prior antidepressant exposure in male bipolar did not result in a lasting reduction in 5-HT1A binding as a result of deficient NUDR repressor function in 5-HT1A-expressing raphe nuclei neurons. Such an effect might contribute to a poorer clinical response to antidepressants. In our prior MDD sample, higher pretreatment 5-HT1A BPF was associated with nonremission after one year of naturalistic treatment (37) and the GG genotype predicts poorer antidepressant treatment response (38).
Over half of our sample of bipolar patients had a history of suicide attempt, but we did not identify an effect of attempter status on 5-HT1A BPF. Our finding of higher 5-HT1A BPF in raphe nuclei in depressed bipolar disorder males is consistent with postmortem studies reporting higher dorsal raphe nucleus 5-HT1A binding in male suicides with MDD using a 5-HT1A agonist probe, [H-3]8-OH-DPAT (39, 40) and higher 5-HT1A mRNA in male depressed suicides in frontopolar cortex and hypothalamus (41).
Our post-hoc finding of no difference in 5-HT1A BPF between female bipolar disorder patients and female controls may be related to sex-specific characteristics of the 5-HT1A receptor often observed in basic (42-46) and clinical (39, 47-49) studies. It was recently reported using PET in vivo in healthy females that 5-HT1A BPND in raphe nuclei increases between the follicular phase and luteal phase of the menstrual cycle (50). Yet, only when using BPF, not BPND, as the outcome measure do we find sex differences in 5-HT1A (19), as reported in postmortem studies of 5-HT1A (39, 47); therefore this is unlikely to explain the lack of difference in 5-HT1A BPF between female bipolar patients and controls.
There has been apparent disagreement in the [C-11]WAY100635 PET literature regarding the direction of difference between depressed patients and controls in 5-HT1A availability for binding, with other groups reporting lower binding in raphe nuclei (9) and specific forebrain regions (9, 11, 33). Yet, we have discussed crucial differences in PET image processing and modeling methodologies and that our outcome measure is BPF, not BPND (10, 19). Use of BPND for estimation of available 5-HT1A using [C-11]WAY100635 assumes no between group differences in the free fraction of ligand in the nondisplaceable compartment, fND. Yet, fND is related to fP, and we now report a significant between-group difference in fP in bipolar depression. Moreover, we have shown that despite the very low concentration of 5-HT1A receptors in cerebellar gray matter and vermis, BPND (=BPP/VND) is exquisitely sensitive to small changes in VND when, as in the case of [C-11]WAY100635, VND is <<1 (19). When we employ SRTM and cerebellar gray instead of white matter, we find BPND is 5-14% lower in bipolar depression throughout the 13 ROIs. We believe that because of the very low specific and nonspecific binding of the [C-11]WAY100635 compound in the cerebellum, this approach is prone to error. Finally, BPF is the outcome measure that estimates Bmax/KD, as it is the only expression of specific distribution volume that depends exclusively on receptor parameters. The other outcome measures require assumptions that either fND or fP is not different between groups.
There are several limitations to the present study. In particular, the washout period of antidepressants before scanning was limited to 14 days for ethical reasons, and 10 (7 female, 3 male) of the 32 bipolar patients had stopped the last antidepressant(s) between 15 and 32 days before PET. This washout time may be insufficient for the effects of antidepressant treatment on the 5-HT system to fully abate. Prior substance and alcohol use disorder and present anxiety disorder comorbidity in some of the sample are also limitations, although we have demonstrated (data not shown) that neither can explain the finding of higher 5-HT1A BPF in the bipolar males. Also, although this is a relatively large sample for PET imaging in bipolar disorder, the numbers are not large enough to test if the higher raphe nuclei 5-HT1A availability in bipolar depression is explained by presence of the C(-1019)G polymorphism.
In summary, we found higher 5-HT1A BPF in midbrain raphe nuclei and to a lesser extent across other brain regions in bipolar disorder within a major depressive episode compared with healthy volunteers. Lack of correlation of 5-HT1A BPF and mood severity measures suggests that future studies should assess bipolar subjects in a remitted state to determine whether higher 5-HT1A BPF is a state or trait effect. Such a potential trait effect on raphe autoreceptors appears related partly to the presence of the higher expressing G allele and future studies need to verify this possibility in a larger sample.
Acknowledgements
We would like to thank the staff of the Brain Imaging Division and Clinical Evaluation Core of the Conte Translational Center for the Neurobiology of Suicidal Behavior, the Columbia Kreitchman PET Center, and the Radioligand Laboratory for expert help. This work was supported by United States Public Health Service grants MH62185 (Conte Center), K08-MH67015 (GMS), and the American Foundation for Suicide Prevention.
Footnotes
Financial Disclosures:
Dr. Sullivan has been on speakers’ bureaus for Pfizer and GSK, owned stock in Pfizer, and has a patent application for use of tianeptine, all unrelated to the current manuscript.
Dr. Oquendo received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to the current manuscript, and was the recipient of a grant from Eli Lilly to support a year of salary for the Lilly Suicide Scholar, Enrique Baca-Garcia, M.D., Ph.D.
Dr. Ogden, Dr. Kumar, Mr. Simpson, and Mr. Huang reported no biomedical financial interests or potential conflicts of interest.
Dr. Mann is principal investigator on PET Imaging grants from GSK and Novartis, unrelated to the current manuscript.
Dr. Parsey has received PET Imaging grants from Novartis Pharmaceuticals, Sepracor, Inc., Pfizer, and Eli Lilly Company, unrelated to the current manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woods SW. The economic burden of bipolar disease. J Clin Psychiatry. 2000;61(Supp 13):38–41. [PubMed] [Google Scholar]
- 3.Goodwin FK, Jamison KR. Manic-depressive illness : bipolar disorders and recurrent depression. 2nd ed. Oxford University Press; Oxford ; New York: 2007. [Google Scholar]
- 4.Marangell LB, Bauer MS, Dennehy EB, Wisniewski SR, Allen MH, Miklowitz DJ, et al. Prospective predictors of suicide and suicide attempts in 1,556 patients with bipolar disorders followed for up to 2 years. Bipolar Disord. 2006;8:566–575. doi: 10.1111/j.1399-5618.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 5.Judd LL, Akiskal HS. Depressive episodes and symptoms dominate the longitudinal course of bipolar disorder. Curr Psychiatry Rep. 2003;5:417–418. doi: 10.1007/s11920-003-0077-2. [DOI] [PubMed] [Google Scholar]
- 6.Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 7.Thase ME. Bipolar depression: diagnostic and treatment considerations. Dev Psychopathol. 2006;18:1213–1230. doi: 10.1017/S0954579406060585. [DOI] [PubMed] [Google Scholar]
- 8.Blier P, Pineyro G, el Mansari M, Bergeron R, de Montigny C. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann N Y Acad Sci. 1998;861:204–216. doi: 10.1111/j.1749-6632.1998.tb10192.x. [DOI] [PubMed] [Google Scholar]
- 9.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 10.Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang YY, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JBW. SCID-I/P, Version 2.0. Biometrics Research, New York State Psychiatric Institute; New York: 1994. Structured clinical interview for DSM-IV Axis I disorders, patient edition. [Google Scholar]
- 13.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 15.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 16.Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1:131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- 17.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 18.Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, et al. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tisssue input functions. J Cereb Blood Flow Metab. 2000;20:1111–1133. doi: 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 21.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 25.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 26.Huang YY, Battistuzzi C, Oquendo MA, Harkavy-Friedman J, Greenhill L, Zalsman G, et al. Human 5-HT1A receptor C(-1019)G polymorphism and psychopathology. Int J Neuropsychopharmacol. 2004;7:441–451. doi: 10.1017/S1461145704004663. [DOI] [PubMed] [Google Scholar]
- 27.Wu S, Comings DE. A common C-1018G polymorphism in the human 5-HT1A receptor gene. Psychiatr Genet. 1999;9:105–106. doi: 10.1097/00041444-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol. 2008;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- 29.Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, et al. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- 30.Rabiner EA, Wilkins MR, Turkheimer F, Gunn RN, Udo de Haes J, de Vries M, et al. 5-Hydroxytryptamine1A receptor occupancy by novel full antagonist 2-[4-[4-(7-chloro-2,3-dihydro-1,4-benzdioxyn-5-yl)-1-piperazinyl]butyl]-1, 2-benzisothiazol-3-(2H)-one-1,1-dioxide: a[11C][O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-py ridinyl)cyclohexanecarboxamide trihydrochloride (WAY-100635) positron emission tomography study in humans. J Pharmacol Exp Ther. 2002;301:1144–1150. doi: 10.1124/jpet.301.3.1144. [DOI] [PubMed] [Google Scholar]
- 31.Ogden RT, Tarpey T. Estimation in regression models with externally estimated parameters. Biostatistics. 2006;7:115–129. doi: 10.1093/biostatistics/kxi044. [DOI] [PubMed] [Google Scholar]
- 32.Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954:173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- 33.Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naudon L, El Yacoubi M, Vaugeois JM, Leroux-Nicollet I, Costentin J. A chronic treatment with fluoxetine decreases 5-HT(1A) receptors labeling in mice selected as a genetic model of helplessness. Brain Res. 2002;936:68–75. doi: 10.1016/s0006-8993(02)02548-9. [DOI] [PubMed] [Google Scholar]
- 36.Raap DK, Evans S, Garcia F, Li Q, Muma NA, Wolf WA, et al. Daily injections of fluoxetine induce dose-dependent desensitization of hypothalamic 5-HT1A receptors: reductions in neuroendocrine responses to 8-OH-DPAT and in levels of Gz and Gi proteins. J Pharmacol Exp Ther. 1999;288:98–106. [PubMed] [Google Scholar]
- 37.Parsey RV, Olvet DM, Oquendo MA, Huang YY, Ogden RT, Mann JJ. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31:1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- 38.Le Francois B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 39.Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–442. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, et al. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- 42.Carrasco GA, Barker SA, Zhang Y, Damjanoska KJ, Sullivan NR, Garcia F, et al. Estrogen treatment increases the levels of regulator of G protein signaling-Z1 in the hypothalamic paraventricular nucleus: possible role in desensitization of 5-hydroxytryptamine1A receptors. Neuroscience. 2004;127:261–267. doi: 10.1016/j.neuroscience.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 43.Flugge G, Pfender D, Rudolph S, Jarry H, Fuchs E. 5HT1A-receptor binding in the brain of cyclic and ovariectomized female rats. J Neuroendocrinol. 1999;11:243–249. doi: 10.1046/j.1365-2826.1999.00317.x. [DOI] [PubMed] [Google Scholar]
- 44.Maswood S, Stewart G, Uphouse L. Gender and estrous cycle effects of the 5-HT1A agonist, 8-OH-DPAT, on hypothalamic serotonin. Pharmacol Biochem Behav. 1995;51:807–813. doi: 10.1016/0091-3057(95)00038-x. [DOI] [PubMed] [Google Scholar]
- 45.Raap DK, DonCarlos L, Garcia F, Muma NA, Wolf WA, Battaglia G, et al. Estrogen desensitizes 5-HT(1A) receptors and reduces levels of G(z), G(i1) and G(i3) proteins in the hypothalamus. Neuropharmacology. 2000;39:1823–1832. doi: 10.1016/s0028-3908(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 46.Schiller L, Jahkel M, Oehler J. The influence of sex and social isolation housing on pre- and postsynaptic 5-HT1A receptors. Brain Res. 2006;1103:76–87. doi: 10.1016/j.brainres.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 47.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- 48.Gelfin Y, Lerer B, Lesch KP, Gorfine M, Allolio B. Complex effects of age and gender on hypothermic, adrenocorticotrophic hormone and cortisol responses to ipsapirone challenge in normal subjects. Psychopharmacology (Berl) 1995;120:356–364. doi: 10.1007/BF02311184. [DOI] [PubMed] [Google Scholar]
- 49.Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, et al. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39:1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 50.Jovanovic H, Cerin A, Karlsson P, Lundberg J, Halldin C, Nordstrom AL. A PET study of 5-HT1A receptors at different phases of the menstrual cycle in women with premenstrual dysphoria. Psychiatry Res. 2006;148:185–193. doi: 10.1016/j.pscychresns.2006.05.002. [DOI] [PubMed] [Google Scholar]


