Abstract
An option for fertility preservation for women facing a cancer diagnosis involves the cryopreservation of ovarian tissue for later re-transplantation or in vitro culture, with in vitro culture preferred to avoid reintroduction of the cancer. Small, immature follicles survive the freeze-thaw process, and can be matured through in follicle maturation (IFM) that involves an initial growth of the follicle and subsequent maturation of the oocyte. The ovarian tissue can be cryopreserved in two forms: (i) cortical strips consisting of follicles and surrounding stroma (Cryo-Ov) or (ii) individually isolated follicles (Cryo-In). The aim of this study was to assess the follicle growth and oocyte maturation for follicles that were cryopreserved either as strips or individually using a slow-freezing cryopreservation method. The two follicle groups, together with non-cryopreserved control follicles, were grown in an alginate-based three-dimensional culture system for twelve days. The overall survival, size increase and antrum formation rates were comparable among the three groups. At day 12 of culture, Androstenedione levels were decreased in the Cryo-Ov group relative to the other two, and the ratio of progesterone to estradiol was increased in the two cryopreserved groups relative to the control. Both Gja1 (known as connexin 43) and Gja4 (known as connexin 37) mRNA expression were decreased at day 6 in the cryopreserved groups relative to controls, and by day 12, Gja1 was similar for all 3 groups. Moreover, Cryo-In resulted in lower GVBD rate indicating some impaired oocyte development. Overall, the present study demonstrated that mouse preantral follicles, either within ovarian tissues or individually isolated, could be successfully cryopreserved by the slow-freezing method, as evidenced by post-thaw follicle development and steroidgenesis, oocyte maturation and molecular markers for oocyte and/or granulosa cells connection.
Introduction
The improved treatments for cancer have allowed more patients to survive their disease and have disease-free lives. However, some of these lifesaving treatments, such as radiation and/or chemotherapy, can either cause acute ovarian failure or a chronic ovarian insufficiency resulting in an early menopause and associated loss of reproductive capacity (Agarwal and Chang 2007; Lee et al. 2006). Ovarian tissue cryopreservation provides an opportunity for long-term preservation of fertility for women of all ages that are having their cancer treated with damaging radiation and/or chemotherapy. Cryopreserved ovarian tissue has been used to produce offspring in mice after orthotopic grafting of frozen-thawed ovaries (Cox et al. 1996; Gunasena et al. 1997; Hani et al. 2006; Kagawa et al. 2007; Migishima et al. 2003). Recently, orthotopic transplantation has been employed with humans, and has led to embryonic development (Oktay et al. 2004) and live births (Demeestere et al. 2006; Demeestere et al. 2007; Donnez et al. 2004; Meirow et al. 2005). Although this approach is promising, it also contains a risk of reintroducing malignant cells into a patient (Shaw et al. 1996).
An alternative to orthotopic grafting involves in follicle maturation (IFM). The follicle is the functional unit within the ovary, and its primary role is to support the oocyte. Follicles at multiple stages of development are present in the adult mouse ovary: primordial follicle (one-layer flat, squamous granulosa cells (GCs), 30-50 μm), primary follicles (one-layer cuboidal GCs, 50-90 μm), secondary follicles (two layers of GCs, basal lamina membrane and laminar theca cells, 100-130μm), preantral follicles (multilayer GCs and theca cells, 150-200 μm), antral follicles (fluid-filled cavity adjacent to the oocyte) and preovulatory follicles (a mature follicle ready to ovulate upon the luteinizing hormone (LH) surge and extrude a fertilizable mature oocyte). Until the preovulatory stage, the follicle contains a primary oocyte that is arrested in prophase of meiosis I. IFM consists of in vitro growth of immature follicle (IVGF) followed by in vitro oocyte maturation (IVM) and in vitro fertilization (IVF). The ovary contains many more immature follicles than mature follicles, and these immature follicles are better able to survive the freeze-thaw process. Successful in vitro growth of cryopreserved follicles is a critical step in adopting novel fertility preservation techniques in the clinical setting. Although primordial follicles can have survival rate of 70-80% following cryopreservation because of their small size and relative quiescence (Gook et al. 1999; Hovatta et al. 1996; Newton et al. 1996), they cannot yet be brought to full maturation in vitro with current culture systems. However, several culture systems have been developed to support preantral follicle growth in vitro to produce meiotically competent oocytes that can be fertilized and produce offspring in mice (Cortvrindt et al. 1996b; Eppig and Schroeder 1989; Lenie et al. 2004; Xu et al. 2006a).
Cryopreservation of ovarian follicles can take two forms: (i) cryopreservation of ovaries that contain enclosed follicles; (ii) individual preantral follicles isolated from ovarian cortex. The extreme conditions that ovaries and follicles encounter during cryopreservation procedures, such as direct chilling injury, low nonphysiological temperatures and concentrated cryoprotectant exposure, can damage the follicle integrity and result in cryoinjury of the follicle after thawing (Vanhoutte et al. 2004). Cryopreservation techniques have been applied to pre-antral follicles. For both cryopreserved ovaries and follicles, in vitro cultured cryopreserved follicles are capable of producing mature oocytes, blastocysts, and in some cases, offspring (Cortvrindt et al. 1996a; dela Pena et al. 2002; Hasegawa et al. 2006; Newton and Illingworth 2001). It is important to point out that the cryopreservation of isolated preantral follicles is different from that of the ovarian tissue fragments, as tissues are more complex structures with a compact stroma tissue and different cell types.
The aim of this study was to investigate IFM for secondary follicles that have been cryopreserved either as individual follicles (Cryo-In), or within an ovary (Cryo-Ov). Non-cryopreserved follicles served as a control, and follicles were cultured within alginate hydrogels for 12 days. Alginate hydrogels are widely used in tissue engineering. Recently we used alginate to create a three-dimensional matrix that maintained follicular cell-cell and cell-matrix connection, and yielded mature oocytes. It is possible that the gap junctions that connect granulosa cells and the oocyte, and among the granulosa cells could be temporarily disrupted by the slow-freezing method. Maintaining both gap junctions between both cells types is critical for proper follicle development and absolutely necessary for oocyte maturation. Given the importance of these connections, the abundance of two major gap junction transcripts, Gja 4 and Gja1, were characterized between cryopreserved and fresh follicle during the in vitro development.
The growth and development of cryopreserved mouse follicles were also characterized by the follicle diameter, steroid production, and antrum formation. Oocyte quality was characterized by the ability to resume meiosis and extrude a polar body in preparation for fertilization. These studies thus identified the survival and potential for growth for early secondary follicles, which could ultimately be applied for fertility preservation in women and girls facing a cancer diagnosis.
Materials and Methods
Animals and Materials
Ovaries were removed from prepubertal, 12-day-old female F1 hybrids (C57BL/6j × CBA/Ca), which has been used for fertility studies by several groups (Liu et al. 2001) (Cortvrindt et al. 1996a). Animals were housed in a temperature- and light-controlled environment (12h light:12h dark) and provided with food and water ad libitum. Animals were fed Teklad Global irradiated 2919 chow, which does not contain soybean or alfalfa meal and therefore contains minimal phytoestrogens. Animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the established IACUC protocol at Northwestern University.
Alginate Hydrogel Preparation
Sodium alginate (55-65% guluronic acid) was provided by FMC BioPolymers (Philadelphia, PA). Alginate was dissolved in deionized water to a concentration of 1% (w/v) and then purified with activated charcoal (0.5g charcoal/ g alginate) to remove organic impurities and improve the purity of the alginate. Following charcoal treatment, alginate solution was sterile filtered through 0.22μM filters, lyophilized within Steriflip conical tubes (Millipore, Billerica, MA) and sterile aliquoted. Aliquots of charcoal-stripped and sterilized sodium alginate were reconstituted with sterile 1×PBS to 0.25% (w/v) alginate.
Cryopreservation
Ovaries and follicles were slowly frozen using a modified method of Newton and Illingworth (Newton and Illingworth 2001). Briefly, the ovary (2-3 mm3) was free from fat tissue and bursa under dissecting microscope before cryopreservation. Two-layered secondary follicles (100-130 μm, type 4) were mechanically isolated from ovaries as described before (West et al. 2007; Xu et al. 2006b). The cryoprotectant was made up of Leibovitz (L15) medium (Invitrogen, Grand island, NY) supplemented with 10% (v/v) heat inactivated fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), 0.1 mol/l sucrose and 1.5 mol/l dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO). Follicles or ovaries were first equilibrated in the cryoprotectant solution for 30 minutes at 4 °C with gentle shaking. After that, either 30 secondary follicles or 2 ovaries were transferred into a 1.8 ml cryovial with 1 ml pre-cold cryoprotectant solution. The vials were cooled in a programmable freezer (Freeze Control CL8800, Cryologic, Australia) as follows: (1) cooled from 4°C at −2°C/min to −9°C; (2) equilibrated for 6 minutes at −9°C; (3) seeded manually; (4) held for 4 minutes at −9°C; (5) cooled at −0.3°C/min to −40°C; (6) plunged into liquid nitrogen and stored for future use. Individual freeze were carried out on three different times for both follicles and ovaries.
Thawing
The vials containing either ovaries or follicles were first left for 30 seconds at room temperature (∼22°C), and then plunged into a 37°C water bath with gentle shaking. In all cases, vials were removed from water bath as soon as they are totally thawed. Dilution of the cryoprotectant was carried out in a stepwise manner that has been validated to minimize the damage of cell function. Briefly, ovaries or follicles were transferred into Petri dishes containing 3ml of L15 supplemented with (1) 1 mol/l DMSO / 0.1 mol/l sucrose /10% FBS for 10 minutes; (2) 0.5 mol/l DMSO / 0.1 mol/l sucrose /10% FBS for 10 minutes; (3) 0.1 mol/l sucrose /10% FBS for 10 minutes; (4) 10%FBS for 5 minutes. All of dilutions were carried out at room temperature. Finally, ovaries or follicles were moved into αMEM (Invitrogen, Carlsbad, CA) containing 1% FBS at 37°C, 5% CO2 for 30 minutes before follicles isolation or encapsulation.
Follicle Isolation, Encapsulation, and Culture
Two-layered secondary follicles (100-130 μm, type 4) were mechanically isolated from either fresh or cryopreserved ovaries by using the same methods as described before (West et al. 2007; Xu et al. 2006b). Briefly, follicles were isolated in L15 media containing 1% FBS under sterile conditions using 28 gauge needles and dissecting microscope with a thermo stage (37°C). Individual follicles (obtained from fresh, cryopreserved ovaries or frozen-thawed follicles) were maintained in αMEM/1% FBS at 37°C, 5% CO2 for 30 minutes before encapsulation. Only those follicles displaying the following characteristics during the 30 minutes pre-incubation period were selected for encapsulation and culture: 1) diameter of 100-130 μm; 2) intact with some attached, fibroblast-like theca cells; 3) a visible, immature oocyte that was round and centrally located within the follicle.
Single follicles were pipetted into the middle of each alginate droplet (∼5 μl) (Fig. 1A) that was on a polypropylene mesh (0.1 mm opening, McMaster-Carr, Atlanta, GA). The mesh was inverted to suspend the droplet over the encapsulation solution (140 mM NaCl / 50mM CaCl2 (Fig. 1B). By quickly shaking the mesh, the alginate bead with follicle dropped into the encapsulation solution (Fig. 1C). Alginate beads were left inside of the encapsulation solution for 2 minutes to cross-link the alginate, and then rinsed in culture media (αMEM, 10 mIU/ml rFSH [A.F. Parlow, NHPP, NIDDK], 3 mg/ml bovine serum albumin [MP Biomedicals, Inc. Ohio], 1 mg/ml bovine fetuin [Sigma-Aldrich, St. Louis, MO], 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium [Sigma-Aldrich, St. Louis, MO]). Alginate beads containing a single follicle were plated one follicle per well in 96-well plates in 100 μl of culture media. Fetuin, dialyzed extensively against embryo culture-grade water and lyophilized, was added to prevent zona pellucida (ZP) hardening. Throughout isolation, encapsulation and plating, follicles were maintained at 37°C and pH 7.
Figure 1.
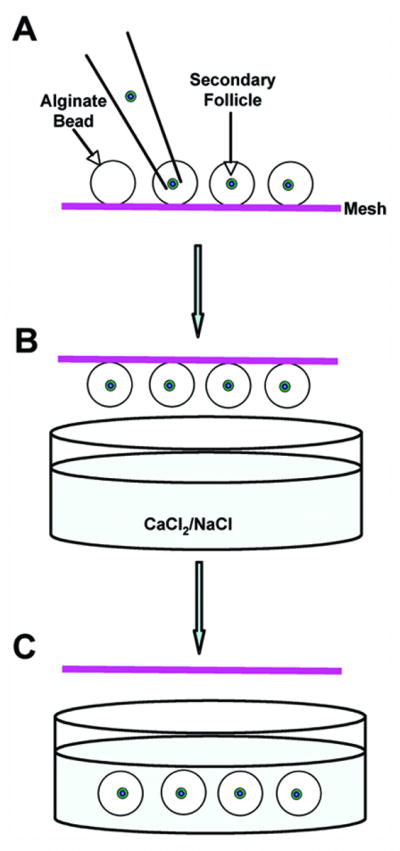
A follicle was transferred into the middle of an alginate droplet (A) that was on a polypropylene mesh. The mesh was inverted over the calcium chloride solution, but not in contact with the solution (B). By shaking the mesh quickly, the alginate bead with follicle was forced to drop into the calcium chloride solution for gelation (C).
Encapsulated follicles were cultured at 37°C in 5% CO2 for 12 days. Every other day, half of the media (50 μl) was exchanged and stored at -80°C for steroid assay. Follicle survival and diameter were assessed using an inverted Leica DM IRB microscope with transmitted light and phase objectives (Leica, Bannockburn, IL). The diameter of follicles containing oocytes was measured in duplicate from the outer layer of theca cells using ImageJ 1.33U (NIH, USA) and based on a calibrated ocular micrometer. Follicles were designated dead if the oocyte was no longer surrounded by a granulosa cell layer or if the granulosa cells had become dark and fragmented and the follicle decreased in size.
Follicle Retrieval
Encapsulated follicles were retrieved from the alginate bead after either 6 days (for RNA extraction) or 12 days (for RNA extraction or oocyte maturation) culture. The culture media was replaced by 100 μl L15 medium containing 10 units/ml alginate lyase (Sigma, St. Louis, MO) for 30 minutes at 37°C. Follicles were removed from the degraded alginate bead and all remaining alginate was removed using a new IVF dish containing L15 medium with 1% FBS. Follicles for RNA isolation were transferred into clean tube with a minimal amount of media, and frozen at -80°C for future use. Follicles for maturation were carried out as followed.
Oocyte Maturation
After 12 days of culture, follicles were transferred to maturation media composed of αMEM, 10% FBS, 1.5 IU/ml human chorionic gonadotropin and 5 ng/ml epidermal growth factor (EGF, Sigma-Aldrich, St. Louis, MO) for 16 hours at 37°C, 5% CO2. Oocytes were then denuded from the surrounding cumulus cells by treating with 0.3% hyaluronidase (Sigma-Aldrich, St. Louis, MO) and gentle aspiration through a polished drawn glass pipette. The oocytes were considered to germinal vesicle breakdown (GVBD) if germinal vesicle was not visible. If a polar body was present in the perivitelline space, the oocytes were classified as metaphase II (MII). Fragmented or shrunken oocytes were classified as degenerated (DG).
Ovary and Follicle Fixation and HE Staining
Fresh ovaries were fixed after they were removed from 12-days old mice whereas the cryopreserved were fixed following the thawing procedures detailed as above. Individual follicle was fixed inside the alginate bead. The fixation was carried out at 4°C for overnight in a solution composed of 4% paraformaldhyde (PFA) (Fisher Sci, Fair Lawn, NJ) with 0.1 M sodium cacodylate (Sigma-Aldrich, St. Louis, MO), 0.1 M sucrose and 10 mM CaCl2, pH=7.4. After fixation, only for follicles within the alginate beads, a 0.5% Alcian Blue/0.25% HAc stain solution was used to stain the bead, which facilitated tracking samples during the paraffin embedding and sectioning process. All samples were dehydrated in ascending concentrations of ethanol (50%-100%), and embedded in paraffin by an automated tissue processor (Leica, Manheim, Germany). Serial 5 μm sections were cut and stained with hematoxylin and eosin.
RNA Isolation and Real-Time PCR
Total RNA was purified from whole follicles by using Stratagene Absolutely RNA Microprep Kit (Cedar Creek, TX) according to the manufacturer's procedure. Total RNA was reverse transcribed into first-strand cDNA (SuperScript First-Stand Kit, Invitrogen, Carlsbad, CA) using random hexamer primers and stored at -20°C. Real-time PCR was used to compare the mRNA expression levels of Gap junction protein, alpha 4 (Gja4, also known as connexin 37) and Gap junction protein alpha 1 (Gja1, also known as connexin 43). Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as an endogenous control. All real-time PCR experiments were performed using Taqman probes. RT reactions run in the absence of reverse transcriptase served as a negative control.
Steroid Assays
Androstenedione, 17β-estradiol and progesterone were measured in conditioned media collected on follicle culture day 12 using commercially available radioimmunoassay kits (androstenedione and 17β-estradiol, Diagnostic Systems Laboratories, INC, Webster, TX; progesterone, Diagnostic Products Corporation, Los Angeles, CA). The condition media obtained from each time point of the specific cultures were pooled together. Four independent measurements for each steroid at each time point were performed. The sensitivities for the androstenedione, estradiol and progesterone assays are 0.1 ng/ml, 10 pg/ml and 0.1 ng/ml, respectively. Media collected from wells containing no follicles was used as the assay control. The hormone assays were performed at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Statistical Analysis
Four independent cryopreservations and cultures of 20-30 follicles each were performed for follicle isolation, size measurement, survival, oocyte maturation and steroid productions. Another three independent cultures were performed to collect follicles for RNA isolation and real-time PCR. Twelve to eighteen follicles were collected from each group at each culture time point (day 6 and day 12). Data were analyzed using a one-way ANOVA followed by a paired t-test. A p-value of less than 0.05 was considered statistically significant. All statistical calculations were done with the software GraphPad Prism version 4.00.
Results
Ovary and Follicle Morphology
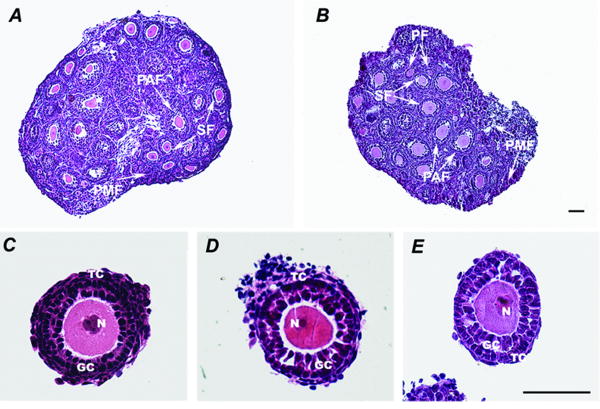
Morphological differences between the fresh and cryopreserved ovaries and follicles were determined by examining the tissue sections obtained. The cryopreserved ovaries (Fig. 2B) appeared healthy and were morphologically similar to the fresh ovaries (Fig. 2A). Cryopreserved ovaries had morphologically normal follicles in different stages, ranging from primordial, primary, and secondary follicles to preantral follicles. Individual follicles (isolated from fresh, cryopreserved ovaries or individual frozen-thawed) maintained their integrity with two intact layers of granulosa cells and a thin layer of theca cells. Oocyte morphology is also quite similar among the three groups of follicles. The oocyte maintains a round shape and a central nucleus.
Figure 2.
The fresh ovary (A) and cryopreserved ovary (B) were morphologically similar. Both of the ovaries contained different class follicles, from primordial, primary, and secondary follicles to preantral follicles. Individual follicles isolated from fresh (C) and cryopreserved ovary (D), or individual frozen-thawed (E) maintained their integrities with two intact layers of granulosa cells, a thin layer of theca cells and a central round oocyte with nucleus. PMF, primordial follicle; PF, primary follicle; SF, secondary follicle; PAF, preantral follicle; GC, granulosa cells; TC, theca cells; N, oocyte nuclear. Bar=100μm.
Folliculogenesis
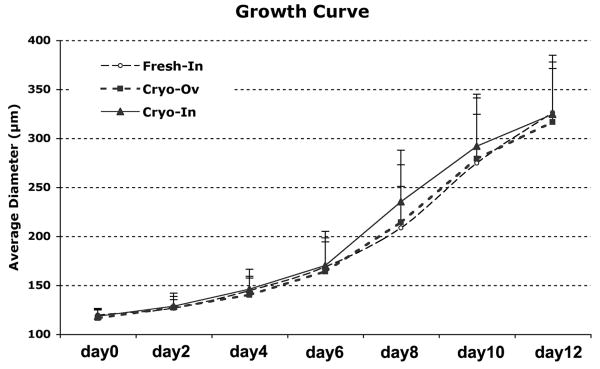
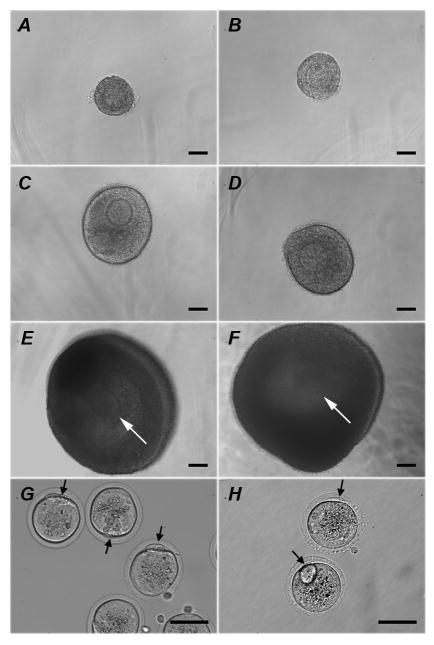
The overall survival, size increase and antrum formation rates among the fresh individual follicles, follicles isolated from cryopreserved ovaries (Cryo-Ov) and cryopreserved individual follicles (Cryo-In) were comparable, which suggested that both cryopreserved follicles could successfully be grown in vitro in an alginate hydrogel (Table 1 and Fig. 3). Both Cryo-Ov and Cryo-In maintained their three-dimentional structures in alginate, developed the laminar-like theca cell layers (Fig. 4C and 4D) at day 6, and formed antral cavities with a cumulus-oocyte-complex (COC) (Fig. 4E and 4F) at day 12.
Table 1.
Survival rates, follicle size measurement and antrum rates for two-layered secondary follicles cultured in alginate scaffold in vitro.
| Groups | N | Survival (%)* | Follicle Diameter (μm)* | Antrum (%) | |
|---|---|---|---|---|---|
| Day0 | Day12 | ||||
| Fresh-In | 96 | 78.0±7.7 | 119.5±6.7 | 326.2±44.8 | 78.9 |
| Cryo-Ov | 92 | 71.7±10.9 | 116.5±8.1 | 316.3±61.3 | 76.9 |
| Cryo-In | 109 | 73.7±13.8 | 118.1±7.0 | 324.2±60.2 | 78.2 |
N = starting follicle number.
Values are the average ± SD of multiple follicles from three or four independent cultures.
Figure 3.
Two-layered secondary follicles from three groups grew continuously during 12 days culture. No statistically significant differences among three groups were observed at each time point.
Figure 4.
Follicles isolated from cryopreserved ovary (Cryo-Ov) (A, C, E) and individually frozen-thawed (Cryo-In) (B, D, F) maintained their 3-dimentional structures in alginate over a 12-day culture period and formed antral cavities with cumulus-oocyte-complex (white arrow) inside. Representative follicle pictures at day 0 (A, B), day 6 (C, D) and day12 (E, F) of culture. Mature oocytes (black arrows indicating first polar body) can be obtained from both groups, Cryo-Ov (G) and Cryo-In (H). Bar=100μm.
Steroid Secretion
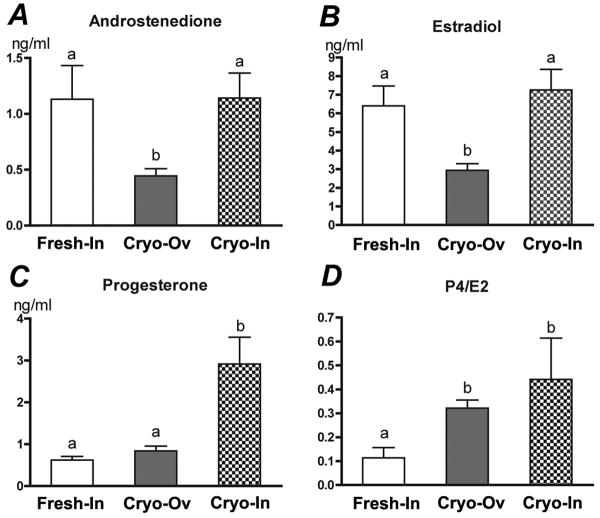
During follicle development, granulosa and theca cells secreted ovarian hormones including androstenedione, estradiol and progesterone. At the end of culture, follicles from Cryo-Ov secreted significantly less androstenedione (Fig. 5A) and estradiol (Fig. 5B), and Cryo-In follicles secreted significantly more progesterone (Fig. 5C). The control follicles, non-cryopreserved, had the lowest ratio of progesterone to estradiol (Fig. 5D).
Figure 5.
Steroid secretion profiles of three groups' follicles on the day 12 of culture. Statistical significance was observed between groups with different letters (p<0.05).
Characterization of Differential Gene Expression
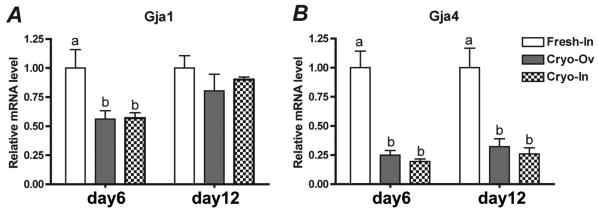
The differential mRNA expression levels of gap junction protein genes (Gja4 and Gja1, known as Connexin 37 and 43) in each group were compared using real-time PCR. Both Gja4 and Gja1 expressions were significantly down-regulated in follicles that have been cryopreserved (Cryo-Ov and Cryo-In) at day 6 of culture (Fig. 6A). Gja4 expression was continuously down-regulated by a factor of four in cryopreserved follicles at day 12 of culture, while Gja1 expression was not significantly different among the three groups (Fig. 6B).
Figure 6.
Relative gene expression levels characterized by real-time PCR. At day 6 of culture, both Gja1 and Gja4 expressions were significantly down-regulated in follicles that have been cryopreserved (Cryo-Ov and Cryo-In) (A). At day12, Gja1 expression was not significant different among three groups, while Gja4 expression was continuously down-regulated in follicles cryopreserved (B). Statistical significance was observed between groups with different letters (p<0.05).
Oocyte Meiotic Competence
After 12 days of culture, follicles were separated from alginate and induced with hCG and EGF for 16 hours. The quality of oocytes obtained from in vitro cultured follicles was measured by their ability to resume meiosis. MII stage oocytes were obtained from both Cryo-Ov (Fig. 4G) and Cyro-In (Fig. 4H). Oocytes from Cryo-In showed the highest GV rate and the lowest GVBD rate. However, no significant differences in the MII oocyte rate were found among the three groups (Table 2).
Table 2.
Meiotic competence of oocytes from two-layered secondary follicles cultured in alginate.
| Groups | N | MII* (n) | GVBD (n) | GV (n) | DG (n) |
|---|---|---|---|---|---|
| Fresh-In | 66 | 59.1% (39) | 83.3% (54) a | 1.5% (1) a | 15.2% (10) a |
| Cryo-Ov | 65 | 68.3% (41) | 92.3% (60) a | 4.6% (3) a | 3.1% (2) b |
| Cryo-In | 77 | 64.1% (34) | 68.8% (53) b | 14.3% (11) b | 16.9% (13) a |
Different letters within each column indicate statistically significant differences (p<0.05). N = surviving follicle number; MII = metaphase II; GVBD = germinal vesicle breakdown; GV = germinal vesicle; DG = degenerate; n=number.
The percent of MII oocytes was calculated as a proportion of oocytes undergoing GVBD.
Discussion
Cryopreservation is an essential step in any protocol in which long-term storage of tissue is required. Yet, tissue injury may result from the use of strong cryoprotectants as well as from the extreme temperatures used to preserve the tissue. Determining the alterations in follicle growth and maturation that result from cryopreservation is critical in developing this innovative technology for use in future fertility preservation options. The studies presented herein investigated the differences for in vitro follicle growth and maturation between fresh and cryopreserved follicles.
Cryopreservation of ovarian follicles can take two forms: either within ovarian tissue or individually isolated follicles. When the morphology of these follicles was compared, the cryopreserved follicles, either isolated from cryopreserved ovaries (Cryo-Ov) or individually cryopreserved (Cryo-In), had similar granulosa cell structure and integrity when compared to the control follicles. Oocyte morphology was also quite similar among the three groups of follicles, which maintained a round shape and a central nucleus (Fig. 2). It was important to notice that some of follicles, either within ovary or isolated, failed to survive through the cryopreservation process and had morphologies consistent with cryo-injury, such as collapsed oocyte, dark granulosa cells (GCs), extruded GCs indicating basement membrane disruption or clear space between oocyte and GCs that indicated complete disconnection between the cells. Between 70-90% preantral follicles in the Cryo-In group survived, having a post-thaw survival rate comparable with previous reports (Carroll and Gosden 1993; Carroll et al. 1990; Cortvrindt et al. 1996a). As for the Cryo-Ov group, some follicles had similar damage as mentioned above; however, it is difficult to distinguish whether follicles were damaged by the cryopreservation or by the mechanical isolating process performed after thawing.
Follicle development in culture was characterized by multiple functional endpoints, such as their survival and antrum formation rate, follicle size increase, hormone secretion pattern, and oocyte maturation rate. The overall survival, size increase (Table 1 and Fig. 3) and antrum formation rates (Table 1 and Fig. 4) among three groups were comparable which suggested that both sources of cryopreserved follicles can successfully be grown in vitro in an alginate hydrogel. These comparable rates of antral cavity formation indicated that the follicles were maturing and developing normally, even the follicles that were exposed to extreme temperatures and harmful cryoprotectant agents. We did not observe a pattern of slow growth with cryopreserved follicles that was mentioned by other investigates (Cortvrindt et al. 1996a). The normal growth rates may result from the three-dimensional culture system that maintains the follicle architecture (West et al. 2007; Xu et al. 2006b).
In vivo, androgen (measured as androstenedione) is secreted by theca cells, and estrogen (measured as estradiol) is produced by granulosa cells. Progesterone is maintained at a low level until after ovulation and corpus luteum formation. Follicle development can be assessed by measuring its ability to maintain steroid production and their proper ratios. In the current study, the fresh and cryopreserved follicles had similarities as well as significant differences. In particular, the Cryo-Ov had significantly decreased androstenedione and estradiol secretion relative to Cryo-In and control follicles. According to the two-cell/two-gonadotropin theory, FSH-stimulated estrogen synthesis in granulosa cells is dependent on a supply of LH-stimulated androgens from theca cells (Hillier et al. 1994). The lower androstenedione and estradiol production might indicate that the theca cells were reduced, possibly as a result of the loss of stroma and theca cells during cryopreservation and/or the isolation process after thawing (Gook et al. 1999; Hreinsson et al. 2003; Oktay et al. 1997). Appropriate granulosa cell differentiation was reflected in the ratio of progesterone and estradiol. At day 12 of culture, the progesterone concentration was increased approximately three-fold in Cryo-In. The ratios of progesterone to estradiol were significantly greater in both cryopreserved follicles (Cryo-Ov and Cryo-In) relative to control, which may suggest premature luteinization is occurring in the cryopreserved follicles.
The successful cryopreservation of secondary follicles requires not only the survival of oocyte and its surrounding somatic cells, but also the maintenance of gap junctions between the granulosa cells and the oocyte, and among the granulosa cells (Eppig 1977). Gja4 (also known as connexin 37) and Gja1 (also known as connexin 43) are two major types of gap junction proteins found within a follicle, with Gja4 acting at heterologous gap junctions between the oocyte and cumulus cells and Gja1 acting within homologous gap junctions among granulosa cells (Gittens et al. 2005; Gittens and Kidder 2005; Veitch et al. 2004). While both gap junctions are critical for proper follicle development, the heterologous junctions are absolutely necessary for oocyte maturation (Gittens and Kidder 2005; Li et al. 2007). Although the Gja1 mRNA abundance was slightly decreased in both cyropreserved preantral follicles at day 6, its expression levels were comparable among three groups at day 12, while the Gja4 expression levels in both cryopreserved preantral follicles were down regulated at day 6, which persisted to day 12. This indicated that the freezing and thawing process resulted in injuries to some oocytes and granulosa cells. Since Gja1 is mainly expressed in granulosa cells, as they proliferate during follicle development, they may compensate for this injury by removing damaged cells and proliferating to produce more healthy granulose cells. However, oocytes may not be able to be rescued, which could result in continuously decreased Gja4 levels during the culture in both cryopreserved follicles. Nonetheless, the mRNA expression changes may not always be correlated to proteins changes, and future studies may need to directly assess these gap junction proteins by immuno-histochemsitry in the future.
The final goal of any fertility preservation is to obtain healthy, mature oocytes. Follicles from Cryo-Ov and Cryo-In had similar rates for survival, growth, antrum formation, and steroid secretion, which may initially suggest that cryopreservation of either intact ovaries or individual follicles produce similar follicles. In the present study, the Cryo-In had a lower GVBD rate, and 14.3% oocyte of this group retained GV stage after hCG stimulation. No differences in oocyte maturation were observed between the control follicles and Cryo-Ov follicles. The greater rates of GBVD for Cryo-OV suggest that the ovary may provide the follicles and oocytes with an environment that limits damage from the harmful cryoprotectants. The same cryoprotectants and equilibration time were applied to both Cyro-Ov and Cyro-In.
These results provide a baseline for translation to human tissue; however, two issues that must be considered include the cryopreservation method and the form of the tissue. Slow freezing systems are automated and provide a reliable and consistent method for cryopreservation. More recently, vitrification has emerged as a promising cryopresrvation technique and does not require specialized equipment; however, the timing of the individual steps must be precise to limit cell death due to the higher concentrations of cryoprotectants that are used with vitrification relative to slow freezing. Additionally, achieving the necessary cooling rates with vitrification can be challenging for tissue relative to individual cells. In regards to the tissue form, we observed that the Cryo-Ov produced 25% greater GVBD stage oocytes relative to Cryo-In. Although the percentage change is substantial, the translation to human follicles must consider several other factors. The efficiency of isolating preantral follicles from cryopreserved human ovarian tissue is low, as most follicles have degenerated (Abir et al. 2001; Hreinsson et al. 2003). In addition, some researchers have also observed the poor survival of the stromal cells (Gook et al. 1999; Hreinsson et al. 2003) isolated from cryopreserved ovarian cortical strips. Lastly, human cells and tissue may have different requirements for cryopreservation components that can penetrate the membranes without reducing cell survival.
In conclusion, this study demonstrated that mouse secondary follicles, either within ovarian tissues or individually isolated, could be successfully cryopreserved by the slow-freezing method. Post-thaw follicles could successfully be grown in vitro in an alginate hydrogel with comparable survival and antral rates compared to fresh follicles, and yielded mature eggs. Individually cryopreserved mouse preantral follicles had some indication of premature luteinization and resulted in lower GVBD rate. The successful cryopreservation of secondary follicles relies on numerous factors, such as the cryoprotectant agents used, freezing protocol and seeding and thawing temperatures. The future optimization of these factors will be needed to fine tune cryoperservation for application for human preantral follicles (Amorim et al. 2003).
Acknowledgments
The authors would like to thank Erin West and Laxmi Kondapalli for editorial assistance. Steroid assays were performed at University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, supported by NIH U54 HD28934. This research was supported by the oncofertility consortium NIH RL1-HD058295 and NIH PL1-EB008542.
Footnotes
This research was supported by the oncofertility consortium: NIH RL1-HD058295 and NIH PL1-EB008542
References
- Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril. 2001;75(1):141–6. doi: 10.1016/s0015-0282(00)01668-x. [DOI] [PubMed] [Google Scholar]
- Agarwal SK, Chang RJ. Fertility management for women with cancer. Cancer Treat Res. 2007;138:15–27. doi: 10.1007/978-0-387-72293-1_2. [DOI] [PubMed] [Google Scholar]
- Amorim CA, Gonçalves PB, Figueiredo JR. Cryopreservation of oocytes from pre-antral follicles. Hum Reprod Update. 2003;9(2):119–29. doi: 10.1093/humupd/dmg014. [DOI] [PubMed] [Google Scholar]
- Carroll J, Gosden RG. Transplantation of frozen-thawed mouse primordial follicles. Hum Reprod. 1993;8(8):1163–7. doi: 10.1093/oxfordjournals.humrep.a138221. [DOI] [PubMed] [Google Scholar]
- Carroll J, Whittingham DG, Wood MJ, Telfer E, Gosden RG. Extra-ovarian production of mature viable mouse oocytes from frozen primary follicles. J Reprod Fertil. 1990;90(1):321–7. doi: 10.1530/jrf.0.0900321. [DOI] [PubMed] [Google Scholar]
- Cortvrindt R, Smitz J, Van Steirteghem AC. A morphological and functional study of the effect of slow freezing followed by complete in-vitro maturation of primary mouse ovarian follicles. Hum Reprod. 1996a;11(12):2648–55. doi: 10.1093/oxfordjournals.humrep.a019187. [DOI] [PubMed] [Google Scholar]
- Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996b;11(12):2656–66. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- Cox SL, Shaw J, Jenkin G. Transplantation of cryopreserved fetal ovarian tissue to adult recipients in mice. J Reprod Fertil. 1996;107(2):315–22. doi: 10.1530/jrf.0.1070315. [DOI] [PubMed] [Google Scholar]
- dela Pena EC, Takahashi Y, Katagiri S, Atabay EC, Nagano M. Birth of pups after transfer of mouse embryos derived from vitrified preantral follicles. Reproduction. 2002;123(4):593–600. doi: 10.1530/rep.0.1230593. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Buxant F, Robin V, Fernandez SA, Centner J, Delbaere A, Englert Y. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod. 2006;21(8):2010–4. doi: 10.1093/humrep/del092. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist. 2007;12(12):1437–42. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Mouse oocyte development in vitro with various culture systems. Dev Biol. 1977;60(2):371–88. doi: 10.1016/0012-1606(77)90135-x. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod. 1989;41(2):268–76. doi: 10.1095/biolreprod41.2.268. [DOI] [PubMed] [Google Scholar]
- Gittens JE, Barr KJ, Vanderhyden BC, Kidder GM. Interplay between paracrine signaling and gap junctional communication in ovarian follicles. Journal of Cell Science. 2005;118(Pt 1):113–22. doi: 10.1242/jcs.01587. [DOI] [PubMed] [Google Scholar]
- Gittens JE, Kidder GM. Differential contributions of connexin37 and connexin43 to oogenesis revealed in chimeric reaggregated mouse ovaries. Journal of Cell Science. 2005;118(Pt 21):5071–8. doi: 10.1242/jcs.02624. [DOI] [PubMed] [Google Scholar]
- Gook DA, Edgar DH, Stern C. Effect of cooling rate and dehydration regimen on the histological appearance of human ovarian cortex following cryopreservation in 1, 2-propanediol. Hum Reprod. 1999;14(8):2061–8. doi: 10.1093/humrep/14.8.2061. [DOI] [PubMed] [Google Scholar]
- Gunasena KT, Villines PM, Critser ES, Critser JK. Live births after autologous transplant of cryopreserved mouse ovaries. Hum Reprod. 1997;12(1):101–6. doi: 10.1093/humrep/12.1.101. [DOI] [PubMed] [Google Scholar]
- Hani T, Tachibe T, Shingai S, Kamada N, Ueda O, Jishage K. Fertility of mice receiving vitrified adult mouse ovaries. Reproduction. 2006;131(4):681–7. doi: 10.1530/rep.1.01030. [DOI] [PubMed] [Google Scholar]
- Hasegawa A, Mochida N, Ogasawara T, Koyama K. Pup birth from mouse oocytes in preantral follicles derived from vitrified and warmed ovaries followed by in vitro growth, in vitro maturation, and in vitro fertilization. Fertil Steril. 2006;86 4:1182–92. doi: 10.1016/j.fertnstert.2005.12.082. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol. 1994;100(1-2):51–4. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, Lass A, Winston RM. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod. 1996;11(6):1268–72. doi: 10.1093/oxfordjournals.humrep.a019370. [DOI] [PubMed] [Google Scholar]
- Hreinsson J, Zhang P, Swahn ML, Hultenby K, Hovatta O. Cryopreservation of follicles in human ovarian cortical tissue. Comparison of serum and human serum albumin in the cryoprotectant solutions. Hum Reprod. 2003;18(11):2420–8. doi: 10.1093/humrep/deg439. [DOI] [PubMed] [Google Scholar]
- Kagawa N, Kuwayama M, Nakata K, Vajta G, Silber S, Manabe N, Kato O. Production of the first offspring from oocytes derived from fresh and cryopreserved pre-antral follicles of adult mice. Reprod Biomed Online. 2007;14(6):693–9. doi: 10.1016/s1472-6483(10)60670-0. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- Lenie S, Cortvrindt R, Adriaenssens T, Smitz J. A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol Reprod. 2004;71(5):1730–8. doi: 10.1095/biolreprod.104.028415. [DOI] [PubMed] [Google Scholar]
- Li T, Colley D, Barr K, Yee S, Kidder G. Rescue of oogenesis in Cx37-null mutant mice by oocyte-specific replacement with Cx43. Journal of Cell Science. 2007;120(23):4117–4125. doi: 10.1242/jcs.03488. [DOI] [PubMed] [Google Scholar]
- Liu J, Van der Elst J, Van den Broecke R, Dhont M. Live offspring by in vitro fertilization of oocytes from cryopreserved primordial mouse follicles after sequential in vivo transplantation and in vitro maturation. Biol Reprod. 2001;64(1):171–8. doi: 10.1095/biolreprod64.1.171. [DOI] [PubMed] [Google Scholar]
- Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353(3):318–21. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- Migishima F, Suzuki-Migishima R, Song SY, Kuramochi T, Azuma S, Nishijima M, Yokoyama M. Successful cryopreservation of mouse ovaries by vitrification. Biol Reprod. 2003;68(3):881–7. doi: 10.1095/biolreprod.102.007948. [DOI] [PubMed] [Google Scholar]
- Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod. 1996;11(7):1487–91. doi: 10.1093/oxfordjournals.humrep.a019423. [DOI] [PubMed] [Google Scholar]
- Newton H, Illingworth P. In-vitro growth of murine pre-antral follicles after isolation from cryopreserved ovarian tissue. Hum Reprod. 2001;16(3):423–9. doi: 10.1093/humrep/16.3.423. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, Opsahl M, Rosenwaks Z. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363(9412):837–40. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- Oktay K, Nugent D, Newton H, Salha O, Chatterjee P, Gosden RG. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil Steril. 1997;67(3):481–6. doi: 10.1016/s0015-0282(97)80073-8. [DOI] [PubMed] [Google Scholar]
- Shaw JM, Bowles J, Koopman P, Wood EC, Trounson AO. Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Hum Reprod. 1996;11(8):1668–73. doi: 10.1093/oxfordjournals.humrep.a019467. [DOI] [PubMed] [Google Scholar]
- Vanhoutte L, Cortvrindt R, Nogueira D, Smitz J. Effects of chilling on structural aspects of early preantral mouse follicles. Biol Reprod. 2004;70(4):1041–8. doi: 10.1095/biolreprod.103.020933. [DOI] [PubMed] [Google Scholar]
- Veitch GI, Gittens JE, Shao Q, Laird DW, Kidder GM. Selective assembly of connexin37 into heterocellular gap junctions at the oocyte/granulosa cell interface. Journal of Cell Science. 2004;117(Pt 13):2699–707. doi: 10.1242/jcs.01124. [DOI] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28(30):4439–48. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006a;12(10):2739–46. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006b;75(6):916–23. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]