Abstract
Changes in hippocampal CA1 dendritic spine density and synaptic number across the estrous cycle in female rats correlate with increased hippocampal-dependent cognitive performance in a manner that is dependent on estrogen receptors (ERs). Two isoforms of the estrogen receptor, α and β are present in the rat hippocampus and distinct effects on cognitive behavior have been described for each receptor. The present study generated a profile of synaptic proteins altered by administration of estradiol benzoate, the ERα selective agonist PPT (1,3,5-tris (4-hydroxyphenyl)-4-propyl-1H-pyrazole) and the ERβ selective agonist DPN (2,3-bis (4-hydroxyphenyl) propionitrile) alone and in combination in comparison to vehicle in the CA1 region of the dorsal hippocampus. In the stratum radiatum, estradiol, DPN, and PPT increased PSD-95 and AMPA-type glutamate receptor subunit GluR1. Only DPN administration regulated expression of AMPA receptor subunits GluR2 and GluR3, increasing and decreasingly levels respectively. DPN also increased GluR2 expression in the other lamina of the CA1. These results support previous reports that estradiol and isoform specific agonists differentially activate ERα and ERβ to regulate protein expression. The distinct effects of DPN and PPT administration on synaptic proteins, suggest that the desired therapeutic outcome of estrogen may be accomplished by using specific estrogen receptor agonists. Moreover, the effects of estradiol treatment on PSD-95 expression are consistent with a growing body of evidence that this postsynaptic protein is a key marker of estrogen action related to spine synapse formation.
Keywords: Hippocampus; Synaptic proteins; Estradiol benzoate; PPT (1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole); DPN (2,3-bis(4-hydroxyphenyl) propionitrile); Estrogen receptor agonists
1. Introduction
Estradiol, derived from endogenous or exogenous sources, has many enhancing effects on neuronal plasticity and behavior related to cognition and mood (Korol, 2004; Hajszan and MacLusky, 2006). In addition, estradiol has neuroprotective as well as neurogenic actions in models of ischemic brain injury, neurodegeneration, and aging (Brann et al., 2007; De Nicola et al., 2009). Estrogen sensitivity has been described in the stratum radiatum of the CA1 region of the hippocampus, where high levels of estradiol result in increased dendritic spine density and synapse number (Gould et al., 1990; Woolley and McEwen, 1993), spine size and shape (Woolley et al., 1996; Li et al., 2004), and neurotransmission (Woolley et al., 1997; Scharfman et al., 2003; LeDoux et al., 2009) and modulate hippocampal-dependent learning and memory (Luine et al., 2003; Sandstrom and Williams, 2004).
In hippocampal neurons, estradiol mediates changes in synaptic protein expression in vitro (Lee et al., 2004b) and in vivo (Brake et al., 2001; Lee et al., 2004c) that parallel changes in synaptic number and efficacy (Woolley, 1998). Estradiol-induced synaptogenesis is dependent on a classical estrogen receptor (ER) (McEwen et al., 1999). Two ER isoforms have been identified in the hippocampus: α and β Milner et al., 2001; Milner et al., 2005) but their distribution does not completely overlap. ERα is present in nuclear and extranuclear sites in principal and inhibitory neurons (Milner et al., 2001). Extranuclear ERβ is present in principal cells and in a few inhibitory cells (Milner et al., 2005).
Activation of both ERα and β with estradiol or specific agonists has been implicated in learning and memory. In rats, both DPN and PPT treatment enhanced memory (Frye et al., 2007). In mice, EB and DPN improved performance on cognitive tests in wildtype but not ERβ knock-out (BERKO) mice (Walf et al., 2008). Loss of ERβ in BERKO mice results in deficits in CA1 LTP and hippocampal related memory (Day et al., 2005). However, over-expression of ERβ, which reduces spine formation in the mouse hippocampus (Szymczak et al., 2006), may also negatively impact memory. ERα also contributes to hippocampal plasticity as restoration of ERα expression in ERα knockout mice rescued both estrogen responsiveness and hippocampal related memory (Foster et al., 2008).
We compared the effects of administration of agonists selective for ERα (PPT) or ERβ (DPN) or estradiol benzoate (EB) to vehicle in ovariectomized female rats on synaptic protein levels. Synaptic proteins levels in the CA1 stratum radiatum were evaluated by quantitative densitometric immunohistochemistry (Pierce et al., 1999). Postsynaptic proteins examined include PSD-95, spinophilin, and AMPA-type glutamate receptor subunits GluR1-3 and presynaptic proteins include synaptophysin, vesicular GABA transporter (VGaT), and vesicular glutamate transporter 1 (VGluT1).
2. Results
2.1 EB, DPN and PPT differentially regulate expression levels of pre- and postsynaptic proteins
Pre- and postsynaptic protein expression levels were examined after administration of EB, and DPN or PPT alone and in combination and compared to vehicle in the CA1 stratum radiatum of the dorsal hippocampus (Fig. 1 A, B) of ovariectomized female rats. Each steroid was administered twice, separated by 24 hours, and tissue was collected 72 hours after the first dose. The vehicle group consists of animals administered DMSO or sesame oil; results from these animals were pooled because no differences in labeling were detected (p > 0.05). Using quantitative densitometry, levels of presynaptic proteins, including synaptophysin, VGluT1, and VGaT, and postsynaptic proteins, including spinophilin, PSD-95 and the AMPA receptor subunits GluR1-3 (Fig. 1 B) were measured in all groups. We also measured GluR1-3 and PSD-95 expression in the other layers of the CA1, the CA3, and the dentate gyrus. Interestingly, the CA1 stratum radiatum showed more changes in protein expression after steroid administration than the CA3 or dentate gyrus. This study demonstrated differential regulation of protein expression by specific ER agonists, which resulted in both increased and decreased labeling for synaptic proteins, depending on the subtype of ER activated.
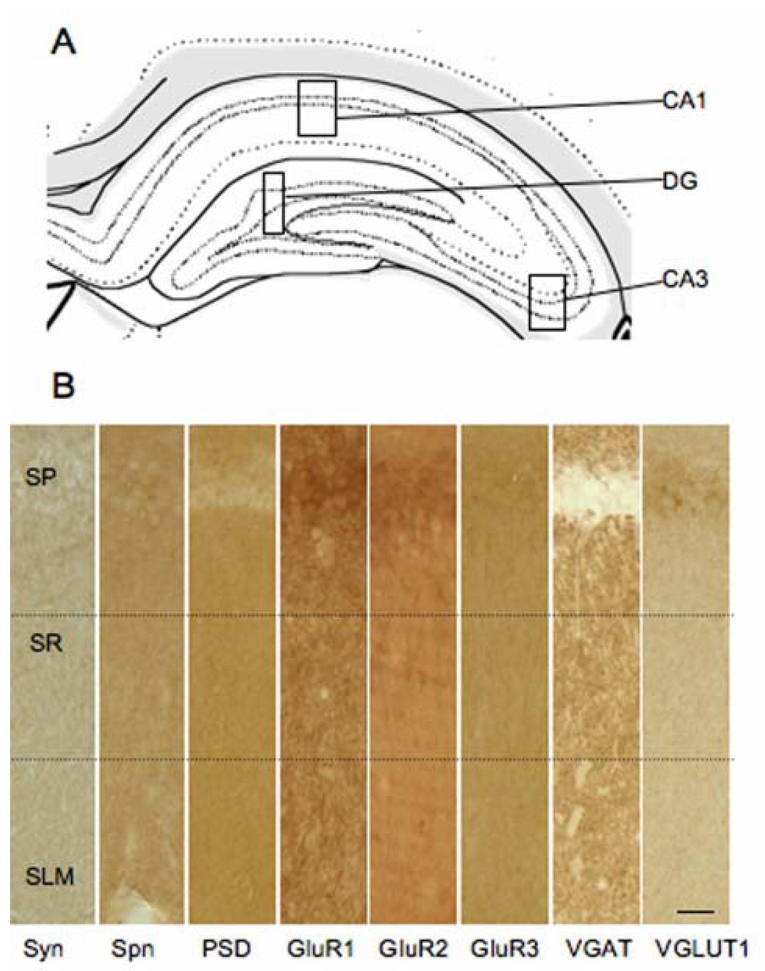
Figure 1.
Examination of synaptic protein expression in the hippocampal formation. A: Schematic representation of hippocampal formation with boxes to demonstrate the areas of the CA1, CA3 and dentate gyrus (DG) analyzed [59]. B: Distribution of synaptic proteins in the CA1 region of the hippocampus. Representative light photomicrographs of synaptophysin (Syn), spinophilin (Spn), PSD-95, GluR1, GluR2, GluR3, VGaT, and VGlut1 peroxidase labeling. Dotted lines demonstrate the medial portion of the stratum radiatum (SR) relative to the strata pyramidale (SP) and lacunosum-moleculare (SLM). Scale bar = 20 µm.
2.2 PSD-95 levels, but not synaptophysin or spinophilin levels, change in response to EB, DPN, and PPT
Synaptophysin is a membrane component of neuronal synaptic vesicles and is expressed in many of the terminals throughout the CA1 stratum radiatum of the dorsal hippocampus (Fig. 1 B) (Fykse et al., 1993). Neither EB nor the estrogen receptor agonists had a significant effect on synaptophysin levels in the stratum radiatum when compared to control (F (4, 20) = 0.9096, p > 0.05; Fig. 2A).
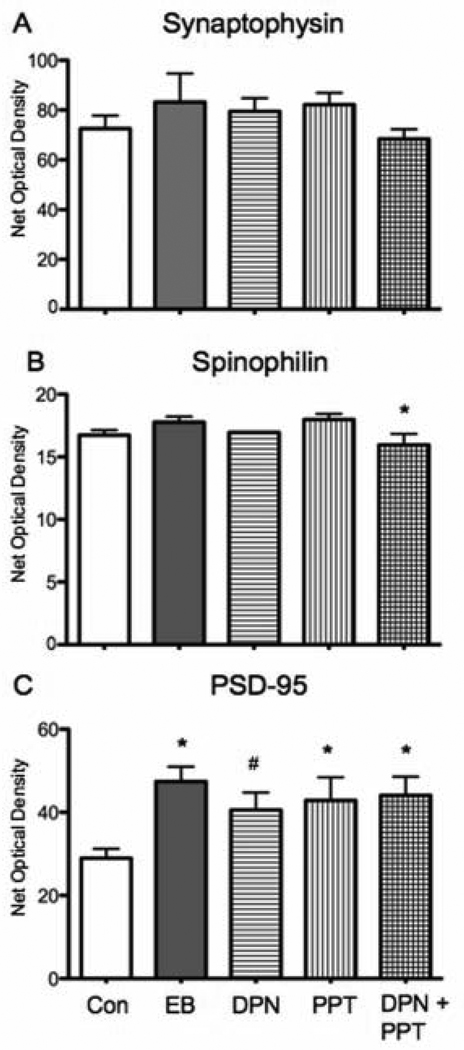
Figure 2.
Effects of selective ER agonist administration on synaptophysin, spinophilin, and PSD-95 immunoreactivity in the CA1 stratum radiatum of the dorsal hippocampus. A: Synaptophysin expression was not altered by any of the steroid regimens when compared to vehicle (Veh). B: Labeling for spinophilin was not altered in any treatment group compared to vehicle. In comparison to EB treatment, DPN plus PPT significanly reduced synatophysin levels (post hoc test * p < 0.05). C: PSD-95 immunoreactivity was significantly higher in EB, PPT alone and DPN + PPT treated hippocampi compared to vehicle (post hoc test * p < 0.05). In the DPN alone group there was a trend for increased PSD-95 expression (post hoc test # p = 0.061). For this and all subsequent figures error bars represent SEM.
Spinophilin is a dendritic, actin-binding protein present primarily in excitatory neurons (Feng et al., 2000). It is expressed throughout the CA1 stratum radiatum of the dorsal hippocampus (Fig. 1B). Neither EB, DPN or PPT administration alone or in combination resulted in a significant change in spinophilin levels relative to vehicle treatment (F(4, 20) = 2.47, p > 0.05, Fig. 2B). However, when an additional post hoc test was used to compare DPN, PPT and DPN plus PPT to EB treatment, DPN plus PPT treatment was significantly reduced in comparison to EB (F(4,20) = 2.47, p < 0.05).
PSD-95 is a scaffolding protein involved in the organization glutamate receptors and other constituents of the postsynaptic density (Kim and Sheng, 2004). PSD-95 labeling was diffusely distributed throughout the CA1 but also outlined dendritic profiles in stratum radiatum (Fig. 1 B). Comparison of EB, PPT alone or DPN plus PPT to control showed increased levels of PSD-95 in all groups (F (4, 19) = 3.646, p < 0.05, Fig. 2C). Post hoc analysis confirmed that PSD-95 labeling in the EB, PPT and DPN plus PPT was significantly increased compared to vehicle (p < 0.05 for all comparisons). In addition, there was a trend (p = 0.061) for DPN alone to increase PSD-95.
2.3 Expression of neurotransmitter transporters, VGluT1 and VGaT, were not altered by steroid replacement
VGluT1 and VGaT were examined to assess changes in the relative number of excitatory and inhibitory terminals, respectively. VGluT1 is the predominant form of glutamate transporter found in the postnatal brain (Nakamura et al., 2005) and is expressed exclusively in terminals of glutamatergic neurons (Herzog et al., 2001). VGaT expression is restricted to terminals and cell bodies of GABAergic interneurons (Chaudhry et al., 1998). Punctate staining for both VGaT and VGluT1, presumed to represent axon terminals, outlining dendrite profiles was apparent in the CA1 stratum radiatum of the dorsal hippocampus (Fig. 1 B). Steroid administration had no effect of the levels of VGluT1 or VGaT in the CA1 stratum radiatum (VGluT1: F(4, 20) = 0.7749, p > 0.05); VGaT: F(4,20) = 1.493; Fig. 3).
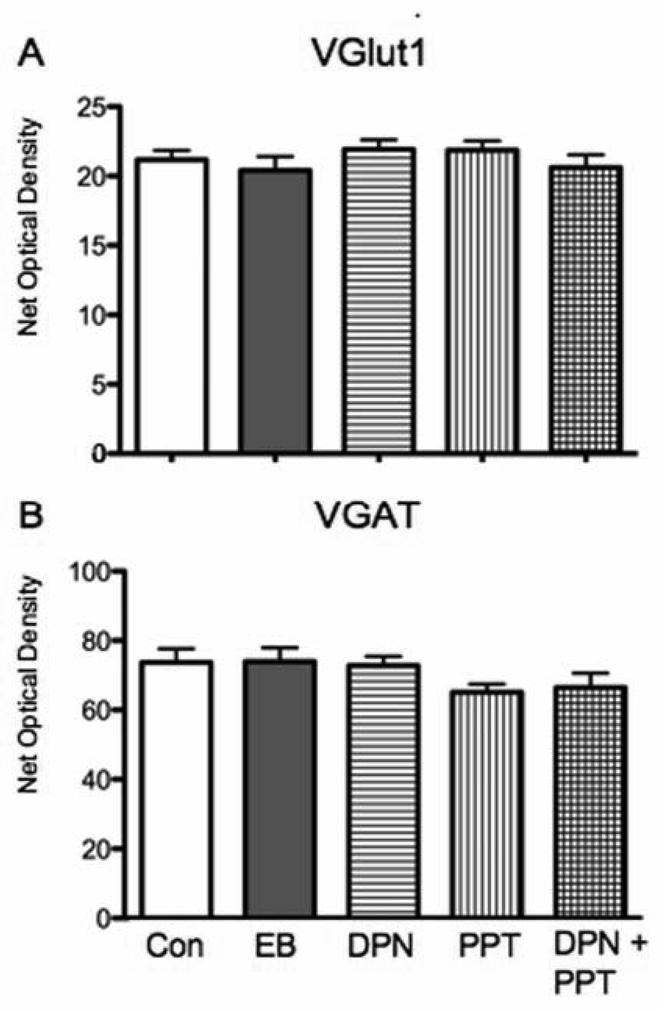
Figure 3.
Expression of vesicular transporters in stratum radiatum of CA1 did not change with any of the steroid regimens. EB, PPT alone, DPN alone and PPT + DPN did not significantly alter VGluT1 or VGaT expression levels in comparison to vehicle.
2.4 AMPA receptor subunits, GluR1-3 were differentially regulated by EB, PPT and DPN
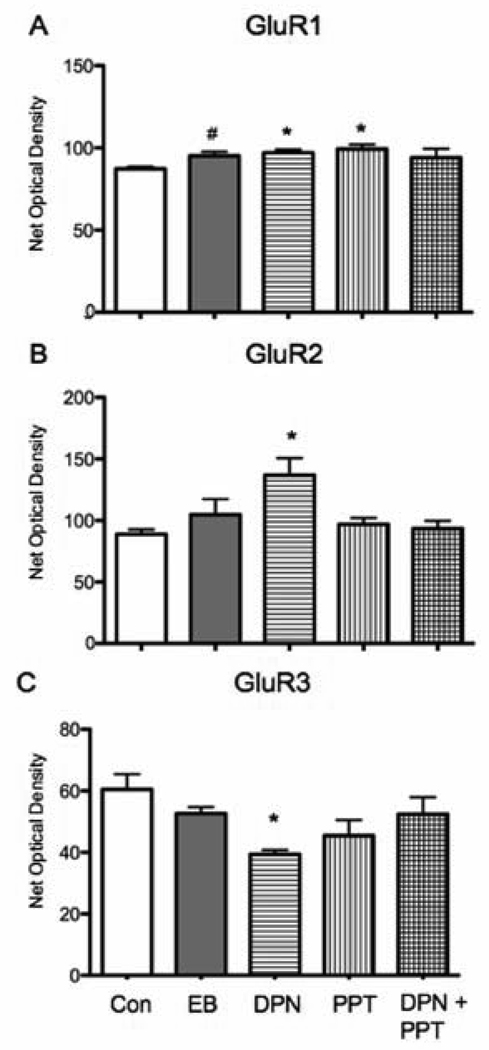
GluR1, 2, 3 have different roles in the properties of AMPA receptors and their movement between synaptic and extrasynaptic sites during synaptic transmission and changes in their expression alter subunit composition of AMPA receptors and consequently synaptic efficacy (Newpher and Ehlers, 2008). In the CA1 stratum radiatum of the dorsal hippocampus, GluR1, 2, and 3 immunoreactivity appeared primarily as diffuse peroxidase labeling (Fig 1 B), representative of their widespread expression. There was a small increase in GluR1 levels with DPN or PPT administration alone compared to vehicle (F(4,19) = 2.723, p > 0.05). Post hoc analysis confirmed that DPN and PPT significantly increased GluR1 expression (p < 0.05 for both comparisons) but DPN plus PPT administration did not (p > 0.05) (Fig. 4A). There was a trend for EB administration to increase GluR1 levels (p = 0.054). In contrast, DPN but not PPT increased GluR2 and GluR3 expression levels. GluR2 labeling significantly increased after DPN administration but did not change after EB, PPT or DPN plus PPT in comparison to vehicle administration (F(4,18) = 3.591, p < 0.05, Fig. 4B). Post hoc analysis confirmed that DPN caused a significant decrease in GluR2 (p < 0.05) while EB, PPT alone or DPN plus PPT had no effect on GluR2 levels (p > 0.05). The reverse effect of DPN was detected for GluR3 expression, DPN decreased GluR3 labeling (F(4, 20) = 2.696, p > 0.05) and post hoc analysis confirmed there was a significant decrease (p < 0.05). Similar to GluR2, GluR3 expression was not altered by EB, PPT alone or DPN plus PPT (p > 0.05, Fig. 4C).
Figure 4.
Estradiol and ER selective agonists differentially regulated AMPA receptor subunits GluR1-3 in CA1 stratum radiatum. A: The level of GluR1 immunoreactivity was significantly increased relative to vehicle by PPT alone and DPN alone (post hoc test * p < 0.05). There was a trend for EB to increase the levels of GluR1 immunoreactivity (post hoc test # p = 054). B: GluR2 labeling was significantly increased by DPN alone (post hoc test * p < 0.05) but not by EB, PPT alone or DPN + PPT when compared to vehicle. C: A significant decrease in the levels of GluR3 immunoreactivity was observed with DPN alone (post-hoc test * p < 0.05) but not administration of EB or PPT alone.
2.5 PSD-95 and GluR2 expression in other hippocampal areas differed in their steroid sensitivity
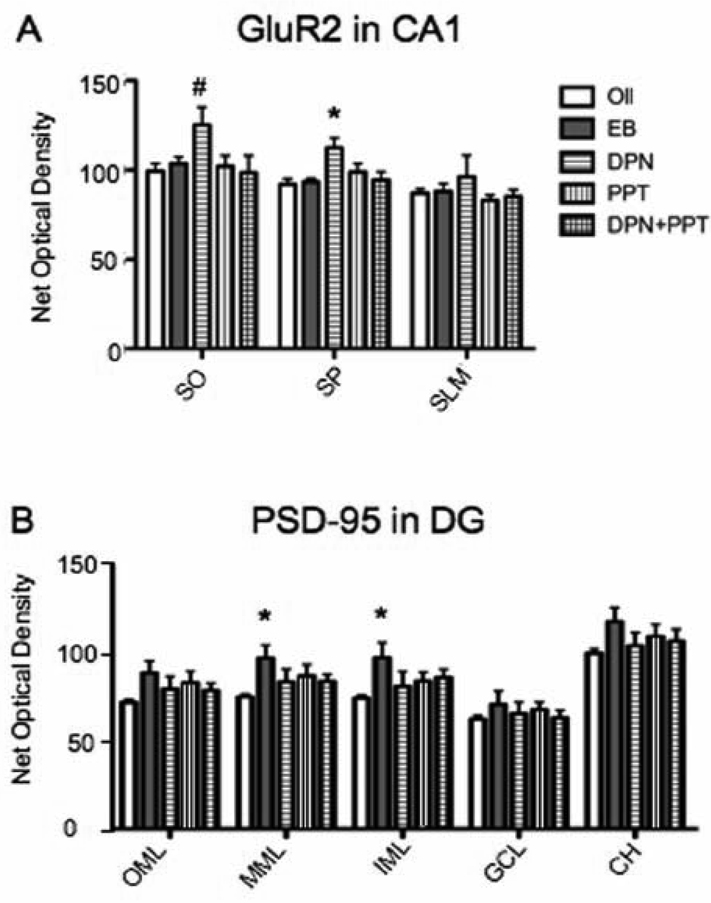
We also examined PSD-95 and GluR2 expression in the other lamina of the CA1 in addition to the dentate gyrus and CA3, because of the profound effects that these proteins have on synaptic physiology (ref). In addition, synaptic protein expression fluctuates with estrogen levels in mice in all hippocampal regions (Li et al., 2004; Spencer et al., 2008) and estrogens opioid peptide expression in the dentate gyrus and CA3 regions (Torres-Reveron et al., 2008; Torres-Reveron et al., 2009). Although GluR1 and GluR3 expression was also not altered by any of the treatments in any of the other hippocampal subregions (data not shown), DPN administration had wide spread effects on GluR2 levels in the lamina of the CA1 in the dorsal hippocampus. DPN increased GluR2 expression significantly in the stratum pyramidale (F(4,18) = 4.488, p < 0.05; post hoc test p < 0.05) and the stratum oriens (F(4,19) = 2.202, p > 0.05, post hoc test p < 0.05) (Fig. 5A). Examination of PSD-95 expression in the other lamina of the CA1 and the CA3 did not reveal any significant changes due to the effects of EB, DPN or PPT. However, in the dentate gyrus of the dorsal hippocampus post hoc comparisons suggest there was a significant increase in PSD-95 expression in the inner and middle molecular layers (IML: F(4,20) = 1.787, p > 0.05, post hoc p < 0.05; MML: F(4,20) = 1.89, p > 0.05, post hoc p < 0.05) (Fig. 5B).
Figure 5.
Steroid replacement alters GluR2 and PSD-95 labeling selectively in other CA1 layers and in the dentate gyrus. A: DPN administration significantly increased GluR2 immunoreactivity is in the CA1 stratum pyramidale (SP) and stratum oriens (SO) (post hoc test * p < 0.05). There was no effect of DPN in the stratum lacunosum-moleculare (SLM) and the other steroid regimens had no effect in any of the layers. B: In the dentate gyrus, compared to vehicle, EB increased PSD-95 levels in the inner molecular layer (IML) and in the middle molecular layer (MML) (post hoc test * p < 0.05). There was no effect of any treatment on the outer molecular layer (OML), granule cell layer (GCL) or central hilus (CH).
3. Discussion
We examined the ability of ERα and ERβ activation to mimic estradiol induced changes in synaptic proteins and identified some novel effects of individual ER activation the levels of AMPA-type glutamate receptor subunits. Quantitative immunohistochemistry revealed differential effects of ERα and ERβ activation on the expression of synaptic proteins. The alterations in the profile of synaptic proteins present in the CA1, particularly PSD-95, GluR2 and GluR3, along with previous reports of EB regulation of NMDA-type glutamate receptor subunits (Cyr et al., 2001) suggest the widespread potential of ER actions to affect glutamatergic transmission in the CA1 region of the hippocampus.
These results along with previous reports demonstrate that expression of a large number of postsynaptic proteins is regulated by steroids. Although fewer presynaptic proteins have been studied, in general protein expression in terminals appears to be less sensitive to steroid regulation. Interestingly, estrogen has been reported to increase the number of multisynaptic boutons (Woolley et al., 1996), suggesting that rearrangements of presynaptic structures occurs while postsynaptic increases may require new protein expression or decreased protein turnover.
Methodological considerations
The steroid replacement paradigm has previously been shown to increase spine density and synaptic number in the medial portion of the apical dendrites in the CA1 stratum radiatum. In addition, the doses of DPN and PPT have been demonstrated to be in the effective range of DPN and PPT (Lund et al., 2005; Gonzales et al., 2008; Morissette et al., 2008); however, the DPN plus PPT treated animals may have experienced pharmacological effects due to the higher levels of steroids administered with the combined treatment (Gonzales et al., 2008). As expected, uterine weights were increased by EB, PPT and DPN plus PPT administration; however, uterine weights after PPT were not as large as after EB, suggesting that in this steroid replacement paradigm, PPT does not fully reproduce EB effects on ERα in the periphery as previously reported (Harris et al., 2002). This may due to dose or decreased length of the treatment period. Previously we have shown that estrogen receptors mediate estrogen-induced increases in spines and synapses using this estradiol replacement paradigm (McEwen et al., 1999).
ER selective agonists can mimic select actions of EB
ERα or ERβ activation differentially regulated protein expression when agonists were administered separately or in tandem. Previous reports suggest that estradiol replacement can increase spinophilin protein in vitro (Lee et al., 2004b) and in vivo (Brake et al., 2001; Lee et al., 2004c). Synaptophysin is also upregulated long term estradiol replacement in ovariectomized female rats (Sharma et al., 2007) and decreased by inhibition of estradiol synthesis in hippocampal cultures (Prange-Kiel et al., 2006). However, synaptophysin and sphinophilin expression were not altered by steroid replacements compared to vehicle in this study, which may be due to the timing or the dose of steroid administered. In contrast, all steroid regimens resulted in increased expression of PSD-95 protein. Similar findings have been reported in rat neuronal cell line, where rapid actions of estradiol increase translation of PSD-95 (Akama and McEwen, 2003), while long-term estradiol administration increases PSD-95 transcription (Akama K and McEwen BS personal observation). In mice, increased levels of PSD-95 are detected during proestrus and after ERβ activation (Liu et al., 2008; Spencer et al., 2008). In total, estradiol may regulate the balance between transcription, translation, and degradation but this remains to be tested in future studies.
Interestingly, alterations in PSD-95 were also detected in the dentate gyrus. Here estradiol alone increased PSD-95 in the inner two thirds of the molecular layer. Interestingly DPN plus PPT did not increase PSD-95, perhaps due to the different binding affinities of each steroid for its receptor and inability to equally activate both receptors at the same time as EB can. The molecular layers receive excitatory inputs from a number of brains areas. Notably, processes from pyramidal basket cells, mossy cell axons and projections from the entorhinal cortex are among the substrates that form synapses with granule cells dendrites in the molecular layer. The inner third of the molecular layer receives excitatory inputs from the mossy cells in the hilus (Soriano and Frotscher, 1994; Buckmaster et al., 1996; Wenzel et al., 1997) and cholinergic projections from the septal nuclei (Wheal and Miller, 1980). The middle third of the molecular layer contain projections from the entorhinal cortex that form asymmetric synapses with the dendritic spines of granule cells (Witter, 1993). The mossy cells, cholinergic systems and entorhinal cortex are also estrogen sensitive brain areas, multiplying the number of pathways through which estrogen can alter the function of the hippocampal formation.
Estradiol has effects, neuroprotective and cognitive, related to its ability to modulate excitatory and inhibitory tone. We examined whether estrogens could alter the expression of vesicular transporters that would parallel the estradiol mediated increase in synapse number. However, we did not see any significant changes in the expression of VGluT1 or VGaT after steroid replacement. It is possible the proteins levels are not upregulated to accommodate changes in dendritic spines and synapses, rather a redistribution of protein due to alterations in protein synthesis and degradation or transformation from single to multi-synaptic boutons that shares the same protein pool may occur. Estradiol also drives movement of vesicles into the readily releasable pool (Hart et al., 2007), which may contribute to its effects on plasticity without altering vesicle number or content.
ER agonists differentially alter the expression of AMPA receptor subunits
Estradiol regulates the number of asymmetric or excitatory synapses in the CA1 stratum radiatum. While estradiol mediated synaptogenesis is dependent on NMDA receptors in vivo (Woolley and McEwen, 1994) and in vitro (Murphy and Segal, 1996) and inhibition of AMPA receptors has no effect (Woolley and McEwen, 1994), we nonetheless wondered if specific actions of ERβ or ERα, since both have been implicated in memory, (Frye et al., 2007; Walf et al., 2008), could regulate the expression of AMPA receptor subunits. Activation of ERβ with DPN treatment did alter the expression of AMPA receptor subunits increasing GluR2 and decreasing GluR3. DPN also had more widespread effects in the CA1 region as it also increased GluR2 in the stratum oriens and stratum pyramidale.
Previously, estradiol has been shown to increase NMDA receptor transmission (Woolley et al., 1997; Gureviciene et al., 2003) and expression of the subunits NR2B and NR1 but has no effect on NR2A (Cyr et al., 2001). In addition, PPT but not DPN mimicked the estrogen induced increased in NR2A/B binding and expression (Morissette et al., 2008). Interestingly, estrogen has been reported to increase binding for NMDA receptor but had no overall effect on binding for AMPA receptors (Woolley et al., 1997; Cyr et al., 2001). One explanation suggests that estrogen mediated synaptogenesis results in silent synapses that contain NMDA but not AMPA (Woolley, 1998). Estrogen also enhances hippocampal LTP (Warren et al., 1995; Cordoba Montoya and Carrer, 1997; Foy et al., 1999; Smith and McMahon, 2005) and LTP requires both NMDA receptors for induction and AMPA receptors for continuation.
Excitatory synapses also contain a concentration of AMPA-receptors, which mediate fast synaptic transmission and likely neuron-neuron interactions related to learning and memory. GluR1, 2, 3 are co-expressed with ERs in the hypothalamus, amygdala, and septum although estrogen regulates AMPA receptor expression only in the hypothalamus (Diano et al., 1997). Although colocalization of ERs and AMPA-receptor subunits has not been demonstrated in the hippocampus, our findings, particularly the effects of ERβ activation, suggest another mechanism for estradiol to alter calcium dynamics and excitability and alter synaptic plasticity involved in cognition and neuroprotection (Zhao et al., 2004). Specific activation of ERβ by DPN can regulate GluR2 and GluR3 expression while activation of ERα by estradiol or PPT does not regulate these subunits and may block ERβ regulation as shown by co-administration of DPN and PPT. The ability of ERα and β to antagonize the other’s affects when coexpressedhas been reported (Hall and McDonnell, 1999; Matthews et al., 2006). In contrast, EB, DPN, and PPT administration alone modestly increased GluR1 expression.
In the adult hippocampus, most AMPA receptors exist as heteromers of GluR1/GluR2 and GluR2/GluR3 subunits (Craig et al., 1993) and, since the expression of GluR3 is relatively low, more than 70% of GluR2 is associated with GluR1 (Wenthold et al., 1996). Another AMPA receptor subunit GluR4, forms complexes with both GluR1 and GluR2 or 3 but is expressed at lower levels in principal cells in the CA1 and was not examined here (Wenthold et al., 1996). GluR1 regulates activity dependent delivery of AMPA receptors to the synapse (Shi et al., 1999). GluR2 regulates Ca2+ permeability (Hume et al., 1991; Sommer et al., 1991) and glutamate-induced redistribution of surface AMPA receptors to internal pools (Lee et al., 2004a). A large increase in GluR2 at the same time that GluR1 is only modestly increasing and GluR3 in decreasing suggests several possible outcomes for AMPA receptor composition and function. Decreased GluR3 could reduce the available pool of GluR2/3 for constitutive insertion in the synapse and increased GluR2 could enhance activity dependent insertion of GluR1/2, shifting the complement of synaptic AMPA receptors. The formation of GluR2 homomers may also increase. Together, the effects of increased GluR2 could dramatically reduce Ca2+ influx and decrease the potential of excitatory events, such as increased glutamate release, to have adverse effects. Increased GluR2 could also delay a restoration in the balance of NMDA and AMPA receptor activity as an increase in AMPA receptor activity is associated with the termination of estrogen enhancement of LTP (Smith and McMahon, 2005).
In comparing the actions of DPN and PPT to EB, we found that DPN affected the expression of more synaptic proteins than PPT or even EB. Although both ERα and ERβ have been identified at similar extranuclear sites in hippocampal pyramidal cells (Milner et al., 2001; Milner et al., 2005), it has not been established that ERα and β are colocalized in the same compartments. ERα and ERβ bind estradiol with similar affinity despite having only moderately similar ligand binding domains (AF-2). Their DNA binding domains are highly conserved but their N-terminal domains (AF-1) share poor homology (Kuiper et al., 1996). Because hippocampal ERs are extranuclear, it is likely that these differences in the N-terminal domains, which mediate receptor interactions with signaling pathways, result in receptor specific effects on protein expression through alterations in gene transcription and protein translation or degradation. Downstream convergence of signaling pathways could mediate protein effects that are similar for each receptor. It has also been reported that ERβ actions can antagonize effects of ERα (Hall and McDonnell, 1999; Matthews et al., 2006; Gonzales et al., 2008). In these reports and our experiments, low doses of DPN or PPT were used to avoid nonspecific activation of the other receptor. PPT is 410 fold more selective for ERα than ERβ, whereas DPN is 72 fold more selective for ERβ than ERα; however, at high doses both DPN and PPT can have effects on either ER (Stauffer et al., 2000; Meyers et al., 2001). Simultaneous activation of ERβ and ERα may result in no net change in the expression of some proteins. The reverse may also be true, when estradiol activates ERα and ERβ in tandem effects can include synaptogenesis and potentiation of LTP. Our findings further suggest that other effects of the ERα and β subtypes can be unmasked by treatment with specific agonists and these effects may have potential for clinical understanding. The effects of estradiol, DPN and PPT may also be related to their ability to regulate neurotrophins such as brain-derived neurotrophic factor, which has also been shown regulate GluR1 and 2 (Cyr et al., 2001; Caldeira et al., 2007a; Caldeira et al., 2007b).
While the relative contributions of ERβ versus ERα on gene transcription versus protein translation or degradation awaits further study, reports on the differential effects of ERα and β activation on intracellular Ca2+ dynamics, phosphorylation of signaling molecules and neuroprotection suggest each receptor has differential contributions to neuronal physiology, synaptic plasticity, and hippocampal-dependent behavior (Day et al., 2005; Szymczak et al., 2006; Zhao and Brinton, 2007; Foster et al., 2008; Walf and Frye, 2008; Walf et al., 2008). Although selective agonists of ERα and β did not completely mimic the actions of EB examined here, both individual and simultaneous actions of ERα and β may contribute to estrogen’s effects on hippocampal plasticity and memory. These reports, along with our results suggest that targeted estrogen replacement that selectively activates ERα and β may offer therapeutic opportunities to specifically enhance the beneficial effects of estrogen.
4. Experimental Procedures
4.1 Animals
Adult female Sprague Dawley rats (250–275g) from Charles River Laboratories (Wilmington, MA) were housed three to a cage with ad libitum access to food and water and with 12:12 light/dark cycles. Ovariectomy was performed following previously published protocols (Becker et al., 2005). All procedures were approved by the Rockefeller University Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines.
4.2 Steroid replacement
Steroid administration regimens followed a protocol previously used by our laboratory to reproduce the modifications of dendritic spines in CA1 normally produced during the estrous cycle (Woolley and McEwen, 1993). Three day after ovariectomy, rats received 100 µl s.c. injection of either 10µg of estradiol benzoate (Sigma, St. Louis, MO), ERα specific agonist 1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT) at 1 mg/kg (Tocris, Ellisville, MO), ERβ specific agonist 2,3-bis(4-hydroxyphenyl) propionitrile (DPN) at 1 mg/kg (Tocris, Ellisville, MO), a combination of both agonists in DMSO, oil or DMSO. These doses of PPT and DPN have been reported to alter behavior and glutamate receptor binding (Lund et al., 2005; Morissette et al., 2008). Rats received 2 injections of vehicle or drug 24 hrs apart and were perfused 48 hrs after the last injection. Biological availability of the steroids was monitored through changes in uterine weights. The average weight of uteri from oil or DMSO-administered animals was 0.233±0.022 grams. After the various steroid regimens the uteri weighed 0.563±0.036 grams for EB, 0.333±0.009 grams for PPT alone, and 0.312±0.079 grams for DPN alone and 0.290+0.008 grams for PPT plus DPN (N = 5 per group). EB, PPT and DPN plus PPT administration resulted in a significant increase in uterine weight compared to vehicle (F(5, 20) = 109.1, p < 0.0001; post hoc test p < 0.05), while DPN did not (p > 0.05).
4.3 Immunohistochemistry
For quantitative light microscopic localization of synaptic proteins, serial dilutions for each antibody were established and a linear function of antibody concentration against labeling intensity was obtained using densitometry, as previously described (Chang et al., 2000; Torres-Reveron et al., 2008). The dilution that produced slightly less than half-maximal labeling was chosen for use to allow for variations in labeling intensity (Chang et al., 2000). To insure identical labeling conditions during immunocytochemistry (Pierce et al., 1999), sections of each treatment group were rinsed in PB, coded with hole punches in the cortex and pooled into single containers. The tissue was processed according to the avidin-biotin complex (ABC) method (Hsu et al., 1981). Briefly, sections were incubated in: (1) PB to remove cryoprotectant; (2) 1% sodium borohydride in PB, 30 min to neutralize free aldehydes; (3); 0.5% bovine serum albumin (BSA) in Tris-saline solution (TS; 0.9% NaCl in 0.1 M Tris, pH 7.6) to block non-specific antibody binding, 30 min; (4) the primary antiserum in 0.1% BSA/TS either without Triton (GluR1-3) or with 0.1% Triton (PSD-95, synaptophysin, spinophilin, VGaT, VGluT1, 1 day at room temperature (~23 °C) followed by 1–4 days at 4°C; (5) 1:400 of anti-mouse or rabbit biotinylated-IgG, 30 min; (6) a 1:100 dilution of peroxidase-avidin complex (Vectastain Elite Kit), 30 min; and (7) 3,3’-diaminobenzidine (DAB) and H2O2 in TS, 6 min. All incubations were separated by washes of TS. Sections were mounted on gelatin coated slides, dehydrated in ascending concentrations of alcohols, and cover-slipped with D.P.X. neutral mounting medium (Sigma).
4.4 Primary Antiserum
The antibody to synaptophysin (Mouse, 1:300,000, Sigma) was generated using clone SVP-38 (Wiedenmann and Franke, 1985) and detects a single band of 38 kD by western blot (Brake et al., 2001). The antibody to spinophilin (Rabbit, 1:500,000, Patrick Allen, Yale University) has been characterized by western blotting (Allen et al., 1997) and in knock out mice (Stafstrom-Davis et al., 2001). Characterization of the antibody to VGluT1 (Guinea Pig, 1: 20,000, Chemicon) has been described by Boulland (Boulland et al., 2007). Specificity of the VGaT antibody (Rabbit, 1:15,000, Synaptic Systems, Gottingen, Germany) has been described by Takamori (Takamori et al., 2000). The antibody to PSD-95 (Mouse, 1:15, 000, Sigma) recognized a single band of 95 kD by western blot (manufacturer’s technical information). Antibodies to GluR1 (Rabbit, 1:1000, Calbiochem, La Jolla, CA), GluR2 (Mouse, 1:500, Chemicon), and GluR3 (Mouse, 1:1000, Chemicon) have been characterized by western blot and in knock-out mice (Wenthold et al., 1992; Molnar et al., 1993; Medvedev et al., 2008).
4.5 Analysis
Sections were analyzed and photographed on a Nikon Eclipse 80i light microscope equipped with bright-field and differential interference optics and a Micropublisher digital camera (Q imaging, Barnaby, BC). Photomicrographs were prepared by adjusting levels, brightness and contrast in Adobe Photoshop CS and final figures were assembled in Adobe PowerPoint 11.3.
For quantitative densitometry, images of regions of interest (R.O.I.) were captured using a Dage MTI CCD-72 camera and NIH Image 1.50 software. The mean gray value (of 256 gray levels) for each selected R.O.I. was determined as previously described (Pierce et al., 1999; Torres-Reveron et al., 2008; Torres-Reveron et al., 2009). To compensate for background staining and control for variations in illumination level between images, the average pixel density for 3 regions within the corpus callosum was subtracted. Tissue from control and experimental animals were processed together in the same crucibles. Sections from each animal were selected from the dorsal midseptotemporal level of the hippocampal formation (AP −3.90 to −4.20 Bregma; Swanson approximately level 32 (Swanson, 2000) and matched sections were selected processing with each antibody. A single hippocampi from each animal with the best morphology and consistent peroxidase labeling was included in the analysis. Optical density values were measured using NIH image. Net optical density values obtained after subtracting background values were converted to a percentage scale of 256 preset gray values ranging from 0 to 100%. A planned comparison was performed to compare steroid administration to vehicle treatment. A one-way ANOVA followed by Bonferroni’s Multiple Comparison Test with a 95% confidence level using Prism 5 for Mac Os X (Graphpad Software, Inc., La Jolla, CA). For this analysis, p values of < 0.05 in the ANOVA and planned comparison test were considered significant. ANOVA results with p values greater than 0.05 also were examined by post-hoc for significant differences. Post hoc p values between 0.05 and 0.065 were considered to be a trend towards significance.
Acknowledgements
Thanks to Scott Herrick for technical assistance.
Grant sponsor: NIH grants NS007080 (B.S.M.), T32 DK07313 (B.S.M.), DA08259 (T.A.M), HL18974 (T.A.M.)
Abbreviations
- GABA
(γ-Aminobutyric acid)
- AMPA
(alpha-amino-3-(3-hydroxy-5-methylisoxazole-4-yl)propionate)
- PPT
(1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole)
- DPN
(2,3-bis(4-hydroxyphenyl) propionitrile)
- ERα and β
(estrogen receptor alpha and beta)
- PSD-95
(post-synaptic density protein)
- VGluT1
(vesicular glutamate transporter 1)
- VGaT
(vesicular GABA transporter)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci U S A. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Boulland JL, Ferhat L, Tallak Solbu T, Ferrand N, Chaudhry FA, Storm-Mathisen J, Esclapez M. Changes in vesicular transporters for gamma-aminobutyric acid and glutamate reveal vulnerability and reorganization of hippocampal neurons following pilocarpine-induced seizures. J Comp Neurol. 2007;503:466–485. doi: 10.1002/cne.21384. [DOI] [PubMed] [Google Scholar]
- Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, Greengard P, McEwen BS. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142:1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Wenzel HJ, Kunkell DD, Schwartzkroin PA. Axon arbors and synaptic connections of hippocampal mossy cells in the rat in vivo. J Comp Neurol. 1996;366:271–292. doi: 10.1002/(sici)1096-9861(19960304)366:2<270::aid-cne7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007a;35:208–219. doi: 10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho R, Correia SS, Backos DS, Carvalho AL, Esteban JA, Duarte CB. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem. 2007b;282:12619–12628. doi: 10.1074/jbc.M700607200. [DOI] [PubMed] [Google Scholar]
- Chang PC, Aicher SA, Drake CT. Kappa opioid receptors in rat spinal cord vary across the estrous cycle. Brain Res. 2000;861:168–172. doi: 10.1016/s0006-8993(99)02461-0. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba Montoya DA, Carrer HF. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res. 1997;778:430–438. doi: 10.1016/s0006-8993(97)01206-7. [DOI] [PubMed] [Google Scholar]
- Craig AM, Blackstone CD, Huganir RL, Banker G. The distribution of glutamate receptors in cultured rat hippocampal neurons: postsynaptic clustering of AMPA-selective subunits. Neuron. 1993;10:1055–1068. doi: 10.1016/0896-6273(93)90054-u. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Rev. 2001;37:153–161. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- Day M, Sung A, Logue S, Bowlby M, Arias R. Beta estrogen receptor knockout (BERKO) mice present attenuated hippocampal CA1 long-term potentiation and related memory deficits in contextual fear conditioning. Behav Brain Res. 2005;164:128–131. doi: 10.1016/j.bbr.2005.05.011. [DOI] [PubMed] [Google Scholar]
- De Nicola AF, Pietranera L, Beauquis J, Ferrini MG, Saravia FE. Steroid protection in aging and age-associated diseases. Exp Gerontol. 2009;44:34–40. doi: 10.1016/j.exger.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Diano S, Naftolin F, Horvath TL. Gonadal steroids target AMPA glutamate receptor-containing neurons in the rat hypothalamus, septum and amygdala: a morphological and biochemical study. Endocrinology. 1997;138:778–789. doi: 10.1210/endo.138.2.4937. [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci U S A. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockout mice. Mol Ther. 2008;16:1587–1593. doi: 10.1038/mt.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fykse EM, Takei K, Walch-Solimena C, Geppert M, Jahn R, De Camilli P, Sudhof TC. Relative properties and localizations of synaptic vesicle protein isoforms: the case of the synaptophysins. J Neurosci. 1993;13:4997–5007. doi: 10.1523/JNEUROSCI.13-11-04997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales KL, Tetel MJ, Wagner CK. Estrogen receptor (ER) beta modulates ERalpha responses to estrogens in the developing rat ventromedial nucleus of the hypothalamus. Endocrinology. 2008;149:4615–4621. doi: 10.1210/en.2008-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureviciene I, Puolivali J, Pussinen R, Wang J, Tanila H, Ylinen A. Estrogen treatment alleviates NMDA-antagonist induced hippocampal LTP blockade and cognitive deficits in ovariectomized mice. Neurobiol Learn Mem. 2003;79:72–80. doi: 10.1016/s1074-7427(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ. Neurologic links between epilepsy and depression in women: is hippocampal neuroplasticity the key? Neurology. 2006;66:S13–S22. doi: 10.1212/wnl.66.66_suppl_3.s13. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endo. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology. 2002;143:4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253:1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kuiper G, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J Neurosci. 2009;29:1475–1486. doi: 10.1523/JNEUROSCI.4688-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004a;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience. 2004b;124:549–560. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Romeo RD, Svenningsson P, Campomanes CR, Allen PB, Greengard P, McEwen BS. Estradiol affects spinophilin protein differently in gonadectomized males and females. Neuroscience. 2004c;127:983–988. doi: 10.1016/j.neuroscience.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Matthews J, Wilhen B, Tujague M, Wan J, Strom A, Gustafsson JA. Estrogen receptor (ER) beta modulates ERalpha mediated transcriptional activity by altering the recruitment of c-Fos and c-Jun to estrogen-responsive elements. Mol Endocrinol. 2006;20:534–543. doi: 10.1210/me.2005-0140. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Tanapat P, Weiland NG. Inhibition of dendritic spine induction on hippocampal CA1 pyramidal neurons by a nonsteroidal estrogen antagonist in female rats. Endocrinology. 1999;140:1044–1047. doi: 10.1210/endo.140.3.6570. [DOI] [PubMed] [Google Scholar]
- Medvedev NI, Rodriguez-Arellano JJ, Popov VI, Davies HA, Tigaret CM, Schoepfer R, Stewart MG. The glutamate receptor 2 subunit controls post-synaptic density complexity and spine shape in the dentate gyrus. Eur J Neurosci. 2008;27:315–325. doi: 10.1111/j.1460-9568.2007.06005.x. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Molnar E, Baude A, Richmond SA, Patel PB, Somogyi P, McIlhinney RA. Biochemical and immunocytochemical characterization of antipeptide antibodies to a cloned GluR1 glutamate receptor subunit: cellular and subcellular distribution in the rat forebrain. Neuroscience. 1993;53:307–326. doi: 10.1016/0306-4522(93)90198-o. [DOI] [PubMed] [Google Scholar]
- Morissette M, Le Saux M, Di Paolo T. Effect of oestrogen receptor alpha and beta agonists on brain N-methyl-D-aspartate receptors. J Neuroendocrinol. 2008;20:1006–1014. doi: 10.1111/j.1365-2826.2008.01754.x. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Hioki H, Fujiyama F, Kaneko T. Postnatal changes of vesicular glutamate transporter (VGluT)1 and VGluT2 immunoreactivities and their colocalization in the mouse forebrain. J Comp Neurol. 2005;492:263–288. doi: 10.1002/cne.20705. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Kurucz OS, Milner TA. Morphometry of a peptidergic transmitter system: dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus. 1999;9:255–276. doi: 10.1002/(SICI)1098-1063(1999)9:3<255::AID-HIPO6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Fester L, Zhou L, Lauke H, Carretero J, Rune GM. Inhibition of hippocampal estrogen synthesis causes region-specific downregulation of synaptic protein expression in hippocampal neurons. Hippocampus. 2006;16:464–471. doi: 10.1002/hipo.20173. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Mehra RD, Dhar P, Vij U. Chronic exposure to estrogen and tamoxifen regulates synaptophysin and phosphorylated cAMP response element-binding (CREB) protein expression in CA1 of ovariectomized rat hippocampus. Brain Res. 2007;1132:10–19. doi: 10.1016/j.brainres.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25:7780–7791. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Soriano E, Frotscher M. Mossy cells of the rat fascia dentata are glutamate immunoreactive. Hippocampus. 1994;4:65–69. doi: 10.1002/hipo.450040108. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom-Davis CA, Ouimet CC, Feng J, Allen PB, Greengard P, Houpt TA. Impaired conditioned taste aversion learning in spinophilin knockout mice. Learn Mem. 2001;8:272–278. doi: 10.1101/lm.42101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson KE, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the rat brain. 2 Edition. San Diego, CA: Academic Press; 2000. [Google Scholar]
- Szymczak S, Kalita K, Jaworski J, Mioduszewska B, Savonenko A, Markowska A, Merchenthaler I, Kaczmarek L. Increased estrogen receptor beta expression correlates with decreased spine formation in the rat hippocampus. Hippocampus. 2006;16:453–463. doi: 10.1002/hipo.20172. [DOI] [PubMed] [Google Scholar]
- Takamori S, Riedel D, Jahn R. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. J Neurosci. 2000;20:4904–4911. doi: 10.1523/JNEUROSCI.20-13-04904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Drake CT, McEwen BS, Milner TA. Ovarian steroids modulate leu-enkephalin levels and target leuenkephalinergic profiles in the female hippocampal mossy fiber pathway. Brain Res. 2008;1232:70–84. doi: 10.1016/j.brainres.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome LF, Luine VN, Drake CT, McEwen BS, Milner TA. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positionedfor direct modulation by ovarian steroid receptors. Neuroscience. 2009;159:204–216. doi: 10.1016/j.neuroscience.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008;73:997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Yokotani N, Doi K, Wada K. Immunochemical characterization of the non-NMDA glutamate receptor using subunit-specific antibodies. Evidence for a hetero-oligomeric structure in rat brain. J Biol Chem. 1992;267:501–507. [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel HJ, Buckmaster PS, Anderson NL, Wenzel ME, Schwartzkroin PA. Ultrastructural localization of neurotransmitter immunoreactivity in mossy cell axons and their synaptic targets in the rat dentate gyrus. Hippocampus. 1997;7:559–570. doi: 10.1002/(SICI)1098-1063(1997)7:5<559::AID-HIPO11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Wheal HV, Miller JJ. Pharmacological identification of acetylcholine and glutamate excitatory systems in the dentate gyrus of the rat. Brain Res. 1980;182:145–155. doi: 10.1016/0006-8993(80)90837-9. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Witter MP. Organization of the entorhinal-hippocampal system: A review of current anatomical data. Hippocampus. 1993;3 Suppl:33–44. [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Wenzel HJ, Schwartzkroin PA. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. J Comp Neurol. 1996;373:108–117. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]