Summary
The Division of Lung Diseases of the National Heart, Lung and Blood Institute (NHLBI) recently held a workshop to identify gaps in our understanding and treatment of childhood lung diseases and to define strategies to enhance translational research in this field. Leading experts with diverse experience in both laboratory and patient-oriented research reviewed selected areas of pediatric lung diseases, including perinatal programming and epigenetic influences;mechanisms of lung injury, repair, and regeneration; pulmonary vascular disease (PVD); sleep and control of breathing; and the application of novel translational methods to enhance personalized medicine. This report summarizes the proceedings of this workshop and provides recommendations for emphasis on targeted areas for future investigation. The priority areas identified for research in pediatric pulmonary diseases included: (1) epigenetic and environmental influences on lung development that program pediatric lung diseases, (2) injury, regeneration, and repair in the developing lung, (3) PVD in children, (4) development and adaptation of ventilatory responses to postnatal life, (5) nonatopic wheezing: aberrant large airway development or injury? (6) strategies to improve assessment, diagnosis, and treatment of pediatric respiratory diseases, and (7) predictive and personalizedmedicine for children.
Keywords: epigenetics, pediatric, respiratory, lung disease
INTRODUCTION
Lung diseases are the fourth leading cause of death and disability in the United States. Historically, much focus has been on the treatment of lung diseases once symptoms are manifest and disease is well established. More recently, it has been recognized that many diseases start early in life, even in utero. Lung development is rapid in the fetus; lungs must be mature enough to support air breathing at birth, or respiratory difficulties will ensue. Evidence now suggests that in addition to genetic factors, pre- and postnatal environmental exposures exert specific long-term effects on lung structure and function which persist and adversely affect lung function and respiratory health into adulthood. The mechanisms of these early influences on lung health are not well understood. Recognizing the relationship of early life events on lung health and disease, the National Heart, Lung, and Blood Institute (NHLBI) convened a Working Group of extramural experts, entitled the “Pediatric Pulmonary Diseases Strategic Planning Workshop” on July 9–10, 2008 in order to develop a strategic plan, which would make recommendations to NHLBI about gaps in current knowledge, and priority setting for new research directions that would capitalize on scientific opportunities. This pediatric plan would synergize with the NHLBI Strategic Plan. A charge to the working group was to identify methods to facilitate translation of basic research findings into practice to better diagnose, treat, and prevent pulmonary diseases in children. A brief summary of these discussions, including identified gaps in knowledge and potential strategies to enhance translational research in pediatric lung diseases, are provided below.
PRIORITY 1: EPIGENETIC AND ENVIRONMENTAL INFLUENCES ON LUNG DEVELOPMENT THAT PROGRAM PEDIATRIC LUNG DISEASES
Background
Early life events including epigenetic and environmental influences likely play a significant role to program early lung development and influence later susceptibility to chronic lung disease. An increased understanding of developmental plasticity, the ability to develop in various ways depending upon environment, is likely to provide additional insights that could lead to new preventive approaches. This information would be especially helpful for infants who are at increased risk for specific lung diseases on the basis of personal characteristics or environmental exposures.
Recent studies show an important role for epigenetic mechanisms of gene regulation in early embryonic development.1 However, there is scarce information about epigenetic regulation of normal cell differentiation in the developing or postnatal lung,2,3 although a link to chronic diseases is becoming apparent. Methylation of cytosines in CG enriched regions of the genomic DNA is the best characterized epigenetic mechanism of lung gene regulation. DNA methylation plays a role in regulation of tumor suppressor genes,4 fibrosis,5 and asthma related genes.6,7 Other epigenetic mechanisms include covalent modification of the N-terminal domain of histones by acetylation, methylation, phosphorylation, or ubiquitination. In the lung, abnormally high histone acetylated regions were linked to active genes in asthma.8 Differential methylation of histones also modulates the expression of surfactant protein A by glucocorticoids.9 Another epigenetic mechanism consists of ATP-dependent complexes that utilize energy derived from ATP hydrolysis to change contacts between histones and DNA, mediating nucleosome sliding, remodeling, or histone replacement. These complexes may be recruited to specific promoters by interactions with cell/tissue specific transcription factors, altering the accessibility of the transcriptional machinery to DNA. These complexes play an important role, for example, in the activation of TGF-β (transforming growth factor-β) and steroid receptor target genes that are key to the development of several lung diseases.10 Finally, there is a potential role for noncoding RNA in controlling epigenetic events. Although not all noncoding RNA are epigenetic in nature, for example, post-transcriptional regulation by micro-RNA, some cooperate with components of the chromatin remodeling and DNA methylation machinery to produce stable epigenetic gene silencing.11 As the epigenetics research field evolves, the role of these mechanisms in modulation of gene expression in normal lung development and pre-disposition to chronic lung diseases needs to be explored.
Multifactorial Origin of Chronic Lung Disease
Twin studies reveal that genetic, nutritional, hormonal, and environmental factors are important modulators of lung disease, even in single gene disorders such as cystic fibrosis (CF). Environmental factors that influence lung development have been identified by epidemiologic studies and characterized using animal models. While there have been extensive studies of the in utero and postnatal effects of environmental tobacco smoke (ETS)12,13 and nutritional deficiencies (e.g., vitamins A and D),14 there is a scarcity of information regarding other environmental exposures that may affect fetal or early lung development. Existing research suggests a number of possible environmental exposures which might affect the epigenome in these early critical periods of development including diet, drugs, and toxins (e.g., air pollution) as well as the social environment.15,16 Whether these exposures could cause subtle epigenetic effects on developmental genes at any time during gestation, and predispose to certain postnatal or adult diseases needs to be evaluated.17,18 Recent advances in genomics and the development of new tools has facilitated the discovery of DNA variants that may contribute to chronic lung diseases. The availability of information on both genetic and environmental influences on lung development provides an unprecedented opportunity to quantify the contribution of gene–environment interactions to lung development.19 Furthermore, new methods that identify DNA modifications at the genome-wide level could be employed to address whether environmental factors detrimental to lung development operate by epigenetic mechanisms.20
Viral Respiratory Infections Affect Lung Health in Early Life
Severe viral infections cause considerable morbidity in infancy, and have been associated with the onset of long-term diseases such as asthma, and more recently, chronic obstructive pulmonary disease.21 Although the majority of children who develop virus-induced wheeze in infancy recover, up to a third of children develop persistent wheezing suggestive of asthma.22 More severe respiratory syncytial virus (RSV) infections, especially those requiring hospitalization, appear to be a risk factor for asthma.23,24 Children with a family history of atopy who wheeze with rhinovirus infections in infancy have a particularly high risk of recurrent wheeze25 and sub-sequent asthma.26,27
A variety of mechanisms have been proposed to link bronchiolitis and asthma. Causal explanations include virus-induced damage to the lung during a crucial period in lung development,28 or imprinting of the epithelial/mesenchymal unit by virus-induced inflammation in infancy.29 Recent evidence suggests that severe RSV infections can induce host epithelial production of chemokines, for example, CCL5, leading to an increased risk of subsequent lung disease.30 Additional explanations for an association between viral infections and asthma include abnormalities of the host immune system (e.g., reduced interferon or dendritic cell responses) that could predispose to the constellation of severe viral respiratory infections, atopy, and asthma.31,32
Research Opportunities
Despite recent advances in defining epigenetic and environmental mechanisms that modify lung development early in life, there are substantial gaps in our knowledge of the following: (1) the environmental factors that modify lung development in utero, (2) the environmental postnatal factors that affect lung development early in life, (3) the epigenetic alterations in utero or postnatally that are induced by environmental influences, and (4) the epigenetic and environmental modifiers that contribute to postnatal lung diseases such as bronchopulmonary dysplasia (BPD), CF, sickle cell, asthma, and chronic lung disease. These questions provide multiple opportunities for further study.
- Epigenetic influences on lung programming. Research is needed to:
- Characterize the chromatin modification signatures in normal lung development and diseased states and in specific cell compartments.
- Determine whether in utero and early postnatal environmental exposures or nutritional factors cause epigenetic alterations in the lung.
- Determine whether specific viral pathogens induce epigenetic modifications and functional consequences to airway cells.
- Develop animal models (in addition to murine) to evaluate the role of in utero and early postnatal environmental influences (e.g., infection, diet, pollutants) on lung development.
- Translation of early life epigenetic programming to specific disease states. Additional studies are needed to:
- Develop high-throughput tools to detect and characterize epigenetic and environmental modifications in the lung using animal models and large clinical studies.
- Use animal models to determine whether altering epigenetic influences (e.g., reverting chromatin state) with pharmacotherapy in early life can alter the incidence or progression of lung disease.
- Apply knowledge gained from animal models of epigenetic and environmental influences to longitudinal studies of the development of lung diseases in humans.
- Evaluate if changes in epigenetic and/or phenotypic signatures predict susceptibility, disease progression, or response to therapies for lung disease in early life.
PRIORITY 2: INJURY, REGENERATION, AND REPAIR IN THE DEVELOPING LUNG
Background
Lung injury in infants and children may alter postnatal lung growth and development to variable degrees. Complete recovery often occurs; however, the determinants of appropriate and complete lung repair have not been well described. Lung repair depends on interaction with the immune and circulatory systems, nutritional status, and continued interaction with the environment, including toxicants and infections. The therapies provided to children during lung injury may not only have beneficial effects but may also have adverse long-term effects. Supplemental oxygen, positive pressure respiratory support, and corticosteroids are common therapies for prematurely born infants which may permit survival but also impair subsequent alveolar development and augment immune responses to future infectious and noninfectious pulmonary insults.33 There is a gap in our knowledge regarding the developmental aspects of the lung’s response to injury and the process of repair which might alter clinical care if better understood.
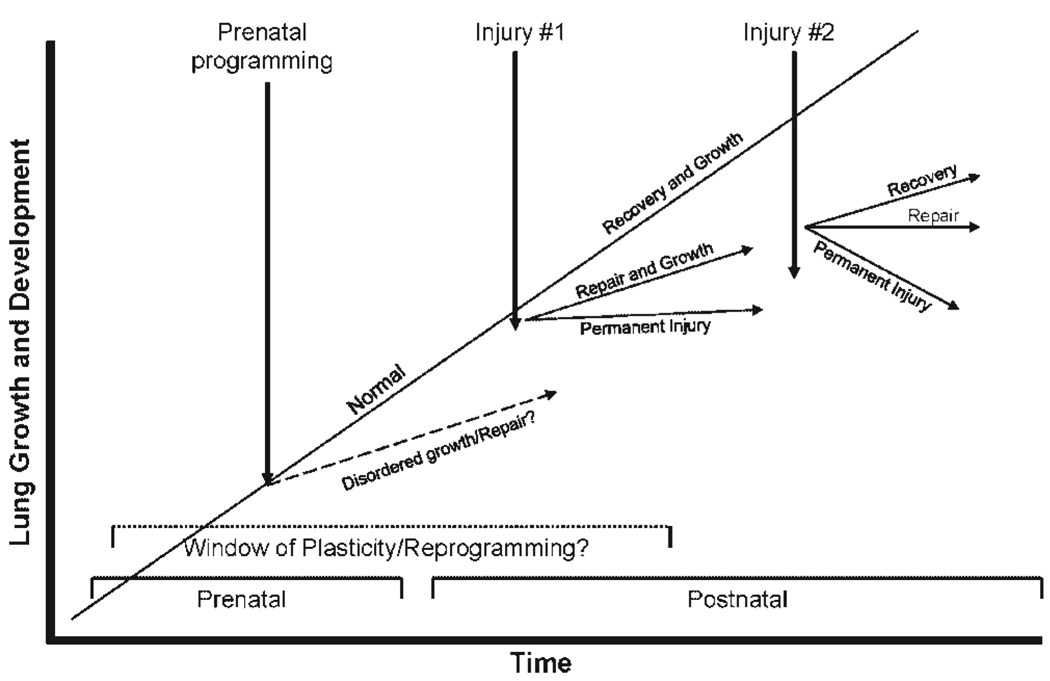
The repair process is influenced by a variety of factors, including the nature, duration, and recurrence of the injury, for example, aspiration events; the adaptive ability of the lungs to persistent injury, for example, infections, mechanical ventilation, oxygen, nutritional deprivation; exposure to pharmacologic agents, and remodeling in asthma; and the age at which injury occurs. It is unclear if the nature and degree of fibrosis in various compartments of the lung, that is, distal and proximal airways versus acinar regions, changes with postnatal age and previous injury. We have yet to define the windows of pre- and postnatal life when reprogramming of immune and growth processes is possible. Figure 1 depicts different responses to serial lung injury depending on the age at which developmental plasticity and reprogramming of growth and repair processes stop. It is also unclear when the lung’s adaptive capabilities, such as compensatory lung growth, become limited. Recent evidence that primates can develop new alveoli through young adulthood challenges the concept that plasticity of the respiratory repair responses wanes with age.34 The finding that alveolar simplification and emphysema is associated with nutritional deficits and that alveolarization occurs with refeeding [3] provides support for the ability of the postnatal lung to regenerate. In addition, stem and progenitor cells, including bone marrow-derived cell populations and progenitors that are intrinsic to the lung, play critical roles in normal lung development, the response to prenatal and postnatal lung injury, capacity to repair the lung following injury, and subsequent susceptibility to late lung disease.35
Fig. 1.
Responses to serial lung injury are dependent on periods of developmental susceptibility and plasticity before and after birth.
There are numerous clinical entities that reflect lung injury and incomplete recovery in childhood. These include premature infants who develop chronic lung disease which persists for years after birth, children with recurrent aspiration events, children with recurrent respiratory infections and subsequent fibrosis and bronchiectasis, those with bronchiolitis obliterans following infection or transplantation, children with ARDS/ALI (acute respiratory distress syndrome/acute lung injury), and infants and children with chronic interstitial pneumonitis. The clinical recovery patterns and determinants of recovery in children with these various diagnoses have not been examined in detail. Most outcomes reported are based on clinical and physiologic measures and less often on tissue histology.36
Barriers to better understanding of repair include imprecise definitions of outcomes, crude correlations between individual interventions and outcomes, and the inability to serially measure lung physiology, structure, and tissue responses in injury. The NHLBI has expressed interest in those initiatives that can be translated to the clinical arena within the next 5 years. However, the invasive nature of repeated broncho-alveolar lavage and lung biopsies precludes clinical research about lung repair in children in the near future and mandates that further basic investigations about the developmental nature of lung repair occur in animal models. Useful surrogate age-specific biomarkers are clearly needed.
Research Opportunities
- Biomarkers and indicators of lung repair through animal models encompassing different age groups and various models of lung injury, there is need to determine the following:
- Biomarkers that characterize lung repair, fibrosis, ongoing and compensatory growth, and remodeling of various lung regions in response to different insults.
- Interactions between mechanical, immune, and hypoxic/hyperoxic states on rate and degree of lung repair and age dependence of these interactions.
- Clinically applicable ways to ascertain postnatal compensatory lung growth.
- Indicators derived from sampling of the upper airway that accurately reflect components of the repair processes in the lung.
- Methods to clinically characterize features of lung recovery, such as regeneration of functional ciliated airway epithelial cells and alveolar-capillary integrity and surface area.
- Roles of genes, gene networks, and the programming of the processes regulating lung injury and repair during development. Identification of the developmental antecedents of adult lung disease.
- Identification of distinct populations of bone marrow-derived or intrinsic lung progenitor cells that may serve as biomarkers for disease, mediate the pathogenesis of lung disorders or provide novel therapeutic approaches to childhood respiratory illnesses.
-
Development of clinical phenotypes of childhood lung injury for longitudinal studies.
Using cohorts of children with specific forms of lung injury, develop longitudinal data through patient registries and/or clinical research networks to identify determinants of recovery/repair despite similar high-risk entry features, for example, nutritional interventions, oxygen therapy, pharmacologic agents. Due to the relatively small numbers of patients with each form of lung injury, data collection among centers and networks will be necessary to accrue sufficient patient numbers for meaningful conclusions.
-
Develop interdisciplinary expertise in the developmental, cellular, molecular, and physiologic bases of lung injury, repair, and recovery.
Convene meetings with experts in lung development, injury, repair, regeneration, and recovery in order to interface with one another and develop a state of the art assessment and basic research agenda on this complex topic.
Synergize multidisciplinary training opportunities to enhance both research and career development related to the pathogenesis of childhood pulmonary diseases.
PRIORITY 3: PULMONARY VASCULAR DISEASE (PVD)
Background
Despite advances over the past decades, PVD continues to cause significant morbidity and mortality in diverse pulmonary, cardiac, and hematologic disorders of child-hood. Structure and function of the pulmonary circulation can be altered by primary aberrations of lung growth and development [such as lung hypoplasia, pulmonary hemangiomatosis, arteriovenous malformations, anomalous pulmonary venous return, pulmonary veno-occlusive disease (PVOD), and others], or secondary to injury associated with acute respiratory failure, chronic lung disease after premature birth, chronic hypoventilation, congenital heart disease, and chronic hemolysis.37,38 Whereas the impact of pulmonary hypertension (PH) on the clinical course of children with congenital heart disease, persistent pulmonary hypertension of the newborn (PPHN), and idiopathic pulmonary arterial hypertension (IPAH) is well appreciated, the contribution of PH to the course and ultimate outcome of children with lung disease is often overlooked or underestimated.
PH is often a “silent” contributor to morbidity and mortality of many chronic lung disorders in pediatrics, including BPD, CF, sickle cell disease (SCD), and various interstitial lung diseases (ILD). For example, 42% of pediatric patients with ILD have evidence of PH early in their clinical course.39 Progressive PH, which appears to predict early death in adults with SCD, is already detected by echocardiogram in 10–20% of children with SCD.40 In general, clinical strategies that anticipate the development of PH may allow earlier recognition and more aggressive therapy, thereby slowing the development of PH in many chronic lung parenchymal and vascular diseases.
Despite some similarities, many aspects of PVD in children are often distinct from adult PH. First, pediatric PH is intrinsically linked to issues of lung growth and development, including many prenatal and early postnatal influences. The development of PH in the neonate and young infant is often related to impaired functional and structural adaptation of the pulmonary circulation during transition from fetal to postnatal life. Second, the timing of pulmonary vascular injury is a critical determinant of the subsequent response of the developing lung to such adverse stimuli as hyperoxia, hypoxia, hemodynamic stress, inflammation, and others. Third, abnormalities of the lung circulation are significant beyond the adverse hemodynamic effects of PH alone. The developing lung circulation plays critical roles in lung organogenesis and development of the distal airspace, maintenance of lung structure, metabolism, gas exchange, the ability to tolerate increased workloads imposed by exercise, and other stressors. Disruption of lung vascular growth can impair distal airspace structure during development and contributes to the pathobiology of diverse lung diseases.41 Fourth, there are apparent differences in function, structure, genetics, and perhaps responsiveness to therapies between adults and children with PH. Therapeutic strategies for adult PH have not been sufficiently studied in children especially regarding potential toxicities or optimal dosing, and age-appropriate endpoints for clinical use and research are lacking in this population.
Research Opportunities
-
Priorities for laboratory-based research on normal development and structural and functional abnormalities of the pulmonary circulation, including the basic biology of disorders associated with PH in newborns, infants, and children.
Mechanisms of lung angiogenesis and vasculo-genesis including the role of progenitor or stem cells.
Vascular–alveolar cross-talk: role in development and disease pathobiology.
Developmental physiology, regulation of lung vascular tone, reactivity, and permeability.
Hemolysis and thrombosis in the developing lung circulation.
Role of the bronchial circulation in development and disease.
Pulmonary venous development, including mechanisms of PVOD and PV stenosis.
Identification of new strategies and targets for therapy, including: rho kinase inhibitors, statins, phosphodiesterase inhibitors, elastase inhibitors, epidermal growth factor receptor inhibitors, tyrosine kinase inhibitors, immunosuppression (mycophenolate), cell cycle inhibitors (rapamycin), VIP (vasoactive intestinal peptide), adrenomedullin, PPARγ (peroxisome proliferator-activated receptor) rosiglitazone, serotonin antagonists, gene therapy, progenitor cell therapy, cell-based gene therapy, soluble guanylate cyclase activators/stimulators, and others.
-
Priority areas to address gaps in our clinical knowledge include the following:
Define natural history, epidemiology, and course of pediatric PVD in diverse diseases.
Determine outcome measures for clinical assessments of PH: molecular, biochemical, cellular, physiologic (e.g., exercise tests, vasoreactivity), and imaging approaches.
Develop novel imaging or physiologic approaches to assess lung vascular and alveolar growth (surface area).
Study age-specific pharmacokinetic, pharmacodynamic, and efficacy studies for drug therapies and predict pharmacogenetic, responsiveness, and risk for drug toxicities.
Develop evidence-based clinical care guidelines for disease- and age-specific diagnostic approaches and interventions.
Determine impact of early interventions on long-term outcomes.
-
Develop interdisciplinary approaches to integrate basic and clinical research and to effectively apply findings in the clinical setting to improve outcomes of children with PVD.
Establish a pediatric PH network with expertise in PH, to characterize the phenotypes of these disorders, define practice patterns and identify potential outcome measures for interventional studies, develop and promote clinical care guidelines, evaluate biomarkers and collect genetic/genomic or proteomic samples, and institute effective multicenter interventional studies.
Develop a consortium of pediatric pathologists with expertise in PVD and lung development to coordinate lung tissue collection and optimize the preparation of lungs for rigorous diagnostic and investigational studies of human tissue.
Explore novel approaches to the multidisciplinary training and development of clinician-scientists in clinical research skills, including rotations across disciplines and between centers with specialized expertise in different areas.
Determine strategies to best interface with Clinical and Translational Science Award (CTSA) programs to encourage interdisciplinary approaches, and better utilization of core labs in genomics, proteomics, and bioinformatics.
PRIORITY 4: DEVELOPMENT AND ADAPTATION OF VENTILATORY RESPONSES TO POSTNATAL LIFE
Background
Conditions in which sleep-disordered breathing occurs are frequent across the pediatric age spectrum and are associated with both significant morbidity and mortality. Sleep disordered-breathing in such conditions as apnea of prematurity, SIDS, obstructive sleep apnea, and even the prevalence of sleep associated respiratory disturbances in primary neurologic or pulmonary diseases (e.g., asthma, chronic lung disease of infancy, SCD, spina bifida, CF) is still poorly defined. Furthermore, the mechanisms of these common disorders are poorly understood,42–44 and the effects of the sleep and gas exchange disturbances have not been systematically explored in children.43–47 The gas exchange perturbations such as recurring hypoxia or hyperoxia can lead to disparate effects depending on the developmental stage at which they occur. For example, hyperoxia during early life can permanently alter respiratory control; intermittent fetal hypoxia will persistently augment ventilatory output into adulthood; and early postnatal intermittent hypoxia will permanently attenuate the baroreflex responses.48,49 These metaplasticity-related phenomena modulate the neural networks underlying sleep and respiratory control. Therefore, innovative multidisciplinary research regarding the mechanisms of causation and end-organ responses to early life abnormal gas exposure and respiratory control abnormalities are needed. Knowledge of these mechanisms may improve the diagnosis, and treatment of sleep disorders in children.50,51
Research Opportunities
Research is needed to better understand the effect of intermittent hypoxia, hyperoxia, hypercapnia and asphyxia (hypoxia and hypercapnia), sleep arousal and fragmentation, upper airway loading, and respiratory muscle development and adaptation. These abnormalities will need to be studied across development in fetal, postnatal term and preterm infancy, childhood, and adolescence. Other variables are gender and ethnic. Specific research topics are: (a) cellular and molecular mechanisms of oxygen sensing; (b) carotid body and other peripheral chemosensitive cells; (c) redox signaling during development; (d) membrane, cytosol, mitochondria, and ER determinants of susceptibility; (e) eupneic mechanisms and their plasticity/programming; (f) central nervous system (CNS) targets; (g) cardiovascular programming and re-programming; (h) sleep generators; (i) interactions between sleep and breathing; and (j) determinants of lymphadenoid proliferation in the upper airway.
This complex research agenda will require focused efforts on:
Identification and validation of genomic and proteomic diagnostic markers of disease and of disease susceptibility to specific sleep breathing disorders.
Collaborative clinical networks for translational epigenetics and whole genome scanning of selected disorders of respiratory control and sleep (e.g., CCHS [congenital central hypoventilation syndrome], SIDS [sudden infant death syndrome], obstructive sleep apnea).
Programs to study the complex biology of upper airway growth and pathology aiming to characterize multiple disease entities associated with upper airway dysfunction in children.
Identification of fetal and childhood determinants of adult sleep-disordered breathing and functional re-programming; that is, early antecedents of adult disease and its modifiers.
Development of therapies specifically targeted toward prevention and treatment of end-organ injury associated with sleep breathing disorders, and development of safe respiratory stimulants for clinical situations currently lacking pharmacological approaches.
Development of molecular imaging techniques permitting identification and monitoring of patients at high risk for complications associated with sleep disordered breathing.
PRIORITY 5: NONATOPIC WHEEZING: ABERRANT LARGE AIRWAY DEVELOPMENT OR INJURY?
Background
Although the molecular mechanisms and long-term consequences of allergic asthma are being actively studied, much less is known about nonatopic forms of wheezing. However, the preponderant form of recurrent airway obstruction during the preschool years is nonatopic wheezing,52 which is associated with significant morbidity and health care cost; almost half of all children hospitalized for “asthma” or “wheezing” are less than 6 years old and nonatopic wheezing is likely the most common cause.53 Recent studies have shown that abnormalities in lung function during a presymptomatic period in young infants tracks with late studies of decreased airflow in older adolescents and young adults.52
The two most common clinical presentations of non-atopic wheezing have been dubbed “typical” and “atypical.” 54 In the typical form, acute episodes of airway obstruction are triggered by viruses, especially rhninovirus 27 followed by periods with few, if any, symptoms. These children do not usually have a family history of asthma or personal history of atopic dermatitis. The airway cellular pattern observed is often neutrophilic inflammation,55 but increased epithelial loss, thickened basement membrane, increased numbers of vessels and of eosinophils appear to be common features of both atopic and nonatopic wheezing.56 Diminished airway function, measured shortly after birth or during the first year of life, is associated with nonatopic wheezing,57 but the airway segments involved and the mechanisms causing alterations in airway function are unknown. Maternal smoking can cause deficits in lung and airway development in utero.58 Neonatal and postneonatal deficits in immune responses predict the risk of subsequent wheezing associated with viral infections.59 Recurrent wheezing, occurring especially during the fall/winter period, often persists until the early school years, but usually remits thereafter in nonatopic children.60 However, deficits in airway function and airway hyperresponsiveness can be detected during the school years and beyond in these patients.52
Atypical wheezing is associated with persistent symptoms often not triggered by viral infection. In children with this clinical presentation, CF should always be excluded. Congenital malformations of the large airways (e.g., tracheo-bronchomalacia) and food aspiration with gastro-esophageal reflux or abnormal deglutition are common risk factors for these atypical forms. Bronchoscopy and other invasive procedures are used for diagnosis in these atypical forms, but systematic longitudinal studies and randomized trials on the usefulness of such procedures are lacking. As a consequence, no evidence-based guidelines are available that could allow physicians to refer these patients to tertiary centers where these procedures can be safely performed.
Research Opportunities
The prenatal and postnatal determinants of airway function and size are not known. A better under-standing of the regulation of airway growth result in early interventions and/or prevention of nonatopic wheezing.
The pathophysiology and structure–function relationships that contribute to the high incidence of non-atopic wheezing early in life are not known. Improved physiologic measurements, imaging of airway structures and computational modeling of wheeze generation are required to understand the multiple mechanisms that may contribute to this infant airway phenotype.
The alterations in innate immune responsiveness that predispose to excessive neutrophilic inflammation in nonatopic wheezing are not well understood. Genetic and epigenetic studies of mediators known to be involved in normal and abnormal responses to viruses could help elucidate the immune mechanisms of nonatopic wheezing.
Reliable diagnostic tests of food aspiration with or without gastro-esophageal reflux are urgently needed to assess the prevalence of these conditions in pediatric populations and their role in atypical forms of wheezing in early life.
Randomized trials of the risks and benefits of antireflux medication in the treatment of atypical nonatopic wheezing are also needed.
PRIORITY 6: STRATEGIES TO IMPROVE ASSESSMENT, DIAGNOSIS, AND TREATMENT OF PEDIATRIC RESPIRATORY DISEASE
Background
Respiratory diseases continue to be the leading causes of morbidity and mortality in children.61,62 This group of disorders is highly diverse, and includes asthma, pneumonia, bronchiolitis, disorders of sleep, complications of tobacco exposure, acute lung injury, recurrent aspiration, BPD, PVDs including PH, chronic respiratory failure requiring long-term, mechanical ventilation, BPD, neuromuscular diseases, apparent life threatening episodes of infancy, chest complications of SCD, ILD, congenital anomalies of the respiratory tract, and CF. Whereas the mechanisms which determine these disorders are poorly understand, even the natural history of most of them also remains unresolved. There is an immediate need for innovative research across all disciplines directed toward improved assessment, diagnosis, and treatment of child-hood lung diseases. There are no diagnostic criteria for many pediatric lung diseases, and the full spectrum of disease for many pediatric respiratory disorders is unknown. In addition, information regarding treatment approaches across centers is not available, making assessments of outcomes difficult. There is an increasing appreciation of the heterogeneity of lung involvement in different patients, further complicating assessment and treatment.63 Evidence highly supports the conclusion that factors which impair fetal and childhood lung growth can set the stage for life-long functional derangements which persist into adult life.64
Research Opportunities
Research is needed to develop noninvasive collection methods that can be integrated with genomic and environmental data to identify specific phenotypes within pediatric respiratory disorders. Approaches to be considered include identification of molecular, biochemical, and cellular biomarkers in blood, sputum, urine, and exhaled breath condensates; assessment of lung function across development, such as lung clearance index; acoustic evaluation of breath sounds and cough; improved approaches to noninvasive imaging that allow topographic assessment of pulmonary ventilation, perfusion, and structure; and robust statistical techniques including cluster analysis of a range of phenotypic characteristics to better define specific diseases. Another potentially fertile area of research is investigation of the respiratory epithelium incorporating novel fluorescent-based histochemistry, flow cytometric characterization of resident inflammatory cells, and ultra-specific molecular techniques to identify the complete microbiome. Studies of respiratory secretions and mucociliary clearance are also needed across a number of pediatric respiratory disorders. Research in patient reported outcomes, quality of life, pulmonary drug delivery, and treatment adherence are also important in preparation for therapeutic trials.
Establishment of disease registries would offer many fundamental opportunities for research in pediatric respiratory disorders. A key step toward reversing this gap in knowledge should align efforts across a wide geographic and socio-cultural spectrum, as could be accomplished through a national registry. The broad purpose of this effort will be to reduce mortality and morbidity of childhood respiratory diseases through tracking of specific outcomes, exposure assessment, genomics, treatment approaches, and standardized biomarker analysis. A national registry will leverage the opportunity for the banking and study of difficult to obtain tissue specimens and facilitate longitudinal studies. Ultimately, new knowledge obtained by these methods can be applied to the development and conduct of meaningful clinical trials in disorders and application of quality improvement techniques to treatment. Registries may be disease specific or inclusive of a variety of conditions.
Studies aimed at elucidating the effects of early injury to the respiratory system to the development of disease in later life should also be pursued. Questions to be pursued should include whether or not there is a particularly vulnerable period of lung development whereby specific exposures or injuries result in long-term perturbations, and how these affects are modified across diverse at risk populations.
Recognition of the crucial need for controlled clinical trials of specific therapeutics in well characterized patients.
PRIORITY 7: PERSONALIZED AND PREDICTIVE MEDICINE FOR PEDIATRIC PULMONARY DISEASE
Background
The recent development of methods for high throughput DNA analysis and association of specific variants or interacting pathway variants with disease phenotypes provides the opportunity to personalize respiratory disease risk assessment and prediction for children.65,66 Pediatric respiratory diseases contribute more to child-hood disease burden and health care costs than any other group of tissue-specific diseases.67 In addition, a substantial proportion of adult respiratory diseases that might be prevented or ameliorated with early recognition originates in childhood. The opportunity to predict and personalize care for pediatric and adult respiratory diseases offers the possibility to develop diagnostic tools and diverse nutritional, behavioral, environmental, and pharmacological intervention and treatment strategies to improve health outcomes and reduce health costs. However, accurate, individualized prediction of risk requires genetic information including replicated, statistically valid genotype–phenotype correlation and knowledge of genomic architecture, credible biologic evidence of a pathogenetic role for the identified variants, identification of environmental triggers for specific respiratory phenotypes, and definition of developmentally based époques for specific respiratory disease susceptibility. 68 Genetic prediction also carries ethical and emotional consequences, the possibility of stigmatization, and lack of absolute precision. Despite these problems, the benefits of substantial reduction in pulmonary disease burden for both children and adults through personalized and predictive medicine for children led us to suggest specific research questions. Answering these questions will require multidisciplinary teams that will likely include, at a minimum, experienced pediatric pulmonary, otolaryngology, neonatology, cardiovascular, and imaging clinicians, informationalists (e.g., genetic epidemiologists, informaticians, data management experts), molecular, and developmental biologists familiar with lung specific pathways that regulate both normal development and pulmonary response to injury and environmental stimuli, imaging scientists (including physicists, nanotechnologists), geneticists, immunologists, environmental and social scientists, regulatory and Institutional Review Board experts, and, most importantly, pediatric pulmonary trainees and trainees in other disciplines who are focused on reducing the health burden of pediatric and adult pulmonary diseases. Because childhood asthma is the most prevalent pediatric pulmonary disease for which large, carefully phenotyped and genetically defined cohorts, environmental information, and longitudinal data are available, it provides a major opportunity for rapid scientific progress and public health impact.69,70 Because phenotype and genetic information, prenatal and postnatal environmental assessment, and defined developmental status at birth are available for the large number of affected children, neonatal respiratory distress syndrome and BPD provide opportunities for rapid acquisition of new knowledge. 71 The neonatal period also provides the opportunity to assess the contribution of prenatal and perinatal programming to pediatric and adult lung outcomes.64,72 Nongenetic biologic responses, for example, the stress response, can permanently imprint pathways that regulate pulmonary development and response to environment in utero and during early childhood through epigenetic mechanisms that may be transmitted to progeny and will require new intervention strategies.15,20 Finally, oxygen-related injury (hypoxia or hyperoxia) is a disease process that represents a final common pathway for multiple pathogenic respiratory mechanisms (e.g., SCD, sleep) and provides a third area of focus for development of tools for personalized and predictive pediatric medicine for children.73–76
Research Opportunities
Pharmacogenetics of asthma response: several already studied asthma drug target genes provide the opportunity to develop a gene chip to permit prediction of optimal acute and long-term drug therapy for asthma.
Environmental triggers of asthma: characterization of environmental triggers can benefit from recent molecular and environmental methods for characterizing the environmental microbiome and quantitating longitudinal exposure to potential asthma triggers.
Developmental biomarkers of asthma phenotype: specific proteomic and/or cellular signatures will provide developmental époque specific biomarkers of the asthma phenotype.
Fetal/perinatal programming and asthma: fetal/perinatal programming contributes to risk for the asthma phenotype. Studies of pathways of fetal/perinatal programming (e.g., stress pathways that permanently imprint the hypothalamic–pituitary axis and the autonomic nervous system possibly through epigenetic modifications) will provide clinically useful targets for diagnosis and interventions.
Genetics and neonatal respiratory distress: genetic disruption of type 2 cell metabolism is associated with irreversible neonatal respiratory distress. Studies to identify rare, genetically disruptive variants will improve diagnosis and treatment of genetically based neonatal respiratory distress.
Lung injury, hypoxia, and hyperoxia: intermittent hypoxia increase risk of stroke in patients with SCD, while hyperoxia can induce lung injury in newborn infants. Studies of genetic variants and/or biomarkers that predict risk of chronic intermittent hypoxia or hyperoxia could be rapidly translated into clinical practice.
SUMMARY
Extensive discussions during this recent NHLBI work-shop on translational approaches to pediatric pulmonary diseases have identified key gaps in our knowledge and approaches, and provide recommendations for the development of novel laboratory and clinical strategies to address these needs. The need for developing registries and highly collaborative, interdisciplinary networks between centers was highlighted. Information from adult trials to support pediatric applications for new and even routine therapies should continue to be utilized, but it should also be stressed that there is a need to conduct new randomized double-blind trials in children to generate pharmacodynamic, kinetic, safety, and ultimately, efficacy data. Larger, multicenter trials, given the paucity of numbers from any of these rare diseases and developmental differences across childhood in pharmacodynamics, kinetics, and their responses will require interactions with FDA, pharmaceutical industry, and multiple centers in order to inform clinical practice for children with lung disease. In addition, the need to develop an academic workforce through enhancement of educational and training approaches is emphasized in order to develop and retain clinician–scientists in pediatric pulmonary disease. Some of these problems were clearly delineated in a recent American Thoracic Society consensus statement.77 Hopefully, this NHLBI workshop should provide new impetus and stimulate future directions for improving our ability to understand and treat childhood lung diseases.
Footnotes
This report is being simultaneously published in the Proceedings of the American Thoracic Society.
REFERENCES
- 1.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Cortese R, Hartmann O, Berlin K, Eckhardt F. Correlative gene expression and DNA methylation profiling in lung development nominate new biomarkers in lung cancer. Int J Biochem Cell Biol. 2008;40:1494–1508. doi: 10.1016/j.biocel.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Millien G, Beane J, Lenburg M, Tsao PN, Lu J, Spira A, Ramirez MI. Characterization of the mid-foregut transcriptome identifies genes regulated during lung bud induction. Gene Expr Patterns. 2008;8:124–139. doi: 10.1016/j.modgep.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu HS, Chen TP, Hung CH, Wen CK, Lin RK, Lee HC, Wang YC. Characterization of a multiple epigenetic marker panel for lung cancer detection and risk assessment in plasma. Cancer. 2007;110:2019–2026. doi: 10.1002/cncr.23001. [DOI] [PubMed] [Google Scholar]
- 5.Sanders YY, Pardo A, Selman M, Nuovo GJ, Tollefsbol TO, Siegal GP, Hagood JS. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008 Nov;39(5):610–618. doi: 10.1165/rcmb.2007-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adcock IM, Tsaprouni L, Bhavsar P, Ito K. Epigenetic regulation of airway inflammation. Curr Opin Immunol. 2007;19:694–700. doi: 10.1016/j.coi.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J, Jacot W, Yssel H, Vignola AM, Humbert M. Epigenetic inheritance of fetal genes in allergic asthma. Allergy. 2004;59:138–147. doi: 10.1046/j.1398-9995.2003.00359.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhavsar P, Ahmad T, Adcock IM. The role of histone deacetylases in asthma and allergic diseases. J Allergy Clin Immunol. 2008;121:580–584. doi: 10.1016/j.jaci.2007.12.1156. [DOI] [PubMed] [Google Scholar]
- 9.Islam KN, Mendelson CR. Glucocorticoid/glucocorticoid receptor inhibition of surfactant protein-a (sp-a) gene expression in lung type ii cells is mediated by repressive changes in histone modification at the sp-a promoter. Mol Endocrinol. 2008;22:585–596. doi: 10.1210/me.2007-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi Q, He W, Zhang XH, Le HV, Massague J. Genome-wide impact of the brg1 swi/snf chromatin remodeler on the transforming growth factor beta transcriptional program. J Biol Chem. 2008;283:1146–1155. doi: 10.1074/jbc.M707479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Prescott SL. Effects of early cigarette smoke exposure on early immune development and respiratory disease. Paediatr Respir Rev. 2008;9:3–9. doi: 10.1016/j.prrv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Pinkerton KE. Detrimental effects of tobacco smoke exposure during development on postnatal lung function and asthma. Birth Defects Res C Embryo Today. 2008;84:54–60. doi: 10.1002/bdrc.20114. [DOI] [PubMed] [Google Scholar]
- 14.Litonjua AA, Weiss ST. Is vitamin d deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 16.Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environ Mol Mutagen. 2008;49:46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- 17.Gicquel C, El-Osta A, Le Bouc Y. Epigenetic regulation and fetal programming. Best Pract Res Clin Endocrinol Metab. 2008;22:1–16. doi: 10.1016/j.beem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Nijland MJ, Ford SP, Nathanielsz PW. Prenatal origins of adult disease. Curr Opin Obstet Gynecol. 2008;20:132–138. doi: 10.1097/GCO.0b013e3282f76753. [DOI] [PubMed] [Google Scholar]
- 19.Sampsonas F, Archontidou MA, Salla E, Karkoulias K, Tsoukalas G, Spiropoulos K. Genetic alterations of glutathione s-transferases in asthma: do they modulate lung growth and response to environmental stimuli? Allergy Asthma Proc. 2007;28:282–286. doi: 10.2500/aap.2007.28.3002. [DOI] [PubMed] [Google Scholar]
- 20.Miller RL, Ho SM. Environmental epigenetics and asthma: current concepts and call for studies. Am J Respir Crit Care Med. 2008;177:567–573. doi: 10.1164/rccm.200710-1511PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303:671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The group health medical associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 23.Cohen R, Bacharier L, Zheng J, Schweiger T, Yin-DeClue H, Christie C, Ramkumar T, Schechtman K, Strunk R, Castro M. Outcomes following severe respiratory syncytial virus bronchiolitis in infancy: a prospective cohort study. 2008 (submitted) [Google Scholar]
- 24.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 25.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gern JE, Rosenthal LA, Sorkness RL, Lemanske RF., Jr Effects of viral respiratory infections on lung development and childhood asthma. J Allergy Clin Immunol. 2005;115:668–674. doi: 10.1016/j.jaci.2005.01.058. quiz 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramkumar T, Bacharier L, Zheng J, Schweiger T, Yin-DeClue H, Christie C, Schechtman K, Strunk R, Castro M. Elevated levels of CCL5 mrna in nasal epithelium post severe rsv bronchiolitis in early life is predictive of developmental of asthma. 2008 (submitted) [Google Scholar]
- 31.Silver E, Yin-DeClue H, Black W, Schechtman K, Grayson M, Bacharier L, Castro M. Lower levels of plasmacytoid dendritic cells are associated with a diagnosis of asthma 6 years after severe respiratory syncytial virus bronchiolitis. Pediatr Allergy Immunol. 2008 doi: 10.1111/j.1399-3038.2008.00818.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type iii interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 33.O’Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza a virus. Am J Respir Crit Care Med. 2008;177:1103–1110. doi: 10.1164/rccm.200712-1839OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyde DM, Blozis SA, Avdalovic MV, Putney LF, Dettorre R, Quesenberry NJ, Singh P, Tyler NK. Alveoli increase in number but not size from birth to adulthood in rhesus monkeys. Am J Physiol Lung Cell Mol Physiol. 2007;293:L570–L579. doi: 10.1152/ajplung.00467.2006. [DOI] [PubMed] [Google Scholar]
- 35.Warburton D, Perin L, Defilippo R, Bellusci S, Shi W, Driscoll B. Stem/progenitor cells in lung development, injury repair, and regeneration. Proc Am Thorac Soc. 2008;5:703–706. doi: 10.1513/pats.200801-012AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massaro D, Massaro GD, Baras A, Hoffman EP, Clerch LB. Calorie-related rapid onset of alveolar loss, regeneration, and changes in mouse lung gene expression. Am J Physiol Lung Cell Mol Physiol. 2004;286:L896–L906. doi: 10.1152/ajplung.00333.2003. [DOI] [PubMed] [Google Scholar]
- 37.Rosenzweig EB, Widlitz AC, Barst RJ. Pulmonary arterial hypertension in children. Pediatr Pulmonol. 2004;38:2–22. doi: 10.1002/ppul.20051. [DOI] [PubMed] [Google Scholar]
- 38.Abman SH. Cor pulmonale and pulmonary complications of cardiac disease. In: Taussig LM, Landau LI, Le Souef PM, Martinez FD, Morgan WJ, Sly PD, editors. Pediatric respiratory medicine. 2nd edition. Philadelphia: Mosby Elsevier; 2008. pp. 735–757. [Google Scholar]
- 39.Sondheimer HM, Lung MC, Brugman SM, Ikle DN, Fan LL, White CW. Pulmonary vascular disorders masquerading as interstitial lung disease. Pediatr Pulmonol. 1995;20:284–288. doi: 10.1002/ppul.1950200505. [DOI] [PubMed] [Google Scholar]
- 40.Onyekwere OC, Campbell A, Teshome M, Onyeagoro S, Sylvan C, Akintilo A, Hutchinson S, Ensing G, Gaskin P, Kato G, et al. Pulmonary hypertension in children and adolescents with sickle cell disease. Pediatr Cardiol. 2008;29:309–312. doi: 10.1007/s00246-007-9018-x. [DOI] [PubMed] [Google Scholar]
- 41.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 42.Audero E, Coppi E, Mlinar B, Rossetti T, Caprioli A, Banchaabouchi MA, Corradetti R, Gross C. Sporadic autonomic dysregulation and death associated with excessive serotonin autoinhibition. Science. 2008;321:130–133. doi: 10.1126/science.1157871. [DOI] [PubMed] [Google Scholar]
- 43.Farahani R, Kanaan A, Gavrialov O, Brunnert S, Douglas RM, Morcillo P, Haddad GG. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr Pulmonol. 2008;43:20–28. doi: 10.1002/ppul.20729. [DOI] [PubMed] [Google Scholar]
- 44.Kumar GK, Prabhakar NR. Post-translational modification of proteins during intermittent hypoxia. Respir Physiol Neurobiol. 2008 July 4; doi: 10.1016/j.resp.2008.05.017. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol. 2008;104:1287–1294. doi: 10.1152/japplphysiol.00644.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Douglas RM, Miyasaka N, Takahashi K, Latuszek-Barrantes A, Haddad GG, Hetherington HP. Chronic intermittent but not constant hypoxia decreases naa/cr ratios in neonatal mouse hippocampus and thalamus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1254–R1259. doi: 10.1152/ajpregu.00404.2006. [DOI] [PubMed] [Google Scholar]
- 47.Kheirandish L, Gozal D, Pequignot JM, Pequignot J, Row BW. Intermittent hypoxia during development induces long-term alterations in spatial working memory, monoamines, and dendritic branching in rat frontal cortex. Pediatr Res. 2005;58:594–599. doi: 10.1203/01.pdr.0000176915.19287.e2. [DOI] [PubMed] [Google Scholar]
- 48.Gozal D, Reeves SR, Row BW, Neville JJ, Guo SZ, Lipton AJ. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med. 2003;167:1540–1547. doi: 10.1164/rccm.200208-963OC. [DOI] [PubMed] [Google Scholar]
- 49.Soukhova-O’Hare GK, Ortines RV, Gu Y, Nozdrachev AD, Prabhu SD, Gozal D. Postnatal intermittent hypoxia and developmental programming of hypertension in spontaneously hypertensive rats: the role of reactive oxygen species and l-ca2+ channels. Hypertension. 2008;52:156–162. doi: 10.1161/HYPERTENSIONAHA.108.110296. [DOI] [PubMed] [Google Scholar]
- 50.Khalyfa A, Capdevila OS, Buazza MO, Serpero LD, Kheirandish-Gozal L, Gozal D. Genome-wide gene expression profiling in children with non-obese obstructive sleep apnea. Sleep Med. 2008 Feb; doi: 10.1016/j.sleep.2007.11.006. epub. [DOI] [PubMed] [Google Scholar]
- 51.Shah ZA, Jortani SA, Tauman R, Valdes R, Jr, Gozal D. Serum proteomic patterns associated with sleep-disordered breathing in children. Pediatr Res. 2006;59:466–470. doi: 10.1203/01.pdr.0000198817.35627.fc. [DOI] [PubMed] [Google Scholar]
- 52.Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, Martinez FD. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52:946–952. doi: 10.1136/thx.52.11.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ. National surveillance for asthma—united states, 1980–2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 54.Martinez FD, Godfrey S. Wheezing disorders in the preschool child. New York: Martin Dunitz, Taylor&Francis Group; 2003. [Google Scholar]
- 55.Krawiec ME, Westcott JY, Chu HW, Balzar S, Trudeau JB, Schwartz LB, Wenzel SE. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med. 2001;163:1338–1343. doi: 10.1164/ajrccm.163.6.2005116. [DOI] [PubMed] [Google Scholar]
- 56.Turato G, Barbato A, Baraldo S, Zanin ME, Bazzan E, Lokar-Oliani K, Calabrese F, Panizzolo C, Snijders D, Maestrelli P, et al. Nonatopic children with multitrigger wheezing have airway pathology comparable to atopic asthma. Am J Respir Crit Care Med. 2008;178:476–482. doi: 10.1164/rccm.200712-1818OC. [DOI] [PubMed] [Google Scholar]
- 57.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stocks J, Dezateux C. The effect of parental smoking on lung function and development during infancy. Respirology. 2003;8:266–285. doi: 10.1046/j.1440-1843.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 59.Gern JE, Brooks GD, Meyer P, Chang A, Shen K, Evans MD, Tisler C, Dasilva D, Roberg KA, Mikus LD, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–78. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 61.Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, Sutton SR. Annual summary of vital statistics:2006. Pediatrics. 2008;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- 62.Braman SS. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 63.Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature. 2005;434:777–782. doi: 10.1038/nature03490. [DOI] [PubMed] [Google Scholar]
- 64.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Broeckel U, Maresso K, Kugathasan S. vii Functional genomics and its implications for molecular medicine. Pediatr Clin North Am. 2006;53:807–816. doi: 10.1016/j.pcl.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Lee C, Morton CC. Structural genomic variation and personalized medicine. N Engl J Med. 2008;358:740–741. doi: 10.1056/NEJMcibr0708452. [DOI] [PubMed] [Google Scholar]
- 67.Healthy people, 2010. 08/26/2008]. Available from: http://www.healthypeople.gov/document/html/volume2/24respiratory.htm.
- 68.Collaco JM, Vanscoy L, Bremer L, McDougal K, Blackman SM, Bowers A, Naughton K, Jennings J, Ellen J, Cutting GR. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA. 2008;299:417–424. doi: 10.1001/jama.299.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guerra S, Martinez FD. Asthma genetics: from linear to multifactorial approaches. Annu Rev Med. 2008;59:327–341. doi: 10.1146/annurev.med.59.060406.213232. [DOI] [PubMed] [Google Scholar]
- 70.Martinez FD. Genes, environments, development and asthma: a reappraisal. Eur Respir J. 2007;29:179–184. doi: 10.1183/09031936.00087906. [DOI] [PubMed] [Google Scholar]
- 71.Garmany TH, Wambach JA, Heins HB, Watkins-Torry JM, Wegner DJ, Bennet K, An P, Land G, Saugstad OD, Henderson H, et al. Population and disease-based prevalence of the common mutations associated with surfactant deficiency. Pediatr Res. 2008;63:645–649. doi: 10.1203/PDR.0b013e31816fdbeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wright RJ. Suppl 3 Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatr Perinat Epidemiol. 2007;21:8–14. doi: 10.1111/j.1365-3016.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 73.Haddad GG. Tolerance to low o2: lessons from invertebrate genetic models. Exp Physiol. 2006;91:277–282. doi: 10.1113/expphysiol.2005.030767. [DOI] [PubMed] [Google Scholar]
- 74.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116:2307–2314. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 76.Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5:274–282. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lands LC, Allen J, Cloutier M, Leigh M, McColley S, Murphy T, Wilfond B. Ats consensus statement: research opportunities and challenges in pediatric pulmonology. Am J Respir Crit Care Med. 2005;172:776–780. doi: 10.1164/rccm.200405-661ST. [DOI] [PubMed] [Google Scholar]