Abstract
Functional magnetic resonance imaging was used to identify regions involved in working memory (WM) retrieval. Neural activation was examined in two WM tasks: an item recognition task, which can be mediated by a direct-access retrieval process, and a judgment of recency task that requires a serial search. Dissociations were found in the activation patterns in the hippocampus and in the left inferior frontal gyrus (LIFG) when the probe contained the most recently studied serial position (where a test probe can be matched to the contents of focal attention) compared to when it contained all other positions (where retrieval is required). The data implicate the hippocampus and the LIFG in retrieval from WM, complementing their established role in long-term memory. Results further suggest that the left posterior parietal cortex (LPPC) supports serial retrieval processes that are often required to recover temporal order information. Together, these data suggest that the LPPC, the LIFG, and the hippocampus collectively support WM retrieval. Critically, the reported findings support accounts that posit a distinction between representations maintained in and outside of focal attention, but are at odds with traditional dual-store models that assume distinct mechanisms for short- and long-term memory representations.
INTRODUCTION
Models of working memory (WM) often posit specialized memory stores for the products of recent cognitive operations. For instance, Baddeley's (2000) WM model assumes three stores: a phonological store, a visual–spatial sketchpad, and an episodic buffer. However, the evidence for short-term representations being functionally distinct from long-term representations has been challenged on several grounds (e.g., Surprenant & Neath, in press; Nairne, 1996; Crowder, 1993; Wickelgren, 1973). Alternative approaches eschew the notion of specialized WM stores in favor of unified accounts in which the representations of recent events are governed by the same principles as representations in long-term memory (LTM) (e.g., Nairne, 1996).
Although the notion of distinct WM stores remains controversial, it, nonetheless, appears necessary to draw a distinction between representations that are being actively processed—those upon which attention is focused— and the potentially larger set of representations formed as the by-products of recent processing (e.g., McElree, 1998, 2001, 2006; Cowan, 2005; Wickelgren, Corbett, & Dosher, 1980). Although several indirect lines of evidence motivate this distinction (see Cowan, 2005), measures of the speed of accessing information (reviewed below) provide the most direct evidence for a unique representational state associated with the focus of attention. These measures suggest that the contents of focal attention can be accessed without engaging the type of retrieval operations required to access representations that reside in memory proper, be it WM or LTM (McElree, 1998, 2001, 2006).
We sought in this study to identify the neural mechanisms that support WM retrieval and to specifically address whether they overlap with those that are known to mediate retrieval from LTM. Part of our logic involved identifying the neural correlates underlying the existing behavioral evidence that dissociates access to representations maintained in focal attention to those that need to be retrieved from WM. Specifically, we reasoned that as the behavioral evidence indicates that only conditions involving the latter engage retrieval operations, neural activation specific to these conditions should serve to uniquely identify regions involved in WM retrieval.
A second aspect of our experimental logic for investigating WM retrieval mechanisms involved manipulating the nature of the to-be-retrieved information as a means of eliciting different retrieval operations. Investigations of memory retrieval have demonstrated that the nature of the information required for a task can determine what type of retrieval operation is deployed. Specifically, access to an item's representation in WM is typically direct, with retrieval cues contacting memory representations in a unitary manner without a search through irrelevant memories (McElree, 1998, 2006; Clark & Gronlund, 1996; McElree & Dosher, 1989). In contrast, recovering relational information—either temporal or spatial order information—requires a relatively slow, serial search through an ordered set of memory representations (McElree, 2001; McElree & Dosher, 1989, 1993). We capitalized on the latter findings as a means of identifying the brain regions supporting serial retrieval: Activation in those regions should parametrically vary with the required number of serial operations needed for successful retrieval.
Neural Basis of WM
Research has identified a remarkably consistent network of brain regions involved in verbal WM tasks (e.g., Chein, Ravizza, & Fiez, 2003; D'Esposito et al., 1998). These include regions hypothesized to be involved in memory storage [e.g., left posterior parietal cortex (LPPC; supramarginal gyrus—BA 40)], speech-based rehearsal processes [e.g., Broca's area (BA 44/45), with contributions from premotor, pre-SMA, and cerebellar areas], and executive control processes [e.g., dorsolateral prefrontal cortex (BA 9/46)]. Nonetheless, the functional role of these regions remains controversial (Chein et al., 2003) and, in order to further understand how these regions contribute to WM, it is necessary to focus on dissociating the contribution of these regions to the distinct cognitive processes of encoding, maintenance, and retrieval that are operative in WM tasks. Without doing so, it is doubtful we can gain a complete understanding of the functional role of different regions implicated in WM tasks, and whether these regions are distinct from those involved in LTM.
Although less is known about the specific role of regions that contribute to WM, regions that support LTM processes are well established: The medial-temporal lobes (MTL) are known to be crucial for the formation and retrieval of long-term episodic memories (see Eichenbaum, Yonelinas, & Ranganath, 2007; Mayes, Montaldi, & Migo, 2007; Davachi, 2006 for recent reviews). In addition, the left inferior frontal gyrus (LIFG) has been implicated in retrieval and selection of long-term representations (Miller & Cohen, 2001; Thompson-Schill, D'Esposito, Aguirre, & Farah, 1997; Demb, Desmond, Wagner, & Chandan, 1995) as well as the strategic retrieval of phonological information (Gold, Balota, Kirchhoff, & Buckner, 2005). However, the LIFG is also known to support WM maintenance operations (Chein et al., 2003; Smith & Jonides, 1999). Similarly, there are indications that the MTL may contribute to WM processes as well: Hippocampal activation has been noted during WM tasks (Blumenfeld & Ranganath, 2006; Cabeza, Dolcos, Graham, & Nyberg, 2002; Davachi & Wagner, 2002; Ranganath & D'Esposito, 2001; Stern, Sherman, Kirchhoff, & Hasselmo, 2001), and patients with MTL damage show some WM impairments (Hannula, Tranel, & Cohen, 2006; Nichols, Kao, Verfaellie, & Gabrieli, 2006; Olson, Moore, Stark, & Chatterjee, 2006). These findings suggest that the MTL and the LIFG may be important for WM processes in some fashion in addition to their role in LTM.
Present Study
We examined neural activation in two paradigms used extensively in behavioral time-course studies of WM retrieval: An item recognition task was used to investigate the retrieval of item information (McElree, 2006), and a judgment of recency (JOR) task was used to investigate the recovery of temporal order information (McElree & Dosher, 1993; Hacker, 1980; Muter, 1979). Critically, in both tasks, the recency of probe items was parametrically varied in order to (i) identify regions that are involved in retrieval of information outside of focal attention, (ii) determine whether these regions are unique to WM or overlap with those that are known to support LTM retrieval, and (iii) investigate the distinct neural mechanisms that support direct-access (i.e., retrieval of item information) and serial search operations (i.e., recovery of temporal order information).
Previous designs have not allowed for WM retrieval operations to be examined separately from encoding operations because the tasks were blocked (e.g., Marshuetz, Smith, Jonides, DeGuits, & Chenevert, 2000), and WM studies have typically used extensive maintenance delay periods. By contrast, in the present study, participants were only cued as to which retrieval operation they should perform (item recognition or JOR; see Figure 2) after the encoding phase. In addition, participants could not predict which serial position (SP) would be tested in each trial. Thus, this design equated encoding across the tasks and across SPs within each task, allowing us to isolate retrieval differences specific to each retrieval task and to each SP. Additionally, probes were presented shortly after (750 msec delay period) study items to eliminate or minimize engagement in maintenance rehearsal operations, so that retrieval specific differences in neural activation across tasks and SPs could be examined without confounding effects of encoding and maintenance operations.
Figure 2.
A sample sequence for an experimental trial. Each trial began with a fixation point, followed by a study list of five letters. After the fifth study letter, participants were presented with a visual mask that cued either an item recognition (IR) or a judgment of recency ( JOR) trial. Two test probes were presented followed by the visual mask. (A) In IR trials, one probe was from the current study list and one probe was new. Participants chose the letter that was in the current study list. (B) In JOR trials, both probes were from the current study list, and participants chose the letter that was more recent.
Our strategy for identifying regions recruited in WM retrieval was to compare activation during trials for which a decision can be made on the basis of information still in focal attention to the activation observed during trials when a decision requires a retrieval operation. Behavioral evidence suggests that this can be accomplished by comparing trials that involve test probes from the most recently studied list position—a case where no other item intervenes between study and test—to trials when the test probes are drawn from earlier study positions. This is the case because decisions about the former can be achieved solely by accessing information resident in focal attention, whereas decisions about the latter require retrieval operations. Support for this claim has come from investigations of several tasks requiring processing of sequentially presented information (item recognition, paired associate recognition, judgments of recency, n-back discriminations, and sentence processing; see McElree, 2006), in which it has been consistently found that the speed of accessing representations from different study positions shows a sharply dichotomous pattern. Specifically, responses to the most recently studied item—a representation of which is plausibly maintained in focal attention at test time—are approximately 30% to 50% faster than responses to representations recently displaced from focal attention (Öztekin & McElree, 2007; McElree, 1996, 1998, 2006; McElree & Dosher, 1989, 1993; Wickelgren et al., 1980).
Crucially, these dichotomous patterns, as illustrated in Figure 1 with data reported in McElree and Dosher (1989), are derived from the response-signal speed–accuracy tradeoff (SAT) procedure. Unlike conventional reaction time (RT) measures, the SAT procedure provides separate measures of the quality (strength, fragility, or analogous construct) of the memory representation and the speed with which it can be accessed. Although both response accuracy and RT systematically decline as test items are drawn from more remote study positions, it is the direct measures of access speed derived from the SAT procedure that show the dichotomous pattern of fast access for information in focal attention and slower access for all other information that must be retrieved from memory. That the observed speed advantage uniquely implicates privileged access for information in focal attention receives support from several convergent lines of evidence, including findings that the advantage tracks with the number of items concurrently encoded, with rehearsal operations in a controlled rehearsal study, and with items that subjects were precued to retrieve prior to a test (McElree, 2006).
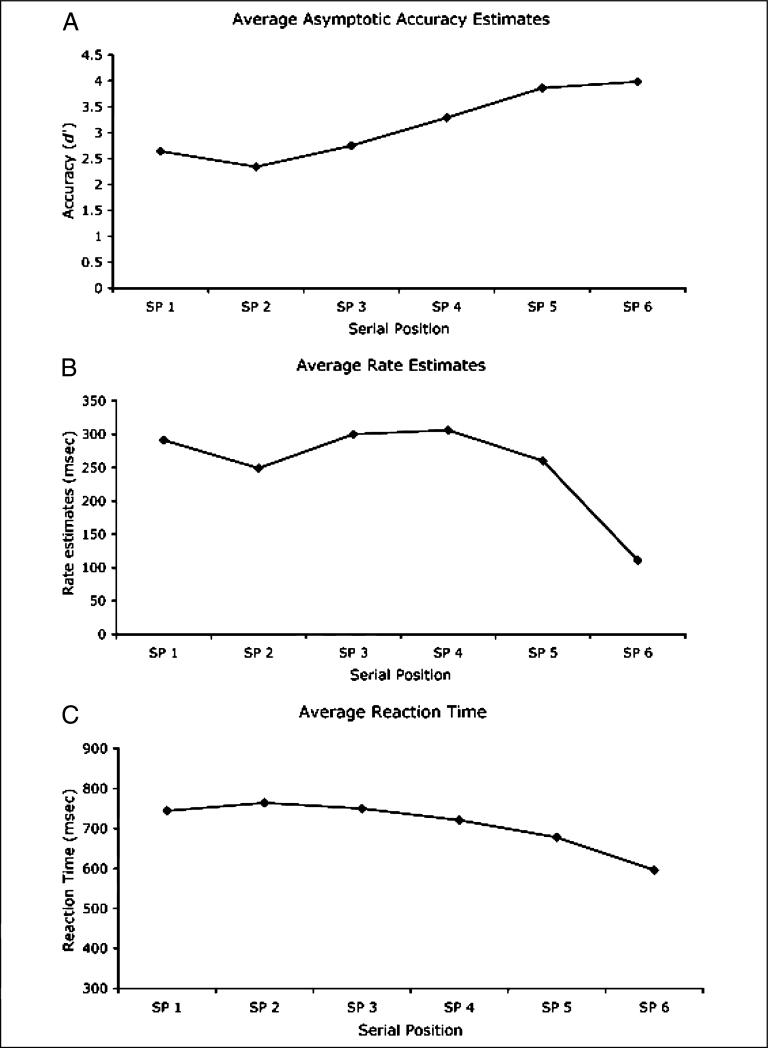
Figure 1.
Illustration of the speed of processing advantage for the last serial position in a speed–accuracy tradeoff (SAT) version of a six-item probe recognition task (A and B), and the corresponding reaction time (RT) patterns for an RT version of the same task (C). The estimates and data from all panels come from experiments reported in McElree and Dosher (1989). (A) The asymptotic accuracy estimates (in d′ units) for each of the six serial positions, which were derived from fits of an exponential approach to a limit retrieval function to the full time-course SAT data (see McElree & Dosher, 1989). These SAT asymptotes reflect the highest level of accuracy obtained with maximal retrieval time, and here exhibit a standard serial position profile, with accuracy increasing as the test probe is drawn from more recent positions, coupled with a modest primacy effect. (B) Rate of informational accrual estimates (msec) that determine the rate of rise of the SAT functions for each serial position. A speed advantage for the last serial position is clearly evident. (C) RT for each serial position from the RT version of the same task. RT patterns largely mirror the asymptotic accuracy patterns in (A) in showing a gradual decline in RT as a function of recency of the test probe. The speed advantage for the last item evident in the unbiased speed estimates shown in (B) also contributes to this RT profile (evidence presented in McElree & Dosher, 1989), but the sharp discontinuity evident in (B) is masked by overall differences in retrieval strength across serial positions. SP = serial position. The presented data were taken from McElree and Dosher (1989).
In line with evidence indicating that the contents of focal attention can be accessed without a retrieval operation, we predicted substantially reduced activation in regions involved in WM retrieval for trials requiring a memory decision about a probe item from the last SP (SP 5). To further investigate the specific role of regions identified to be active during WM retrieval, we also examined how neural activation in these regions varies with the type and complexity of retrieval operation. Specifically, in order to investigate the neural mechanisms underlying retrieving information from WM with either a direct access or serial search operation, we identified regions that significantly contribute more to JOR than item recognition, and further examined how neural activation in JOR was modulated by the cognitive strategies adapted to recover temporal order, or relational, information.
Our means for identifying the cognitive strategies and interpreting neural activation in JOR was based on previous behavioral work on this paradigm: Early models of JOR assumed that participants used an assessment of trace strength as a proxy for recency, by comparing the two probes and selecting the one with the greatest strength (e.g., Yntema & Trask, 1963). Importantly, strength-based models of JOR predict an RT distance effect, which predicts RT to decrease with increasing distance in SP between the two probes. However, further research has indicated that probe distance does not predict JOR RT. Rather, RT is determined by the recency of one of the test probes alone, typically the most recent item in the probe (McElree & Dosher, 1993; Hacker, 1980; Muter, 1979). Specifically, if participants use a backward (recency-based) serial search for JOR, RT will vary with the most recent item in the test probe, with faster RTs as the most recent item is drawn from more recent study positions. If participants use a forward serial search, then RTs will be faster as the probe is drawn from less recent positions. Accordingly, we used participants’ RT patterns to identify their serial search strategies, and then examined brain regions important in serial search by looking for distinct blood oxygen level-dependent (BOLD) activations across participants who applied forward and backward serial search strategies.
METHODS
Participants
Fifteen right-handed adults (7 women, aged 18–28 years) participated in the study. Informed consent was obtained in accordance with the institutional review board at New York University. Participants had normal or corrected-to-normal vision and were paid for their time.
Design and Stimuli
The stimuli were consonants of the English alphabet. The study lists consisted of five consonants randomly selected (without replacement). In JOR trials, the test probes were randomly selected from the current study list. In item recognition trials, one of the test probes was randomly selected from the current study list, and a consonant that had not appeared in the current study list was randomly selected for the other test probe. Critically, the allocation of JOR and item recognition trials was randomized so that participants could not predict the type of trial (JOR or item recognition) during encoding of the study list. Instead, they were cued right before the test probes as to whether they would perform an item recognition or JOR task (see below). For each task, all SPs were tested equally often and randomly. The order of the test probes on the screen was also determined randomly, and each order appeared equally often for all probe types. Hence, the participants could not predict which type of task they would perform and which study positions would be tested while encoding the study list.
Procedure
The sequence of events in a single trial is illustrated in Figure 2. Each trial began with a centered fixation point presented for 500 msec. Following the fixation point, each of the five letters (in lowercase) of the study list was presented one at a time on the center of the screen for 500 msec. After the presentation of the last study letter, a mask, also a task indicator, consisting of non-letter symbols in either red or blue color, was presented on the center of the screen for 750 msec. The blue mask consisting of symbols “#####” cued an item recognition trial, and the red mask consisting of symbols “&&&&&” cued a JOR trial for the participants. Following the mask, two test probes in uppercase were presented on the screen for 3000 msec. In JOR trials, both probes were from the current study list, and the participants chose the letter that was more recent by pressing either the middle or index finger on the button box. In item recognition trials, one of the probes was new, and the other probe was from the study list. Participants chose the letter that was from the study list by pressing the middle or index finger on the button box. The intertrial interval consisted of presentation of a green fixation point (symbol “^”) on the center of the screen for a variable duration (2.25 to 22.5 sec). The order of trials and duration of intertrial interval was optimized with the Optseq2 program (http://surfer.nmr.mgh.harvard.edu/optseq/) for optimal stimulus presentation for event-related functional magnetic resonance imaging (fMRI) designs.
fMRI Protocol
A 3-T scanner acquired functional and anatomical images. We obtained 36 slices (3 mm × 3 mm × 5 mm) oriented perpendicular to the hippocampal axis (TR = 2.25 sec; TE = 30 msec; flip angle = 90°). Following the functional runs, T1-weighted high-resolution anatomical images (MP-RAGE) were obtained for localization.
Image Processing
Image processing and data analysis were performed using SPM2 (www.fil.ion.ucl.ac.uk/spm/). Preprocessing of images consisted of correction of slice acquisition timing across slices, realigning the images to the first volume in each run to correct for head movement, normalization of functional and anatomical images to a standard template EPI, and smoothing images with a 6-mm full-width half-maximum isotropic Gaussian kernel.
Behavioral Data Analysis
For both the item recognition and JOR tasks, accuracy was derived by computing asymmetric d′ for each test probe type. Asymmetric d′ scaling accommodates bias to one of response alternatives, d′ = [z(1|1) – z(1|2)]/21/2, where z is the standard normal deviate of the probability of responding that the test probe was the first alternative, given that the test probe was either the first (1|1) or the second (1|2) alternative.
fMRI Data Analysis
Data analysis was conducted using the General Linear Model implemented in SPM2. Only correct trials were analyzed. Correct trials were sorted according to the conditions of interest (type of task and study position of the test probe) and were modeled using a canonical hemodynamic response function and its temporal derivative. Data across runs were concatenated and modeled as one session with mean signal and scanner drift entered into the model as covariates. For each participant, contrasts of interest were derived using a subject-specific fixed-effects model. For SP contrasts, the trials were modeled from the second TR because the first TR corresponded to the encoding phase and, hence, was identical across trials. Contrast images were then carried onto a second-level random-effects analysis. Regions consisting of at least 16 contiguous voxels that exceeded an uncorrected threshold of p < .001 were considered significant in the neocortex. To account for lower signal-to-noise ratio in the MTL, the threshold was adjusted to p < .005 with a minimum cluster size of 10 contiguous voxels to assess activation here (e.g., Strange, Otten, Josephs, Rugg, & Dolan, 2002; Ojeman et al., 1997).
Regions of interest (ROI) emerging from functional contrasts were further analyzed using the MarsBaR ROI toolbox for SPM (http://marsbar.sourceforge.net/). The event-related time course (measured in percent BOLD signal change) was derived for each region, and percent signal change data across participants were subjected to mixed-effect analysis of variance (ANOVAs), treating “condition” (type of task and study position of the test probe) and “time” (TRs) as repeated measures and “subjects” as a random effect. In case of a condition main effect or a Condition × Time interaction, these effects were followed by additional comparisons on peak percent signal change to reveal the statistical pattern across SPs. For these comparisons, the peak time point (i.e., point of maximum percent signal change) and the two adjacent time points (peak time point plus/minus 1 TR) were averaged to account for potential differences in time to peak across conditions.
RESULTS
Behavioral Data
Item Recognition Task
Accuracy increased as the test probe was drawn from more recent positions [F(4, 56) = 3.478, p < .013, pairwise comparisons were not reliable] and participants also responded faster to more recent probes [F(4, 56) = 8.566, p < .001] (Figure 3A). Pairwise comparisons on RT further indicated that SP 5 was significantly faster than SP 1 [t(14) = 2.452, p < .028] and SP 2 [t(14) = 4.039, p < .001]. Overall, the RT data show the same pattern as the data presented in Figure 1A, which were originally reported in McElree and Dosher (1989).
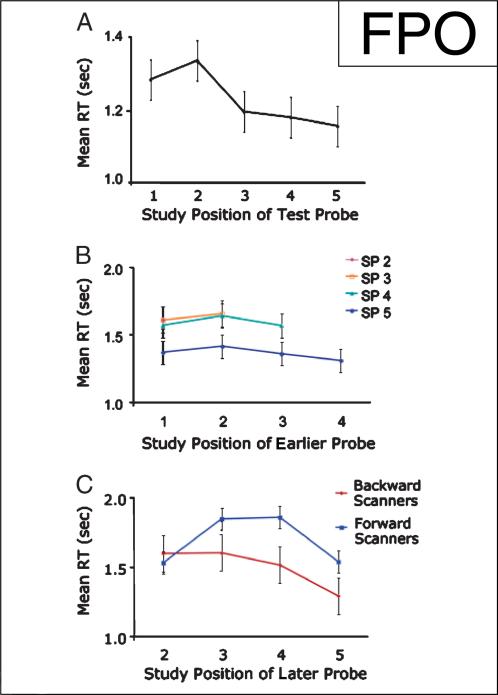
Figure 3.
RT data in item recognition (IR) and judgment of recency ( JOR) tasks. (A) Average (over participants) RT (sec) in IR task. (B) Average (over participants) RT (sec) in JOR task for later Probes 2 to 5 (noted as separate lines in the figure) as a function of study position of earlier probe (SP = study position). Performance was modulated by the recency of the later probe, indicating that participants were engaging in a serial search to recover temporal order information in this task. (C) RT in JOR task as a function of study position of the later probe, broken into backward and forward scanning participants. Backward scanning participants (participants who started scanning from the end of the study list) display faster RTs as the position of the probe was more recent, whereas forward scanning participants (participants who started scanning from the beginning of the study list) show slower RTs as the position of the probe was more recent, with the exception of an RT advantage for the most recent item. As the most recent item can be maintained in current focus of attention, it should not engage a retrieval process. Consistent with this claim, both forward and backward scanning participants exhibit fast RTs for this item, indicating that the serial scan process that was engaged for Positions 2 to 4 to recover temporal order information was not necessary for SP 5, which could directly be matched to the contents
Judgments of Recency Task
Similar SP effects were observed in the JOR task. Accuracy increased as the later item in the test probes was more recent [F(3, 42) = 10.967, p < .001; pairwise comparisons not reliable]. RT also depended on the SP of the later probe, with more recent probes eliciting faster responses [F(3, 42) = 9.853, p < .001] (Figure 3B). Pairwise comparisons indicated that RT was reliably faster when the most recent probe was Position 5 compared to all other positions [t(14) = 3.978, p < .001 for Probe 2; t(14) = 6.227, p < .001 for Probe 3; t(14) = 5.469, p < .001 for Probe 4].
As in previous JOR investigations (McElree & Dosher, 1993; Hacker, 1980; Muter, 1979), RT and accuracy patterns were not consistent with a strength-based model. Holding position of the later (most recent) probe constant, the position of the earlier (least recent) probe did not reliably affect accuracy [p > .171 for Probe 5, p > .281 for Probe 4, F(1, 14) = 3.962, p < .066 for Probe 3] or RT [F(3, 42) = 2.435, p < .078 for Probe 5, p > .171 for Probe 4, p > .318 for Probe 3]. Hence, the factor determining RT was not the distance between the probes but rather the SP of the most recent probe item. Consequently, the rest of the analyses concerning the JOR task were conducted as a function of the position of the later probe, averaging over the position of the earlier probe (e.g., Probe 4 averaged over Probes 41, 42, and 43). (Consequently, there is no SP “1” for the JOR, as all probes with this SP contain a more recent item.)
Examination of individual participants’ data indicated that eight participants used a backward serial search strategy, with RT increasing as the later probe was drawn from less recent positions. Four participants produced a pattern indicating a forward serial search, with RT increasing as the probe was drawn from more recent positions (Figure 2C). This interaction was significant [F(3, 30) = 6.574, p < .002]. Three participants’ data could not be classified with either search strategy, or with a strength-based strategy.
Neuroimaging Data
Focal Attention Effects
To contrast access to information in focal attention with information outside of focal attention, we compared neural activation during probes containing SP 5, where a test probe can be directly matched to the contents of focal attention, to probes containing Positions 1 to 4, where the probes will necessitate a retrieval operation. Table 1 reports regions showing reliably more BOLD activation for SP 1 to SP 4 than SP 5. The reverse analysis revealed no significant activation in either task.
Table 1.
List of Reliable Activations from Voxelwise Comparisons
| Region | BA | x | y | z | Z | Cluster Size |
|---|---|---|---|---|---|---|
| Item Recognition Task | ||||||
| SPs 1–4 > SP 5 | ||||||
| Superior frontal gyrus | 6 | 6 | 6 | 60 | 4.33 | 41 |
| 6 | –30 | 66 | 3.39 | 30 | ||
| –15 | –3 | 57 | 3.60 | 18 | ||
| Middle temporal gyrus | 37 | –57 | –63 | 6 | 3.81 | 35 |
| Thalamus | –15 | –24 | 12 | 3.65 | 22 | |
| Putamen | –18 | 12 | 0 | 3.62 | 26 | |
| Cuneus | 6 | –93 | 3 | 4.22 | 330 | |
| Hippocampus | 30 | –21 | –12 | 2.82 | 11 | |
| Judgments of Recency Task | ||||||
| SPs 2, 3, 4 > SP 5 | ||||||
| Superior frontal gyrus | 6 | –15 | –3 | 66 | 4.28 | 55 |
| Inferior frontal gyrus | 45 | –57 | 21 | 3 | 4.08 | 16 |
| Superior temporal gyrus | 42 | 60 | –30 | 6 | 3.93 | 19 |
| Hippocampus | –33 | –24 | –9 | 3.13 | 39 | |
| (JOR + IR) > Baseline | ||||||
| Superior frontal gyrus | 6 | –3 | 6 | 60 | 4.85 | 99 |
| Middle/inferior frontal gyrus | 9 | –48 | 0 | 39 | 4.98 | 256 |
| Inferior frontal gyrus | 45 | –30 | 27 | 3 | 4.07 | 21 |
| Putamen | –21 | 6 | –3 | 5.37 | 144 | |
| Intraparietal sulcus | 40/39 | –27 | –63 | 39 | 3.98 | 22 |
| –27 | –48 | 45 | 3.63 | 43 | ||
| Inferior occipital gyrus | 18 | –30 | –96 | –6 | 5.37 | 704 |
| 27 | –99 | –9 | 4.98 | 733 |
SP = serial position.
Item recognition
As Figure 4B illustrates, pairwise comparisons on the peak percent signal change revealed that hippocampal activation was significantly reduced for SP 5 trials compared to all other SPs [SP 4: F(1, 14) = 8.177, p < .013; SP 3: F(1, 14) = 6.430, p < .024; SP 2: F(1, 14) = 5.876, p < .029; and SP 1: F(1, 14) = 3.876, p < .069].
Figure 4.
Changes in neural activation depending on study position of the test probe in item recognition task (left column) and JOR (right column). (A) Right hippocampus (30 –21 –12) activation in item recognition task from SP 1 to SP 4 > SP 5 contrast at .005 threshold. (B) Peak percent signal change in this region as a function of study position of the test probe. (C) Left hippocampus (–33 –24 –9) activation in JOR task from SPs 2 to 4 > SP 5 contrast at .005 threshold. (D) Peak percent signal change in this region as a function of study position of the probe. (E) The left inferior frontal gyrus (IFG) (–57 21 3) in JOR task from SPs 2 to 4 > SP 5 contrast at .001 threshold. (F) Peak percent signal change in the left IFG (BA 45) as a function of study position of the probe. In both tasks, the peak percent signal change in these regions indicate diminished activation for study position 5 compared to other probes.
Judgments of recency
Peak percent signal change in the left hippocampus (Figure 4D) revealed diminished activation for Probe 5 trials compared to other probes [pairwise comparisons were not reliable]. The same trend was also evident in the left IFG (BA 45) (Figure 4F) [SP 4: F(1, 14) = 6.634, p < .022; SP 3: F(1, 14) = 6.483, p < .023; SP 2: F(1, 14) = 3.229, p < .094].
Accuracy analysis
As previous work has implicated the MTL in successful memory retrieval (Dobbins, Heather, Wagner, & Schacter, 2003; Eldridge, Knowlton, Furmanski, Bookheimer, & Engel, 2000; Stark & Squire, 2000), we examined whether the MTL also support retrieval success in our JOR task.1 We conducted a voxelwise contrast that assessed regions that showed enhanced neural activation for correct compared to incorrect trials (collapsing across SPs 1–4). This analysis revealed regions in the right parahippocampal gyrus and the right posterior hippocampus. Additionally, we queried the left hippocampal and the inferior frontal gyri ROIs reported above in the JOR task. Only the left hippocampus ROI showed greater activation for correct trials compared to incorrect trials. This pattern was marginally significant across participants [F(1, 12) = 3.886, p < .072]. These findings further indicate the role of the hippocampus in successful recovery of temporal order information necessary in the JOR task.
Time on task effects
Activations reported in both tasks remained significant at p < .005 threshold when RT was modeled as a covariate, confirming that diminished neural activation in the reported regions for the most recently studied test probe is not merely a result of the faster RTs associated with this item compared to other test probes. Hence, judgments involving the most recently studied item engendered less activation in the hippocampus and the LIFG, suggesting that these regions are important in WM retrieval.
Serial Retrieval Effects
We next turn to our second question: For representations that are outside the focus of attention, what are the neural processes associated with the distinct forms of retrieval operations (direct access vs. serial search) that access different types of information (item vs. temporal order information) from WM? To investigate this question, we first identify the regions that show differential neural activation during JOR and item recognition tasks. We next consider the two distinct hypotheses identified in the Introduction, namely, the distance hypothesis (based on the strength-based model) and the serial scan hypothesis (based on serial search models) to identify the specific contribution of these regions to WM retrieval.
Analyses of the regions that were engaged during task performance (Table 1) revealed enhanced activation for JOR compared with item recognition in the left intraparietal sulcus (LIPS, BA 40/39) [F(1, 14) = 15.848, p < .001], the left IFG (BA 45) [F(1, 14) = 12.860, p < .003], and the supplementary motor area [F(1, 14) = 19.167, p < .001].
Test of the strength-based model
In line with previous behavioral work (see Introduction), our behavioral data indicated that participants did not engage in strength-based judgments in our JOR paradigm. Nonetheless, to make sure that the neuroimaging data show the same pattern, and to follow up on previous neuroimaging work that suggested probe distance effects in a temporal order memory paradigm (Marshuetz, Reuter-Lorenz, & Smith, 2006; Marshuetz et al., 2000), we used a parametric analysis to assess differences in activation as a function of probe distance. No reliable activations were found.
Serial search processes
Accordingly, we next examined the regions (among those that showed greater activation for JOR than item recognition) that show neural activation consistent with a serial search retrieval mechanism. To do so, we compared neural activation in these regions for our backward and forward scanning participants (as identified from the RT data in the Behavioral Data section).
Strikingly, examination of activation across SPs revealed that the resultant BOLD activation patterns were reversed for backward and forward scanners in the LIPS (BA 39/40) and the LIFG (BA 45), indicating that activation in these regions was correlated with the number of items that needed to be scanned in memory (Figure 5). A 4 (SP) by 2 (group) ANOVA indicated a main effect of SP [F(3, 30) = 3.408, p < .030], and crucially, a significant interaction between SP and group [F(3, 30) = 3.734, p < .022] for the IPS. That is, for backward scanners, activation decreased as the probe was more recent, whereas for forward scanning participants, activation increased as the probe was more recent, with the exception of the last item, which was maintained in focal attention and did not necessitate a serial scan operation for access.2 The same trend was also evident in the LIFG: The ANOVA results did not reach significance, but there was a marginal difference in the slopes of the two groups [t = –1.919, p < .061].
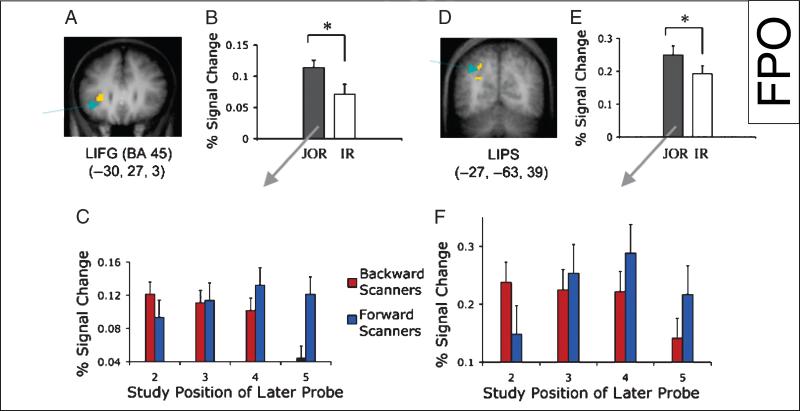
Figure 5.
Neural correlates of serial search mechanisms in JOR task. (A) LIFG (–30 27 3) activation. (B) Peak percent signal change in this region as a function of the type of task ( JOR = judgments of recency; IR = item recognition). (C) Peak percent signal change in this region in JOR as a function of the study position of the probe for backward and forward scanning participants. (D) LIPS (–27 –63 39) activation. (E) Peak percent signal change in this region as a function of the type of task. (F) Peak percent signal change in this region in JOR as a function of study position of the probe for backward and forward scanning participants. Both regions show reversed neural activation for forward and backward scanning participants. For backward scanning participants (participants who start scanning at the end of the list), neural activation shows a linear decrease as the study position of the later probe is more recent, correlated with the number of items scanned in memory. On the contrary, for forward scanning participants (participants who start scanning from the beginning of the list), neural activation linearly increases as the study position of the probe is more recent, with the exception of the most recent item (Position 5). This decline for SP 5 is consistent with the hypothesis that this item was maintained in focal attention and was not subject to a retrieval mechanism.
To assess whether the reported effects might be confounded by different encoding strategies across the two groups of participants, we examined neural activation in the reported LPPC and LIFG regions in the item recognition task. Specifically, if backward and forward scanning participants have different encoding strategies, the pattern found in JOR should also be evident in the item recognition data. However, an SP by Group ANOVA revealed no reliable SP and group interaction in either of the regions, indicating that the reported serial scan findings reflect the distinct retrieval strategies deployed by the backward and forward scanning participants.3
To ensure that left posterior parietal regions were particularly involved in serial retrieval processes, we further conducted an ROI analysis on the supramarginal gyrus—the BA 40 peak (defining a 6-mm sphere around this voxel) reported in Marshuetz et al. (2000) to be more engaged in temporal order memory than item recognition. Indeed, this region was more active in JOR than in item recognition [F(1, 14) = 13.223, p < .003]. Critically, activation in this region also correlated with scanning load [F(3, 30) = 3.622, p < .024 for interaction of SP and group]. These results indicate that the LPPC (specifically the left supramarginal gyrus and the LIPS) were involved in serial search operations in JOR.
DISCUSSION
Focal Attention Effects
Neural activation in several regions, including the hippocampus and the LIFG, was substantially reduced for the most recent item in the memory set. To our knowledge, these data provide the first neuroimaging evidence demonstrating clear dissociations between accessing information in focal attention and retrieving information from memory. Behavioral measures of access speed using SAT have consistently found an analogous dissociation, with the most recent item being accessed at a markedly faster speed than all other positions (McElree, 2006). Hence, the neural evidence aligns with behavioral findings in suggesting that retrieval operations are not required for a small subset of WM representations in focal attention. Interestingly, the signature of focal attention was a lack of activation present during retrieval of items from WM. Thus, the implication is that retrieval processes supported by these regions are not recruited for items within focal attention.
Estimates of the capacity of focal attention across various tasks range from 1 to 4 units of information (Cowan, 2005). However, like previous behavioral findings, our neuroimaging data converge with estimates of focal capacity derived from the speed of processing sequentially presented verbal items (e.g., McElree, 2006; Wickelgren et al., 1980) in indicating that only the last unit in a memory set is resident in focal attention at test time. Presumably, this was the case because, for this information alone, no activity intervened between study and test, enabling the most recent item to stay in the current focus of attention at test.
Research on visual short-term memory (STM) (e.g., Xu & Chun, 2006; Vogel, Woodman, & Luck, 2001) suggests a larger focal attention capacity, typically three to four items. However, these estimates are derived from accuracy measures in tasks requiring the processing of simultaneous multi-object visual displays (e.g., colored shapes). There may be salient differences in the encoding and retention of the information in these tasks and ours that could account for the different estimates. For instance, simultaneously presented multi-object visual displays afford a greater potential for immediate coding of relational information and grouping operations. Furthermore, these lines of research may assess different notions of “capacity.” Research on multi-object visual displays measures the upper limit on encoding of concurrently presented elements, whereas our research measures the ability to access and internally maintain items in focal attention while processing new information that is accessible in the environment. Further research is clearly needed to address these issues, hence, we stress that our findings should be interpreted with respect to the processing of sequentially presented information only.
Although these dissociations were evident in several regions, we limit our discussion to two salient regions, the LIFG and the hippocampus, regions previously implicated in LTM retrieval. As noted, previous work suggests LIFG involvement in the retrieval and/or selection of long-term representations (e.g., Miller & Cohen, 2001; Thompson-Schill et al., 1997; Demb et al., 1995). Our finding of enhanced LIFG activation for SPs 1–4, compared to the absence of such activation for SP 5, indicates that the LIFG is also critical in the selection and/or retrieval of what are traditionally regarded as WM representations. Specifically, our findings identify a ventrolateral portion of the LIFG that has been previously indicated to be involved in controlled episodic and semantic retrieval (see Badre & Wagner, 2007 for a review). The role of the MTL for long-term episodic memory is well established, but recent neuroimaging (e.g., Blumenfeld & Ranganath, 2006; Cabeza et al., 2002; Davachi & Wagner, 2002; Ranganath & D'Esposito, 2001; Stern et al., 2001) and patient studies (e.g., Hannula et al., 2006; Nichols et al., 2006; Olson et al., 2006) suggest that it may have a similar function in the WM domain. Recently, Talmi, Grady, Goshen-Gottstein, and Moscovitch (2005) found enhanced MTL activation for the recognition of the first-two as compared to last-two SPs in a 12-item study list. They suggested that enhanced MTL activation for the first-two compared to the last-two items of a 12-item study list supports the classical distinction between STM and LTM. Our results reveal a similar dissociation in the hippocampus, but crucially, with a smaller set of items within WM span. Hence, the dissociations reported in Talmi et al. (2005) may instead reflect a distinction between memory representations and focal attention, rather than the classical distinction between LTM and STM. Importantly, the involvement of the MTL and the LIFG in WM retrieval supports accounts that claim similar principles operate over the short- and long-term domains (e.g., Nairne, 1996).
In addition to the dissociation across SPs, neural activation in the hippocampus predicted successful performance in the JOR task. Taken together, our results provide evidence pointing to the involvement and importance of the hippocampus in WM retrieval. These findings raise the intriguing possibility that the hippocampus might have the same role in WM as its role in LTM processes.
The hippocampus has been shown to be differentially important for the encoding and retrieval of relational information (Davachi, 2006; Cohen, Poldrack, & Eichenbaum, 1997). Hence, the involvement of this region in our item recognition task is particularly surprising, as no relational information is required in this task. However, although item information alone may be sufficient for a correct judgment in some circumstances, it is quite possible that participants used relational information during some of these trials. There is clear evidence that detailed episodic information (e.g., source memory or list-specific information), which is recovered by a controlled retrieval process, contributes to recognition memory performance in short-term immediate recognition tasks, such as the one used in our study (e.g.,Öztekin & McElree, 2007; McElree & Dosher, 1989). Hence, it is possible that activation in the hippocampus could be reflecting recovery of episodic and/or relational information in our item recognition task. Future work will be necessary to further address the specific contribution of the MTL to the successful recovery of different kinds of information (e.g., item versus relational) from WM.
Serial Retrieval Processes
We examined the retrieval of item and temporal order information for representations outside focal attention to investigate the neural mechanisms supporting different types of WM retrieval operations. Crucially, to isolate activation specifically associated with retrieval processes, we equated encoding across tasks and used a short interval between study and test to prevent rehearsal. Recovery of temporal order information contrasts with the recovery of item information in requiring a slow serial search (McElree & Dosher, 1993). For the former, we found that activation in the LPPC (including both the supramarginal gyrus and the IPS) and the LIFG correlated with the number of items that would have to be serially scanned to make a temporal order judgment. These findings indicate the involvement of these regions in serial scanning operations.
The LIFG was also identified in our focal attention analyses. That the LIFG was active only when retrieval was required and that the number of items to be retrieved from WM modulated activation in this region support the conclusion that this region is involved in WM retrieval. A straightforward interpretation of our findings is that activation in the LIFG might reflect the amount of successive retrieval operations needed to support JOR.
Marshuetz et al. (2000) suggested that activation in the LPPC might reflect encoding of order information, specifically magnitude coding. In contrast, we found that activation in the LPPC was not dependent on the distance between test probes, but rather was modulated by an interaction between the recency of the most recent probe and the participant's search strategy, which likewise suggests its involvement with serial scanning operations.
The specific role of the LPPC in WM processes remains controversial. The LPPC has been argued to be involved in phonological storage operations (e.g., Smith & Jonides, 1998; see Chein et al., 2003 for a review), but recent approaches posit that it might be involved in attentional scanning when attention is switched from one mental representation to another (e.g., Chein et al., 2003). Our findings are consistent with the latter if it is assumed that the serial scan process that recovers temporal order information consists of two distinct operations, serially retrieving representations from memory and switching attention to a retrieved representation. Additionally, our data suggest that the LIFG might be involved in the former. That the LPPC might be involved in switching attention is consistent with the LPPC being active in a wide range of tasks that involve complex cognitive operations such as maintenance and manipulation in WM (Owen, McMillan, Laird, & Bullmore, 2005; Marshuetz et al., 2000), as well as successful encoding of LTMs (Staresina & Davachi, 2006; Uncapher & Rugg, 2005) and making recognition memory judgments (Wagner, Shannon, Kahn, & Buckner, 2005). In these tasks, as in JOR, participants must switch attention between multiple active representations to reach a decision.
Conclusion
Our fMRI results align with measures of retrieval speed in suggesting that information in focal attention can be accessed without engaging the retrieval operations required for information outside of focal attention. Our data also indicate that retrieving information outside focal attention in what is nominally viewed as a short-term or WM task recruits brain regions identified in the retention and retrieval of long-term information, namely, the hippocampus and the LIFG. Further, our analyses of temporal order judgments in the JOR task indicated that the LPPC and the LIFG were involved in serial retrieval operations engaged to recover temporal order information. Collectively, our results suggest that WM retrieval is accomplished with joint contributions from the LPPC, the LIFG, and the hippocampus. These findings are consistent with frameworks that afford a privileged status to attended information, but assume that representations of other recent events are governed by the same principles as LTM representations.
Acknowledgments
This research was supported by grants from National Institute of Mental Health (MH-074692) to L. Davachi and National Science Foundation (BCS-0236732) to B. McElree, and a Dean's Dissertation Fellowship to I.Öztekin. We thank the Seaver Foundation for supporting scanner-related costs.
Footnotes
UNCITED REFERENCES Gronlund, Mark, & Ohrt, 1997 Halferd, Mayberry, & Bain, 1988 Karlsgodt, Shirinyan, van Erp, Cohen, & Cannon, 2005 Sternberg, 1975
This analysis could only be performed on the JOR task due to the insufficient number of incorrect trials across participants in the item recognition task. Additionally, 2 participants who did not have enough number of incorrect trials in the JOR task were excluded from this analysis.
As stated in the Introduction, retrieval mechanisms are operative only for items that are outside the current focus of attention. Hence, no serial scan will be required for test probes involving SP 5 on those trials in which a subject has maintained this item in focal attention. The U-shaped form observed in RT and neural activation patterns for our forward scanning participants follows these predictions. For these participants, the U-shaped form arises because RT is faster and neural activation is less for SP 5 than for other positions, whereas across all other serial positions, RT and neural activation systematically increase as the probe is drawn from later serial positions (i.e., a forward scanner scans the first SP first, whereas a backward scanner scans the fourth serial position first.)
Additionally, we tested whether the diminished neural activation for SP 5 for the regions reported in the Focal Attention Effects section was evident in both backward and forward scanning participants. We did not find a reliable Group by SP interaction effect in any of the examined regions, with the exception of a marginal interaction in the LIFG peak reported in our JOR task [F(1, 10) = 3.522, p < .090]. This interaction is consistent with the finding that the LIFG might be involved in serial scan operations.
REFERENCES
- Baddeley AD. The episodic buffer: A new component of working memory? Trends in Cognitive Sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. Journal of Neuroscience. 2006;26:916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Chein JM, Ravizza JA, Fiez JA. Using neuroimaging to evaluate models of working memory and their implications for language processing. Journal of Neurolinguistics. 2003;16:315–339. [Google Scholar]
- Clark SE, Gronlund SD. Global matching models of recognition memory: How the models match the data. Psychonomic Bulletin & Review. 1996;3:37–60. doi: 10.3758/BF03210740. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5:131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- Cowan N. Working memory capacity (Essays in cognitive psychology) Psychology Press; New York: 2005. [Google Scholar]
- Crowder RG. Short-term memory: Where do we stand? Memory & Cognition. 1993;21:142–145. doi: 10.3758/bf03202725. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. Journal of Neurophysiology. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Chandan J. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Cognitive Brain Research. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Heather RJ, Wagner AD, Schacter DL. Memory orientation and success: Separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Kirchhoff BA, Buckner RL. Common and dissociable activation patterns associated with controlled semantic and phonological processing: Evidence from fMRI adaptation. Cerebral Cortex. 2005;15:1438–1450. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- Gronlund SD, Mark EB, Ohrt DD. Comparison of the retrieval of item versus spatial position information. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23:1261–1274. doi: 10.1037//0278-7393.23.5.1261. [DOI] [PubMed] [Google Scholar]
- Hacker MJ. Speed and accuracy of recency judgments for events in short-term memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1980;6:651–675. [Google Scholar]
- Halferd GS, Mayberry MT, Bain JD. Set-size effects in primary memory: An age-related capacity limitation? Memory & Cognition. 1988;16:480–487. doi: 10.3758/bf03214229. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. Journal of Neuroscience. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Shirinyan D, van Erp TGM, Cohen MS, Cannon TD. Hippocampal activations during encoding and retrieval in a verbal working memory paradigm. Neuroimage. 2005;25:1224–1231. doi: 10.1016/j.neuroimage.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Reuter-Lorenz PA, Smith EE. Working memory for order and the parietal cortex: An event-related functional magnetic resonance imaging study. Neuroscience. 2006;139:311–316. doi: 10.1016/j.neuroscience.2005.04.071. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Smith EE, Jonides J, DeGuits J, Chenevert TL. Order information in working memory: fMRI evidence for parietal and prefrontal mechanisms. Journal of Cognitive Neuroscience. 2000;12:130–144. doi: 10.1162/08989290051137459. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;3:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- McElree B. Attended and non-attended states in working memory: Accessing categorized structures. Journal of Memory and Language. 1998;38:225–252. [Google Scholar]
- McElree B. Working memory and focal attention. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:817–835. [PMC free article] [PubMed] [Google Scholar]
- McElree B. Accessing recent events. In: Ross BH, editor. The psychology of learning and motivation. Vol. 46. Academic Press; San Diego: 2006. pp. 155–200. [Google Scholar]
- McElree B, Dosher BA. Serial position and set size in short-term memory: Time course of recognition. Journal of Experimental Psychology: General. 1989;18:346–373. [Google Scholar]
- McElree B, Dosher BA. Serial retrieval processes in the recovery of order information. Journal of Experimental Psychology: General. 1993;122:291–315. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Reviews of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Muter PA. Response latencies in discriminations of recency. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1979;5:160–169. [Google Scholar]
- Nairne JS. Short-term/working memory. In: Bjork EL, Bjork RA, editors. Memory. Academic Press; San Diego: 1996. pp. 160–169. [Google Scholar]
- Nichols EA, Kao YC, Verfaellie M, Gabrieli JDE. Working memory and long-term memory for faces: Evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:614–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. Journal of Cognitive Neuroscience. 2006;18:1087–1097. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öztekin I, McElree B. Proactive interference slows recognition by eliminating fast assessments of familiarity. Journal of Memory and Language. 2007;57:126–149. [Google Scholar]
- Ranganath C, D'Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proceedings of the National Academy of Sciences, U.S.A. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Staresina B, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. Journal of Neuroscience. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Functional magnetic resonance (fMRI) activity in the hippocampal region during recognition memory. Journal of Neuroscience. 2000;20:7776–7781. doi: 10.1523/JNEUROSCI.20-20-07776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11:337–346. doi: 10.1002/hipo.1048. [DOI] [PubMed] [Google Scholar]
- Sternberg S. Memory scanning: New findings and controversies. Quarterly Journal of Experimental Psychology. 1975;27:1–32. [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal and parahippocampal roles during verbal encoding. Journal of Neuroscience. 2002;15:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant AM, Neath I. The 9 lives of short-term memory. In: Thorn A, Page M, editors. Interactions between short-term and long-term memory in the verbal domain. Psychology Press; Hove, UK: in press. [Google Scholar]
- Talmi D, Grady CL, Goshen-Gottstein Y, Moscovitch M. Neuroimaging the serial position curve: A test of single-store versus dual-store models. Psychological Science. 2005;16:716–723. doi: 10.1111/j.1467-9280.2005.01601.x. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left prefrontal cortex in retrieval of semantic knowledge: A re-evaluation. Proceedings of the National Academy of Sciences, U.S.A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. Encoding and the durability of episodic memory: A functional magnetic resonance imaging study. Journal of Neuroscience. 2005;25:7269–7267. doi: 10.1523/JNEUROSCI.1641-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. Storage of features, conjunctions, and objects in visual working memory. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. The long and the short of memory. Psychological Bulletin. 1973;80:425–438. [Google Scholar]
- Wickelgren WA, Corbett AT, Dosher BA. Priming and retrieval from short-term memory: A speed accuracy trade-off analysis. Journal of Verbal Learning and Verbal Behavior. 1980;19:387–404. [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- Yntema DB, Trask FP. Recall as a search process. Journal of Verbal Learning and Verbal Behavior. 1963;2:65–74. [Google Scholar]