Abstract
(1) Among the DNA-like RNA (dRNA) species of rat brain, 35 per cent remain nuclear, whereas 65 per cent are found in the microsomes and may represent functional messenger RNA. It was estimated that 30,000-300,000 cistrons would thus code for messenger RNA in rat brain.
(2) No nuclear-specific dRNA with sedimentation characteristics similar to microsomal dRNA are detected.
(3) The nuclear large dRNA contain all the dRNA species of the brain.
(4) The presence of microsomal dRNA precursors and of nuclear restricted dRNA in large dRNA suggests the existence of a limited cleavage process and of a selection among the fragments for their transport from the nucleus to the cytoplasm. Such a selective mechanism could represent an important regulation step in the transfer of genetic information.
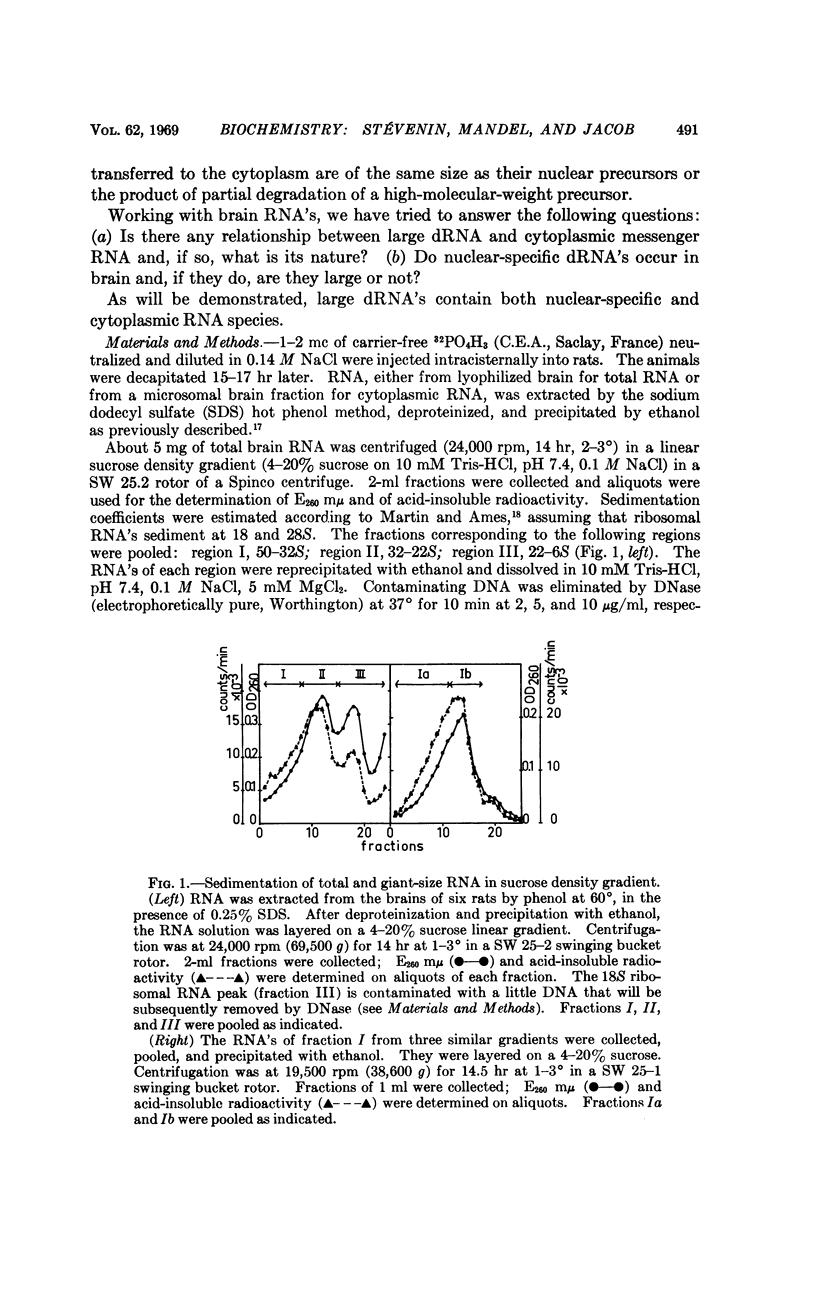
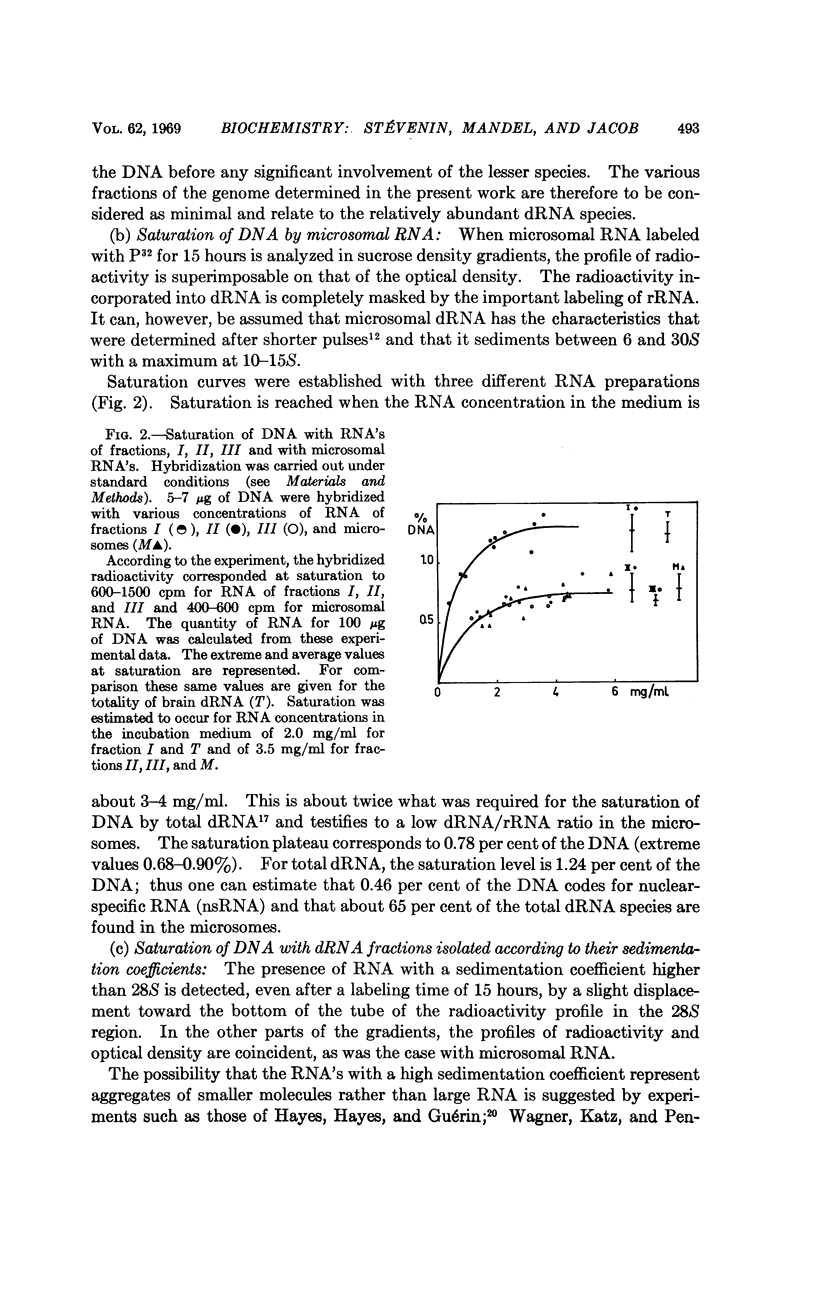
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Parnas H., Hwang M. I., Attardi B. Giant-size rapidly labeled nuclear ribonucleic acid and cytoplasmic messenger ribonucleic acid in immature duck erythrocytes. J Mol Biol. 1966 Sep;20(1):145–182. doi: 10.1016/0022-2836(66)90123-9. [DOI] [PubMed] [Google Scholar]
- Bramwell M. E., Harris H. The origin of the polydispersity in sedimentation patterns of rapidly labelled nuclear ribonucleic acid. Biochem J. 1967 Jun;103(3):816–830. doi: 10.1042/bj1030816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon R. H. The effect of thioacetamide on the maturation of high-molecular-weight ribonucleic acid in tumour cells. Biochem J. 1967 Jul;104(1):186–197. doi: 10.1042/bj1040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church R., McCarthy B. J. Changes in nuclear and cytoplasmic RNA in regenerating mouse liver. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1548–1555. doi: 10.1073/pnas.58.4.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews J., Brawerman G., Morris H. P. Nucleotide sequence homologies in nuclear and cytoplasmic ribonucleic acid from rat liver and hepatomas. Eur J Biochem. 1968 Jan;3(3):284–292. doi: 10.1111/j.1432-1033.1968.tb19528.x. [DOI] [PubMed] [Google Scholar]
- Frenster J. H. Nuclear polyanions as de-repressors of synthesis of ribonucleic acid. Nature. 1965 May 15;206(985):680–683. doi: 10.1038/206680a0. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hadjivassiliou A., Brawerman G. Template and ribosomal ribonucleic acid components in the nucleus and the cytoplasm of rat liver. Biochemistry. 1967 Jul;6(7):1934–1941. doi: 10.1021/bi00859a008. [DOI] [PubMed] [Google Scholar]
- Houssais J. F., Attardi G. High molecular weight nonribosomal-type nuclear RNA and cytoplasmic messenger RNA in HeLa cells. Proc Natl Acad Sci U S A. 1966 Aug;56(2):616–623. doi: 10.1073/pnas.56.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob M., Samec J., Stevenin J., Garel J. P., Mandel P. Polysomes and polysomal RNA of rat brain. J Neurochem. 1967 Feb;14(2):169–178. doi: 10.1111/j.1471-4159.1967.tb05889.x. [DOI] [PubMed] [Google Scholar]
- Jacob M., Stevenin J., Jund R., Judes C., Mandel P. Rapidly-labelled ribonucleic acids in brain. J Neurochem. 1966 Aug;13(8):619–628. doi: 10.1111/j.1471-4159.1966.tb09870.x. [DOI] [PubMed] [Google Scholar]
- Kempf J., Mandel P. RNA de haut poids moléculaire, rapidement marqué, des sarcomes plasmocytaires de la souris. Bull Soc Chim Biol (Paris) 1966;48(2):211–224. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. V. Studies on the properties and distribution of ribonuclease inhibitor in the rat. Biochim Biophys Acta. 1956 Jul;21(1):34–43. doi: 10.1016/0006-3002(56)90091-9. [DOI] [PubMed] [Google Scholar]
- Roberts W. K. Studies on RNA synthesis in Ehrlich ascites cells extraction and properties of labeled RNA. Biochim Biophys Acta. 1965 Nov 8;108(3):474–488. doi: 10.1016/0005-2787(65)90039-0. [DOI] [PubMed] [Google Scholar]
- Scherrer K., Marcaud L., Zajdela F., Breckenridge B., Gros F. Etude des RNA nucléaires et cytoplasmiques à marquage rapide dans les cellules érythropoiétiques aviaires différenciées. Bull Soc Chim Biol (Paris) 1966;48(10):1037–1075. [PubMed] [Google Scholar]
- Scherrer K., Marcaud L., Zajdela F., London I. M., Gros F. Patterns of RNA metabolism in a differentiated cell: a rapidly labeled, unstable 60S RNA with messenger properties in duck erythroblasts. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1571–1578. doi: 10.1073/pnas.56.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer R. W., McCarthy B. J. Evidence for ribonucleic acid molecules restricted to the cell nucleus. Biochemistry. 1967 Jan;6(1):283–289. doi: 10.1021/bi00853a044. [DOI] [PubMed] [Google Scholar]
- Stevenin J., Samec J., Jacob M., Mandel P. Détermination de la fraction du génome codant pour les RNA ribosomiques et messagers dans le cerveau du rat adulte. J Mol Biol. 1968 May 14;33(3):777–793. doi: 10.1016/0022-2836(68)90319-7. [DOI] [PubMed] [Google Scholar]
- Vesco C., Giuditta A. Pattern of RNA synthesis in rabbit brain. Biochim Biophys Acta. 1967 Jul 18;142(2):385–402. doi: 10.1016/0005-2787(67)90620-x. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- Yoshikawa-Fukada M., Fukada T., Kawade Y. Characterization of rapidly labeled ribonucleic acid of animal cells in culture. Biochim Biophys Acta. 1965 Jul 15;103(3):383–398. doi: 10.1016/0005-2787(65)90132-2. [DOI] [PubMed] [Google Scholar]
- Yoshikawa-Fukada M. The nature of the two rapidly labelled ribonucleic acid components of animal cells in culture. Biochim Biophys Acta. 1966 Jul 20;123(1):91–101. doi: 10.1016/0005-2787(66)90162-6. [DOI] [PubMed] [Google Scholar]


