Abstract
EMAP II is an endothelial cell and monocyte activating proinflammatory cytokine, which has been demonstrated to induce endothelial cell apoptosis. In order to analyze its role in disease models linked to inflammation and endothelial cell death, we aimed to develop a neutralizing antibody against mouse EMAP II. Therefore, we generated rat monoclonal anti-mouse EMAP II antibodies by immunization with recombinant full length, mouse pro-EMAP II protein. From fusion with mouse myeloma SP2/0 we could identify by ELISA hybridoma clones, which produce antibodies recognizing both full length and processed EMAP II. We further characterized one antibody, M7/1 and demonstrated its ability to detect both EMAP II forms by Western blotting and to neutralize EMAP II directed migration of human peripheral blood monocytes as well as EMAP II induced tumor and endothelial cell apoptosis. We conclude that this antibody can be useful to both target and analyze murine disease models, in which EMAP II may be involved.
Keywords: CXCR3, neutralizing monoclonal antibodies, hybridoma, monocyte migration, inflammation, apoptosis, endothelial
INTRODUCTION
EMAP II was initially discovered as an endothelial-and monocyte-activating polypeptide from the supernatant of tumor cells based on its ability to induce tissue factor in endothelial cells and in monocytes and to evoke chemotactic migration of blood leukocytes and monocytes (Kao et al., 1992; Kao et al., 1994). It was later identified as an anti-angiogenic molecule, which induces apoptosis in proliferating and hypoxic endothelial cell in vitro and in angiogenic tumor vasculature in vivo (Schwarz et al., 1999; Berger et al., 2000). This apoptotic activity can be explained by the ability of EMAP II to activate the proapoptotic splice variant of the chemokine receptor CXCR3B (Hou et al., 2006) and to compete with VEGF for binding to the VEGF receptor -2 (Awasthi et al., 2009).
Because molecular cloning of EMAPII revealed that the mature 23 kDa form, which was originally isolated from tumor cells, is part of a larger 43 kDa pro-EMAP II form, attempts were focused on identifying proteolytic cleavage mechanism. Depending on cells and assay system being used opposing mechanism, i.e. intracellular (caspases- 3 and -7 or cathepsin) versus extracellular cleavage (matrix metalloproteinases), were identified (Wakasugi and Schimmel, 1999b; Behrensdorf et al., 2000; Shalak et al., 2001; Zhang and Schwarz, 2002; Liu and Schwarz, 2006). Measuring gene expression levels, we and others identified conditions which elicit EMAP II production, including general cellular stress, hypoxia, and apoptosis (Knies et al., 1998; Matschurat et al., 2003). EMAP II is released from cells, by a yet unknown mechanism, as pro- and mature EMAP II proteins in response to various forms of cellular stresses, including glucose starvation and hypoxia (Matschurat et al., 2003; Park et al., 2006) (Barnett et al., 2000; Zhang and Schwarz, 2002). There is still uncertainty regarding which of the EMAP II forms, the mature or the proform exert more potent cytokine activity (Kim et al., 2006).
Although EMAP II is upregulated in many disease models and is an interesting target for understanding molecular mechanisms in tissue remodeling, no knockout approaches have been published so far. This could be explained by the fact that the intracellular 43 kDa EMAP II is as a protein expressed in all cell types and essential part of the tRNA-synthetase multi-enzyme complex or the tyrosine tRNA-synthetase (Quevillon et al., 1997; Wakasugi and Schimmel, 1999a). This essential intracellular function of the EMAP II-containing complexes predict that EMAP II gene deficient embryos may not be viable, even at very early developmental stages. Therefore, alternative strategies such as neutralizing antibodies are required to determine whether the secreted EMAP II forms have a pathogenic role in diseases associated with cellular stresses. Here we describe the development of EMAP II specific neutralizing antibodies. We have produced and characterized a rat monoclonal anti-mouse-antibody based on their reactivity in ELISA, Western blotting and functional in vitro assays.
MATERIALS AND METHODS
EMAP II production
The full length and the mature forms of EMAP II coding region were cloned into pPICZ A vector (Easy Select™ Pichia Expression Kit from Invitrogen), which contains combined His-and Myc-tags at the C-terminus. Expression of these two EMAP II forms was carried out according to manufacturer’s instruction and confirmed by Western blot analysis using rabbit polyclonal antibodies against EMAP II (SA2846). Purification of recombinant EMAP II was performed by affinity chromatography using a Ni Sepharose 6 Fast Flow column (Amersham Biosciences). Protein concentration was determined using BCA protein assay kit from Pierce. In some of the experiments recombinant EMAPII protein from bacterial expression (kind gift of Dr. Marc Mirande) was used.
Establishment of monoclonal antibodies against EMAP II
Rat Monoclonal antibodies against mouse EMAP II were generated by immunizing Lewis rats four times with 200 μg recombinant full length EMAP II for each immunization. Lymphocytes isolated from the spleen and lymph nodes of immunized rats were fused with the mouse myeloma SP2/0, and hybridoma supernatants were tested in enzyme-linked immunosorbent assays (ELISAs) for binding to recombinant full length and mature EMAP II (100 ng/well). For purification of mABs hybridomas were grown in protein-free hybridoma medium (Invitrogen) and antibodies were purified with protein G-Sepharose (Pharmacia, Uppsala, Sweden).
Western blotting
Recombinant EMAP II proteins were loaded in equal amounts and separated by SDS-PAGE using Novex gels (Invitrogen, Carlsbad, CA), followed by immunoblotting for EMAP II as previously described (Petrache et al., 2005). Briefly, samples were mixed with Laemmli buffer, boiled at 95°C for 10 min and loaded onto 15% SDS/PAGE gels. Proteins were separated by electrophoresis and blotted onto nitrocellulose (Pierce). Non-specific binding was reduced by blocking the membrane in TBS/0.1% Tween 20/5% nonfat dry milk. The primary antibody (M7.1 or rat IgG, 140 ng in Phosphate buffer or rabbit anti-EMAP II antiserum SA 2847, diluted 1:20000 in Phosphate buffer) was applied at room temperature for one hour. After washing, the membranes were incubated in either peroxidase-coupled goat anti-rat IgG or goat anti-rabbit IgG (Dianova/Jackson Immuno Research; were diluted 1:10000 and 1:50000, respectively in blocking buffer) for 1 h at room temperature and developed using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech).
Isolation of periphereal mononuclear blood cells
Monocytes were isolated from buffy coats of healthy donors in a two-step-procedure by density-gradient centrifugation and elutriation. Peripheral blood mononuclear cells (PBMCs) were obtained from citrated venous blood (buffy coats) from healthy donors as described previously (Clauss, 2000; Shalak et al., 2001). Blood was diluted 1:3 in Hank’s buffered saline (HBSS) and 25 ml of this cell suspension was layered over 15 ml Ficoll-Paque (Pharmacia, Freiburg, FRG). After centrifugation at 600 × g for 20 minutes cells at the interphase containing PBMC were collected and cell suspension was washed twice with HBSS to remove platelets.
Monocyte migration assay
Migration of monocytes to EMAP II was assessed as previously described with minor modifications (Shalak et al., 2001). Briefly, 104 cells were placed in 100 ml medium into the upper chamber of a 6.5μm diameter, 5.0 μm pore size transwell (Costar, #3421, NY, USA). Collagen coated filters were employed to enhance adhesion. Full length pro-EMAP II with or without purified anti- EMAP II or control IgG (10 μg/ml) antibodies were added to the lower chambers. The assays were conducted over a 4 hr incubation period at 37°C in a CO2 (7.5%) equilibrated incubator. After migration, non-migrating cells were removed by cotton tipped applicators from the upper side of the transwells and membranes of transwell filters were fixed and stained by Hema 3 STAT PACK protocol (Fisher Scientific, # 123-869, VA, USA). Cells which migrated and adhered to the lower side of the membranes were counted by microscopy in 5-6 high power fields/well and the average/standard error of the mean was determined from triplicate measurement.
Cell culture
Human endothelial cells were routinely grown in collagen coated culture flaks at 37 °C in a 95% humidified atmosphere of 5% v/v CO2 in VascuLife EnGS Medium (www.lifelinecelltech.com). Human breast carcinoma (MCF7, ATCC) cells were routinely grown at 37 °C in a 95% humidified atmosphere of 5% v/v CO2 in DMEM medium supplemented with 10% v/v fetal calf serum (FCS), 100 IU penicillin and 100 μg/ml streptomycin, 2 mg/ml fungizone, 2 mM Lglutamine (all Gibco®, Invitrogen).
Detection of Apoptosis by TUNEL
About 20,000 cells were plated per well of a 8-well Lab-Tek™ Chamber Slides (www.nuncbrand.com) and left overnight for attachment. Media was changed to serum starvation medium (DMEM with 0.1% FCS) and apoptosis was induced with recombinant EMAP II with and without pre-treatment with neutralizing anti-EMAP II M 7/1 antibody or isotype matched rat IgG. After 24 hours, cells were fixed in paraformaldehyde (4%) and DNA strand breaks were assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling using a fluorescein detection system according to manufacturer’s instructions (TUNEL Apoptosis Detection Kit, www.millipore.com). Sections were mounted in a solution of Biomeda™ mounting medium and analyzed with a Leitz fluorescence microscope. Quantification was performed by MetaMorph image software (Castro et al., 2000) and results expressed by computing the ratios between TUNEL positive and total (DAPI positive) cells of triplicate measurements.
Statistical analysis
All experiments were performed at least in triplicates. A paired t-test was utilized to establish statistically significant differences between the treatment groups using Microsoft Excel package. Where applicable, mean ± SEM of multiple measurements is reported, as indicated.
RESULTS
Production and analysis of monoclonal antibodies against EMAP II
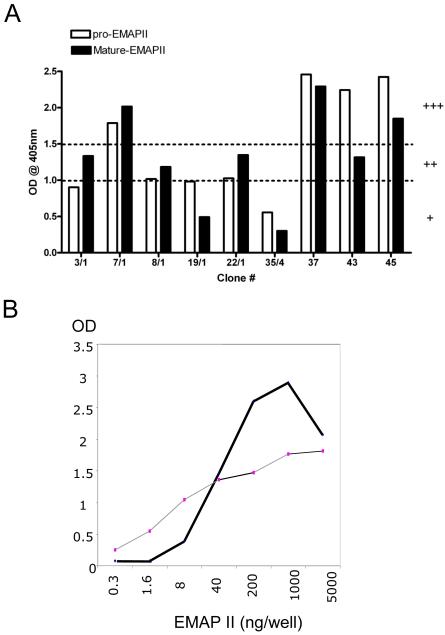
In order to generate monoclonal antibodies against mouse EMAP II 200 μg recombinant full length mouse EMAP II protein was injected in rats 4 times every second week. Two weeks after the last injection, T cells were isolated both from spleen and lymph nodes of immunized rats, taken into tissue culture and fused with the mouse myeloma line SP2/0. From these fused cells several mAB producing hybridoma clones were isolated and established based on analysis with ELISA. Hybridomas positive in ELISA versus recombinant EMAP II were identified, which produced mostly IgG. As depicted in figure 1A supernatants from selected hypridomas showed the ability to detect either one or both EMAP II forms. We identified several candidate clones, which were able to recognize both EMAP II forms but concentrated in the following on hybridoma clone # M 7 (i.e. its sub clone M 7/1) and assigned the corresponding antibody as M 7/1. When this M 7/1 antibody was compared to anti-EMAP II rabbit serum in terms of recognizing serial dilutions of recombinant mature EMAP II immobilized on ELISA plates, the antiserum was able to recognize as little as ca 0.3 ng and M 7/1 antibody as little as ca 2 ng of recombinant protein (figure 1B).
Figure 1. Analysis of anti-EMAP II antibodies by ELISA.
A) Monoclonal anti-EMAP II antibodies recognize full length and processed EMAP II in ELISA. Subclones of monoclonal anti-EMAP II antibodies were tested in ELISA using recombinant EMAP II protein and the response was graded as + (slightly positive; OD <1.0), ++ (medium positive; OD >1.0 & <1.5), +++ (strongly positive; OD >1.5) based on the optical densities. Data shown are from a representative experiments repeated two times independently with similar results. B) Dose dependence of EMAP II. Comparison of EMAP II polyclonal (gray line) and M7/1 monoclonal antibodies (bold line) by ELISA. Shown are the OD values from the ELISA obtained with increasing concentrations of EMAP II.
Characterization of anti-EMAP II antibodies in Western Blot analysis
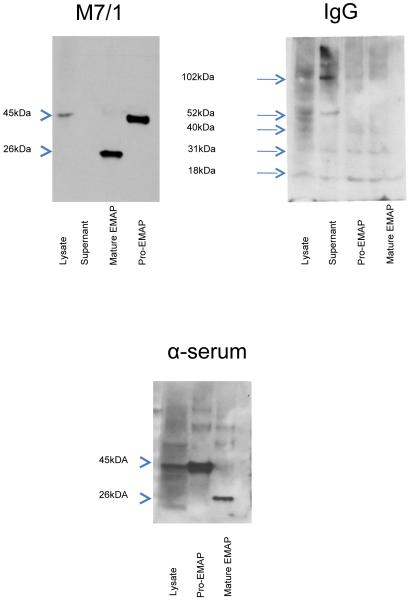
Next we tested whether the monoclonal antibody M 7/1, which generated a strongly positive signal by ELISA, was able to recognize both EMAP II forms as denatured proteins after PAGE and transfer to nitrocellulose. As shown in figure 2A protein A purified mAB M 7/1 (140 ng/ml) strongly reacted against recombinant EMAP II proteins resulting in bands corresponding to a relative molecular weight of 46 kDa for the proform and 25 kDa for the mature form (see arrow heads). In addition, this antibody was capable of detecting the precursor pro-EMAP II (46 kDa) within cell lysates from mouse endothelial cells (10 μg lysate) but not from cell supernatants. In contrast, control rat IgG (Sigma, www.sigmaaldrich.com) neither was able to detect any of the recombinant proteins nor pro-EMAP II from cell lysate (figure 2B). Polyclonal anti-EMAP II antibody recognized both recombinant EMAP II protein as well as pro-EMAP II from cell lysates (figure 2C).
Figure 2. Monoclonal anti-EMAP II antibodies recognize EMAP II by Western blotting.
Shown are Western blots of gels loaded with EMAP II precursor (p43), which were developed with (A) monoclonal anti-EMAP II antibody M7/1 (B) control rat IgG, or (C) polyclonal anti-EMAP II antibodies. In (A) M7/1 and (B) IgG control the pro and mature forms were loaded with 21 and 42 ng/slot, respectively. The (C) anti-serum was further diluted to yield a final concentration of 2.6 ng/slot for the proform and 1.31 ng/slot for the mature form. Note bands of 45 kDa (EMAP II precursor, arrowhead) and 26 kDa (mature-EMAP, arrowhead) for M7/1 and anti-serum, while only unspecific bands were visible with rat control IgG (arrows).
Inhibition of EMAP II-induced migration of monocytes and apoptosis of tumor cells
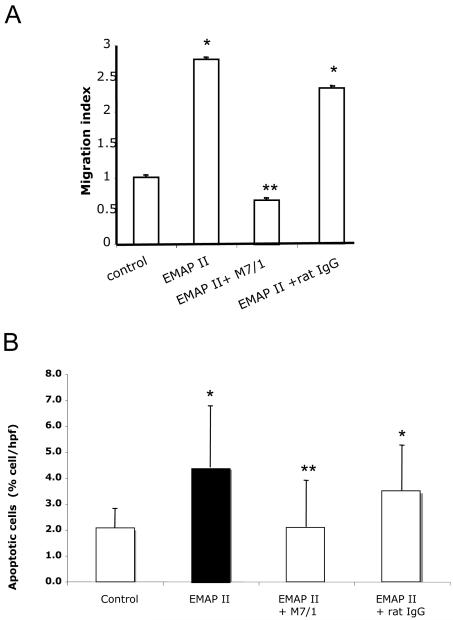
To test the functional effects of the monoclonal anti-EMAP II-binding antibodies, we tested whether EMAP II-induced migration of human peripheral blood monocytes was reduced by neutralizing anti-EMAP II antibodies (1 μg/ml). As shown in figure 3A, migration of monocytes in response to recombinant full length (pro) EMAP II was blocked by anti-EMAP II M 7/1 antibodies but not with control rat IgG.
Figure 3. Effect of M7/1 on EMAP II-induced migration and apoptosis.
A) Inhibition of EMAP II-induced monocyte migration. Migration assays were performed using Boyden chambers with PBMC. EMAPII protein (1 μg/ml) in the lower well induced a significant migration compared to control medium (*p<0.01). Pre-treatment with neutralizing antibody M 7/1 (10 μg/ml) but not with control rat IgG inhibited pro-EMAP II (1 μg/ml)-induced migration (p**<0.05). B) Inhibition of EMAP II-induced apoptosis of tumor cells. Human breast carcinoma cells incubated with pro-EMAPII protein (1 μg/ml) demonstrated a significant apoptosis as shown by TUNEL (*p<0.01). Pre-treatment of MCF7 cells with neutralizing antibody M 7/1 (10 μg/ml) but not with control rat IgG inhibited EMAP II (1 μg/ml)-induced apoptosis (**p<0.05). Data shown are from a representative experiment performed in triplicates and repeated independently two additional times with similar results.
Next, we tested whether the M7/1antibody, was also able to neutralize apoptosis induced by EMAP II. We employed the breast carcinoma cell line MCF-7, previously shown to be susceptible to EMAP II induced apoptosis (http://www.abcam.com/index.html?datasheet=50057). EMAP II-induced apoptosis, assessed by quantification of TUNEL-positive cells was blocked by anti-EMAP II antibody M7/1 but not by control rat IgG (figure 3B). This suggests that the M7/1 can effectively neutralize the pro-apoptotic function of EMAP II.
Neutralization of pro and mature-EMAP II-induced endothelial cell apoptosis
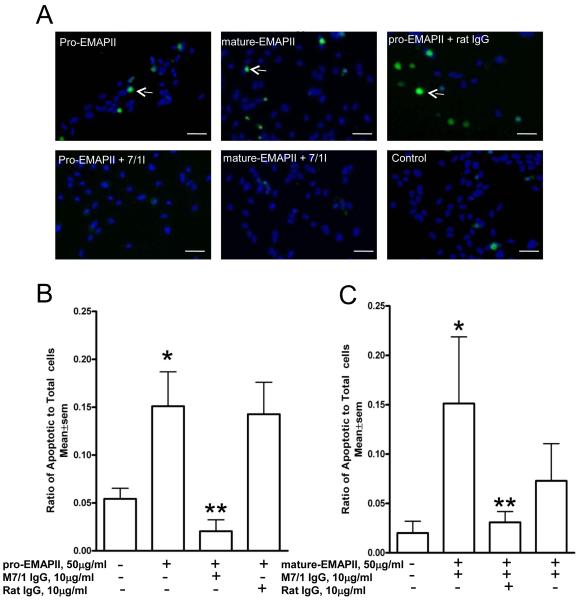
Because mature EMAP II reportedly induces endothelial apoptosis we next we tested whether the M7/1antibody, was also able to neutralize apoptosis induced by EMAP II. EMAP II-induced apoptosis, assessed by quantification of TUNEL-positive cells was significantly (p<0.03) blocked by anti-EMAP II antibody M 7/1 but not by control rat IgG (figure 4). Interestingly we observed that pro-EMAP II at the same molar concentrations as mature EMAP II was also a strong inducer of endothelial apoptosis. Again, M 7/1 antibody was able to completely neutralize this activity (p<0.01). These data demonstrate that the M7/1 can effectively neutralize the pro-apoptotic function of both EMAP II forms and may be a suitable tool to inhibit pathophysiological activities of this protein in mice.
Figure 4. Inhibition of EMAP II-induced apoptosis in endothelial cells.
A) Endothelial cells incubated with pro-EMAPII protein (50 μg/ml) or mature-EMAPII protein (50 μg/ml) demonstrated a significant apoptosis (arrows) as shown by TUNEL (*p<0.01). Pre-treatment of these cells with neutralizing antibody M 7/1 (10 μg/ml) but not with control rat IgG significantly (**p<0.03) inhibited apoptosis induced by both pro and mature EMAPII as shown from representative fluorescent microscope images following TUNEL assay. B) and C) Quantification of TUNEL positive cells by MetaMorph software normalized to total DAPI nuclear positive cells is shown below for pro-EMAPII (B) and mature EMAPII (C). Data shown are from a representative experiment performed in triplicates and repeated independently two additional times with similar results. Scale bar=50μm
DISCUSSION
Our study describes the generation and characterization of rat anti-mouse EMAP II monoclonal antibodies. We were able to raise hybridoma lines producing antibodies with reactivity against recombinant full length (pro-) and mature EMAP II forms (figure 1A). In addition, the ability of clones such as M 7/1 to recognize denatured full length and processed EMAP II suggests that this antibody can substitute the polyclonal rabbit anti-EMAP II antiserum, which we have raised against mature EMAP and have used previously for detection of the differently processed EMAP II forms (Knies et al., 1998; Behrensdorf et al., 2000; Knies et al., 2000), rendering it suitable for a couple of applications including sandwich ELISA. However, none of the assessed clones showed specificity for only the pro or the mature form. This could be best explained by the hypothesis that carboxyterminal domains, which are shared by both EMAP II forms are more immunogenic. Interestingly, when the polyclonal antibody was compared with monoclonal M7/1 cell supernatant for its ability to recognize recombinant EMAP II in ELISA, the polyclonal antibody displayed the ability to detect lower levels of EMAP II than the monoclonal antibody (figure 1B).
When assessed by Western blot analysis, the mAB M 7/1 recognized the recombinant pro and mature EMAP II forms as well as endogenous EMAP II from cell lysates (figure 2). This was reflected with using rabbit antiserum II, which displayed similar bands. However, using the same protein concentrations of lysate and recombinant EMAP II, the antiserum produced stronger bands and more background (data not shown) in line with its higher sensitivity described in figure 1B. Therefore, we reduced the amounts of recombinant EMAP II proteins but not of lysates on the gels in order to obtain comparable bands. Of note, EMAP II antiserum displayed higher background for both recombinant proteins as well as for lysates. These limitations of the EMAP II antiserum make the monoclonal as a good candidate for reproducible analysis of the two EMAP II forms by Western Blot analysis.
EMAP II has been suggested to be a proinflammatory cytokine based on its ability to induce inflammation when injected into the mouse footpad. Because monocytes have been demonstrated to be involved in these activities and previous reports have shown that EMAP II is a chemo-attractant for monocytes (Knies et al., 1998; Knies et al., 2000; Ko et al., 2001; Shalak et al., 2001), we have chosen this activity as a functional assay to probe these EMAP II directed antibodies for neutralization in light of the proinflammatory properties of EMAP II (figure 3A). This activity is believed to be mediated by chemokine receptor activation (Loetscher et al., 1994). Neutralizing antibodies against CXCR3 blocked both EMAP-induced intracellular calcium increase and migration of endothelial progenitors (Hou et al., 2006). In endothelial cells a splice variant of CXCR3, CXCR3B, was demonstrated to mediate the inhibitory effect of antiangiogenic chemokines such as PF4 and IP-10 on endothelial proliferation (Romagnani et al., 2004). Therefore, we have assessed a human breast carcinoma cell line susceptible to EMAP II -triggered apoptosis. In fact, neutralizing EMAP II antibodies effectively inhibited EMAP II-induced apoptosis in MCF7 cells (figure 3B). In this context, EMAP II had been also described to display antiangiogenic activity in growing endothelium (Schwarz et al., 1999), which may be related to the restriction of CXCR3 expression to proliferating endothelium (Romagnani et al., 2001). We have demonstrated here for the first time that pro-EMAP II can also induce endothelial cell apoptosis, in addition to the mature form and that the mAB M 7/1 can efficiently inhibit apoptosis induced by both forms of EMAP II, based on TUNEL assay.
Together, this antibody could be useful to address the proinflammatory and proapoptotic role of EMAP II in conditions of EMAP II upregulation. These may include hypoxic or proinflammatory conditions such as in tumors or in lipopolysaccharide (LPS)-induced acute lung inflammation(Matschurat et al., 2003; Journeay et al., 2007) and maybe used to address the role of tumor-produced EMAP II in tumor angiogenesis regulation (Berger et al., 2000; Keezer et al., 2003). One limitation is that this antibody cannot distinguish between the pro- and the mature-EMAP II form so that the relative contribution of these cannot be addressed. For analytical analysis this means that Western blot analysis need to employed when the differential abundance of the two forms of EMAP II need to be addressed.
In conclusion, our demonstration that the antibody produced from monoclonal hybridoma line M7/1 can recognize both EMAP II forms makes it a suitable tool to address the role of EMAP II as a biomarker in murine disease models. Furthermore, the ability of the M7/1 to neutralize EMAP II-induced monocyte migration and apoptosis in vitro suggests that these antibodies are suitable tools to address the functional involvement of EMAP II in inflammation and apoptosis in disease models in vitro and in vivo.
Acknowledgements
This project was funded by the NIH (R01HL090950-01A1 to IP and MC) and the DFG (SFB 547-C5 to MC). We are grateful to Dr. Marc Mirande at INSERM, France, for a gift of recombinant EMAP II from bacteria.
Abbreviations
- EMAP II
Endothelial Monocyte Activating Polypeptide II
- mAB
Monoclonal antibodies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awasthi N, Schwarz MA, Verma V, Cappiello C, Schwarz RE. Endothelial monocyte activating polypeptide II interferes with VEGF-induced proangiogenic signaling. Lab Invest. 2009;89:38–46. doi: 10.1038/labinvest.2008.106. [DOI] [PubMed] [Google Scholar]
- Barnett G, Jakobsen AM, Tas M, Rice K, Carmichael J, Murray JC. Prostate adenocarcinoma cells release the novel proinflammatory polypeptide EMAP-II in response to stress. Cancer Res. 2000;60:2850–7. [PubMed] [Google Scholar]
- Behrensdorf HA, van de Craen M, Knies UE, Vandenabeele P, Clauss M. The endothelial monocyte-activating polypeptide II (EMAP II) is a substrate for caspase-7. FEBS Lett. 2000;466:143–7. doi: 10.1016/s0014-5793(99)01777-9. [DOI] [PubMed] [Google Scholar]
- Berger AC, Alexander HR, Tang G, Wu PS, Hewitt SM, Turner E, Kruger E, Figg WD, Grove A, Kohn E, Stern D, Libutti SK. Endothelial monocyte activating polypeptide II induces endothelial cell apoptosis and may inhibit tumor angiogenesis. Microvasc Res. 2000;60:70–80. doi: 10.1006/mvre.2000.2249. [DOI] [PubMed] [Google Scholar]
- Castro CM, Yang Y, Zhang Z, Linnoila RI. Attenuation of pulmonary neuroendocrine differentiation in mice lacking Clara cell secretory protein. Lab Invest. 2000;80:1533–40. doi: 10.1038/labinvest.3780163. [DOI] [PubMed] [Google Scholar]
- Clauss M. Molecular biology of the VEGF and the VEGF receptor family. Semin Thromb Hemost. 2000;26:561–9. doi: 10.1055/s-2000-13213. [DOI] [PubMed] [Google Scholar]
- Hou Y, Plett PA, Ingram DA, Rajashekhar G, Orschell CM, Yoder MC, March KL, Clauss M. Endothelial-monocyte-activating polypeptide II induces migration of endothelial progenitor cells via the chemokine receptor CXCR3. Exp Hematol. 2006;34:1125–32. doi: 10.1016/j.exphem.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Journeay WS, Janardhan KS, Singh B. Expression and function of endothelial monocyte-activating polypeptide-II in acute lung inflammation. Inflamm Res. 2007;56:175–81. doi: 10.1007/s00011-006-6162-3. [DOI] [PubMed] [Google Scholar]
- Kao J, Houck K, Fan Y, Haehnel I, Libutti SK, Kayton ML, Grikscheit T, Chabot J, Nowygrod R, Greenberg S, Kuang W-J, Leung DW, Hayward JR, Kisiel W, Heath M, Brett J, Stern DM. Characterization of a novel tumor-derived cytokine. Endothelial- monocyte activating polypeptide II. J Biol Chem. 1994;269:25106–19. [PubMed] [Google Scholar]
- Kao J, Ryan J, Brett G, Chen J, Shen H, Fan YG, Godman G, Familletti PC, Wang F, Pan YC, et al. Endothelial monocyte-activating polypeptide II. A novel tumor-derived polypeptide that activates host-response mechanisms. J Biol Chem. 1992;267:20239–47. [PubMed] [Google Scholar]
- Keezer SM, Ivie SE, Krutzsch HC, Tandle A, Libutti SK, Roberts DD. Angiogenesis inhibitors target the endothelial cell cytoskeleton through altered regulation of heat shock protein 27 and cofilin. Cancer Res. 2003;63:6405–12. [PubMed] [Google Scholar]
- Kim E, Kim SH, Kim S, Kim TS. The novel cytokine p43 induces IL-12 production in macrophages via NF-kappaB activation, leading to enhanced IFN-gamma production in CD4+ T cells. J Immunol. 2006;176:256–64. doi: 10.4049/jimmunol.176.1.256. [DOI] [PubMed] [Google Scholar]
- Knies UE, Behrensdorf HA, Mitchell CA, Deutsch U, Risau W, Drexler HC, Clauss M. Regulation of endothelial monocyte-activating polypeptide II release by apoptosis. Proc Natl Acad Sci U S A. 1998;95:12322–7. doi: 10.1073/pnas.95.21.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knies UE, Kroger S, Clauss M. Expression of EMAP II in the developing and adult mouse. Apoptosis. 2000;5:141–51. doi: 10.1023/a:1009632712876. [DOI] [PubMed] [Google Scholar]
- Ko YG, Park H, Kim T, Lee JW, Park SG, Seol W, Kim JE, Lee WH, Kim SH, Park JE, Kim S. A cofactor of tRNA synthetase, p43, is secreted to up-regulate proinflammatory genes. J Biol Chem. 2001;276:23028–33. doi: 10.1074/jbc.M101544200. [DOI] [PubMed] [Google Scholar]
- Liu J, Schwarz MA. Identification of protease-sensitive sites in Human Endothelial-Monocyte Activating Polypeptide II protein. Exp Cell Res. 2006 doi: 10.1016/j.yexcr.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Loetscher P, Seitz M, Clark Lewis I, Baggiolini M, Moser B. Monocyte chemotactic proteins MCP-1, MCP-2, and MCP-3 are major attractants for human CD4+ and CD8+ T lymphocytes. Faseb J. 1994;8:1055–60. doi: 10.1096/fasebj.8.13.7926371. [DOI] [PubMed] [Google Scholar]
- Matschurat S, Knies UE, Person V, Fink L, Stoelcker B, Ebenebe C, Behrensdorf HA, Schaper J, Clauss M. Regulation of EMAP II by Hypoxia. Am J Pathol. 2003;162:93–103. doi: 10.1016/S0002-9440(10)63801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SG, Kang YS, Kim JY, Lee CS, Ko YG, Lee WJ, Lee KU, Yeom YI, Kim S. Hormonal activity of AIMP1/p43 for glucose homeostasis. Proc Natl Acad Sci U S A. 2006;103:14913–8. doi: 10.1073/pnas.0602045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–8. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevillon S, Agou F, Robinson JC, Mirande M. The p43 component of the mammalian multi-synthetase complex is likely to be the precursor of the endothelial monocyte-activating polypeptide II cytokine. J Biol Chem. 1997;272:32573–9. doi: 10.1074/jbc.272.51.32573. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Annunziato F, Lasagni L, Lazzeri E, Beltrame C, Francalanci M, Uguccioni M, Galli G, Cosmi L, Maurenzig L, Baggiolini M, Maggi E, Romagnani S, Serio M. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest. 2001;107:53–63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–9. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Schwarz MA, Kandel J, Brett J, Li J, Hayward J, Schwarz RE, Chappey O, Wautier JL, Chabot J, Lo Gerfo P, Stern D. Endothelial-monocyte activating polypeptide II, a novel antitumor cytokine that suppresses primary and metastatic tumor growth and induces apoptosis in growing endothelial cells. J Exp Med. 1999;190:341–54. doi: 10.1084/jem.190.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalak V, Kaminska M, Mitnacht-Kraus R, Vandenabeele P, Clauss M, Mirande M. The EMAPII cytokine is released from the mammalian multisynthetase complex after cleavage of its p43/proEMAPII component. J Biol Chem. 2001;276:23769–76. doi: 10.1074/jbc.M100489200. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Schimmel P. Highly differentiated motifs responsible for two cytokine activities of a split human tRNA synthetase. Journal of Biological Chemistry. 1999a;274:23155–23159. doi: 10.1074/jbc.274.33.23155. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science. 1999b;284:147–51. doi: 10.1126/science.284.5411.147. [DOI] [PubMed] [Google Scholar]
- Zhang FR, Schwarz MA. Pro-EMAP II is not primarily cleaved by caspase-3 and -7. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1239–44. doi: 10.1152/ajplung.00141.2001. [DOI] [PubMed] [Google Scholar]