Abstract
Pulmonary vascular resistance (PVR) is an important hemodynamic variable that affects prognosis and therapy in a wide range of cardiovascular and pulmonary conditions. We sought to determine whether a noninvasive estimate of PVR predicts adverse outcomes in patients with stable coronary artery disease. Using Doppler echocardiography we measured the estimated PVR (defined as the ratio of the tricuspid regurgitant velocity [TRV] to the velocity–time integral [VTI] of the right ventricular outflow tract [RVOT]) in 795 ambulatory patients with stable coronary artery disease. Participants were categorized by quartiles of the TRV/VTIRVOT ratio. Hazard ratios (HRs) and 95% confidence intervals were calculated for all-cause mortality, heart failure hospitalization, and adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction, or stroke). After 4.3 years of follow-up there were 161 deaths, 44 deaths from cardiovascular causes, 103 heart failure hospitalizations, and 120 adverse cardiovascular events. Compared with patients in the lowest TRV/VTIRVOT quartile, those in the highest quartile were at increased risk of all-cause mortality (unadjusted HR 1.8, 95% confidence interval 1.3 to 2.5), heart failure hospitalization (unadjusted HR 2.9, 95% confidence interval 2.0 to 4.3), and adverse cardiovascular events (unadjusted HR 2.0, 95% confidence interval 1.4 to 2.9). After multivariate adjustment, patients in the highest quartile were at increased risk of heart failure hospitalizations (adjusted HR 2.5, 95% confidence interval 1.3 to 4.7). In conclusion, a noninvasive estimate of PVR (TRV/VTIRVOT ratio) predicts mortality, heart failure hospitalization, and adverse cardiovascular events in patients with stable coronary artery disease.
Doppler echocardiography has achieved widespread use as a noninvasive means of estimating pulmonary artery pressure.1 We previously demonstrated that an increase in Doppler-estimated pulmonary artery pressures predicts mortality and heart failure hospitalization in patients with stable coronary artery disease.2 Because flow and pressure in the pulmonary circulation can be measured noninvasively, we hypothesized that a previously validated echocardiographic estimate of pulmonary vascular resistance (PVR), the ratio of tricuspid regurgitant velocity (TRV) to the velocity–time integral (VTI) of the right ventricular outflow tract (RVOT), predicts adverse cardiovascular events independently of established risk factors, and provides incremental prognostic value beyond that provided by pulmonary artery pressure in patients with stable coronary artery disease.3
Methods
The Heart and Soul Study is a prospective cohort study investigating the influence of psychosocial factors on cardiovascular events in outpatients with stable coronary artery disease. The enrollment process for the Heart and Soul Study has been previously described.4 Eligible participants had ≥1 of the following: (1) history of myocardial infarction, (2) angiographic evidence of ≥50% diameter stenosis in ≥1 coronary artery, (3) evidence of exercise-induced ischemia by treadmill electrocardiogram or stress nuclear perfusion imaging, or (4) history of coronary revascularization. Subjects were excluded if they deemed themselves unable to walk 1 block or if they were planning to move out of the local area within 3 years.
The study protocol was approved by the following institutional review boards: the University of California San Francisco committee on human research, the research and development committee at the San Francisco Veterans Affairs Medical Center, the medical human subjects committee at Stanford University, the human subjects committee at the Veterans Affairs Palo Alto Health Care System, and the data governance board of the Community Health Network of San Francisco. All participants provided written informed consent.
Between September 2000 and December 2002, a total of 1,024 participants enrolled in the study. Of these, we were unable to determine the TRV/VTIRVOT in 223 participants due to absence of an adequate tricuspid regurgitation envelope and/or suboptimal alignment with the RVOT Doppler signal. Six participants were lost to follow-up, leaving a total of 795 for outcome analysis.
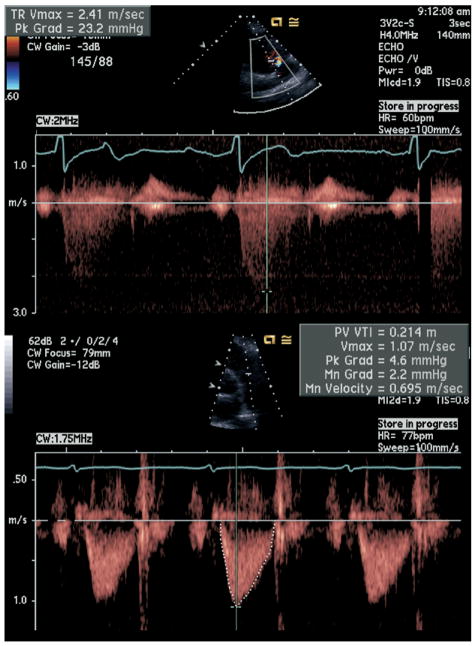
A complete 2-dimensional echocardiogram at rest using an Acuson Sequoia ultrasound system (Siemens Medical Solutions, Mountain View, California) with a 3.5-MHz transducer and Doppler ultrasound examination was performed in all patients. Standard 2-dimensional parasternal short-axis and apical 2- and 4-chamber views during inspiration were obtained. The peak TRV for the present study was the highest measurement obtainable by Doppler imaging among the parasternal, apical, and subcostal views. Pulmonary artery systolic pressure was estimated using the modified Bernoulli equation (ΔP = 4v2). Right atrial pressure was estimated by ultrasonic inspection of the inferior vena cava as previously described5 and was added to the tricuspid regurgitation gradient to determine pulmonary artery systolic pressure. VTIRVOT was obtained by placing a pulse-wave Doppler sample volume in the proximal RVOT at the level of the pulmonic valve, in the parasternal short-axis view, and tracing the outer boundaries of the spectral Doppler signal to obtain VTIRVOT. The sample volume was placed such that the closing but not the opening click of the pulmonic valve was visualized, and the opening valve Doppler signal was equal to or greater than the closing signal. This measurement was repeated up to 3 times if possible and the average value was recorded. TRV/VTIRVOT was then calculated (Figure 1). End-diastolic pulmonary regurgitation gradient was determined as previously described.6 Left ventricular ejection fraction was calculated as (end-diastolic volume − end-systolic volume)/end-diastolic volume. Diastolic dysfunction was defined as the presence of ≥1 of the following: impaired relaxation, defined as a ratio of peak mitral early diastolic to atrial contraction velocity (E/A) of ≤0.75 with systolic dominant pulmonary vein flow; pseudonormal, defined as 0.75 < E/A <1.5 with diastolic dominant pulmonary vein flow; restrictive filling, defined as an E/A ≥1.5 with diastolic dominant pulmonary vein flow. A single technician made all sonographic measurements, and a single experienced cardiologist reader, who was blinded to clinical and laboratory data, evaluated each echocardiogram.
Figure 1.
Noninvasive estimation of PVR by TRV/VTIRVOT (TRV 2.4 m/s, VTIRVOT 0.21 m, TRV/VTIRVOT 2.4 m/s ÷ 0.21 m = 11 s−1). CW = continuous wave doppler; HR = heart rate; Min Grad = minimum gradient; Pk Grad = peak gradient; PV Vmax = maximum velocity.
Baseline demographics, self-reported age, gender, ethnicity, medical history, and smoking status were determined by questionnaire. Participants were weighed and measured without shoes, and body mass index was calculated. All participants were instructed to bring their medication bottles to the study appointment where study personnel recorded all current medications.
We conducted annual telephone interviews with participants or their proxies regarding recent emergency room visits, hospitalizations, or death. Medical records, death certificates, and coroner reports were reviewed by 2 independent and blinded adjudicators. If the adjudicators agreed on the outcome classification, their classification was binding. If they disagreed, a third blinded adjudicator reviewed the event and determined the outcome classification. All-cause mortality was determined by review of death certificates. Death was considered due to cardiovascular causes if the death certificate listed acute myocardial infarction, congestive heart failure, or arrhythmia as the primary cause of death. Sudden death, defined as death occurring unexpectedly within 1 hour of the onset of symptoms, was also considered cardiovascular. Hospitalization for heart failure was defined as a minimum 1-night hospital stay for a clinical syndrome comprising ≥2 of the following: paroxysmal nocturnal dyspnea, orthopnea, increased jugular venous pressure, pulmonary rales, third heart sound, and cardiomegaly or pulmonary edema on chest x-ray. These clinical signs and symptoms must have represented a clear change from the baseline clinical status of the participant and must have been accompanied by failing cardiac output as determined by peripheral hypoperfusion (in the absence of other causes such as sepsis or dehydration) or peripheral or pulmonary edema requiring intravenous diuretics, inotropes, or vasodilators. Cardiovascular events were defined as the composite of cardiovascular death, nonfatal myocardial infarction, or stroke. Nonfatal myocardial infarction was defined as hospitalization for acute myocardial infarction as defined by American Heart Association diagnostic criteria.
We included 795 participants with completed outcome adjudications in the analyses. Because age-, gender-, and race-specific normal ranges for TRV/VTIRVOT have not been established, we categorized TRV/VTIRVOT into quartile groups. Differences in baseline characteristics were compared with the use of analysis of variance for continuous variables and chi-square test for dichotomous variables. We used multivariate logistic regression analysis to calculate hazard ratios (HRs) for quartiles of TRV/VTIRVOT as the primary predictor variable. We report HRs with 95% confidence intervals. Predefined end points were all-cause mortality, cardiovascular death, heart failure hospitalization, and the combined end point (composite of cardiovascular death, nonfatal myocardial infarction, and stroke). To determine the independent association between TRV/VTIRVOT and outcomes, we adjusted for known clinical risk factors (age, gender, smoking status, hypertension, diabetes, body mass index, history of myocardial infarction, history of congestive heart failure, history of chronic obstructive pulmonary disease) and echocardiographic risk factors (left ventricular ejection fraction, pulmonary artery systolic pressure, and end-diastolic pulmonary regurgitation gradient). Statistical analysis was performed using SAS 9.1 (SAS Institute, Cary, North Carolina).
Results
TRV/VTIRVOT in the study population was 5.7 to 36 per second. Baseline characteristics of the 795 study participants by TRV/VTIRVOT are listed in Table 1. Compared with participants in the lowest quartile of TRV/VTIRVOT, those in the highest quartile were more likely to be older, to be men, to have a lower body mass index, and to have a history of congestive heart failure or chronic obstructive pulmonary disease. They also had lower left ventricular ejection fraction, higher pulmonary artery systolic pressure, and higher end-diastolic pulmonary regurgitation gradient. There was no significant difference in diastolic dysfunction across quartiles of TRV/VTIRVOT.
Table 1.
Baseline characteristics of study participants by quartile of tricuspid regurgitation velocity/right ventricular outflow tract velocity–time integral ratio
| TRV/VTIRVOT Quartiles (s−1) |
p Value* | ||||
|---|---|---|---|---|---|
| I (5.7–11) (n = 201) | II (11–13) (n = 200) | III (13–15) (n = 200) | IV (15–36) (n = 200) | ||
| Age (yrs) | 64 ± 11 | 66 ± 11 | 68 ± 10 | 71 ± 10 | <0.0001 |
| Men | 144 (72%) | 162 (81%) | 163 (82%) | 176 (88%) | 0.0006 |
| Body mass index (kg/m2) | 29 ± 5.5 | 28 ± 5.5 | 28 ± 5.0 | 27 ± 4.7 | 0.02 |
| White | 117 (58%) | 118 (59%) | 112 (56%) | 126 (63%) | 0.04 |
| Hypertension | 137 (68%) | 148 (74%) | 140 (70%) | 140 (70%) | 0.6 |
| Myocardial infarction | 104 (52%) | 104 (52%) | 101 (51%) | 118 (59%) | 0.3 |
| Congestive heart failure | 22 (11%) | 30 (15%) | 44 (22%) | 47 (24%) | 0.003 |
| Stroke | 19 (9%) | 27 (14%) | 34 (17%) | 35 (18%) | 0.08 |
| Diabetes mellitus | 51 (25%) | 49 (25%) | 43 (22%) | 48 (24%) | 0.8 |
| Chronic obstructive pulmonary disease | 24 (12%) | 24 (12%) | 36 (18%) | 43 (22%) | 0.02 |
| Revascularization | 124 (62%) | 110 (55%) | 119 (60%) | 126 (63%) | 0.4 |
| Current smoking | 40 (20%) | 43 (22%) | 30 (15%) | 41 (21%) | 0.4 |
| Left ventricular ejection fraction | 63 ± 8.2 | 63 ± 9.2 | 62 ± 9.0 | 59 ± 12 | <0.0001 |
| Pulmonary artery systolic pressure (mm Hg) | 22 ± 5.5 | 26 ± 6.1 | 27 ± 6.5 | 32 ± 11 | <0.0001 |
| End-diastolic pulmonary regurgitation gradient (mm Hg) | 2.9 ± 2.1 | 3.1 ± 2.2 | 3.6 ± 2.4 | 5.0 ± 3.7 | <0.0001 |
| Diastolic dysfunction | 0.3 | ||||
| Impaired relaxation | 97 (84%) | 85 (82%) | 112 (84%) | 103 (79%) | |
| Pseudonormal | 15 (13%) | 15 (14%) | 18 (14%) | 16 (12%) | |
| Restrictive | 3 (3%) | 4 (4%) | 3 (2%) | 11 (8%) | |
| Physically active | 125 (62%) | 145 (73%) | 125 (63%) | 115 (58%) | 0.02 |
| Aspirin use | 164 (82%) | 161 (81%) | 154 (77%) | 138 (69%) | 0.01 |
| β-Blocker use | 109 (54%) | 120 (60%) | 121 (61%) | 123 (62%) | 0.4 |
| Statin use | 124 (62%) | 125 (63%) | 138 (69%) | 128 (64%) | 0.4 |
| Renin–angiotensin inhibitor use | 97 (48%) | 99 (50%) | 107 (54%) | 100 (50%) | 0.8 |
Analysis of variance for continuous variables and chi-square test for dichotomous variables.
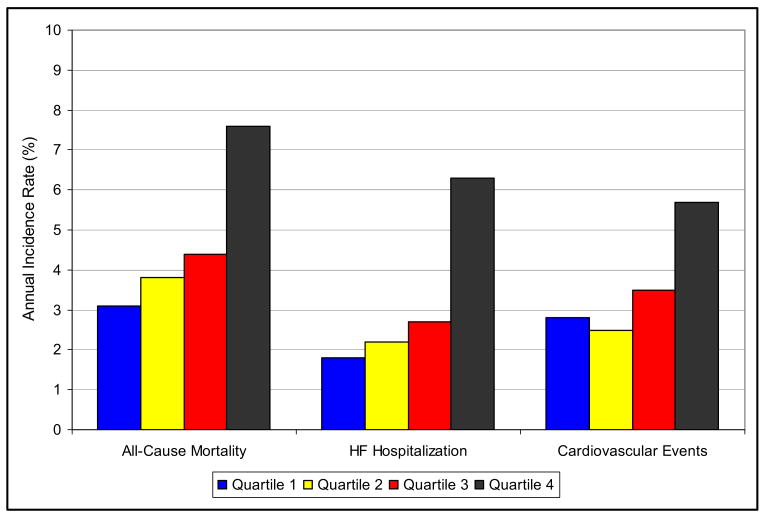
During a mean follow-up of 4.3 years there were 161 deaths, 44 cardiovascular deaths, 103 hospitalizations for heart failure, and 120 combined adverse cardiovascular events. The number of participants with outcome events separated by TRV/VTIRVOT quartile and the corresponding unadjusted HRs are presented in Table 2. Outcome by quartiles of TRV/VTIRVOT is shown graphically in Figure 2.
Table 2.
Adverse cardiovascular outcomes by quartile of tricuspid regurgitation velocity/right ventricular outflow tract velocity–time integral ratio
| Quartiles of TRV/VTIRVOT |
Unadjusted HR (95% CI)* | p Value | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| All-cause mortality | 27 (13%) | 32 (16%) | 38 (19%) | 64 (32%) | 1.8 (1.3–2.5) | 0.0002 |
| Heart failure hospitalization | 15 (8%) | 18 (9%) | 22 (11%) | 48 (24%) | 2.9 (2.0–4.3) | <0.0001 |
| Cardiovascular events (nonfatal myocardial infarction, cardiovascular death, or stroke) | 23 (12%) | 20 (10%) | 28 (14%) | 44 (22%) | 2.0 (1.4–2.9) | 0.0003 |
Quartile I versus IV.
CI = confidence interval.
Figure 2.
Outcome by quartiles I, II, III, and IV of TRV/VTIRVOT (x axis) with annual event rates for adverse cardiovascular events (y axis).
TRV/VTIRVOT was independently predictive of heart failure hospitalization even after adjusting for known clinical and echocardiographic prognostic markers. Multivariate regression analysis adjusted for age, gender, body mass index, ethnicity, history of congestive heart failure, history of stroke, left ventricular ejection fraction, pulmonary artery systolic pressure, and end-diastolic pulmonary regurgitation gradient is presented in Table 3.
Table 3.
Association of tricuspid regurgitation velocity/right ventricular outflow tract velocity–time integral ratio quartiles with adverse cardiovascular events after adjustment for available clinical and echocardiographic risk factors (including left ventricular ejection fraction, pulmonary artery systolic pressure, and end-diastolic pulmonary regurgitation gradient)
| Outcome | Adjusted HR (95% CI)* | p Value |
|---|---|---|
| All-cause mortality | 1.1 (0.6–1.8) | 0.80 |
| Heart failure hospitalization | 2.5 (1.3–4.7) | 0.004 |
| Cardiovascular events (nonfatal myocardial infarction, cardiovascular death, or stroke) | 1.5 (0.8–2.8) | 0.20 |
Quartile I versus IV. Adjusted for age, male gender, body mass index, ethnicity, congestive heart failure, stroke, chronic obstructive pulmonary disease, left ventricular ejection fraction, pulmonary artery systolic pressure, end-diastolic pulmonary regurgitation gradient, physically activity, and aspirin use.
Abbreviation as in Table 2.
Discussion
In the present study we demonstrate that a novel noninvasive estimate of PVR (TRV/VTIRVOT) predicts heart failure hospitalization independently of traditional cardiac risk factors, left ventricular ejection fraction, and pulmonary artery pressures in ambulatory patients with stable coronary artery disease.
Previous studies have shown that Doppler-estimated increases in pulmonary artery systolic and diastolic pressure predict adverse cardiovascular outcomes in stable coronary artery disease.2 The present study extends these findings by showing that, by expressing the relation between pulmonary artery pressure and transpulmonary blood flow, TRV/VTIRVOT retains independent prognostic value even after adjustment for pulmonary artery systolic and diastolic pressures. This suggests that increased PVR is a fundamental hemodynamic determinant of prognosis of patients with stable coronary artery disease.
Our findings are consistent with the hypothesis that chronic ischemic heart disease leads, in a subset of patients, to adverse structural remodeling in the pulmonary vasculature.7 Although it is well documented that longstanding severe pulmonary venous hypertension due to increased left atrial pressure can lead to irreversible pulmonary hypertension,8 to our knowledge, this study is the first to demonstrate independent prognostic significance to increased PVR in patients with stable coronary artery disease. Further studies are required to confirm these findings and to determine whether increased PVR by Doppler echocardiography should be considered an independent target of therapy in ischemic heart disease. Recent data suggesting a beneficial effect of phosphodiesterase V inhibitors in ischemic heart disease may partly reflect the beneficial effects of decreased PVR in this patient population.9
Several important limitations of our study must be considered. First, concurrent invasive hemodynamic measurements were not made in this large prospective cohort study. Second, use of TRV to estimate pulmonary artery systolic pressure may be prone to inaccuracy in certain patients.10 Third, our estimation of PVR by TRV/VTIRVOT does not account for some potentially confounding hemodynamic variables including right atrial pressure and pulmonary capillary wedge pressure. However, previous validation studies correlating TRV/VTIRVOT to invasively measured PVR showed a robust correlation even in patients with increased right atrial pressure and pulmonary capillary wedge pressure.3 Fourth, although after multivariate adjustment, increased PVR was independently predictive of heart failure hospitalization, the HRs for all-cause mortality and adverse cardiovascular events did not reach statistical significance. This may suggest a specific relation between PVR and incident heart failure in this patient population. Fifth, our study population, derived largely from a Veterans Affairs hospital, consisted predominantly of men (80%) and thus our findings cannot readily be extrapolated to women.
Acknowledgments
The Heart and Soul Study was supported by the Department of Veterans Affairs (Epidemiology Merit Review Program) Washington, DC, the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program) Princeton, New Jersey, the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program) New York, New York, and the Nancy Kirwan Heart Research Fund San Francisco, California.
References
- 1.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 2.Ristow B, Ali S, Ren X, Whooley MA, Schiller NB. Elevated pulmonary artery pressure by Doppler echocardiography predicts hospitalization for heart failure and mortality in ambulatory stable coronary artery disease: the Heart and Soul Study. J Am Coll Cardiol. 2007;49:43–49. doi: 10.1016/j.jacc.2006.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol. 2003;41:1021–1027. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 4.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kircher BJ, Himmelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–496. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 6.Ristow B, Ahmed S, Wang L, Liu H, Angeja BJ, Whooley MA, Schiller NB. Pulmonary-regurgitation end-diastolic gradient is a Doppler marker of cardiac status: data from the Heart and Soul Study. J Am Soc Echocardiogr. 2005;18:885–891. doi: 10.1016/j.echo.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 9.Jackson G. Hemodynamic and exercise effects of phosphodiesterase 5 inhibitors. Am J Cardiol. 2005;96(suppl 12B):32M–36M. doi: 10.1016/j.amjcard.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–740. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]