Abstract
BACKGROUND
Magnetic resonance imaging (MRI) can aid clinical assessment of brain changes potentially correlated with Alzheimer disease (AD). MRI traits may improve our ability to identify genes associated with AD-outcomes. We evaluated semi-quantitative MRI measures as endophenotypes for genetic studies by assessing their association with AD in families from the Multi-Institutional Research in Alzheimer Genetic Epidemiology (MIRAGE) Study.
METHODS
Discordant siblings from multiple ethnicities were ascertained through a single affected proband. Semi-quantitative MRI measures were obtained for each individual. The association between continuous/ordinal MRI traits and AD were analyzed using generalized estimating equations. Medical history and Apolipoprotein E (APOE)ε4 status were evaluated as potential confounders.
RESULTS
Comparisons of 214 affected and 234 unaffected subjects from 229 sibships revealed that general cerebral atrophy, white matter hyperintensities (WMH), and mediotemporal atrophy differed significantly between groups (each at P < .0001) and varied by ethnicity. Age at MRI and duration of AD confounded all associations between AD and MRI traits. Among unaffected sibs, the presence of at least one APOEε4 allele and MRI infarction was associated with more WMH after adjusting for age at MRI.
CONCLUSION
The strong association between MRI traits and AD suggests that MRI traits may be informative endophenotypes for basic and clinical studies of AD. In particular, WMH may be a marker of vascular disease that contributes to AD pathogenesis.
Keywords: Alzheimer disease, MRI, genetic, endophenotype, white matter hyperintensities
Introduction
Neuroimaging methods can be helpful in characterizing brain regions affected by Alzheimer disease (AD).1,2 Hippocampal atrophy observed by magnetic resonance imaging (MRI), for example, is considered an early and specific marker of the AD process 3-6 that correlates with impairments in memory function7 and AD pathology.8,9 Cross-sectional and longitudinal measures of cerebral atrophy also differ between AD patients and age-matched controls 5,10-15 and are associated with the rate of cognitive deterioration.16-18 These data suggest that hippocampal and cerebral atrophy could serve as biological markers of early AD pathology.19
White matter hyperintensities (WMH) which are abnormalities of cerebral white matter are frequently seen in normal aging and are commonly attributed to cerebrovascular disease (CVD),20-22 WMH also have been associated with AD,23 but these associations are controversial.24 MRI atrophy and WMH have not been examined in a family-based cohort of patients and unaffected family members who share environmental and genetic backgrounds.
One goal of the Multi-Institutional Research in Alzheimer Genetic Epidemiology (MIRAGE) Study is to identify genes influencing susceptibility to AD and AD-related brain changes measured on MRI scan. In this paper, we evaluate associations between MRI traits and AD in a multi-ethnic group of families comprised of affected and unaffected siblings and demonstrate that these traits may be informative endophenotypes for genetic and other studies aimed at elucidating mechanisms of AD pathogenesis.
Methods
Subject Recruitment and Classification
MIRAGE participants described in this report are AD patients and their siblings who were recruited at 14 sites in the United States and at three sites in other countries (Canada, Germany, and Greece). Families were ascertained through a proband with a diagnosis of probable AD according to National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) criteria.25 Two cognitive tests were used to confirm unaffected status in first-degree relatives through a two-step process. The modified mini-mental state examination (3MS) was used initially to assess absence of dementia.26 A cut-off score of ≥86 on this 100 point scale was required to be considered unaffected, based on data from population studies.27,28 Persons with a 3MS ≤ 83 were considered affected and referred for evaluation. If the 3MS score fell between 83 and 86, the informant questionnaire on cognitive decline (IQ-Code),29-31 an observer-based instrument of possible dementia symptoms, was administered and an IQCode score of < 3.25 was necessary to be categorized as unaffected. Ethnic designation was assigned based on self-description as Caucasian non-Hispanic (CNH), Caucasian Hispanic (CH), African American (AA), and Japanese American (JA). The study protocol was approved by Institutional Review Boards at each site.
Data Collection
Medical history, family history, demographic, and risk factor information, physical trait measurements, and blood samples were collected after obtaining informed consent from the non-demented subjects and a combination of consent or assent along with informed consent by proxy on living demented subjects. Details of data collection procedures, protocols for obtaining family histories, APOE genotyping, and reports of validity of study questionnaires have been published elsewhere.32-36
MRI Traits and Scanning Procedures
MRI traits utilized in this analysis included semi-quantitative measures of generalized cerebral atrophy (CA), bilateral medial temporal atrophy (MTA), severity of WMH, and the presence or absence of cerebral infarcts (INF). All measures were evaluated by a single rater (CD) blinded to age, gender, and affection status. All MRIs were acquired using 1.5 Tesla magnetic field strength scanners and the sequences were modified to suit differences in site machine vender and operating systems.
Semi-quantitative Assessment of CA and WMH
CA was evaluated from the second (T2) echo of a conventional double spin-echo sequence which consisted of a TR 2000, TE 20/100, FOV 24 cm, 1 NEX, and 256 × 192.
WMH were rated from axial FLAIR sequences which consisted of a TE 144 ms, TR: 11,000 ms, TI 2,250 ms, FOV: 22 cm, Flip Angle of 90 deg, 1 NEX and 256 × 192 matrices. When FLAIR imaging was unavailable, WMH measures were obtained from the proton density image of a conventional double echo image. Both CA and WMH were measured using an analog visual scale previously reported37 and subsequently modified to include reference images38 where CA or WMH were quantified according to previously reported methods.39
The scale consisted of a 100-mm straight line on which the origin is labeled “no abnormality” and the terminus is labeled “very severe abnormality.” The rater examined each slice of the brain image and drew a vertical line on the scale corresponding to the assessment of CA or WMH. The extent of abnormality was then determined by measuring the distance from the vertical line to the origin of the scale. As noted above, the scale was supplemented with reference images that were quantified for the percent of brain volume to establish the extent of CA or volume of WMH. Thus, the actual percent of CA or WMH was known for these images and they could be used as a reference for the rating scale.
MTA measures were obtained from axial-oblique 3-dimensional fast spoiled gradient recalled echo (FSPGR) or similar sequence that consisted of a TE 2.9 ms (min), TR 9 ms (min), Flip angle: 15 deg, Slice thickness: 1.5 mm, FOV: 25 cm × 25 cm, Matrix: 256 × 256. This image was obtained in the coronal orientation whenever possible. MTA was rated using a previously published qualitative rating scale.40-42 This scale assesses the extent of bilateral MTA by estimating the combined widths of the choroidal fissure, temporal horn of the lateral ventricle, and the height of the hippocampus in the coronal, oblique orientation to derive five different categories ranging from 0 (no atrophy) to 4 (severe atrophy). The intraclass correlation coefficient of inter-rater reliability measure is .83 (range 82-86).
The presence of INF was determined from the size, location, and imaging characteristics of the lesion. The image analysis system allowed for viewing of all images at three times magnified view to assist in interpretation of lesion characteristics. Signal void, best seen on the T2 weighted image was interpreted to indicate a vessel. Only lesions 3 mm or larger qualified for consideration as INF. INF also were required to have (1) cerebrospinal fluid (CSF) density on the subtraction image and (2) distinct separation from the circle of Willis vessels if the stroke was in the basal ganglia area. Kappa values for this method as determined in our laboratory range from .73 to .90.
A cerebrovascular disease measurement (CVR) was derived from INF and WMH measures. If INF were absent, then CVR was set equal to WMH. If INF were present, then CVR was given a score based on the amount of WMH and presence of INF.
Statistical Analysis Procedures
The influence of APOE genotype was evaluated assuming an additive model. Hence, subjects were grouped according to the number of ε4 alleles (0, 1, or 2). Nine possible education levels were collapsed into four categories: less than 7th grade, 7th through 12th grade, technical through college degree work, and graduate/professional degree work. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height measured in meters. Disease duration was defined as the number of years between earliest observable dementia symptoms as reported by the family and age at MRI in an affected sibling. For unaffected siblings, disease duration equaled zero years. WMH and CA were analyzed as continuous dependent variables and evaluated for normality using Kolmogorov-Smirnov43 and Anderson–Darling statistics44 and normal probability plots. Based on failed tests for normality, WMH was log transformed for all further analyses. MTA was analyzed as an ordinal variable. Associations between each MRI trait and affection status, adjusting for potential confounding variables (ie, ethnicity, education, stroke, APOE ε4 alleles) were assessed using generalized estimating equations (GEE) regression models.45,46 GEE models were used to obtain regression parameter estimates while accounting for correlated responses from sib-ship data. We assumed an exchangeable correlation matrix for all regression models. CA and log WMH were analyzed as normally distributed dependent variables. The association between MTA and affection status was assessed with a continuation ratio logit regression model that is appropriate for an ordinal, non-normal response and results in a range of regressions for the variable. Each logit is the log odds of an individual having the next higher MTA score given sequential progression through less severe atrophy scores. In other words, each logit is the log odds of transitioning to a more severe state of MTA. The evaluation of the association between AD and MRI traits adjusting for potential confounders was conducted with MRI traits as dependent variables. The association between each MRI trait and AD was assessed for potential confounding by comparing the parameter estimate of association from models with and without a potential confounder. We use the notation βAD|confounder to denote the regression estimate of association between MRI trait and AD controlling for a confounder. βAD denotes the regression estimate of association excluding the confounder from the model. If the addition of a confounder changed the association estimate by approximately 10%, then the variable was designated a confounder of the primary association between the MRI trait and AD. Differences in distributions of MRI traits regressed on age at MRI from GEE models plotted by affection status were also evaluated. All analyses were conducted using SAS v. 9.1.
Results
Sample Characteristics
The sample included in the analysis consisted of 448 subjects with complete MRI scan data. Of these subjects, 214 were affected and 234 were unaffected siblings. There were 229 sib-ships of size ≥2 of which 173 families included an AD individual. Affected and unaffected siblings had similar education and ethnic distribution (Table 1). AD siblings had a significantly greater proportion of APOE ε4 alleles (P < .0001) and were slightly older than unaffected siblings.
Table 1.
Demographics
| Affected sibs (n = 214) |
Unaffected sibs (n = 234) |
|
|---|---|---|
| Age at MRI1 | 75.0 (9.1) | 71.6 (9.0) |
| No. (%) | No. (%) | |
| Highest Level of Education2 | ||
| Less than 6th grade | 20 (9) | 12 (5) |
| 7th through 12th grade | 100 (47) | 102 (44) |
| College | 64 (30) | 85 (36) |
| Graduate or professional | 27 (13) | 34 (15) |
| Ethnicity3 | ||
| Caucasian non-Hispanic | 120 (56) | 137 (59) |
| Caucasian Hispanic | 32 (15) | 30 (13) |
| African American | 36 (17) | 35 (15) |
| Japanese American | 23 (11) | 31 (13) |
| APOE4 | ||
| 44, 24 | 42 (20) | 22 (9) |
| 34, 23 | 103 (48) | 96 (41) |
| 33 | 62 (29) | 106 (45) |
Pooled t-test P < .0001.
Two-sided Wilcoxon test P = .066.
Two-sided Wilcoxon test P = .60.
χ2test P < .0001.
Relationship of CA, MTA, and log WMH with AD
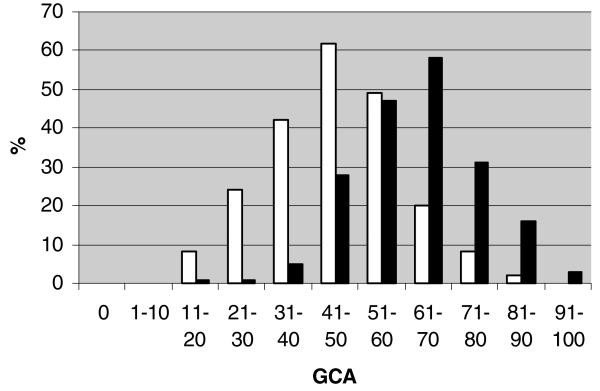
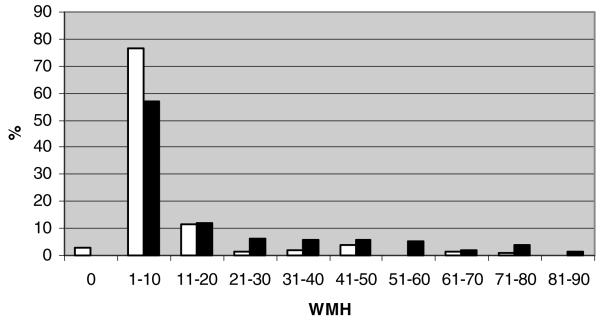
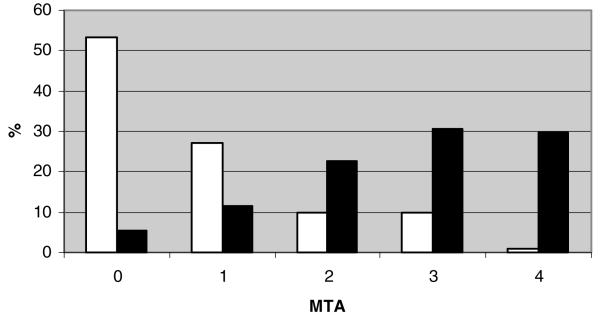
CA scores for affected (n = 208, Shapiro-Wilks P = .62) and unaffected siblings (n = 233, Shapiro-Wilks P = .32) were approximately normally distributed (Fig 1), whereas the distributions of WMH were skewed and non-normal (Fig 2). Natural log transformations of WMH were used for subsequent analyses although they remained somewhat skewed. MTA (Fig 3) and CVR (not shown) measures were also skewed and non-normally distributed. AD siblings had significantly greater CA and MTA (P < .0001 for all atrophy score comparisons), log WMH (P < .0001), and frequency of INF (P = .038) than unaffected siblings (Table 2). The relationships between individual MRI traits and AD remained significant in GEE models addressing the familial correlation structure of the dataset. Based on crude estimates of association, affected siblings consistently had higher CA within the observed age range (βAD = 16.36, s.e. [β] = 1.29, P < .0001) and baseline log WMH levels than unaffected siblings (βAD = 1.14, s.e. [β] = .15, P < .0001). AD status was also associated with worsening mediotemporal atrophy as reflected by positive crude log odds of MTA estimates (MTA 1 vs. 0: βAD = 1.52, s.e. [β] = .36; MTA 2 vs. <2: βAD = 2.38, s.e. [β] = .31; MTA 3 vs. <3: βAD = 1.91, s.e. [β] = .26; MTA 4 vs. <4: βAD = 3.88, s.e. [β] =.73; each at P ≤ .0002).
Fig 1.
Distribution of CA by affection status. Black bars denote affected subjects. White bars denote unaffected subjects.
Fig 2.
Distribution of WMH by affection status. Black bars denote affected subjects. White bars denote unaffected subjects.
Fig 3.
Distribution of MTA by affection status Black bars denote affected subjects. White bars denote unaffected subjects.
Table 2.
Mean MRI Trait Values among Affected and Unaffected Siblings
| Affected Sibs (n = 214) |
Unaffected Sibs (n = 234) |
|
|---|---|---|
| CA (Std. dev.)1 | 62.0 (13.8) | 45.7 (14.1) |
| logWMH (Std. dev.)2 | 2.3 (1.2) | 1.2 (2.0) |
| MTA (Std dev)2,3 | 2.7 (1.2) | .78 (1.0) |
| MRI infarcts | ||
| Present (no.)4 | 49 | 37 |
| Small (%) | 17 | 14 |
| Large (%) | 5 | 2 |
Pooled t-test P < .0001;
Unequal variances t-test P < .0001.
Treated as continuous variable;
χ2 test P = .038.
Age at MRI
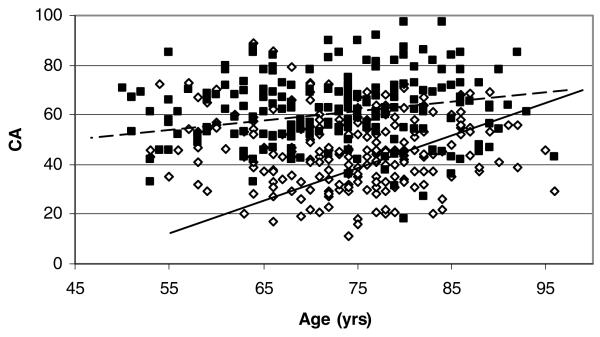
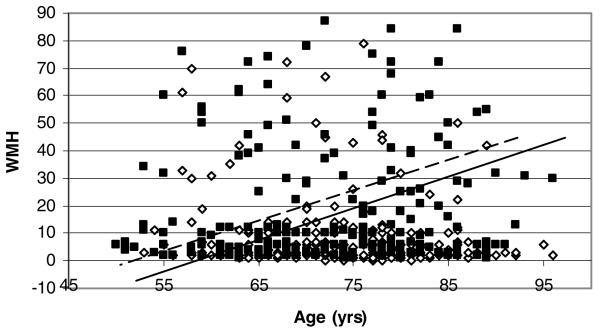
MRI trait measures were strongly influenced by an individual's age at MRI. Age at MRI was positively correlated with log WMH, CA, and MTA among affected (Pearson correlations range from .36 to .51, P = .0027 to <0001) and unaffected (Pearson correlations range from .21 to .39, P < .0001) siblings. Associations between CA, log WMH, and MTA with AD were confounded by age at MRI, but associations remained statistically significant. The association of CA with AD decreased after adjusting for age at MRI (βAD|ageatMRI = 14.33, S.E.[β] 1.35). Age at MRI had greater impact on CA in unaffected =(βage = .85, S.E. (β) = .092) versus affected (βage = .31, S.E. [β] = .12) siblings (Fig 4). The crude association between log WMH and AD decreased after adjusting for age at MRI (βAD|ageatMRI = .89, S.E.[β] = .13). The impact of age at MRI was also greater in unaffected than affected siblings (Fig 5). Age at MRI confounded the association between MTA and AD, but the confounding occurred only in comparisons of MTA ≤ 2. Specifically, continuation ratio logit (odds) of MTA of 1 versus 0 (βAD|ageatMRI = 1.72, s.e. [β] = .40) and 2 vs. < 2 increased after adjusting for age at MRI (βAD|ageatMRI = 2.69, s.e. [β] = .36). The odds of MTA of 3 vs < 3 (βAD|ageatMRI = 1.87, s.e. [β] = .27) or 4 vs. < 4(βAD|ageatMRI = 3.72, s.e. [β] = .73) did not change after adjusting for age at MRI.
Fig 4.
CA versus age. Data points are uncorrected for family structure. GEE regression estimates (Unaffected: Intercept = −14.8, β = .85; Affected: Intercept = 38.7, β = .31) and lines are corrected for family structure. Black squares and dotted line denote affected. White diamonds and solid black line denote unaffected.
Fig 5.
WMH by age. Data points are uncorrected for family structure. GEE regression estimates ((Unaffected: Intercept = −32.5, β = .57; Affected: Intercept = −46.5, β = .87) and lines are corrected for family structure. Black squares and dotted line denote affected. White diamonds and solid black line denote unaffected.
Disease Duration
While age at MRI had the largest impact on confounding the associations between MRI traits and AD, disease duration also affected the association estimates. Disease duration was positively correlated with CA (Pearson correlation = .25, P = .0002), log WMH (Pearson correlation = .19, P = .0069), and MTA (Spearman correlation = .23, P = .0010) among affected siblings. The decrease in associations between CA (βAD|diseaseduration,ageatMRI = 10.81, s.e. [β] = 1.74), log WMH βAD|diseaseduration,ageatMRI = .67, s.e. [β] = .15), and middle severity MTA (MTA 2 vs. <2: βAD|diseaseduration,ageatMRI = 3.01, MTA 3 vs. <3: βAD|diseaseduration,ageatMRI = 1.23) with AD adjusted for age at MRI indicated confounding by duration of disease. The least and most severe MTA associations adjusted for age at MRI were not further confounded by disease duration (MTA 1 vs. 0: βAD|diseaseduration,ageatMRI = 1.59; MTA 4 vs. <4: βAD|diseaseduration,ageatMRI = 3.58).
Ethnicity
Associations between CA and log WMH with AD were similar across ethnic groups with a few exceptions. The association between CA and AD in CHs (βAD|ageatMRI = 8.61, S.E. [β] = 3.67) was weaker than in other ethnic groups combined (βAD|ageatMRI ranges 12.97-16. 36, S.E. [β] ranges 1.79-3.22). Ethnic-specific associations for log WMH with AD were more variable (βAD|ageatMRI = .47, S.E. [β] .24 for AAs to βAD|ageatMRI = 1.17, S.E. [β] = .20 for CNHs). The associations between MTA and AD were similar across ethnic groups. Complete assessment of MTA was not possible due to statistical non-convergence in AAs (MTA 1 vs. 0), CNHs (MTA 4 vs. < 4), and JAs (MTA 4 vs. < 4).
Other Potential Confounders
Separate analyses of affected and unaffected siblings revealed APOE ε4 and INF confounded the association between log WMH and age at MRI. The presence of an APOE ε4 allele was significantly associated with higher log WMH after adjusting for age at MRI in unaffected siblings (βAPOE4,ageatMRI = .46, S.E. = .19, P = .0209). No such effect was observed in affected individuals. Log WMH was significantly associated with INF after adjusting for age in both unaffecteds (βINF,ageatMRI = .82, S.E. [β] = .24, P = .0026) and in affecteds (βINF,ageatMRI = .68, S.E. [β] = .17, P = .0015). BMI, sex, head circumference, and education had negligible effect on the association between log WMH and INF in these analyses. Analyses of all siblings combined were not affected by these potential confounders.
Discussion
Our results using simple semi-quantitative scales suggest that MRI traits related to atrophy (CA and MTA) are significantly associated with AD in families. This concurs with earlier literature suggesting the use of MR measures for differentiating AD from non-AD pathology in unrelated subjects.19 Our results indicate AD siblings had greater amounts of WMH and more frequent presence of infarction than unaffected siblings despite the fact that AD subjects were ascertained through criteria that would be expected to minimize cerebrovascular findings. These associations have been previously noted19,38 suggesting that vascular injury may play a role in the AD process52,53 Age at MRI and duration of AD each confound the association between these three MRI trait measurements and AD and are important variables to consider when comparing cognitively normal subjects to AD patients. Despite the obvious significant mean differences in MRI measures seen between patients with AD and their unaffected siblings, considerable overlap between the two groups is apparent for all measures. While some of this overlap can be explained by measurement error, the shared genetic background of the cases and controls in this study may account for this overlap. Other non-genetic factors could also contribute to the overlap, but our analyses of family data and other reports of MRI traits in twin data indicate that genetic factors play a sizeable role.54,55 Studies of WMH assessing the contribution of genetic and non-genetic factors have attributed the MR distirubtion to primarily genetic factors.56,57 Therefore, these traits could therefore be used as potential endophenotypes to investigate novel genetic risk factors that may influence AD onset. As such, this dataset will be important to future genetic studies within the MIRAGE project.
The MIRAGE Study has assembled one of the largest collections of AA families with AD. Our results suggest that the magnitude of association between WMH and AD may be lower in AAs. It is possible that other risk factors for CVD, such as hypertension and diabetes which are more common in AAs than Caucasians,47,48 may explain this weaker association between AD and WMH. The association between CA and AD is greater in AAs than in Caucasians, adjusting for age at MRI. The results for MTA are less clear, but suggest that MTA is a uniform process related to aging and not to factors associated with ethnicity. These differences are subtle possibly due to the constricted number of samples available for non-Caucasian ethnic groups. Yet, statistical testing is able to take into account the variability in sample size and results still suggest that the utility of MRI measurements in association with AD will vary by ethnic background.
We found that WMH are associated with the presence of infarcts in both affected and unaffected siblings, supporting the hypothesis that WMH reflects vascular disease.49,50 Of interest, APOE ε4 was associated with WMH only among unaffected siblings. This may reflect the increased vascular risk factor of the APOE ε4, an effect that is overwhelmed by the robust association between ε4 and AD in affected individuals.
Age at MRI and duration of disease (as a measure of disease severity) confound the relationship between AD and MRI traits as reported in previous studies.51 This is particularly true for CA and less so for WMH. CA differences between AD and non-AD subjects dissipate while WMH differences are steady with increasing age. This suggests concomitant CVD or white matter degeneration is part of the AD process.52,53 While both processes are possible, there continues to be considerable debate regarding the etiology of WMH in AD.50
Our family-based design which uses non-demented siblings as controls reduces greatly the potential for genetic substructure, or confounding, due to differences in underlying gene frequency distributions in affected and unaffected individuals. Family-based controls are also more likely to be similar to cases than unrelated controls in other pertinent factors including socioeconomic factors, educational, dietary, and access to medical care. If these factors are correlated with MRI traits, differential distribution of these factors among cases and controls could confound the association between MRI traits and AD.
The sensitivity and specificity of AD diagnosis could affect the univariate and regression results. Some overlap of affected an unaffected MRI values may be due to limitations of the measurements to differentiate adequately between those with and without AD. In addition, we may have taken a too conservative approach for defining AD probands. Because the overarching aim of the MIRAGE Study is to identify AD risk factors, AD probands were ascertained based on narrow NINCDA/ADRDA criteria that exclude subjects having any suggestion of vascular dementia. Thus, evidence of CVD, such as WMH or MRI infarct would not be associated with an initial diagnosis of vascular dementia. This implies that CVD in AD subjects is underreported. Another potential caveat of our findings is that some siblings categorized as unaffected actually have AD. If siblings were disproportionately misclassified as unaffected, then some MRI trait associations could have been overestimated. Finally, the sibling controls in our study may not be representative of non-demented siblings of AD cases because healthy volunteer effects could have enriched the reference group for subjects lacking brain atrophy or CVD. Lastly, the study data collected were based on self-report and proxies that may not accurately reflect an individual. Proxy measures, while essential in cognitively challenged populations, may be prone to biases. However, our own validation study suggests that proxy reporting is highly accurate.58
Our results showing strong association between MRI traits and AD in a family-based sample indicate that semi-quantitative MRI measurements will be useful endophenotypes for genetic association and other clinical and basic studies of AD.59 Each MRI trait, measured on either an ordinal or continuous scale, is an improvement over using a dichotomous AD measures as an outcome because the continuous variable contains more information than a dichotomous trait. The broader measurement scale is less sensitive to measurement error than in the binary measure and could improve study power for identifying genes that impact AD development. We expect, therefore, that genetic association findings with MRI measures of neurodegeneration and CVD will provide insights into the pathophysiology of AD.
Additional MIRAGE Study Group investigators are: C. T. Baldwin, and S. Auerbach (Boston University); Drs. A. Akomolafe, Lorin Freedman, and E. Ofili (Morehouse School of Medicine); Dr. H. Chui (University of Southern California); Dr. C. DeCarli (University of California - Davis); Dr. R. Duara (Mt. Sinai Medical Center, Miami); Drs. T. Foroud and M. Farlow (Indiana University School of Medicine); Dr. R. Friedland (Case Western Reserve University); Dr. R. Go (University of Alabama-Birmingham); Dr. A. Kurz (Technical University, Munich, Germany); Dr. T. Obisesan (Howard University); Drs. H. Petrovitch and L. White (Pacific Health Research Institute); Dr. N. Relkin (Cornell University); Dr. M. Sabbagh (Sun Health Research Institute); Dr. D. Sadovnick (University of British Columbia); and Dr. M. Tsolaki (University of Aristotle, Thessaloniki, Greece).
Acknowledgments
This work was supported in part by National Institute on Aging grants R01-AG09029, RO1-HG/AG02213, K24-AG027841, and P30-AG13846.
We are grateful to the contributions of the MIRAGE Study coordinators (P. Morey, B. Adamiak, Dr. A. Ade-Okanlami, L. Cowden, B. Cramer, M. Dandiilidou, E. Dwosh, E.Grady, S. Humphrey, C. Imamura, H. Kolody, N. Long, M. Luis, K. Martelli, and J. Wainwright) and J. Farrell, M. Huyck and D. Johnson for database programming and electronic data capturing support.
References
- 1.Scheltens P, Fox NC, Barkhof F, DeCarli C. Structural MRI in the practical evaluation of dementia: beyond exclusion. Lancet Neurol. 2002;1(1):13–21. doi: 10.1016/s1474-4422(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 2.Kantarci K, Jack CR., Jr Quantitative magnetic resonance techniques as surrogate markers of Alzheimer's disease. NeuroRx. 2004;1(2):196–205. doi: 10.1602/neurorx.1.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seab JP, Jagust WJ, Wong STS, Roos MS, Reed BR, Budinger TF. Quantitative NMR measurements of hippocampal atrophy in Alzheimer's disease. Magn Reson Med. 1988;8:200–208. doi: 10.1002/mrm.1910080210. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 5.DeCarli C, Murphy DG, McIntosh AR, Teichberg D, Schapiro MB, Horwitz B. Discriminant analysis of MRI measures as a method to determine the presence of dementia of the Alzheimer type. Psychiatry Res. 1995;57(2):119–130. doi: 10.1016/0165-1781(95)02651-c. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49(3):786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen RC, Jack CR, Jr, Xu YC, Waring SC, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Kokmen E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54(3):581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- 8.de Leon MJ, Convit A, Tarshish C, DeSanti S, Bobinski M. MRI studies of the hippocampal formation: contributions to the early diagnosis of Alzheimer's disease. In: de Leon M, editor. An Atlas of Alzheimer's Disease. The Partheon Publishing Group; New York: 1999. pp. 3–55. [Google Scholar]
- 9.Jack CR, Dickson DW, Parisi JE, Xu YC, Cha RH, O'Brien PC, Edland SD, Smith GE, Boeve BF, Tangalos EG, Kokmen E, Petersen RC. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47(4):430–439. [PubMed] [Google Scholar]
- 11.Fox NC, Freeborough PA, Rossor MN. Visualisation and quantification of rates of atrophy in Alzheimer's disease [see comments] Lancet. 1996;348(9020):94–97. doi: 10.1016/s0140-6736(96)05228-2. [DOI] [PubMed] [Google Scholar]
- 12.Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer's disease. J Magn Reson Imaging. 1997;7(6):1069–1075. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- 13.Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. Ieee Trans Med Imaging. 1997;16(5):623–629. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- 14.Fox NC, Warrington EK, Rossor MN. Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer's disease [letter] Lancet. 1999;353(9170):2125. doi: 10.1016/S0140-6736(99)00496-1. [DOI] [PubMed] [Google Scholar]
- 15.Jack CR, Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD [see comments] Neurology. 1999;52(8):1687–1689. doi: 10.1212/wnl.52.8.1687. [DOI] [PubMed] [Google Scholar]
- 17.Jack CR, Jr, Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62(4):591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack CR, Jr, Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65(8):1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantarci K, Jack CR., Jr Quantitative magnetic resonance techniques as surrogate markers of Alzheimer's disease. NeuroRx: J Am Soc Exp NeuroTherapeutics. 2004;1:196–205. doi: 10.1602/neurorx.1.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyper-intensity volume: the Framingham Study. Stroke. 2004;35(8):1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 21.Vermeer SE, Den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM, Rotterdam Scan Study Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003;34(2):392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 22.Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Risk factors for mild cognitive impairment in the cardiovascular health study cognition study: part 2. Arch Neurol. 2003;60(10):1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 23.Wu CC, Mungas D, Petkov CI, Eberling JL, Zrelak PA, Buonocore MH, Brunberg JA, Haan MN, Jaqust WJ. Brain structure and cognition in a community sample of elderly Latinos. Neurology. 2002;59:383–391. doi: 10.1212/wnl.59.3.383. [DOI] [PubMed] [Google Scholar]
- 24.Skoog I. Vascular aspects in Alzheimer's disease. J Neural Transm Suppl. 2000;59:37–43. doi: 10.1007/978-3-7091-6781-6_6. [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease:report of the NINCDS-ADRDA work group. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Teng EL, Chui HC. The modified mini mental stat (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 27.Norton MC, Tschanz JA, Fan X, Plassman BL, Welsh-Bohmer KA, West N, Wyse BW, Bretner JC. Telephone adaptation of the Modified Mini-Mental State Exam (3MS). The Cache County Study. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12(4):270–276. [PubMed] [Google Scholar]
- 28.Khachaturian AS, Gallo JJ, Breitner JC. Performance characteristics of a two-stage dementia screen in a population sample. J Clin Epidemiol. 2000;53(5):531–540. doi: 10.1016/s0895-4356(99)00196-1. [DOI] [PubMed] [Google Scholar]
- 29.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 30.Jorm AF, Scott R, Cullen JS, MacKinnon AJ. Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med. 1991;21:785–790. doi: 10.1017/s0033291700022418. [DOI] [PubMed] [Google Scholar]
- 31.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 32.Lautenschlager NT, Cupples LA, Rao VS, Auerbach SA, Becker R, Burke J, Chui H, Duara R, Foley EJ, Glatt SL, Green RC, Jones R, Karlinsky H, Kukull WA, Kurz A, Larson EB, Martelli K, Sadovnick AD, Volicer L, Waring SC, Growdon JH, Farrer LA. Risk of dementia among relatives of Alzheimer's disease patients in the MIRAGE study: what is in store for the oldest old? Neurology. 1996;46:641–650. doi: 10.1212/wnl.46.3.641. [DOI] [PubMed] [Google Scholar]
- 33.Farrer LA, Cupples LA, Blackburn S, Kiely DK, Auerbach S, Growdon JH, Connor-Lacke L, Karlinsky H, Thibert A, Burke JR, Utley S, Chui H, Ireland A, Duara R, Lopez-Alberola R, Larson EB, O'Connell S, Kukull WA. Interrater agreement for diagnosis of Alzheimer's disase: the MIRAGE study. Neurology. 1994;44:652–656. doi: 10.1212/wnl.44.4.652. [DOI] [PubMed] [Google Scholar]
- 34.Demissie S, Green RC, Muchhi L, Tziavas S, Martelli K, Bang K, Coons L, Bourque S, Buchillon D, Johnson K, Smith T, Sharrow N, Lautenschlager N, Friedland R, Cupples LA, Farrer LA. Reliability of information collected by proxy in family studies of Alzheimer's disease. Neuroepidemiology. 2001;20:105–111. doi: 10.1159/000054768. [DOI] [PubMed] [Google Scholar]
- 35.Green RC, Cupples LA, Go RCPG, Benke KS, Edeki T, Griffth PA, Williams M, Hipps Y, Graff-Radford N, Bachman D, Farrer LA, MIRAGE Study Group Risk of dementia among white and African American relatives of patients with Alzheimer's disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 36.Graff-Radford N, Green RC, Go RCPG, Hutton ML, Edeki T, Bachman D, Adamson JL, Griffith P, Willis FB, Williams M, Hipps Y, Haines JL, Cupples LA, Farrer LA. Association between apolipoprotein E genotype and Alzheimer disease in African Americans subjects. Arch Neurol. 2002;59:594–600. doi: 10.1001/archneur.59.4.594. [DOI] [PubMed] [Google Scholar]
- 37.DeCarli C, Fugate L, Falloon J, Eddy J, Katz DA, Friedland RP, Rapoport SI, Brouwers P, Pizzo PA. Brain growth and cognitive improvement in children with human immunodeficiency virus-induced encephalopathy after 6 months of continuous infusion zidovudine therapy. J Acquir Immune Defic Syndr. 1991;4(6):585–592. [PubMed] [Google Scholar]
- 38.Wu CC, Mungas D, Petkov CI, Eberling JL, Zrelak PA, Buonocore MH, Brunberg JA, Haan MN, Jaqust WJ. Brain structure and cognition in a community sample of elderly Latinos. Neurology. 2002;59(3):383–391. doi: 10.1212/wnl.59.3.383. [DOI] [PubMed] [Google Scholar]
- 39.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16(2):274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, Kuiper M, Steinling M, Wolters EC, Valk J. Atrophy of medial temporal lobes on MRI in ‘probable’ Alzheimer's disease and normal aging: diagnostic value and neuropsychological correlates. J Neurol, Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheltens P, Launer LJ, Barkhof F, Weinstein HC, van Gool WA. Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurology. 1995;242(9):557–560. doi: 10.1007/BF00868807. [DOI] [PubMed] [Google Scholar]
- 42.Scheltens P, Pasquier F, Weerts JG, Barkhof F, Leys D. Qualitative assessment of cerebral atrophy on MRI: inter- and intra-observer reproducibility in dementia and normal aging. Eur Neurol. 1997;37(2):95–99. doi: 10.1159/000117417. [DOI] [PubMed] [Google Scholar]
- 43.Stephens MA. EDF Statistics for Goodness of Fit and Some Comparisons. J Am Stat Assoc. 1974;69:730–737. [Google Scholar]
- 44.D'Agostino RB, Stephens MA. Goodness-of-Fit Techniques. Marcel Dekker, Inc; New York: 1986. [Google Scholar]
- 45.Liang KY, Zeger SL. Longitudinal Data Analysis using Generalized Linear Models. Biometrika. 1986;73:13–22. [Google Scholar]
- 46.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 47.Bolen JC, Rhodes L, Powell-Griner EE, Bland SD, Holtzman D. State-specific prevalence of selected health behaviors, by race and ethnicity-Behavioral Risk Factor Surveillance System, 1997. MMWR CDC Surveill Summ. 2000;49(2):1–60. [PubMed] [Google Scholar]
- 48.Ness J, Aronow WS, Ahn C. Risk Factors for Coronary Artery Disease in Older Persons in an Academic Hospital-Based Geriatrics Practice. Coron Artery Dis. 2000;11:437–439. doi: 10.1097/00019501-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Decarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 50.DeCarli C. The role of cerebrovascular disease in dementia. Neurologist. 2003;9(3):123–136. doi: 10.1097/00127893-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Murphy DG, DeCarli CD, Daly E, Gillette JA, MeIntosh AR, Haxby JV, Teichberg D, Schapiro MB, Rapoport SI, Horwitz B. Volumetric magnetic resonance imaging in men with dementia of the Alzheimer type: correlations with disease severity. Biol Psychiatry. 1993;34(9):612–621. doi: 10.1016/0006-3223(93)90153-5. [DOI] [PubMed] [Google Scholar]
- 52.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60(7):1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 53.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62(7):1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 54.Jarvenpaa TM, Laakso P, Rossi R, Koskenvuo M, Kaprio J, Raiha I, Kurki T, Laine M, Frisoni GB, Rinne JO. Hippocampal MRI volumetry in cognitively discordant monozygotic twin pairs. J Neurol Neurosurg Psychiatry. 2004;75(1):116–120. [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen V-P, Huttunen M, LonnQvist, Standertskjold-Nordenstam C-G, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nat Neurosci. 2001;4(12):1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 56.Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D'Agostino RB, DeCarli C. Genetic variation in white matter hyper-intenisty volume in the Framingham Study. Stroke. 2004;35(7):1609–1613. doi: 10.1161/01.STR.0000129643.77045.10. [DOI] [PubMed] [Google Scholar]
- 57.Carmelli D, DeCarli C, Swan GE, Jack LM, Reed T, Wolf PA, Miller BL. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke. 1998;29(6):1177–1181. doi: 10.1161/01.str.29.6.1177. [DOI] [PubMed] [Google Scholar]
- 58.Demissie S, Green RC, Mucci L, Tziavas S, Martelli K, Bang K, Coons L, Bourque S, Buchillon D, Johnson K, Smith T, Sharrow N, Lautenschlager N, Friedland R, Cupples LA, Farrer LA. Reliability of information collected by proxy in family studies of Alzheimer's disease. Neuroepidemiology. 2001;20:105–111. doi: 10.1159/000054768. [DOI] [PubMed] [Google Scholar]
- 59.Gottesman II, Gould TD. The endophenotypes concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]