Abstract
Mammals, including humans, show sex differences in juvenile play behavior. In rodents and nonhuman primates, these behavioral sex differences result, in part, from sex differences in androgens during early development. Girls exposed to high levels of androgen prenatally, because of the genetic disorder congenital adrenal hyperplasia, show increased male-typical play, suggesting similar hormonal influences on human development, at least in females. Here, we report that fetal testosterone measured from amniotic fluid relates positively to male-typical scores on a standardized questionnaire measure of sex-typical play in both boys and girls. These results show, for the first time, a link between fetal testosterone and the development of sex-typical play in children from the general population, and are the first data linking high levels of prenatal testosterone to increased male-typical play behavior in boys.
Sexual differentiation of the mammalian brain occurs under the control of gonadal hormones, particularly androgens, during early development (De Vries & Simerly, 2002; Ehrhardt & Meyer-Bahlburg, 1981; Goy & McEwen, 1980). Manipulating androgens prenatally or neonatally permanently alters brain regions and behaviors that show sex differences (De Vries & Simerly, 2002; Goy & McEwen, 1980; Hines, 2004). For instance, in rodents and nonhuman primates, treating developing females with testosterone or other androgens increases male-typical play, whereas reducing androgens in developing males reduces it (Goy & McEwen, 1980; Hines, 2004). Androgen exerts similar effects on sex-typed reproductive behaviors and on neural sex differences (De Vries & Simerly, 2002).
In humans, sex differences in toy preferences have been observed in children as young as 12 months of age (Servin, Bohlin, & Berlin, 1999; Snow, Jacklin, & Maccoby, 1983), and these differences, along with sex differences in playmate and activity preferences, grow larger as children progress into middle childhood (Golombok & Hines, 2002). The strongest evidence that androgens influence human sexual differentiation comes from studies of play behavior in girls exposed to abnormally high levels of androgens because of congenital adrenal hyperplasia (CAH), a genetic disorder that causes excess adrenal androgen production beginning prenatally (New, 1998). Several research groups have reported that girls with CAH show increased male-typical toy, playmate, and activity preferences (Ehrhardt & Meyer-Bahlburg, 1981; Hines, 2003, 2004; Pasterski et al., 2005). Because girls with CAH are treated postnatally to normalize hormones, this behavioral masculinization is thought to result from prenatal androgen exposure. However, CAH-related disease characteristics, rather than prenatal androgen exposure, could be responsible (Fausto-Sterling, 1992; Quadagno, Briscoe, & Quadagno, 1977).
Studies relating prenatal testosterone to play behavior in typically developing children have produced mixed results. One study, based on a large population sample, reported a positive relationship between maternal testosterone during pregnancy and male-typical play in girls, but not boys (Hines, Golombok, Rust, Johnston, & Golding, 2002). However, it has been suggested that these results could reflect mothers with high testosterone encouraging more male-typical play in their daughters, rather than an effect of testosterone on the developing brain (Cohen-Bendahan, van de Beek, & Berenbaum, 2005). Also, two studies have found no relationship between testosterone measured in amniotic fluid and subsequent sex-typical play (Knickmeyer et al., 2005; van de Beek, van Goozen, Buitelaar, & Cohen-Kettenis, 2008). The first study found no relationship between testosterone and maternal reports of childhood sex-typed activities for 22 girls and 31 boys (age range = 4–6 years), and the second found no relationship between testosterone and observed toy choices in 63 girls and 63 boys (age 13 months). These negative results could reflect small samples or insufficiently sensitive behavioral measures.
Direct measurement of fetal testosterone and of childhood sex-typed behavior in a large sample of girls and boys, using a sensitive, reliable, standardized measure, could clarify the role of testosterone in human sexual differentiation. In the human fetus, testosterone enters the amniotic fluid via diffusion through the fetal skin, and later via fetal urination (Robinson, Judd, Young, Jones, & Yen, 1977). Testosterone measured in amniotic fluid shows variability in both sexes, but is higher on average in male than in female fetuses (Martin, 1985). In the present study, fetal testosterone was measured in amniotic fluid from 212 pregnant women and related to subsequent sex-typed behavior assessed using a standardized measure, designed specifically to detect differences in sex-typed behavior within each sex.
METHOD
Participants
Participants were recruited from a longitudinal study of the effects of fetal testosterone on child development. All mothers had undergone routine amniocentesis in the Cambridge, United Kingdom, region and given birth to healthy singleton infants (Baron-Cohen, Lutchmaya, & Knickmeyer, 2004). Materials for the present study were sent to all 452 available mothers, who were asked to complete a questionnaire about their child’s activities and interests. Complete information was obtained for 112 male and 100 female offspring (mean age = 8.59 years, SD = 0.97 years, range = 6.38–10.30 years). No significant differences were observed for the predictor or control variables between the larger and current sample in this study.
Measures
Outcome Variable
The Pre-School Activities Inventory (PSAI) is a psychometric scale, with established validity and reliability, developed specifically to assess variability in sex-typical behavior within each sex (Golombok & Rust, 1993a, 1993b). It includes 24 items and is completed by a parent to describe the child’s behavior. Higher scores reflect more male-typical behavior, and females with CAH obtain elevated (more male-typical) scores on the PSAI in comparison to unaffected female relatives (Hines, Brook, & Conway, 2004), suggesting sensitivity to the effects of prenatal androgen exposure.
Predictor Variable
Amniotic fluid samples were collected between weeks 11 and 21 of gestation (M = 16.31, SD = 1.88). This timing coincides with the hypothesized critical period for human sexual differentiation, which is thought to occur between approximately weeks 8 and 24 of gestation (Hines, 2004). Fetal testosterone was measured in amniotic fluid via radioimmunoassay with ether extraction using the DPC “Count a Coat” method (Diagnostic Products Corporation, Los Angeles, CA), which uses an antibody to testosterone coated onto propylene tubes and a 125-I labeled testosterone analogue. The detection limit of the assay using the ether-extraction method is approximately 0.1 nmol/L. The coefficient of variation (CV) for between-batch imprecision is 19% at a concentration of 0.8 nmol/L and 9.5% at a concentration of 7.3 nmol/L. The CVs for within-batch imprecision are 15% at a concentration of 0.3 nmol/L and 5.9% at a concentration of 2.5 nmol/L. This method measures total extractable testosterone.
Control Variables
Gestational age at amniocentesis, maternal age, maternal education, and child’s age at PSAI assessment were included for control purposes. Gestational age and child’s age were assessed from medical records. Maternal age and education were assessed by self-report, with education rated on a 5-point scale from 1 (no formal qualifications) to 5 (postgraduate qualification).
RESULTS
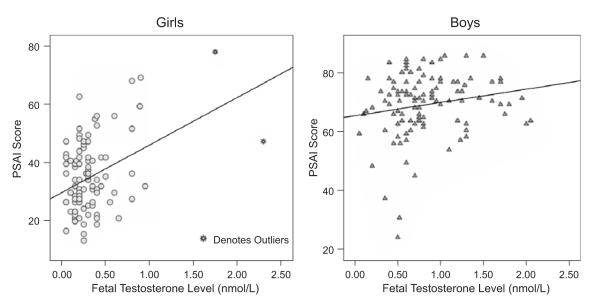
There were two female outlier values for fetal testosterone, but no male outliers (see Fig. 1). Because of the outliers, analyses involving testosterone in females were first conducted by using the full data set and then repeated excluding the outliers. No outliers were observed for PSAI scores or control variables. All variables had acceptable skewness statistics (<1.0).
Fig. 1.
Scatter plot showing the relation between Pre-School Activities Inventory (PSAI) score and fetal testosterone level, among girls (left) and boys (right).
As expected, boys had higher amniotic testosterone than girls in general (boys: M = 0.83, SD = 0.43; girls: M = 0.33, SD = 0.32), t(202.66) = 9.67, p < .001, unequal variance, and with the female outliers removed (girls: M = 0.29, SD = 0.20), t(161.58) = 11.71, p < .001, unequal variance. Boys also had higher PSAI scores (boys: M = 68.95, SD = 10.73; girls: M = 34.95, SD = 12.48), t(210) = 21.32, p < .001. None of the control variables showed a sex difference (see Table 1 for descriptive statistics and correlations of PSAI scores with fetal testosterone and control variables).
TABLE 1.
Descriptive Information and Correlations Between Predictor Variables and Pre-School Activities Inventory (PSAI) Scores
| Group | Fetal testosterone level (nmol/L) |
Gestational age (weeks) |
Child’s age (years) |
Maternal age (years) |
Maternal education level |
PSAI score |
|---|---|---|---|---|---|---|
| Boys and girls combined | ||||||
| n | 212 | 145 | 209 | 191 | 186 | 212 |
| M (SD) | 0.59 (0.46) | 16.31 (1.88) | 8.59 (0.97) | 35.11 (8.02) | 3.11 (1.06) | 52.91 (20.57) |
| Range | 0.05–2.30 | 11.00–21.50 | 6.35–10.29 | 22.81–46.42 | 1–5 | 13–86 |
| Correlation (r) with PSAI | .59** | -.01 | .11 | -.10 | .21** | — |
| Girls | ||||||
| n | 100 | 67 | 99 | 91 | 88 | 100 |
| M (SD) | 0.33 (0.32) | 16.31 (2.32) | 8.44 (1.02) | 35.31 (4.10) | 2.88 (0.90) | 34.95 (12.48) |
| Range | 0.05–2.30 | 11.0–19.0 | 6.35–10.27 | 22.81–46.42 | 2–5 | 13–78 |
| Correlation (r) with PSAI | .42* | -.04 | -.01 | .16 | .08 | — |
| Boys | ||||||
| n | 112 | 78 | 110 | 100 | 98 | 112 |
| M (SD) | 0.83 (0.43) | 16.32 (1.5) | 8.70 (0.93) | 34.90 (4.78) | 3.21 (1.25) | 68.95 (10.73) |
| Range | 0.05–2.05 | 13.0–20.0 | 6.57–10.29 | 23.38–44.14 | 1–5 | 24–86 |
| Correlation (r) with PSAI | .20* | .01 | -.06 | -.20* | .04 | — |
Note. The table presents raw values for fetal testosterone levels. Maternal education level was self-reported and rated on a 5-point scale from 1 (no formal qualifications) to 5 (postgraduate qualification).
p < .05.
p < .01.
Data were first analyzed for both sexes combined by using backward stepwise linear regression using all fetal testosterone values. Any variable that correlated with PSAI scores at p < .2 was entered into the analysis (Altman, 1991). In addition, the influence of suppressor variables (variables that correlated highly with other predictors in the model, p < .01) was investigated. Maternal education, maternal age, child’s age, fetal testosterone levels, sex, and the interaction between sex and fetal testosterone were included in the analysis (entry criterion, p < .05; removal criterion, p > .10). The only significant predictors included in the final model were sex and fetal testosterone, R2 = .73, F(2, 181) = 250.11, p < .001. The results remained the same when the 2 female outliers were excluded from the analysis, and the only predictors retained in the final model were sex and fetal testosterone, R2 = .73, F(2, 180) = 248.63, p < .001.
Within-sex analyses on the full data set indicated that testosterone correlated positively with PSAI scores for both girls (r = .42, p < .001) and boys (r = .20, p < .05). The size of the correlations between PSAI scores and fetal testosterone level did not differ significantly between the sexes (z = 1.75, p > .05; see Fig. 1 and Tables 1 and 2). Without the 2 female outliers, the correlation for girls remained significant (r = .33, p < .001). For within-sex regression analyses, the same predictor variable selection procedure described above was used. For girls, maternal age, child’s age (suppressor), and fetal testosterone levels were included in the analysis. Fetal testosterone level was the only significant predictor retained in the final model, R2 = .11, F(2, 87) = 5.53, p < .01. In the regression analysis without the 2 female outliers, fetal testosterone level was the only variable retained in the final model, R2 = .09, F(1, 87) = 8.76, p < .01. For boys, fetal testosterone level and maternal age were entered in the analysis. The final regression model for boys retained both fetal testosterone level and maternal age, R2 = .08, F(2, 97) = 4.36, p < .05.
TABLE 2.
Final Hierarchical Regression Models for the Pre-School Activities Inventory
| Group and predictor | R2 | β | SE | p |
|---|---|---|---|---|
| Boys and girls combined | .73 | |||
| Fetal testosterone level | .14 | 2.28 | <.01 | |
| Sex | .77 | 1.94 | <.001 | |
| Girls | .11 | |||
| Fetal testosterone level | .27 | 4.17 | <.05 | |
| Maternal age | .18 | 0.09 | >.05 | |
| Boys | .04 | |||
| Fetal testosterone level | .21 | 2.66 | <.05 | |
| Maternal age | .20 | 0.12 | <.05 |
DISCUSSION
We found a significant relationship between fetal testosterone and sexually differentiated play behavior in both girls and boys. The large sample and the specific measure used may account for our ability to detect this relationship, even though two prior studies did not detect it (Knickmeyer et al., 2005; van de Beek et al., 2008).
Because children in the current study were developing typically, and because measures of testosterone were taken directly from the fetal environment, our results strengthen the evidence that testosterone plays a role in sexual differentiation of human behavior. Prior studies linking prenatal testosterone to childhood play have relied on clinical populations or measures of maternal hormone levels. Our study avoids problems of interpretation associated with those approaches.
Our results also differ from prior findings in that we found a relationship between prenatal testosterone and sex-typical play in both boys and girls. In contrast, studies of children with CAH have reported elevated male-typical behavior in girls but not boys (Hines, 2004), and the prior study relating maternal testosterone during pregnancy to childhood behavior found a relationship in girls but not boys (Hines et al., 2002). Thus, our data are the first documentation that androgen exposure prenatally relates to sexually differentiated play behavior in boys and in girls. In addition, the current results support an organizational, as opposed to current, activational role of testosterone, because play behavior is measured in childhood, when concurrent testosterone levels are low.
Prior difficulty finding predicted relationships between testosterone and behavior in boys may reflect the use of approaches that are not well-suited to its detection. Boys with CAH, unlike girls, may adjust testicular androgen production prenatally, thus avoiding marked androgen elevation (Hines, 2003), and testosterone in mothers and daughters correlate, whereas there is no correlation between testosterone in mothers and sons (Harris, Vernon, & Boomsma, 1998), making maternal samples less useful for detecting hormone-behavior relationships in boys. Therefore, studies relating amniotic fluid testosterone to subsequent behavior may be particularly useful for elucidating the role of testosterone in the behavioral development of boys.
Acknowledgments
This work was supported by grants from the Nancy Lurie-Marks Family Foundation and the Medical Research Council (to S.B.-C.) and by National Institutes of Health Grant HD 24542 (to M.H.). B.A. was supported by a scholarship from Trinity College, Cambridge. We are grateful to the families who have taken part in this longitudinal study over many years and to Ian Goodyer, Greg Davis, and Ieuan Hughes for valuable discussions.
REFERENCES
- Altman DG. Practical statistics for medical research. Chapman and Hall; London: 1991. [Google Scholar]
- Baron-Cohen S, Lutchmaya S, Knickmeyer R. Prenatal testosterone in mind. MIT Press; Cambridge, MA: 2004. [Google Scholar]
- Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: Methods and findings. Neuroscience and Biobehavioral Reviews. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB. Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AE, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Vol. 4. Academic Press; San Diego, CA: 2002. pp. 137–191. [Google Scholar]
- Ehrhardt AA, Meyer-Bahlburg HF. Effects of prenatal sex hormones on gender-related behavior. Science. 1981;211:1312–1318. doi: 10.1126/science.7209510. [DOI] [PubMed] [Google Scholar]
- Fausto-Sterling A. Myths of gender. Basic Books; New York: 1992. [Google Scholar]
- Golombok S, Hines M. Sex differences in social behavior. In: Smith PK, Hart CH, editors. Blackwell handbook of childhood social development. Blackwell; Oxford, England: 2002. pp. 117–136. [Google Scholar]
- Golombok S, Rust J. The measurement of gender role behaviour in pre-school children: A research note. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1993a;34:805–811. doi: 10.1111/j.1469-7610.1993.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Golombok S, Rust J. The Pre-School Activities Inventory: A standardized assessment of gender role in children. Psychological Assessment. 1993b;5:131–136. [Google Scholar]
- Goy RW, McEwen BS. Sexual differentiation of the brain. MIT Press; Cambridge, MA: 1980. [Google Scholar]
- Harris JA, Vernon PA, Boomsma DI. The heritability of testosterone: A study of Dutch adolescent twins and their parents. Behavior Genetics. 1998;28:165–171. doi: 10.1023/a:1021466929053. [DOI] [PubMed] [Google Scholar]
- Hines M. Sex steroids and human behavior: Prenatal androgen exposure and sex-typical play behavior in children. In: Panzica G, Melcangi RC, editors. Steroids and the nervous system. Annals of the New York Academy of Sciences Vol. 1007. New York Academy of Sciences; New York: 2003. pp. 272–282. [DOI] [PubMed] [Google Scholar]
- Hines M. Brain gender. Oxford University Press; New York: 2004. [Google Scholar]
- Hines M, Brook C, Conway GS. Androgen and psychosexual development: Core gender identity, sexual orientation and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH) Journal of Sex Research. 2004;41:75–81. doi: 10.1080/00224490409552215. [DOI] [PubMed] [Google Scholar]
- Hines M, Golombok S, Rust J, Johnston KJ, Golding J. Testosterone during pregnancy and gender role behavior of preschool children: A longitudinal, population study. Child Development. 2002;73:1678–1687. doi: 10.1111/1467-8624.00498. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R, Wheelwright S, Taylor K, Raggatt P, Hackett G, Baron-Cohen S. Gender-typed play and amniotic testosterone. Developmental Psychology. 2005;41:517–528. doi: 10.1037/0012-1649.41.3.517. [DOI] [PubMed] [Google Scholar]
- Martin CR. Endocrine physiology. Oxford University Press; New York: 1985. [Google Scholar]
- New MI. Diagnosis and management of congenital adrenal hyperplasia. Annual Review of Medicine. 1998;49:311–328. doi: 10.1146/annurev.med.49.1.311. [DOI] [PubMed] [Google Scholar]
- Pasterski VL, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M. Prenatal hormones and postnatal socialization by parents as determinants of male-typical toy play in girls with congenital adrenal hyperplasia. Child Development. 2005;76:264–278. doi: 10.1111/j.1467-8624.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- Quadagno DM, Briscoe R, Quadagno JS. Effects of perinatal gonadal hormones on selected nonsexual behavior patterns: A critical assessment of the nonhuman and human literature. Psychological Bulletin. 1977;84:62–80. [PubMed] [Google Scholar]
- Robinson J, Judd H, Young P, Jones D, Yen S. Amniotic fluid androgens and estrogens in midgestation. Journal of Clinical Endocrinology. 1977;45:755–761. doi: 10.1210/jcem-45-4-755. [DOI] [PubMed] [Google Scholar]
- Servin A, Bohlin G, Berlin D. Sex differences in 1-, 3-, and 5-year-olds’ toy-choice in a structured play session. Scandinavian Journal of Psychology. 1999;40:43–48. doi: 10.1111/1467-9450.00096. [DOI] [PubMed] [Google Scholar]
- Snow ME, Jacklin CN, Maccoby EE. Sex of child differences in father-child interaction at one year of age. Child Development. 1983;54:227–232. [Google Scholar]
- van de Beek C, van Goozen SHM, Buitelaar JK, Cohen-Kettenis PT. Prenatal sex hormones (maternal and amniotic fluid) and gender-related play behavior in 13-month-old infants. Archives of Sexual Behavior. 2008 doi: 10.1007/s10508-007-9291-z. [DOI] [PubMed] [Google Scholar]