Abstract
Background
The significance of troponin elevation and clinical utility of troponin testing in ambulatory patients with coronary artery disease (CAD) have not been examined. We sought to investigate the prevalence and prognostic value of cardiac troponin T (cTnT) elevation in a population with stable CAD.
Methods
We studied 987 patients with stable CAD enrolled in the Heart & Soul study who had plasma cTnT measurements before performing exercise treadmill testing.
Results
Of the studied population, 58 patients or 6.2% had detectable cTnT levels, ≥0.01 ng/mL (0.01–0.72 ng/mL). During a mean follow-up period of 4.3 (0.1–6.5) years, 58.6% of participants with detectable cTnT had cardiovascular events compared with 22.5% of those without detectable cTnT (hazard ratio [HR] 3.8, 95% CI 2.6–5.4, P <.001). This association remained strong after adjustment for traditional risk factors and C-reactive protein (HR 2.0, 95% CI 1.3–3.1, P = .002). However, after further adjustment for N-terminal pro–B-type natriuretic peptide and echocardiographic parameters of left ventricular function, cTnT elevation was not an independent predictor of cardiovascular events (HR 1.3, 95% CI, 0.8–2.3, P = .28).
Conclusions
In ambulatory patients with stable CAD, the prevalence of cTnT elevation was 6.2%. Cardiac troponin T elevation detected using the conventional troponin assay was associated with increased risk of adverse cardiovascular outcomes, but its prognostic value was not incremental over N-terminal pro–B-type natriuretic peptide and echocardiographic evidence of cardiac abnormalities.
In the setting of acute coronary syndrome, cardiac troponins are sensitive and specific diagnostic markers of myocyte injury secondary to coronary artery disease (CAD), and serve as prognostic markers for future adverse events.1–3 In ambulatory settings, the correlates of troponin elevation have been examined. However, the clinical utility of cardiac troponin testing is less clear. In a general population, Wallace et al4 reported cardiac troponin T (cTnT) elevation in 0.7% of the study population. An association of cTnT elevation with high-risk phenotypes of cardiac disease such as the presence of heart failure, left ventricular (LV) hypertrophy, chronic kidney disease, and diabetes was noted. In a similarly unselected population with regard to cardiovascular disease, Zethelius et al5 showed that cardiac troponin I (cTnI) elevation predicts increased rate of first coronary event and mortality independent of conventional risk factors. Although the prevalence of cTnI was higher than that of cTnT elevation in the study by Wallace et al,4 the cause of cTnI elevation was not explored. The significance of elevated troponins in unselected asymptomatic individuals is unknown.
Despite the significant value of troponin elevation for predicting adverse cardiovascular outcomes,6,7 routine clinical application of troponin testing has not been recommended in asymptomatic patients at high risk for cardiac events. Difficulty in interpreting the origin of myocardial injury and difficulty in selecting specific treatment strategies are the main limitations for advocating routine screening. In the setting of stable CAD, however, where the specificity of troponin elevation for myocardial ischemia is higher and effective treatment strategies are available, troponin testing may be justified.
To our knowledge, no large study has examined the prevalence and significance of troponin elevation in ambulatory population with CAD. We sought to investigate the prevalence and prognostic significance of cTnT elevation in a population with stable CAD using data from the Heart and Soul Study to determine if cTnT testing is potentially justified in this clinical setting.
Methods
Participants
The Heart and Soul Study is a prospective cohort study designed to investigate the prognostic impact of psychosocial factors on patients with CAD. Methods have been described previously.8 Participants were recruited from 2 Veterans Affairs Medical Centers (San Francisco and Palo Alto), 1 university-based medical center (University of California, San Francisco), and 9 public health clinics in San Francisco, CA. One of the following criteria must be met to be eligible for study enrollment: (1) history of myocardial infarction (MI), (2) angiographic evidence of ≥50% stenosis in one or more coronary vessels, (3) evidence of exercise-induced ischemia by treadmill or nuclear testing, or (4) history of coronary revascularization.
All eligible patients were mailed an invitation to attend a baseline study appointment at the San Francisco Veterans Affairs Medical Center. Among those who responded and agreed to participate, individuals were excluded if they had a history of MI in the prior 6 months, deemed themselves unable to walk one block or more, or were planning to move out of the local area within 3 years. A total of 1,024 patients enrolled between September 2000 and December 2002. Participants underwent a baseline study appointment that included a medical history interview, a comprehensive health status questionnaire, a physical examination, and both a resting and treadmill stress echocardiogram. Fasting (12-hour) venous samples were drawn, and plasma and serum were frozen at −70°C. Subjects for whom frozen serum was not available (n = 37) were excluded, resulting in a sample size of 987 participants for the analysis.
Patient characteristics
Age, gender, medical history, and smoking history were obtained by questionnaire. We measured height and weight, and calculated body mass index as kilograms per square meter. Participants were instructed to bring their medication bottles to the study appointment, and study personnel recorded all current medications. Medications were categorized using Epocrates Rx (San Mateo, CA).
Measurement
Cardiac troponin T
Before the study appointment, participants completed an overnight fast except for taking their regularly prescribed medications. Before exercise testing, blood samples were drawn into chilled tubes containing EDTA, and plasma was aliquoted and stored at −70°C. We used immunoassay (Elecsys TnT third-generation assay, Roche Diagnostics, Inc, Indianapolis, IN) to measure cTnT in frozen plasma samples thawed to room temperature.9 The lowest measurable troponin T concentration that can be distinguished from zero was 0.01 ng/mL. This is also the 99th percentile upper reference limit for a healthy population. The lowest level at which the interassay coefficient of variation was <10% was 0.03 ng/mL. The laboratory technician who ran the assays was at a different site and blinded to the characteristics of the patients.
Other laboratory measurements
Kidney function was measured using serum creatinine concentration. N-terminal proB-type natriuretic peptide (NT-proBNP) was measured using the Elecsys 2010 proBNP electrochemiluminescence immunoassay (Roche Diagnostics, Indianapolis, IN). High-sensitivity C-reactive protein (CRP) was measured using the Roche Integra assay.
Electrocardiograms
A resting 12-lead electrocardiogram (ECG) was performed on all participants. The final ECG diagnosis was based on the consensus interpretation by 2 independent reviewers, blinded to patient’s clinical information. Abnormal Q waves indicative of prior MI were defined according to the joint European Society of Cardiology and American College of Cardiology committee10; any Q wave in leads V1 to V3 or a Q wave ≥30 milliseconds in leads I, II, aVL, aVF, or V4 to V6; the Q wave must be present in any 2 contiguous leads and ≥1 mm in depth.
Echocardiograms
We performed a complete resting 2-dimensional echocardiogram and Doppler ultrasound examination, including standard 2-dimensional parasternal short-axis, apical 2- and 4-chamber, and subcostal views using the Acuson Sequoia Ultrasound System (Mountain View, CA). Left ventricular end-diastolic and end-systolic volumes were estimated using modified biplane methods of discs. Left ventricular mass was estimated using the truncated ellipsoid method and indexed to body surface area; LV hypertrophy was defined as ≥102 g/m2 in men and ≥88 g/m2 in women.11 We defined diastolic dysfunction by peak early diastolic filling velocity (E velocity), peak filling velocity at atrial contraction (A velocity), and pattern of pulmonary venous flow: (1) impaired relaxation = E/A ratio <1.0 and systolic-dominant pulmonary vein flow, (2) pseudonormal = E/A ratio ≥1 and <2 and diastolic-dominant pulmonary vein flow; (3) restrictive filling = E/A ratio ≥2 and diastolic-dominant pulmonary vein flow. All echocardiograms were reviewed by one cardiologist (NBS).
Exercise test
In the baseline study, treadmill exercise testing was performed with continuous 12-lead ECG monitoring according to 1 of 3 protocols: standard Bruce, modified Bruce, or manually modified protocol depending on the test supervisor’s impression of the subject’s functional capacity. Participants were encouraged to exercise for as long as possible. An echocardiogram was performed immediately before and after exercise. Inducible ischemia was defined as the presence of more than one new wall motion abnormality at peak exercise. One investigator (NBS) interpreted the stress echocardiograms, blinded to the results of the cTnT assay and the clinical history. Nine hundred two patients underwent the stress testing; 86 patients did not perform the exercise for orthopedic or other mechanical reasons.
Outcomes
Annual telephone interviews were conducted with participants or their proxy to inquire about interval hospitalization or death. For any reported event, medical records, ECGs, death certificates, autopsy, and coroner’s reports were obtained. Each event was adjudicated by 2 independent and blinded reviewers. In the event of disagreement, the adjudicators conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator.
The primary end point was adverse cardiovascular events, defined as the composite of first hospitalization since the baseline study appointment for MI, congestive heart failure (CHF), severe arrhythmias, transient ischemic attack (TIA), stroke, or cardiovascular death (due to MI, congestive heart failure, coronary bypass surgery, percutaneous revascularization, severe arrhythmias, or sudden death). Myocardial infarction was defined using standard diagnostic criteria set by the joint European Society of Cardiology and American College of Cardiology committee.10
Analysis
We used SPSS (version 12.0; SPSS, Chicago, IL) for all statistical analysis. The study population was first divided into 2 groups based on detectable cTnT (≥0.01 ng/mL) or undetectable cTnT (<0.01 ng/mL). We compared the frequency of baseline clinical characteristics and outcome variables between the 2 groups (detectable cTnT vs undetectable cTnT) using 2 or Fisher exact tests for dichotomous variables, t test for normally distributed continuous variables, and Mann-Whitney U test for nonparametric variables. Values for normally distributed variables were expressed as mean ± SD and nonparametric variables as median ± interquartile range.
To examine the prognostic value of troponin for adverse cardiovascular outcome, we evaluated troponin as a dichotomous variable (detectable vs undetectable) in an unadjusted Cox proportional hazards regression model, followed by a multivariate Cox regression model adjusted for all baseline clinical and echocardiographic variables in a stepwise fashion. The discriminative ability of models with and without cTnT testing for adverse cardiovascular events was evaluated by plotting receiver operating characteristic (ROC) curves.
Funding sources
This study was supported by the Department of Veterans Affairs (Epidemiology Merit Review Program), the National Heart, Lung and Blood Institute [R01 HL079235], the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), the Nancy Kirwan Heart Research Fund, and a loan of equipment from Siemens Corporation (Mountain View, CA). The cTnT assays were funded by Roche Diagnostics Corporation, Indianapolis, IN. The authors are solely responsible for the design and conduct of the study, all study analyses, and the drafting and editing of the paper and its final contents.
Results
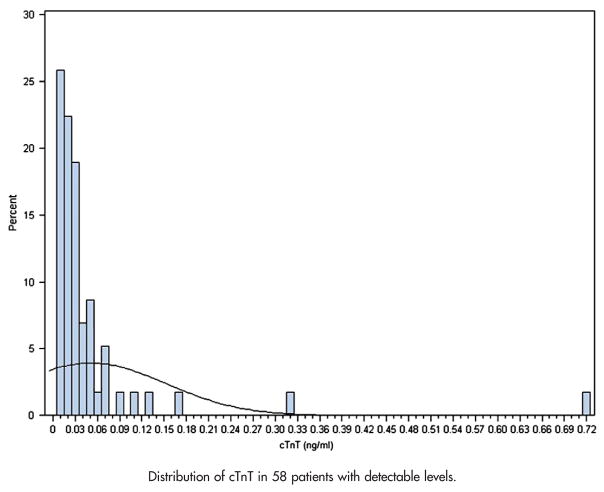
We found that in our study population of patients with stable CAD, the prevalence of any cTnT elevation was 6.2% (58/987). The level of elevation ranged from 0.01 to 0.72 ng/mL (Figure 1). Among the patients with cTnT elevation, 23 patients, or 2.3%, of all studied patients had levels >0.03 ng/mL. The cTnT levels for the remaining 35 patients, or 3.5%, of the studied population of 987 were between 0.01 and 0.03 ng/mL. Of the 987 study participants, 978 (>99%) were followed for a mean of 4.3 years (range 0.1–6.5 years).
Figure 1.
Distribution of cTnT in 58 patients with detectable levels.
Thirty-three baseline clinical and echocardiographic variables were examined for differences between those with and without detectable cTnT (Tables I and II). The group with detectable cTnT was found to be older, had slightly lower body mass index, and had a higher prevalence of diabetes and heart failure. On laboratory tests, those with cTnT elevation had higher NT-proBNP, CRP, and serum creatinine compared to those without elevation. On the echocardiogram, the LV ejection fraction was lower, and the LV mass, LV end-diastolic, end-systolic volumes, and pulmonary arterial systolic pressure were higher. Moderate to severe diastolic dysfunction and resting wall motion abnormalities were more prevalent in those with cTnT elevation. On treadmill exercise testing, the group with cTnT elevation had lower exercise tolerance and was more likely to develop exercise-induced ischemia by stress echocardiography compared to the group with undetectable cTnT (Table II). Among the 16 variables with values that differ significantly between the 2 cTnT groups, NT-proBNP levels showed the largest difference between the 2 groups. There was a 7-fold higher median level in the group with detectable cTnT than the level in the undetectable group (Table I). The prevalence of elevated NT-proBNP levels defined as levels equal or above the median level for the entire study cohort (175 pg/mL) was 2-fold higher among the subjects with detectable cTnT as compared to those without detectable cTnT (89.7% vs 47.5%).
Table I.
Baseline clinical characteristics of participants with detectable and undetectable cTnT
| Undetectable cTnTb 0.01 ng/mL (n = 929) | Detectable cTnT =0.01 ng/mL (n = 58) | p | |
|---|---|---|---|
| Demographics | |||
| Age, y (mean ± SD) | 66 ± 11 | 70 ± 12 | .01 |
| Male sex, n (%) | 753/929 (81.0) | 51/58 (87.9) | .19 |
| Body mass index (mean ± SD) | 29 ± 5 | 27 ± 5 | .03 |
| White, n (%) | 566/928 (61.0) | 29/58 (50.0) | .10 |
| CHD risk factors | |||
| Hypertension, n (%) | 649/927 (70%) | 46/58 (79.3%) | .13 |
| Systolic blood pressure, mm Hg (mean ± SD) | 133 ± 21 | 138 ± 22 | .09 |
| Diastolic blood pressure, mm Hg (mean ± SD) | 75 ± 11 | 75 ± 12 | .73 |
| Hypercholesterolemia, n (%) | 617/923 (66.8) | 37/58 (63.8) | .70 |
| Diabetes, n (%) | 227/927 (24.5) | 32/58 (55.2) | <.001 |
| Current smoking, n (%) | 188/927 (20.3) | 8/58 (13.8) | .23 |
| Medication use | |||
| β-Blocker, n (%) | 534 (57.5) | 33 (56.9) | .93 |
| ACEI/ARB, n (%) | 468 (50.4) | 37 (63.8) | .05 |
| Aspirin, n (%) | 723 (77.8) | 39 (67.2) | .06 |
| Statins, n (%) | 595 (64.1) | 38 (65.5) | .82 |
| Previous cardiovascular events | |||
| MI, n (%) | 493/924 (53.4) | 34/57 (59.6) | .36 |
| Stroke, n (%) | 127/926 (13.7) | 13/58 (22.4) | .07 |
| Revascularization, n (%) | 543/927 (58.6) | 3/58 (60.3) | .79 |
| Angina frequency, one or more angina a week, n (%) | 170/927 (18.4) | 9/58 (15.5) | .35 |
| Heart failure, n (%) | 152 (16.5) | 21 (36.2) | <.001 |
| Laboratory tests | |||
| NT-proBNP, pg/mL (median ± IQR) | 161 ± 324 | 1098 ± 2407 | <.001 |
| CRP, mg/dL (median ± IQR) | 2.1 ± 3.9 | 3.1 ± 7.0 | .002 |
| Serum creatinine, mg/dL (mean ± SD) | 1.07 ± 0.34 | 2.30 ± 0.95 | <.001 |
| ECGs | |||
| Pathologic Q waves on ECG | 172/913 (18.8) | 14/57 (24.6) | .29 |
| Resting heart rate (mean ± SD) | 68 ± 13 | 68 ± 12 | .72 |
CHD, coronary heart disease; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; IQR, interquartile range.
Table II.
Comparison of the resting and exercise echocardiographic characteristics of the groups with detectable cTnT and undetectable cTnT
| Undetectable cTnTb 0.01 ng/mL (n = 929) | Detectable cTnT =0.01 ng/mL (n = 58) | P | |
|---|---|---|---|
| LV ejection fraction, % (mean ± SD) | 62 ± 10 | 56 ± 11 | <.001 |
| Moderate to severe diastolic dysfunction, n (%) | 89/873 (10.2) | 13/52 (25.0) | <.001 |
| LV mass index, g/m2 (mean ± SD) | 97 ± 25 | 133 ± 35 | <.001 |
| LV end-diastolic volume index, mL/m2 (mean ± SD) | 51 ± 18 | 64 ± 25 | <.001 |
| LV end-systolic volume index, mL/m2 (mean ± SD) | 20 ± 13 | 30 ± 19 | <.001 |
| Pulmonary arterial systolic pressure, mm Hg (mean ± SD) | 27 ± 13 | 31 ± 14 | .03 |
| Resting wall motion abnormalities, n (%) | 274/923 (29.7) | 30/56 (53.6) | <.001 |
| Exercise inducible ischemia, n (%) | 199 (23.3) | 18 (37.5) | .03 |
| Exercise capacity (METs) (mean ± SD) | 7 ± 3 | 5 ± 2 | <.001 |
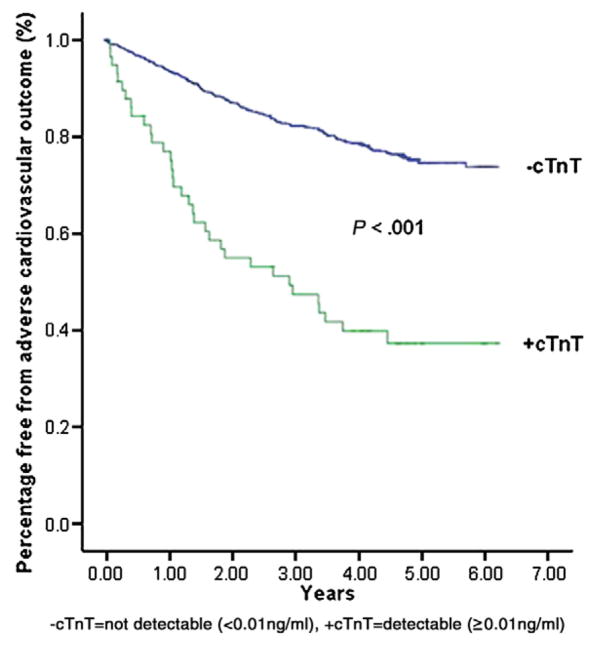
During the average 4.3 years of follow-up, a greater percentage of participants with detectable cTnT achieved the primary end point, adverse cardiovascular events, than those without detectable cTnT (58.6.% vs 22.5%, P <.001) (Figure 2). Among events that occurred with higher frequency in the group with elevated cTnT were hospitalizations for congestive heart failure, MI, and severe dysrhythmias (Table III). In univariate Cox proportional hazards regression analysis, detectable cTnT elevation (>0.01 ng/mL) significantly predicted the primary end point of MI, heart failure, severe arrhythmia, stroke, transient ischemic attack, or cardiovascular death (hazard ratio [HR] 3.8, 95% CI 2.6–5.4, P <.001). After excluding patients with renal insufficiency (serum creatinine ≥1.2 mg/dL), a previous history of heart failure, current echocardiography showing a LV ejection fraction <55%, and pseudonormalized or restrictive diastolic filling patterns, subjects with cTnT elevation (n = 6) were significantly more likely to experience adverse cardiac events than those without cTnT elevation (n = 431) (HR 5.2, 95% CI 1.6–16.7, P = .006).
Figure 2.
Kaplan-Meier survival curves showing cumulative percentage free from adverse cardiovascular events.
Table III.
Proportion of patients with cardiovascular events
| Undetectable cTnT (n = 920) | Detectable cTnT (n = 58) | P | |
|---|---|---|---|
| Surgical or percutaneous revascularization, n (%) | 127 (13.8) | 12 (20.7) | .15 |
| CHF hospitalization, n (%) | 100 (10.9) | 26 (44.8) | <.001 |
| Acute MI, n (%) | 82 (8.9) | 13 (22.4) | <.001 |
| Severe dysrhythmias, with or without defibrillation, n (%) | 67 (7.3) | 10 (17.2) | .006 |
| Stroke or TIA, n (%) | 36 (3.9) | 4 (6.9) | .27 |
| Cardiovascular deaths, n (%) | 39 (4.2) | 12 (20.7) | <.001 |
| Adverse cardiovascular events*, n (%) | 207 (22.5) | 34 (58.6) | <.001 |
| All cause deaths, n (%) | 164 (17.7) | 32 (55.2) | <.001 |
The composite of first hospitalization since the baseline study appointment for MI, congestive heart failure, severe arrhythmias, transient ischemic attack, stroke, or cardiovascular deaths.
In multivariate Cox regression analysis, adjustment was made for serum creatinine concentration, baseline clinical characteristics, and CRP; cTnT elevation remained predictive of adverse cardiovascular events (HR 2.0, 95% CI 1.3–3.1, P = .0002). However, after additional adjustment for NT-proBNP and echocardio-graphic variables, cTnT elevation was not independently predictive of events (HR 1.3, 95% CI 0.8–2.3, P = .28) (Table IV). In ROC analysis, the addition of cTnT to the clinical model incorporating all variables in Tables I and II did not change the discriminative ability for adverse cardiac events (c statistics for the model without cTnT = 0.7938, with cTnT = 0.7940).
Table IV.
Unadjusted and adjusted HRs of cTnT elevation for predicting adverse cardiovascular events
| Adverse cardiovascular event |
||
|---|---|---|
| HR (95% CI) | P | |
| Unadjusted | 3.8 (2.6–5.4) | <.001 |
| Model 1: cTnT and serum creatinine | 3.2 (2.1–4.9) | <.001 |
| Model 2: model 1 and traditional risk factors* | 2.1 (1.4–3.3) | .008 |
| Model 3: model 2 and CRP | 2.0 (1.3–3.1) | .002 |
| Model 4: model 3 and NT-pro BNP | 1.4 (0.9–2.2) | .15 |
| Model 5: model 3 and all Table II variables | 1.4 (0.7–2.8) | .32 |
| Model 6: model 4 and all Table II variables | 1.3 (0.8–2.3) | .28 |
All variables in Table I except NT-proBNP, CRP, and creatinine.
Discussion
In an ambulatory population of patients with stable CAD, we found that the prevalence of cTnT elevation was 6.2%. Detectable cTnT was associated with an increased risk of cardiovascular events, independent of creatinine, traditional cardiovascular risk factors, and CRP. However, the predictive value of cTnT elevation was not independent of NT-proBNP or echocardiographic measures of cardiac structure and function.
Prevalence of troponin elevation in stable CAD
The prevalence of cTnI in an ambulatory CAD population has not been studied previously. Given that our study cohort was composed of a heterogeneous group with stable CAD and a spectrum of risk profiles that are commonly encountered in general outpatient cardiology, the 6.2% prevalence of cTnT elevation is likely representative of most CAD outpatients in a predominantly male population. In contrast, the detection of cTnT in the general population with an overall low risk profile was found to be 0.7% in the Dallas Heart Study.4 On the other hand, among the patients enrolled in the Val-HeFT trial with stable, symptomatic heart failure, predominantly secondary to myocardial ischemia, cTnT elevation was detected in 10.4% of the studied population.6 Given the significant discordance between cTnT and cTnI levels, as demonstrated by a FRISC II substudy12 and the ADHERE registry,13 we could not extrapolate the prevalence of cTnI in the setting of stable CAD based on our finding on cTnT. However, the 27% prevalence rate of cTnI elevation reported by Zethelius et al5 in a community-based study with an overall lower risk profile of CHD than our study cohort suggests that the prevalence of cTnI elevation in an ambulatory population with stable CAD may be even higher.
Correlates of cTnT elevations in stable CAD
Consistent with the findings of Wallace et al4 in the Dallas Heart Study, our study showed that elevated levels of NT-proBNP, serum creatinine levels, increased LV mass index, and diabetes were all associated with cTnT elevation in an ambulatory population of CAD. Furthermore, it appears that most variables that were associated with cTnT elevation were indicators of cardiac functional and structural abnormalities; prior history of heart failure, NT-proBNP level, reduced LV ejection fraction, increased LV end-diastolic, end-systolic volumes and PASP, significant diastolic dysfunction, regional wall motion abnormalities. The overwhelming presence of elevated NT-proBNP levels among the elevated cTnT patients suggested that cTnT elevation in the setting of stable CAD is likely to be predominantly mediated by elevated LV filling pressure and increased LV wall stress.14–16
In addition to being a marker of high risk profile for CAD and cardiac structural and functional abnormalities,17 renal failure has also been found to cause elevated troponins secondary to impaired renal clearance of troponin fragments and loss of membrane integrity with leakage of free cytosolic troponins.18 Although we could not determine the exact role of renal insufficiency in contributing to cTnT elevation in our study cohort, the association between cTnT and cardiovascular events was independent of serum creatinine.
Prognostic value of cTnT elevation in stable CAD
Elevation of troponins has been shown to portend increased mortality and adverse outcomes in various clinical settings other than acute coronary syndrome, such as acute and stable heart failure, and ambulatory patients with end-stage renal failure.6,7,13 The prognostic value of cTnT elevation in these settings was independent of clinical risk factors, suggesting detection of subclinical myocyte injury that could not be identified by traditional scheme of risk prediction. Among an ambulatory, heterogeneous population composed of 31% of patients with coronary heart disease, Zethelius et al reported independent prognostic value of cTnI elevation in univariate and multivariate analyses adjusted for selected clinical characteristics, smoking, total and high-density lipoprotein cholesterol, systolic blood pressure, body mass index, and fasting gluocse.5
Our study differed significantly from previous studies in that we recruited only those with stable CAD and included other biomarkers and echocardiographic parameters of cardiac function and structure in the multivariate regression analysis. We found that cTnT elevation predicts adverse cardiovascular outcome independent of traditional baseline clinical risk factors but not markers of cardiac functional and structural abnormalities. Based on the observed strong association, between NT-proBNP levels and cTnT elevation at baseline, the attenuated prognostic value of cTnT elevation by NT-proBNP levels and echocardiographic parameters of abnormal cardiac function and structure is likely to be real and unlikely to be a false negative (type II) error due to overadjustment of covariaties in the context of a relatively small number of detectable cTnT. With the advent of newer generations of cTnT assays, such as the highly sensitive cTnT assay (ECLIA; Elecsys 2010 analyzer, Roche Diagnostics, Germany), it remains to be seen if cTnT detected by such assays provides incremental prognostic value over other markers of cardiac structural and functional abnormalities.
Several limitations must be considered when interpreting our results. First, the male predominant nature of our population raises a question about the validity of generalizing our results to a more female predominant population. The issue of how gender composition affects the prevalence of cTnT elevation in an ambulatory setting needs further elucidation. Second, false-positive cTnT elevation can occur secondary to assay imprecision, antibody interference, or skeletal muscle troponin detection such as in rhabdomyolysis or polymyositis. However, the reported frequencies of false-positive cTnT elevations are low and insignificant.19
Conclusions
We found that the prevalence of cTnT elevation in an ambulatory population with stable CAD was 6.2%. Cardiac troponin T elevation predicted adverse cardiovascular outcomes, independent of traditional risk factors and CRP. However, the association between cTnT and cardiovascular events was explained by other measures of cardiac functional and structural abnormalities. Therefore, routine cTnT testing does not provide incremental prognostic value over other clinical and biochemical biomarkers.
References
- 1.Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342–9. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 3.Goodman SG, Steg PG, Eagle KA, et al. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: lessons from the Global Registry of Acute Coronary Events (GRACE) Am Heart J. 2006;151:654–60. doi: 10.1016/j.ahj.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Wallace TW, Abdullah SM, Drazner MH, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113:1958–65. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 5.Zethelius B, Johnston N, Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: a community-based cohort study. Circulation. 2006;113:1071–8. doi: 10.1161/CIRCULATIONAHA.105.570762. [DOI] [PubMed] [Google Scholar]
- 6.Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–9. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 7.Khan NA, Hemmelgarn BR, Tonelli M, et al. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation. 2005;112:3088–96. doi: 10.1161/CIRCULATIONAHA.105.560128. [DOI] [PubMed] [Google Scholar]
- 8.Bibbins-Domingo K, Gupta R, Na B, et al. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. Jama. 2007;297:169–76. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Troponin T STAT (short turn around time), cardiac T. Elecsys (package insert) 2004;9:1–5. [Google Scholar]
- 10.Alpert JS, Thygesen K, Antman E, et al. Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 11.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 12.Venge P, Lagerqvist B, Diderholm E, et al. Clinical performance of three cardiac troponin assays in patients with unstable coronary artery disease (a FRISC II substudy) Am J Cardiol. 2002;89:1035–41. doi: 10.1016/s0002-9149(02)02271-3. [DOI] [PubMed] [Google Scholar]
- 13.Peacock WFt, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–26. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 14.Missov E, Calzolari C, Pau B. Circulating cardiac troponin I in severe congestive heart failure. Circulation. 1997;96:2953–8. doi: 10.1161/01.cir.96.9.2953. [DOI] [PubMed] [Google Scholar]
- 15.La Vecchia L, Mezzena G, Ometto R, et al. Detectable serum troponin I in patients with heart failure of nonmyocardial ischemic origin. Am J Cardiol. 1997;80:88–90. [PubMed] [Google Scholar]
- 16.Logeart D, Beyne P, Cusson C, et al. Evidence of cardiac myolysis in severe nonischemic heart failure and the potential role of increased wall strain. Am Heart J. 2001;141:247–53. doi: 10.1067/mhj.2001.111767. [DOI] [PubMed] [Google Scholar]
- 17.Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol. 2002;13:1918–27. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 18.Diris JH, Hackeng CM, Kooman JP, et al. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation. 2004;109:23–5. doi: 10.1161/01.CIR.0000109483.45211.8F. [DOI] [PubMed] [Google Scholar]
- 19.Apple FS, Parvin CA, Buechler KF, et al. Validation of the 99th percentile cutoff independent of assay imprecision (CV) for cardiac troponin monitoring for ruling out myocardial infarction. Clin Chem. 2005;51:2198–200. doi: 10.1373/clinchem.2005.052886. [DOI] [PubMed] [Google Scholar]