Abstract
Due to the heterogeneous nature of most brain injuries, the contributions of gray and white matter involvement to motor deficits and recovery potential remain obscure. We tested the hypothesis that duration of hand motor impairment and recovery of skilled arm and hand motor function depends on the volume of gray and white matter damage of the frontal lobe. Lesions of the primary motor cortex (M1), M1 + lateral premotor cortex (LPMC), M1 + LPMC + supplementary motor cortex (M2) or multi-focal lesions affecting motor areas and medial prefrontal cortex were evaluated in rhesus monkeys. Fine hand motor function was quantitatively assessed pre-lesion and for 3–12 months post-lesion using two motor tests. White and gray matter lesion volumes were determined using histological and quantitative methods. Regression analyses showed that duration of fine hand motor impairment was strongly correlated (R2 > 0.8) with the volume of gray and white matter lesions, with white matter lesion volume being the primary predictor of impairment duration. Level of recovery of fine hand motor skill was also well correlated (R2 > 0.5) with gray and white matter lesion volume. In some monkeys post-lesion skill exceeded pre-lesion skill in one or both motor tasks demonstrating that continued post-injury task practice can improve motor performance after localized loss of frontal motor cortex. These findings will assist in interpreting acute motor deficits, predicting the time course and expected level of functional recovery, and designing therapeutic strategies in patients with localized frontal lobe injury or neurosurgical resection.
Introduction
It seems intuitive that motor deficits will increase and the potential for recovery of function will decrease with greater injury to the frontal lobe motor areas. However, both human and animal studies suggest that the correlation of lesion volume and lasting motor deficits is rather weak (e.g., (Binkofski, et al., 2001; Miyai, et al., 1999; Prabhakaran, et al., 2008)). In humans, supratentorial lesions induced by stroke and traumatic brain injury typically affect both gray and white matter of the cerebral cortex, and major subcortical structures. This post-injury condition makes it difficult to interpret whether movement deficits and recovery are related primarily to cortical or subcortical structures. For example, studies in patients have shown that recovery is strongly correlated with initial impairment of upper limb function, but effects of lesion size are unclear with one study showing no effect (Binkofski, et al., 2001) and another reporting a correlation with subcortical, but not cortical lesion size (Prabhakaran, et al., 2008). In contrast, other studies in stroke patients found strong correlations between infarct volume and either motor deficits (Pineiro, et al., 2000) or severity of weakness of upper and lower limbs (Mohr, et al., 1993; Pineiro, et al., 2000). However, Mohr et al. (1993) also noted that lesion location was poorly correlated with specific syndromes of focal weakness as similar lesions often produced different syndromes and quite different lesions could produce the same syndrome. Thus, inter-individual variability in deficits and recovery in humans with similar types of brain lesions may be rather high. Overall, the relationship between recovery of hand motor function and the size of lesions affecting cortical and subcortical gray and white matter areas of the brain remains unclear. Clarifying some of these issues in a study of controlled lesions of different size in an animal model with highly developed dexterous movements would enhance our ability to predict potential clinical outcomes in humans following isolated frontal cortical injury and may assist in developing more effective therapeutic strategies.
Observations in animal models of brain injury also suggest that correlation of lesion volume with impairment and recovery of function is weak. For example, temporary (1 hour) middle cerebral artery (MCA) occlusion in rats produced variable sized lesions that did not correlate well with recovery of motor or brain function (Weber et al., 2008). Other methods of producing brain lesions also have variable effects on recovery (Alaverdashvili, et al., 2008; Metz, et al., 2005; Whishaw, 2000), although some studies have reported that volume of tissue damage is the primary determinant of motor impairment (Gonzalez and Kolb, 2003). Studies in adult subhuman primates have shown that localized surgically and chemically induced gray matter lesions of primary motor cortex (M1) of the frontal lobe produce lasting deficits in highly trained wrist/hand/digit movements (Hoffman and Strick, 1995; Murata, et al., 2008) and grip strength (Black, et al., 1971). Lesion location within the M1 hand area may also affect the types of hand movement control deficits observed (Friel, et al., 2005). However, there have been no studies comparing the effects of progressively larger lesions of frontal lobe motor areas controlling upper limb motion. Clinically, such investigations would be of significant value for assessing the consequences of localized frontal lobe lesion size on fine hand motor control.
A significant amount of previous work has also shown that rhesus monkeys have a remarkable ability to recover upper limb and hand function after large lesions to the lateral precentral motor areas that initially cause hemiparesis (Bucy, 1949; Denny-Brown, 1950; Denny-Brown, et al., 1975; Travis, 1955a; Vilensky and Gilman, 2002), especially if forced to use the contralesional limb (Murata, et al., 2008; Ogden and Franz, 1917). However, the precise effects of gray matter lesion volume involving frontal lobe motor areas on the initial motor deficit of dexterous movements and the subsequent long-term recovery process are unclear because the previous classical, non-human studies did not quantitatively evaluate lesion volume or fine hand/digit movements.
The primary aim of the present work was to characterize the effects of isolated motor cortex lesions of different volumes in a non-human primate animal model with similar cortical structure to humans (Geyer, et al., 2000; Picard and Strick, 1996; Roland and Zilles, 1996; Zilles, et al., 1995) and highly developed distal upper extremity motor function. Indeed, the unique and direct corticospinal projection to lower motor neurons in higher-order primates has long been recognized to play an essential role in the production of finely coordinated dexterous hand movement (Heffner and Masterton, 1975; Heffner and Masterton, 1983; Kuypers, 1981; Lemon and Griffiths, 2005; Schieber, 2007). Such studies are likely to provide important insights relevant to recovery from brain injury in the human because the imposed lesions targeted selective removal of the gray matter of frontal motor cortex while attempting to minimize involvement of adjacent structures including the subcortical white matter. Thus, it was possible with this approach to assess specifically whether the gray matter lesion volume correlated closely with motor function after the lesion. This model also enabled us to determine if the volume of damage to white matter immediately below cortex affected the initial level of deficit and subsequent recovery process. Specifically, we tested the hypothesis that greater lesion volume of motor cortex would produce larger and longer duration fine hand motor deficits initially, but that long-term recovery would be poorly correlated with lesion volume because of reorganization of intact neural structures following the lesion.
Methods
Experimental Animals
Ten adult rhesus monkeys (Macaca mulatta – SDM38, 45, 46, 48, 50, 55, 56, 64, 67, and 70) were subjects for these experiments (Table 1). The monkeys were housed, cared for, and maintained in a United States Department of Agriculture (USDA) approved and inspected facility. All behavioral and surgical protocols were approved by the University of South Dakota (USD) Institutional Animal Care and Use Committee (IACUC), and conducted in accordance with USDA, National Institutes of Health, and Society for Neuroscience guidelines for the ethical treatment of experimental animals. Prior to beginning the study, each monkey was evaluated by a primate veterinarian and judged to be healthy and free of any neurological deficit. Proximal and distal movements and range of motion at the joints in both upper extremities of all animals were normal with the exception of SDM55. In this case the interphalangeal joints of digit 3 were permanently extended. However, this animal was able to perform precision opposition with digits 1 and 2 to successfully acquire the food rewards in both motor tests.
Table 1.
Characteristics and Experimental Parameters of Monkeys
| Monkey | Age1 (yrs.) | Sex | HI2 | Les. Cat.3 | Areas lesioned | Post- lesion dur (mo.) | GMLV4 (mm3) | WMLV5 (mm3) |
|---|---|---|---|---|---|---|---|---|
| SDM38 | 20.7 | F | 6R | F1 | M1 | 12 | 101.96 | 9.62 |
| SDM45 | 4.9 | M | 21.3R | F2 | M1+LPMC | 6 | 212.64 | 23.02 |
| SDM48 | 6.8 | F | 6R | F2 | M1+LPMC | 12 | 220.31 | 23.12 |
| SDM55 | 11.8 | M | 20L | F2 | M1+LPMC | 12 | 207.67 | 20.51 |
| SDM64 | 13.6 | F | 95.3L | F2 | M1+LPMC | 6 | 217.92 | 43.03 |
| SDM70 | 7.2 | M | 4.4R | F2 | M1+LPMC | 6 | 143.27 | 7.76 |
| SDM50 | 8.7 | F | 79R | F3 | M1+LPMC+M2 | 12 | 430.91 | 53.66 |
| SDM56 | 9.2 | M | 39.3L | F3 | M1+LPMC+M2 | 6 | 407.54 | 64.01 |
| SDM67 | 13.8 | F | 1.1R | MF | M1+LPMC+ M2+pSMA | 12 | 236.19 | 135.37 |
| SDM46 | 7.0 | M | 96L | MF | M1+LPMC+ M2+pSMA+ M3+PFC | 3 | 811.07 | 190.26 |
Age at time of lesion;
Handedness Index ((percentage of initial reaches and retrievals with preferred hand – 50)*2 - (Nudo, et al., 1992); R-right hand preferred, L – left hand preferred);
lesion category – F1 (M1 – primary motor cortex), F2 (M1+LPMC – lateral premotor cortex), F3 (M1+LPMC+M2 – supplementary area), MF (multi-focal lesion), M3 (rostral cingulate motor area), pSMA (pre-supplementary motor area), PFC – prefrontal cortex);
GMLV – gray matter lesion volume;
WMLV – white matter lesion volume
Experimental Apparati
Two apparati were used to test fine hand/finger motor function: the modified movement assessment panel (mMAP; Darling, et al., 2006) and the modified dexterity board (mDB – Pizzimenti et al., 2007). Both devices were attached to the monkey’s cage and allow control, without restraint, of which hand the monkey could use to perform the tasks to permit pre- and post-lesion testing of each hand. The monkeys were allowed to move freely about the cage between trials and food targets were used to minimize training requirements. The mMAP was adapted from the movement assessment panel originally developed by Gash and colleagues (Gash, et al., 1999) to permit recording of forces applied as the monkey grasps and lifts a lifesaver candy (in our studies, a carrot chip with a hole punched from the center is used) from a flat surface (easiest task) and threaded over straight (medium difficulty) and curved rods (highest difficulty) (Darling, et al., 2006). The mDB is similar to a typical “Kluver board” used in many previous studies (Kermadi, et al., 1997; Mason, et al., 1998; Nudo, et al., 1992; Nudo and Milliken, 1996) but modified such that the food pellet is always presented at the same location for reaching with one hand. Our mDB contained 4 wells of varying diameter (A - 10 mm, B - 13 mm, C - 19 mm and D - 25 mm) routed (1 cm deep) into the surface and a shallow “dimple” (well E) to hold the pellet but not restrict digit motions needed to acquire the pellet (Pizzimenti et al., 2007). Different levels of fine-digit motor control were required depending on the size of the well with the shallow dimple (well E) as the easiest task and well A as the most difficult.
Data Acquisition
Forces applied during manipulation of the carrot chip in the mMAP task were recorded at 200 samples/s using Datapac 2k2 (Run Technologies). Movements of the hand during the mMAP task were recorded using a single digital video camera (Sony, model DCR-DVD301) placed directly in front of the cage. These recordings were used for qualitative ratings of the movement strategy and to assess success/failure on each trial.
Quantitative video recordings of hand movements during the mDB task were used to assess spatial and temporal variables (e.g., accuracy and duration of the initial reach, grip aperture at touchdown of the hand, etc.) as described previously (Pizzimenti, et al., 2007). These recordings used four digital video cameras interfaced with the SIMI Motion data acquisition package (SIMI Reality Motion Systems, Unterschleissheim, Germany). Video data collection began when the portal door was opened to allow the monkey to reach toward the food pellet and continued until the pellet was retrieved into the cage, the pellet was knocked off of the platform, or a 60 s time limit had expired. Video collection was manually triggered and single trial video clips of each trial were manually created and verified. Details of the video collection protocol, data acquisition, and data analysis are provided in our previous work (Pizzimenti, et. al, 2007)
Behavioral Procedures
Prior to an experimental session the monkey was food restricted for 18–24 hours. The initial training sessions for the mDB used a “standard” rectangular dexterity board to assess the preferred hand of each monkey (since the monkey could choose to use either hand with this device) as described previously (Nudo, et al., 1992). There were 150 trials over three days (30 in each of 4 wells and 30 from the flat surface) with the pellets put into wells or on the flat surface in random order. The number of initial reaches and subsequent reaches with each hand on each trial was assessed from video recording of the two sessions and used to compute an index of hand preference as described previously (Nudo, et al., 1992) (Table 1).
Training with the mMAP and mDB devices commenced after hand preference was determined and was performed in block fashion for each task. Full testing sessions with the mDB included 5 retrieval attempts for each of the wells (A–E) for both limbs proceeding from the easiest well (E) to the most difficult (A), for a total of 25 trials with each hand. During post-lesion tests, the more impaired hand (contralateral to the surgically induced lesion) was always tested first to ensure high motivation. Full testing sessions with the mMAP included blocks of 5 trials at each difficulty level with each hand, thereby giving the monkey 30 opportunities to retrieve carrot chips, 15 with each hand. During the first few post-lesion tests, the more impaired hand was tested first on the flat surface task (easiest task) to ensure high motivation. Once the monkey began successfully retrieving the carrot chips, the more impaired hand was tested first on the curved rod task to ensure high motivation on this difficult task. Overall, when considering both tests, a single testing session consisted of only 40 reaches for each hand.
Pre-lesion data were collected every 1–3 weeks with a variable number of training and testing sessions according to each monkey’s ability to learn the task and perform consistently (Table 2). The final five pre-lesion experiments demonstrated relatively stable levels of performance before lesions were made (see Results). Post-lesion data were collected from both limbs during weekly experimental sessions for the first two months after the surgery and every two weeks thereafter. After initial training, it was only during the pre- and post lesion experimental sessions that subjects had exposure to the mMAP and mDB devices.
Table 2.
Total number of pre-lesion testing and training sessions and ratios of post-lesion maximum skill (over 5 consecutive testing sessions) to pre-lesion skill (over final 5 pre-lesion testing sessions) in the mDB (manipulation skill for two wells, reach skill for the best well) and mMAP (curved rod) tasks.
| Monkey | Lesion Category | Number of pre- lesion testing/training sessions | Manipulation Skill Recovery Ratio | Reach Skill Recovery Ratio for mDB (1st well) | ||
|---|---|---|---|---|---|---|
| mDB (1st well1) | mDB (2ndwell) | mMAP | ||||
| SDM38 | F1 | 16 | 0.89(D) | 1.34(C) | 1.40 | 0.76 |
| SDM45 | F2 | 10 | 1.18(C) | 0.96(A) | 1.66 | 0.98 |
| SDM48 | F2 | 6 | 0.48(E) | 1.29(B) | 1.16 | 1.51 |
| SDM55 | F2 | 13 | 1.09(E) | 1.41(C) | 0.91 | 1.05 |
| SDM64 | F2 | 15 | 0.88(D) | 0.52(C) | 0.50 | 0.41 |
| SDM70 | F2 | 15 | 1.09(B) | 1.19(A) | 0.95 | 1.57 |
| SDM50 | F3 | 13 | 0.85(E) | 1.34(C) | 1.16 | 1.37 |
| SDM56 | F3 | 13 | 0.5(D) | 0.55(C) | 0.34 | 1.56 |
| SDM67 | MF | 12 | 0.43(C) | 0.56(B) | 2.28 | 1.15 |
| SDM46 | MF | 12 | 0.00(D) | 0.00(A) | 0.00 | 0.00 |
The letters in these columns correspond to the well of the mDB device A - 10 mm, B - 13 mm, C - 19 mm and D - 25 mm, E – small dimple to hold pellet in place) on which the monkey had the highest skill (1st well) and a smaller well (2nd well) on which skill was lower.
Surgical Procedure
The planned surgical lesions included the arm areas of primary motor cortex (M1) (category F1 lesion); M1 + the adjacent lateral premotor cortex (LPMC) (category F2 lesion); M1 + LMPC + the supplementary motor cortex (M2) (category F3 lesion). Two additional lesions cases are also presented that initially involved the surgical removal of motor cortex, but spread rostrally to involve medial prefrontal cortex (multifocal lesion). All lesions were placed in the hemisphere contralateral to the preferred limb. Preoperatively, each monkey was injected with either glycopyrrolate (0.2 mg/kg IM) or atropine sulfate (0.05mg/kg), immobilized with ketamine hydrochloride (10mg/kg), intubated then placed on a mechanical respirator where it was anesthetized with isoflurane inhalation (1.2–1.5%) and a surgical grade air/oxygen mixture. The monkey was placed into a head holding device and administered mannitol (1.5–2g/kg) intravenously. Using sterile surgical technique, a skin incision, bone flap and dural flap was made over the lateral frontal convexity of the cerebral hemisphere and the frontal lobe exposed. After aseptic cortical exposure, the animal was transferred to intravenous ketamine anesthetic sedation for electrophysiological mapping of the frontal motor cortices. Intracortical microstimulation (ICMS) was used to localize the arm areas of M1, LPMC and M2 (Morecraft, et al., 2002, Morecraft, et al., 2001). To accomplish this, a tungsten electrode (impedance 0.5–1.5 MΩ) was inserted 200 μm below the pial surface then advanced at 500 μm intervals. Movements were evoked using a train duration of 50 ms and pulse duration of 0.2 ms delivered at 330 Hz. Current intensity ranged between 0.1 and 90 μA. Threshold currents were determined and the evoked movements were recorded when agreed upon by two observers. After defining the location and borders of the forelimb representation(s) and non-excitable cortex (i.e., the rostral part of area 6DR and area 8Bm laterally, and the pre-SMA medially), the animal was returned to isoflurane anesthesia and subpial aspiration/electrocoagulation, as applied in human neurosurgery for tissue resection, was then used to remove the gray matter (Fig. 1). Specifically, tissue removal was performed using an angled glass pipette or angled Frazier surgical suction tube that was attached to a vacuum source. Large and medium-sized arteries directly within the cortical field to be resected were cauterized. Following electrocoagulation, the tip of the micropipette or suction tube was carefully placed on the cortical surface using microscopic guidance and the gray matter gently removed until the underlying subcortical white matter field was identified.
Figure 1.
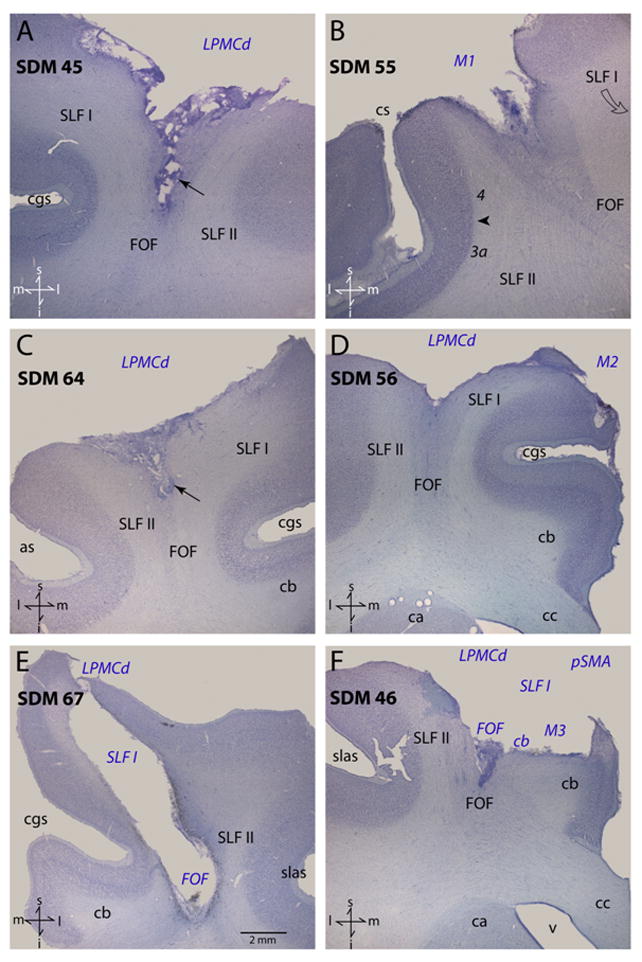
Plate of photomicrographs illustrating Nissl-stained coronal sections through representative cases analyzed in this study. In each panel the region of extirpated cortex and involved subcortical pathways are identified by the blue italicized conventions. The arrows in panels A and C identify the tapered portion of the subcortical lesion that part the adjacent SLF I and SLF II. The arrow head in panel B identifies the boundary between Brodmann’s areas 4 and 3a. The scale bar in panel E corresponds to all panels. Directional orientation is indicated in the lower left corner of each panel with abbreviated directions: superior (s), inferior (i), medial (m) and lateral (l). Abbreviations: as, spur of the arcuate sulcus; ca, caudate nucleus; cb, cingulum bundle; cc, corpus callosum; cgs, cingulate sulcus; cs, central sulcus; FOF, fronto-occipital fasciculus; i, inferior; l, lateral; LPMCd, dorsal lateral premotor cortex; m, medial; M1, primary motor cortex; M2, supplementary motor cortex; pSMA, pre-supplementary motor cortex; s, superior; slas, superior limb of the arcuate sulcus; SLF I, superior longitudinal fasciculus I; SLF II, superior longitudinal fasciculus II; v ventricle.
Following resection, the dura was sutured closed and the bone flap replaced and anchored to the cranium. The galea aponeurotica and temporalis muscle was reattached with 3-0 silk and the skin closed using sterile staples. Each animal was carefully monitored postsurgically throughout the survival period. Buprenorphin (0.5mg/kg) was administered postoperatively for 48–72 hours. Twenty four hours prior to the surgery, and for at least 9 days post-surgery, each animal was administered amoxicillin or penicillin as a prophylactic and postoperative antibiotic. In all cases, a second surgery was performed 33–34 days prior to sacrifice to inject neural tract tracers into arm areas of spared, intact motor cortices to study reorganization of output from these areas following brain injury. Findings from these studies will be published in future reports (e.g., McNeal et al., accepted with review).
Histological Procedures
Following the predetermined survival period, each monkey was deeply anesthetized with an overdose of pentobarbital (50 mg/kg or more) and perfused transcardially with 0.9% saline. Saline infusion was followed by 2 liters of 4% paraformaldehyde in 0.1M phosphate buffer at pH 7.4 (PB), then one liter each of 10% and 30% sucrose in 0.1M PB for cryoprotection. The brain was removed, blocked into cortical, brainstem and spinal cord regions, placed in 30% sucrose in 0.1M PB, and stored for 2 to 5 days at 4 °C. Post-mortem digital electronic images were taken for data reconstruction. The tissue was then processed for histochemical and immunohistochemical visualization (Morecraft, et al., 2004, Morecraft, et al., 2007). Specifically, the cortical tissue was frozen sectioned in the coronal plane on a sliding microtome (American Optical 860, Buffalo, NY, USA) at a thickness of 50 μm in cycles of 10, forming 10 complete series of evenly spaced tissue sections respectively. SDM56 was cut at a thickness of 60 μm in cycles of 10. One series of tissue sections (with each section spaced 500 μm) was processed for Nissl substance using our previously described histochemical methods (Morecraft, et al., 2004, Morecraft, et al., 1992) and used for the lesion volume analysis in the present report. Additional series of tissue sections were used for immunohistochemical visualization of neural tract tracers for additional neuroanatomical studies.
Estimation of Lesion Volume
The methods for estimating gray and white matter lesion volume were described previously (see Figs. 3 and 4 of Pizzimenti et al. 2007), but have been modified slightly by examining every Nissl stained tissue section at 500 μm intervals through the lesion site (instead of 1000 μm intervals). This additional data permitted more accurate volume measures of the various lesion sizes. It has long been known that atrophic distortion occurs over time as a result of brain tissue removal (Bucy, 1964; Denny-Brown, et al., 1975; Graham and Landtos, 2002; Warabi, et al., 1990) and this phenomena leads to altered volumetric representation of tissue loss at autopsy. To minimize this effect on estimating gray and white matter lesion volume we superimposed an outline of the lesion site onto the undamaged hemisphere to calculate the total gray matter and total white matter volume of the lesion. This was accomplished using metrically calibrated images of the cortical surface produced immediately before and after the lesion that included principal anatomical landmarks (e.g., central, arcuate and cingulate sulci) to assist the postmortem reconstruction and transfer of the lesion site boundaries onto a surface image of the non-lesioned hemisphere (e.g., see Figs. 2, 3). Specifically, gray matter lesion volume was estimated by marking the medial, lateral and inferior boundaries of the lesion site on anatomically matched Nissl stained tissue sections through the non-lesioned hemisphere and tracing the boundaries as closed contours using Stereo Investigator software (Microbrightfield, Colchester, VT) and an Olympus BX-52 microscope (Leeds Precision, Minneapolis, MN) equipped with a computer controlled MAC 5000 motorized microscope stage (Ludl Electronic Products, Hawthorne, NY). The Cavalieri estimator in the Stereo Investigator software (Microbrightfield, Colchester, VT) was applied to estimate the total area of the lesion in each of a systematic series of tissue samples spaced 500 μm apart. The volume of the lesion was calculated based on the number of samples and area within each sample (Gundersen and Jensen, 1987). This stereological method has been shown to reliably calculate the volume of cortical and subcortical brain structures (Jelsing, et al., 2005; van der Worp, et al., 2001). The superimposed lesion on the intact hemisphere was also used to evaluate and report the specific cytoarchitectonic areas involved in the lesion using Nissl stained sections through the intact hemisphere. The cytoarchitectonic criteria used to evaluate the frontal lobe was based upon the original report of Barbas and Pandya (1987); see also Figs 1 and 2 of Morecraft et al. (2004).
Figure 2.
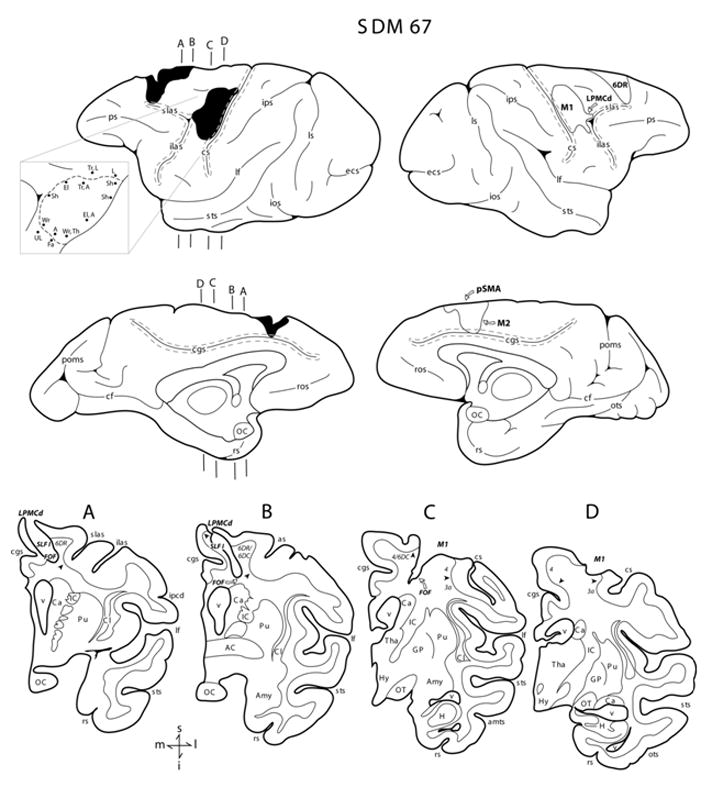
Line drawings of the lateral and medial surfaces of the hemisphere in case SDM67. Representative coronal sections through the lesioned hemisphere are shown below the medial surfaces. The left hemisphere illustrates the location of the cortical lesion (blackened area) which extended onto the medial wall to the pre-supplementary motor cortex (pSMA) and the rostral portion of M2 and the right hemisphere shows the location of the superimposed lesion (outlined area) that was used to calculate the respective gray and white matter lesion volumes. Coronal panels A through D are through the lesion site in the left hemisphere. In each coronal section, the region of lesioned cortex and involved subcortical pathways are identified by the bold italicized conventions. Pertinent Brodmann’s areas are indicated on the coronal sections immediately below the gray matter and the respective boundaries are identified by the arrow heads. The pullout on the left hemisphere illustrates the microstimulation map. On the map each black dot represents a stimulation point with the affiliated movement(s) observed following stimulation. Abbreviations: A, arm; ac, anterior commissure; amts, anterior medial temporal sulcus; Amy, amygdala; Ca, caudate nucleus; cf, calcarine fissure; cgs, cingulate sulcus; Cl, claustrum; cs, central sulcus; ecs, ectocalcarine sulcus; El, elbow; Fa, face; GP, globus pallidus; H, hippocampus; hy, hypothalamus; IC, internal capsule; ilas, inferior limb of the arcuate sulcus; ios, inferior occipital sulcus; ipcd, inferior precentral dimple; ips, intraparietal sulcus; L, leg; lf, lateral fissure; ls, lunate sulcus; OC, optic chiasm; OT, optic tract; ots, occipito-temporal sulcus; poms, medial parieto-occipital sulcus; ps, principle sulcus; Pu, putamen; rs, rhinal sulcus; ros, rostral sulcus; Sh, shoulder; slas, superior limb of the arcuate sulcus; sts, superior temporal sulcus; Th, thumb; Tha, thalamus; To, tongue; Tr, trunk; UL, upper lip; v ventricle; Wr, wrist. For other abbreviations see Figure 1.
Figure 3.
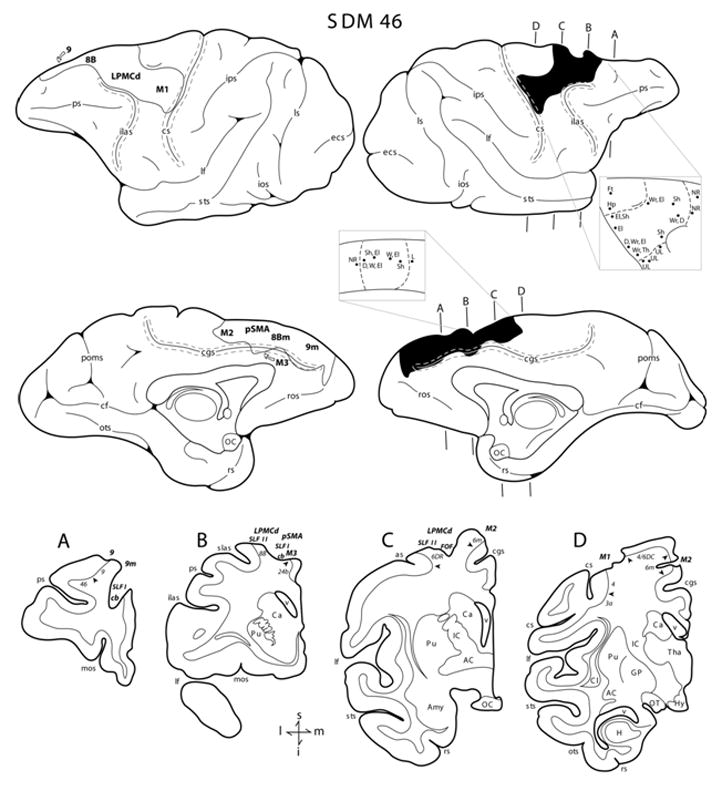
Line drawings of the lateral and medial surfaces of the hemisphere in case SDM46. Representative coronal sections through the lesioned hemisphere are shown below the medial surfaces. The right hemisphere illustrates the location of the cortical lesion (blackened area) which extended onto the medial wall to the pre-supplementary motor cortex (pSMA), the rostral portion of M2 and prefrontal region (areas 8Bm and 9m). The right hemisphere shows the location of the superimposed lesion (outlined area) that was used to calculate the respective gray and white matter lesion volumes. Coronal panels A through D are through the lesion site in the right hemisphere. In each coronal section, the region of lesioned cortex and involved subcortical pathways are identified by the bold italicized conventions. Pertinent Brodmann’s areas are indicated on the coronal sections immediately below the gray matter and the respective boundaries are identified by the arrow heads. The pullout on the right hemisphere illustrated the microstimulation map. On the map each black dot represents a stimulation point with the affiliated movement(s) observed following stimulation. Abbreviations: D, digit; Ft, foot; Hp, hip; M3, rostral cingulate motor cortex; mos, medial orbital sulcus; NR, no response. For other abbreviations see Figures 1 and 2.
The same general method was used to estimate the white matter lesion area and volume (Pizzimenti et al. 2007) with the exception that the analyzed sections were spaced 500 μm apart. Briefly, the external boundary of the white matter region of interest (ROI) coincided with the plane of interface between layer VI and the subcortical white matter. Similarly, the internal boundary of the white matter ROI corresponded to the depth of the lesion as determined from Nissl stained sections through the lesioned hemisphere. This was also determined by transferring this information from the lesioned hemisphere to a matching coronal section in the non-lesioned hemisphere (e.g., see Figs. 2, 3). Subcortical structures and fiber pathways were identified using the atlas and nomenclature of Schmahmann and Pandya (2006). Gray and white matter lesion volumes are reported in Table 1.
mMAP Data Analysis
Force data from the mMAP task were analyzed by visually identifying the first touch of the carrot chip or plate/rod supporting the carrot chip to the end of force application (i.e., when the carrot chip was removed from plate supported by the load cell or the rod) on each trial using recordings of applied forces in Datapac 2k2. The accompanying video data was also analyzed to verify these times and to identify trial outcome (see equation 1). The duration and total applied 3-dimensional absolute impulse (see equation 1) were computed for each trial. The total absolute impulse was computed by integrating the rectified 3-dimensional force signals over the duration of force application. It represents the total force-time impulse applied during target acquisition and is larger if greater forces are applied over longer durations (Darling, et al., 2006). Low impulse on successful trials (outcome ≥ 2) represents greater skill because the subject uses less force and removes the carrot chip faster.
After pre-lesion data collection was completed, performance scores normalized to individual monkey’s abilities (i.e., ranges of pre-lesion impulse and duration) for each trial at each difficulty level were computed (see equation 2). Also note in the performance score calculations that if the monkey attempts, but fails to successfully acquire the carrot chip (i.e., if outcome < 2) that higher scores are given if the monkey exerts larger impulses for longer time periods while attempting to acquire it. This occurred primarily in post-lesion trials when the monkey may abandon the attempt quickly and have very low applied impulse and duration (compared to during pre-lesion testing), which would result in high performance scores even though it was in fact a very poor attempt. With this scoring mechanism, a higher score is awarded for strong attempts (but limited to a maximum score of 200, which is also the minimum score for a successful trial). The minimum score for an attempt was set at 50 to clearly discriminate it from a trial with no attempt.
| (1) |
TAImp (n) – Total Absolute Impulse of trial n
∫ - integral over duration of trial t with respect to time (dt)
Fx - Force applied in left/right direction
Fy - Force applied in anterior-posterior direction
Fz - Force applied in vertical direction
| (2) |
Where:
PSmMAP(n) – performance score on mMAP trial n
Outcome(n) – success on trial n (0 for no attempt with the correct hand, 1 for unsuccessful attempt with the correct hand, 2 if the carrot chip is successfully grasped and lifted over the rod but then dropped and not removed from the food chamber, 3 if the carrot chip is successfully grasped and lifted over the rod but then dropped and removed from the food chamber, 4 for successful acquisition without dropping the carrot chip)
MinTAImp – minimum single trial pre-lesion total absolute impulse within a difficulty level for either hand
MaxTAImp - maximum single trial pre-lesion total absolute impulse within a difficulty level for either hand
TAImp Range – MaxTAImp – MinTAImp
Dur(n) – duration of trial n
MinDur – minimum single trial duration during pre-lesion tests with either hand within a difficulty level
MaxDur - maximum single trial duration during pre-lesion tests with either hand within a difficulty level
DurRange – MaxDur – MinDur
mDB Data Analysis
Temporal characteristics of reaching, manipulation, and 3D locations of the tips of the index finger and thumb were determined from the digital video files as described previously and used to compute a performance score on each pre- and post-lesion trial (Pizzimenti et al. 2007). Measurements taken from video were used to compute reach duration, accuracy, grip aperture, manipulation duration and manipulation attempts (# of times contact between the pellet and a digit was lost and then re-established) on each trial. These measurements were normalized to the performance ranges for each monkey prior to the lesion (i.e., to maximum/minimum reach and manipulation duration, least/most accurate reach, largest/smallest grip aperture, maximum/minimum number of manipulation attempts) and used to compute a performance score for each trial (see equation 1 of Pizzimenti et al. 2007). Note that the mDB performance score is zero if there is no attempt to acquire the food pellet and is higher for shorter reach and manipulation durations, better accuracy, smaller grip aperture and fewer grasp attempts (maximum of 1000 for a single trial with the best score for each component among pre-lesion scores). Also, the minimum score of a trial in which the monkey attempted to retrieve the pellet was awarded 50 to ensure it is clearly better than a no attempt. The minimum score for a successful trial was 200.
Analysis of Hand Motor Skill
Highly skilled reaching and grasping behavior should demonstrate consistent high performance scores with low variability. To quantify overall motor skill in our tasks we computed the mean and then divided it by the standard deviation of performance scores over 5 consecutive testing sessions (i.e., 25 trials over an approximately 5 week period) (Pizzimenti, et al., 2007). This measure of skill was computed for the performance score on the mMAP curved rod task (depends primarily on duration and forces applied during manipulation) and for the manipulation, reach and overall scores on the mDB task (wells with highest pre-lesion skill and a smaller diameter 2nd well with a lower pre-lesion skill). Reach and manipulation performance scores were computed as described previously (Pizzimenti, et al., 2007). Note that higher mean performance scores (low duration, impulse on mMAP; low duration, high accuracy and fewer lost contacts with the food pellet in the mDB tasks) and low trial-to-trial variability of performances over 5 consecutive testing sessions will result in higher skill values. These skill measures were computed for the last 5 pre-lesion test sessions and for the 5 consecutive test sessions when skill was highest during post-lesion recovery.
We also qualitatively analyzed each monkey’s contralesional hand/digit motions in the mDB and mMAP tasks to determine whether the motions appeared similar in pre and post-lesion successful trials. Specifically, we compared individual pre-lesion trials and post-lesion trials that received similar performance scores (about equal to the average performance scores in the last 5 pre-lesion test sessions) when possible (i.e., in monkeys that showed recovery close to pre-lesion performance scores). In monkeys that showed poor recovery, we compared the post-lesion trials with the highest performances scores to pre-lesion trials with average performance scores. These qualitative assessments focused on wrist and hand posture and digit motions associated with grasping and manipulation of the food targets. We were particularly interested to determine whether the monkey’s strategy changed post-lesion to use additional digits or different digits to perform the task. We also assessed specifically whether the appearance of grasp of the food targets appeared similar pre and post-lesion.
Statistical Analysis
Post-lesion hand motor performance by each monkey was assessed from changes in the performance scores relative to pre-lesion scores in the two tasks. We evaluated severity of lesion effects on hand motor function from measures of the duration (in weeks) from the time of the lesion until: (1) the first attempt to perform each task (i.e., a reach out of the cage and attempt to manipulate the food target for a performance score > 0), (2) the first successful acquisition, and (3) the first testing session with 5 successful acquisitions on the mMAP (curved rod, most difficult, task) and mDB (best well and 2nd well) tasks. The magnitude of hand motor deficits after the lesion was also assessed from the pre- to initial post-lesion changes in: (1) average mMAP curved rod task performance scores, (2) average impulses and durations in the mMAP curved rod task over the last 5 pre-lesion test sessions, (3) average mDB manipulation and reach scores for the best well and (4) average mDB manipulation durations and number of manipulation attempts for the 2nd well. Pre-lesion average values were computed from the last 5 pre-lesion test sessions. Initial post-lesion average values were computed from either the first 5 post-lesion test sessions or, for monkeys that had not made consistent successful acquisitions within the first 5 weeks, from post-lesion session 1 until the first post-lesion session in which the monkey successfully acquired the food targets on all trials for the curved rod mMAP task or the particular well on the mDB task. These were used to establish whether the monkeys showed clear decrements after the lesion in manipulation performance in terms of performance scores and higher impulses, longer durations and greater numbers of manipulation attempts (loss of contact of the digit and food pellet, which necessitates re-establishing contact). Multiple and single linear regression analyses were used to determine whether the severity of lesion effects on hand motor function (dependent variables) depended on gray and white matter lesion volumes.
The level of recovery of fine motor skill was assessed by taking the ratio of the highest skill attained in 5 consecutive post-lesion testing sessions (until the 2nd surgery was performed to inject neurotracers) to the skill level in the final 5 pre-lesion testing sessions. Skill was defined as the mean performance score divided by the standard deviation (s.d.) of performance scores over 25 trials in 5 consecutive test sessions(Pizzimenti, et al., 2007). Thus, the recovery skill ratio ranged from 0 (if there were no post-lesion attempts at the task, indicating no recovery of reach/grasp) to 1 (if the highest pre-lesion skill equaled post-lesion skill) or even higher values if the highest post-lesion skill exceeded pre-lesion skill. We used a skill ratio rather than a difference score to evaluate recovery because performance was not assessed in an absolute manner (i.e., performance scores of each monkey were normalized to the range of pre-lesion performances on each component of the performance score for that monkey). Moreover, we expected that skill exhibited during recovery would be proportional to the volume of brain damage.
Results
Histological Lesion Site Analysis
A brief description of the microscopic evaluation of the lesion site in three cases with M1, M1 + LPMC and M1 + LPMC + M2 lesions (SDM38, SDM48 and SDM50) was given in our previous paper describing the mDB apparatus (Pizzimenti et al., 2007). The other cases with these planned lesions (M1 + LPMC: SDM45, SDM64, SDM70, SDM55; M1 + LPMC + M2: SDM56) had similar microscopic evaluations but with some minor differences (see McNeal et al., accepted with review for detailed lesion evaluations of SDM45, SDM48, SDM70 and SDM55 and supplementary material for detailed lesion evaluations of SDM38, SDM64, SDM50 and SDM56). In all cases presented in the current report, the volume of the gray matter lesion increased as a function of the number of cortical areas involved in the lesion increased (Table 1) and microscopic evaluation demonstrated complete removal of the cortical gray matter through the main body of the lesion site (i.e., layers I–VI) (Figs. 1, 2, 3; supplementary figures S1, S2, S4; see McNeal et al., accepted with review). In general, the edge of the gray matter lesion site was either perpendicular to the laminar axis of the gray matter (i.e., a vertical ledge) or it gradually tapered. Thus, the tapered form involved only the supragranular layers (i.e., layers I–III) which when present, occurred on only the perimeter of the lesion site. In all cases the underlying basal ganglia nuclei (caudate and putamen) and thalamus were spared. A detailed description of the lesion sites is provided below for the two multi-focal lesions cases and for the four planned lesion categories (F1, F2, F3) in the supplementary material.
SDM67 (Multi-focal Lesion)
With respect to the lateral frontal region, the arm area of M1 was fully removed with the exception of the cortex forming the lower one half of the anterior bank of the central sulcus (Fig. 2C,D). Ventrally, the face area of M1 was spared as well as the dorsally located leg representation. The lesion extended rostrally, involving gray matter of the peri-arcuate cortex around the arcuate spur. Deep to the lateral frontal lesion site, white matter damage extended inferiorly forming an inverted “V” shape at which the apex ended at transverse levels including the cortex forming the cingulate cortex (Fig 2C). In the coronal dimension, this location corresponded to a plane through the inferior third of the central sulcus. The inferior portion of the white matter lesion clearly included the upper one half of the FOF (Fig. 2C) (i.e., see p. 541, section 81 of Schmahmann and Pandya 2006) but spared the cingulate bundle and SLF I medially, and SLF II laterally. Subcortical white matter associated with the rostrally located LPMCd and LPMCv was spared. With respect to the dorsal and medial region of the hemisphere, there was no gray matter observable in the rostral and dorsal part of area 6DR. The lesion site extended onto the medial surface of the hemisphere to involve the deep layers (i.e., layers V and VI) of the very rostral and dorsal part of M2 (area 6m) (Fig. 2A), and all cortical layers of a large portion of the pre-supplementary motor area (Fig. 2, medial surface). For approximately 2.5 mm, the lesion extended into the cingulate sulcus to involve the cortex forming the upper bank of the cingulate sulcus, while sparing the cortex lining the lower bank of the cingulate sulcus (Fig. 2, medial surface). Below the medial part of the lesion, white matter damage extended inferiorly involving all coronal sections from the rostral tip of the superior limb of the arcuate sulcus to the spur of the arcuate sulcus (Fig. 2A,B). Rostrally, the dorsal one half of the SLF I was involved in the lesion while caudally, the entire SLF I and FOF were damaged (Figs. 1E, 4A,B). In all sections, the SLF II was found to be spared (Fig. 1E, 4A–D). Also in the caudal segment of the lesion, in addition to involvement of the SLF I, the lesion extended slightly inferiorly to involve the upper most part of the cingulum bundle (specifically where it wraps around the cortex forming the fundus of the cingulate sulcus) and the dorsal most part of the corpus callosum (Fig. 2A) (i.e., see p. 537, section 49 of Schmahmann and Pandya, 2006).
Figure 4.
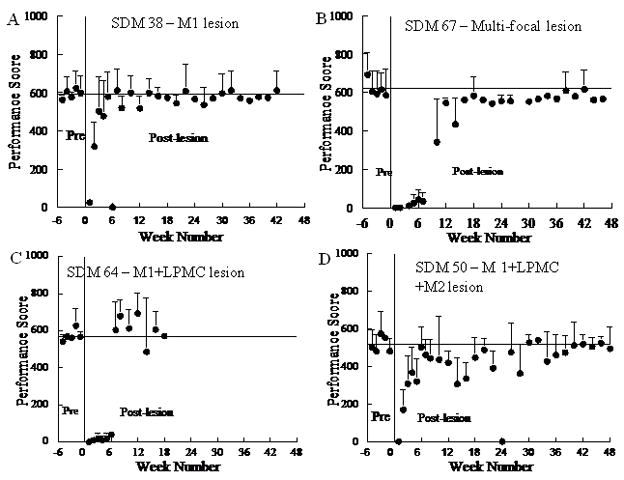
Pre and post-lesion performance scores on the mMAP curved rod task for 4 monkeys. The solid vertical line shows the time of the surgically induced lesion. The solid horizontal line shows average performance scores for the last 5 pre-lesion tests. Each plotted point is the mean performance score for 5 trials. Error bars are 1 s.d.
SDM46 (Multi-focal Lesion)
With respect to the lateral frontal region, the arm area of M1 was fully removed with the exception of the cortex forming the lower one half of the anterior bank of the central sulcus (Fig. 3D). Ventrally, the face area of M1 was spared as well as the dorsally located leg representation. The surgical lesion extended rostrally, involving gray matter of the peri-arcuate cortex including the upper portion of the dorsal bank of the superior limb of the arcuate sulcus (Fig. 3B,C). Deep to the lateral frontal lesion site, white matter damage extended inferiorly involving the SLF I and upper portion of the FOF and SLF II (Fig. 3B,C). White matter located beneath LPMCv (area 6V) was spared. Dorsally, the lesion extended to unintentionally involve the gray matter in area 6DR including the prefrontal cortex on the dorsal convexity of the superior frontal lobule (i.e., areas 8B and 9) (Fig. 3A–B). The lesion site also extended onto the medial surface of the hemisphere to involve the arm and face regions of M2 (area 6m), the pre-supplementary motor area (pSMA), area 8Bm and area 9m (Fig. 3, medial surface). In the rostral part of the cingulate sulcus cortex lining both the upper and lower banks of the cingulate sulcus was absent (Fig. 3A, B) which included part of the rostral cingulate motor cortex (M3) above the genu of the corpus callosum (Fig. 3B). More caudally cortex lining the cingulate sulcus was spared (Fig. 3C,D). In the prefrontal region, the cingulum bundle was also affected (Fig. 3A) and at caudal prefrontal levels, the lesion involved the dorsal part of the cingulum bundle (Fig. 3B).
Overall Lesion Site Analysis
Gray and white matter lesion volumes increased with involvement of more regions of motor cortex as expected (Table 1). Gray matter lesion volumes were similar among 4 of the 5 monkeys with M1 and LPMC lesions, but SDM70 had a smaller lesion because ICMS of LPMCd did not elicit upper limb movements (and was not removed –see McNeal et al., accepted with review). The multi-focal lesions produced high gray and white matter lesion volumes compared to the Category F1 to F3 lesion cases. SDM67 had a gray matter lesion more than double the volume that of SDM38, with white matter involvement more than 10X that of SDM38 (Table 1). Similarly, SDM46 had a gray matter lesion approximately double that of the SDM50 and SDM56, with a white matter lesion 3–4X greater than in SDM50 and SDM56. White matter lesion volumes averaged 11.7% of gray matter lesion volumes for Category F1-F3 cases and these volumes were well correlated (r = 0.77, p < 0.05). Thus, the amount of white matter removed increased proportionally with the amount of gray matter removed in these cases. However, for category MF lesions (i.e., SDM46, 67) the white matter lesion volumes were, respectively, 23% and 57% of gray matter lesion volume. These larger lesions, and the range of white matter lesion volumes in category F1-F3 cases permitted us to evaluate the effects of additional white matter damage on hand fine motor recovery.
Behavioral Analysis
A large range of hand/digit movement deficits and rates of recovery were observed following the lesions. Observations of behavior in the cage in the days immediately following the lesion indicated that the contralesional arm was not used even for postural tasks in most monkeys, although some began to use the arm for such tasks during the first few days following the lesion. The first post-lesion test of hand function was at one week post-lesion and most monkeys (6/10) exhibited a severe deficit in that they failed to make an attempt (i.e., performance score = 0) in both the mMAP and/or mDB tasks in this testing session (Table 3, Fig. 4B,C,D). Three monkeys had at least one unsuccessful attempt at one week post-lesion (SDM38 on both tasks, SDM70 on the mMAP curved rod task and SDM55 on the mDB task). Surprisingly, two monkeys with category F2 lesions (SDM45, SDM70) had successful acquisitions on both the mMAP and mDB tasks in the first testing session after the lesion, with SDM45 being successful on all trials in both tasks at that time.
Table 3.
Post-lesion duration of impairment in the modified dexterity board (mDB – any well) and modified movement assessment panel (mMAP – flat surface, curved rod) tasks.
| Post-lesion week of | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Monkey | 1st attempt | 1st success | Consistent Success | ||||||
| mDB | mMAP (flat) | mMAP (curved) | mDB | mMAP (flat) | mMAP (curved) | mDB | mMAP (flat) | mMAP (curved) | |
| SDM38 | 1 | 1 | 1 | 1 | 1 | 2 | 4 | 2 | 5 |
| SDM45 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| SDM48 | 2 | 2 | 2 | 2 | 2 | 2 | 5 | 1 | 4 |
| SDM55 | 1 | 1 | 2 | 2 | 1 | 4 | 4 | 1 | 5 |
| SDM64 | 4 | 2 | 2 | 4 | 2 | 7 | 6 | 3 | 8 |
| SDM70 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 |
| SDM50 | 2 | 2 | 3 | 4 | 2 | 3 | 22 | 4 | 4 |
| SDM56 | 2 | 2 | 2 | 2 | 2 | 3 | 6 | 3 | 7 |
| SDM67 | 7 | 3 | 3 | 8 | 3 | 9 | 19 | 4 | 10 |
| SDM46 | 3 | 2 | NA1 | NA | 6 | NA | NA | 7 | NA |
not applicable (no attempts or successes)
All monkeys except SDM46 showed excellent recovery of hand motor skill in both the mDB and mMAP tasks (Table 2). SDM46 showed some recovery on the mMAP task by successfully retrieving carrot chips from the flat surface and straight rod after the lesion (e.g., Table 3, Fig. 5), but this monkey never attempted the curved rod task after the lesion. Analysis of force recordings showed that strength was not a major problem as this monkey exerted much larger forces during the straight rod task after the lesion than during successful performances on the straight and curved rod tasks before the lesion (Fig. 5). However, qualitative analysis of video during the mMAP task and another task similar to the mDB task showed that this monkey’s ability to coordinate hand/digit movements was severely impaired throughout the post-lesion period. Indeed, on the mDB task this monkey only made 3 reach attempts after the lesion (none on the wells with the best pre-lesion skill or a 2nd smaller well) and only made very minimal attempts to actually grasp and manipulate the food pellet.
Figure 5.
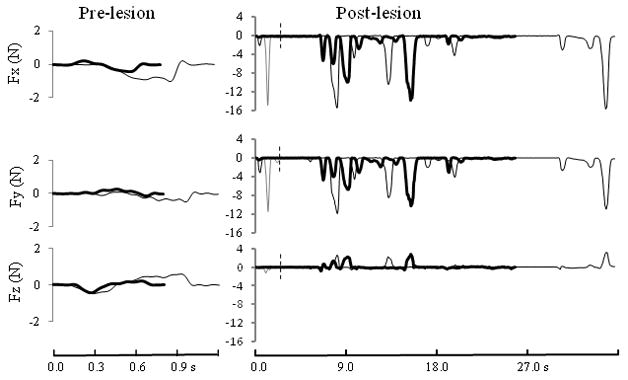
Forces exerted in the left/right (Fx), anterior-posterior (Fy) and vertical (Fz) directions during retrieval of a carrot chip in the mMAP straight rod task by SDM46. On the left side are shown data from two successful pre-lesion trials that had the best (black line) and worst (gray line) performance scores in the last 5 testing sessions. On the right side are shown the first post-lesion attempt (thin light gray line ending at vertical dashed line), highest post-lesion impulse (thin dark gray line) and the only post-lesion successful trial in this task (thick black line). Note that the forces exerted even in the first post-lesion attempt were much larger than those exerted during successful pre-lesion trials.
The magnitude of fine hand motor performance decline in the first several weeks varied among monkeys in relation to lesion volume. On the mMAP curved rod task, initial post-lesion performance scores averaged 41.5% (range: 0–87.6%) lower than those for the last 5 pre-lesion test scores. Initial post-lesion impulses and durations averaged, respectively, 170% (range: 20% – 390%) and 130% (range: 50%–220%) higher than in the last 5 pre-lesion tests. Similarly, on the mDB best well task, initial post-lesion reach and manipulation scores averaged, respectively, 59.0% (range: 0 – 119%) and 51.2% (range: 0 – 93.4%) of those for the last 5 pre-lesion test scores. Initial post-lesion manipulation durations and attempts averaged, respectively, 100% (range: −10 – 210%) and 50% higher than in the last 5 pre-lesion tests for the 2nd well. These declines in manipulation performance scores on the mMAP and mDB tasks showed a strong dependence on white matter lesion volume whereas the reach scores in the mDB task showed a weaker dependence on lesion volume (Table 4). Analysis of components of the reach and manipulation scores on the mDB task revealed that reaching ability (based on reach duration, accuracy and grip aperture at touchdown) generally recovered sooner and closer to pre-lesion skill levels than recovery of manipulation ability (based on manipulation duration and number of manipulation attempts) (e.g., Fig. 6, Table 2). However, it should be noted that the post-lesion testing session with the first successful acquisition occurred in the same session as the first post-lesion attempt was made in 6 monkeys, indicating that most monkeys did not even attempt the task until some manipulation ability had returned. The monkey with the smallest lesion (SDM38) had post-lesion increases in reach duration, errors and grip apertures that declined over the first 4–6 weeks after the lesion (Fig. 6A,D,G). Thus, reaches were slower and less accurate. In contrast, SDM45 had a lesion about 2X the volume of SDM38, but exhibited longer reach durations with smaller reach errors (and grip apertures only slightly increased) during the first 4 post-lesion weeks, indicating reaching movements were slowed to make them more accurate (Fig. 6B,E,H). SDM67 (large MF lesion) had no attempts over the first 10 weeks post-lesion on well B with long reach durations and large errors over weeks 12–24 when only small numbers of attempts were made on this well (Fig. 6C,F).
Table 4.
Coefficients of determination for relationships between motor performance decline in the first several weeks after the lesion and lesion volume.
| mDB (best well) | mDB (2nd well) | mMAP (curved rod) | |||
|---|---|---|---|---|---|
| Reach | Manip | Reach | Manip | ||
| MLR1 | 0.653* | 0.774* | 0.276 | 0.695* | 0.568* |
| SLR-GM2 | 0.269* | 0.406* | 0.255 | 0.364* | 0.350* |
| SLR-WM3 | 0.615* | 0.761* | 0.244 | 0.682* | 0.567* |
| SLR-GM+WM4 | 0.358* | 0.510* | 0.271 | 0.457* | 0.422* |
multiple linear regression with gray and white matter lesion volumes as independent (predictor) variables
single linear regression with gray matter lesion volume as the independent variable
single linear regression with white matter lesion volume as the independent variable
single linear regression with sum of gray and white matter lesion volumes as the independent variable
p < 0.05
Figure 6.
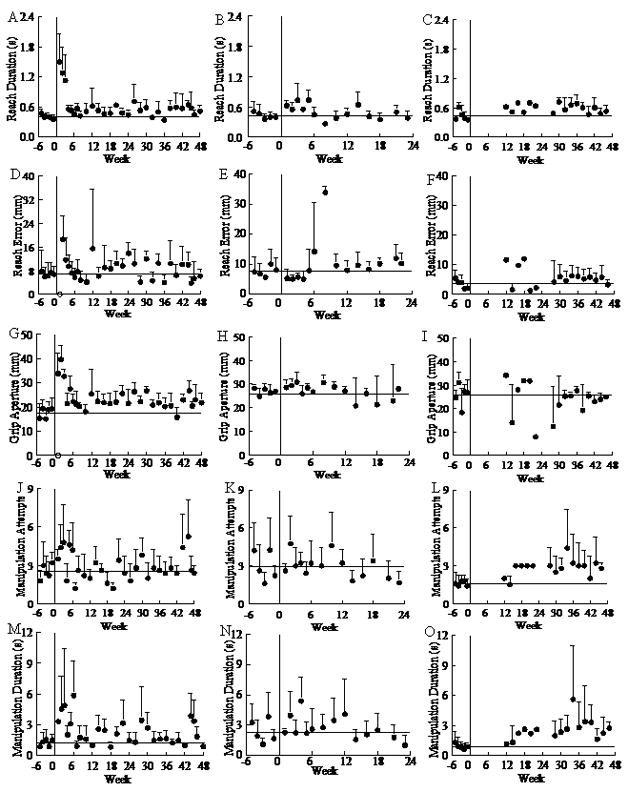
Pre- and post-lesion performance on individual components of the mDB task for SDM38 (left side – A, D, G, J, M) on well D, SDM45 (middle – B, E, H, K, N) on well A and SDM67 (right side – C, F, I, L, O) on well B. Each symbol on a graph shows the mean performance on individual test sessions for one component of the mDB performance score for one monkey. Error bars are 1 S.D.
Recovery of manipulation proceeded on a slower schedule, and was generally more variable than reaching. SDM38 had increases in manipulation attempts and duration over the first six post-lesion weeks (Fig. 6J,M) and these remained generally higher and more variable than pre-lesion values throughout 48 weeks post-lesion. In contrast, the number of manipulation attempts appeared to stabilize at pre-lesion levels by 3 weeks post-lesion for SDM45 while manipulation duration remained elevated until 14 weeks post-lesion and then decreased and became less variable (Fig. 6K,N). SDM67 remained at higher than pre-lesion levels in manipulation attempts and duration throughout 12 – 48 weeks post-lesion (Fig. 6L,O), consistent with the large multi-focal lesion. However, SDM45 recovered faster and to generally higher skill levels than SDM38 (Table 2), despite a larger lesion. Thus, the relationship between lesion volume and recovery of skill is complex for smaller volume lesions.
Time required for recovery of hand motor function was strongly correlated with white matter lesion volume. The post-lesion duration until the first attempt to grasp the food target and first successful acquisition on the mDB task and the mMAP curved rod task were strongly related to lesion volume as shown by multiple linear regression with white and gray matter lesion volumes as predictor variables (Fig. 7A, 8A, Table 5). Additional regression analyses showed a stronger dependence on white than on gray matter lesion volume (Fig. 7B, 8B, Table 5). Similarly, post-lesion duration to the first successful acquisition was also highly correlated with lesion volume, with an even stronger dependence on white matter lesion volume than post-lesion duration until the first attempt (Table 5).
Figure 7.
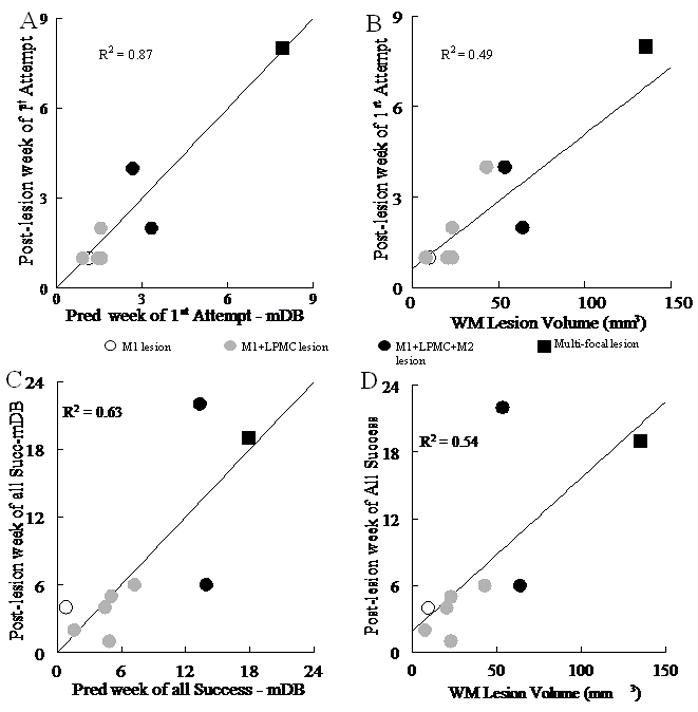
Recovery on the mDB task. A and B show post-lesion week of 1st attempt (on the best well - with highest pre-lesion skill) plotted against: predicted week of first attempt from gray and white matter lesion volumes by multiple linear regression (A) and white matter (WM) lesion volume by single linear regression (B). Post-lesion week of all successes (on the best well) is plotted against predicted week of all successes from gray and white matter lesions by multiple linear regression (C) and white matter lesion volume by single linear regression (SLR) (D). Each plotted point is data from a single monkey with type of lesion indicated by the symbol (see legend in center of figure). Coefficients of determination for the plotted relationships (p < 0.005) and for gray matter volume (D) are shown.
Figure 8.
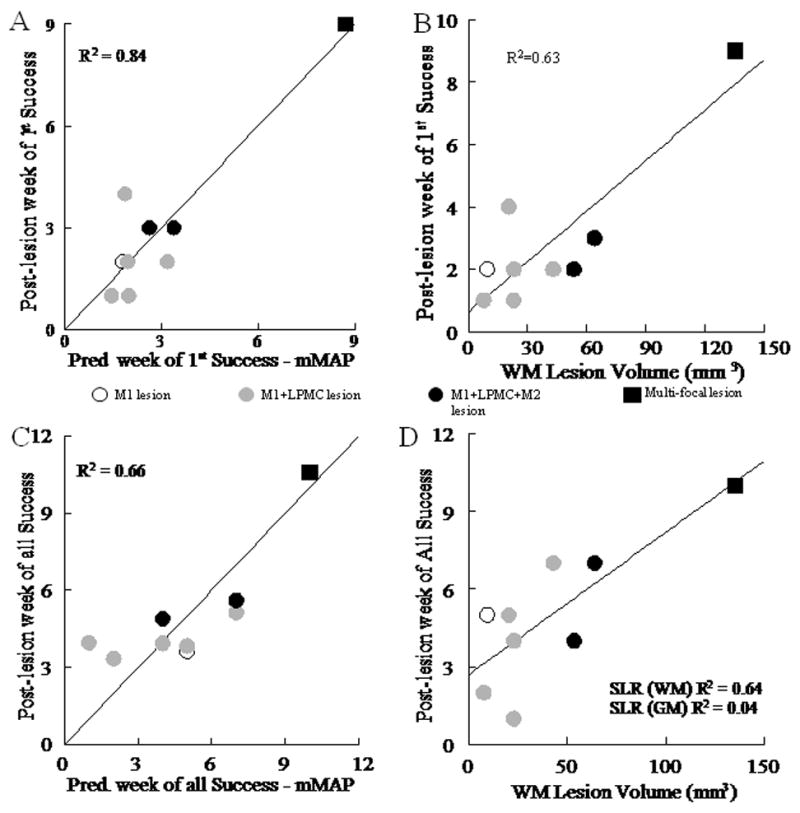
Recovery on the mMAP task. Post-lesion week of first success on the mMAP curved rod task is plotted against: the predicted week of first success from gray and white matter lesion volume by multiple linear regression (A) and white matter (WM) lesion volume by single linear regression (B). Post-lesion week of all successes on the mMAP curved rod task is plotted against: predicted week of all successes from gray and white matter lesion volume (C) by multiple linear regression and white matter lesion volume by single linear regression (SLR - D). Each plotted point is data from a single monkey with type of lesion indicated by the symbol (see legend on figure). Coefficients of determination for the plotted relationships (p < 0.005) and for gray matter volume (D) are shown.
Table 5.
Coefficients of determination for multiple and single linear regressions for dependence of post-lesion duration until the first manipulation attempts (att.) and successes (succ.) on gray and white matter lesion volumes in the mDB and mMAP task.
| mDB (any well) | mDB (best well) | mMAP (curved rod) | ||||
|---|---|---|---|---|---|---|
| 1st att. | 1st succ. | 1st att. | 1st succ. | 1st att. | 1st succ. | |
| MLR1 | 0.804* | 0.862* | 0.866* | 0.947* | 0.874* | 0.843* |
| SLR-GM2 | 0.026 | 0.080 | 0.349* | 0.021 | 0.837* | 0.030 |
| SLR-WM3 | 0.419* | 0.847* | 0.491* | 0.870* | 0.728* | 0.791* |
| SLR-GM+WM4 | 0.078 | 0.265* | 0.273* | 0.349* | 0.870* | 0.172 |
multiple linear regression with gray and white matter lesion volumes as independent (predictor) variables
single linear regression with gray matter lesion volume as the independent variable
single linear regression with white matter lesion volume as the independent variable
single linear regression with sum of gray and white matter lesion volumes as the independent variable
p < 0.05
Lesion volume was also correlated with the duration of post-lesion moderate hand function deficits. We characterized such deficits as failure to successfully acquire the food target in all 5 trials of a testing session on the mDB best well, 2nd well and mMAP curved rod tasks within a single testing session. Gray and white matter lesion volumes correlated well with the post-lesion duration until there were successful acquisitions in all 5 trials for both tasks (Fig. 7C, 8C, p < 0.001). As with duration of severe deficits, post-lesion duration until this level of recovery showed a stronger dependence on white matter than on gray matter lesion volume (Fig. 7D, 8D).
As expected, lesion volume correlated inversely with recovery of motor skill. Recovery of manipulation skill was strongly correlated with white matter lesion volume on the best well and 2nd well in the mDB task (Fig. 9A, B, Table 6, p < 0.001 with SDM46) and with gray matter lesion volume in the mMAP curved rod task (Fig. 9C, Table 6, p < 0.05 with SDM46). However, these correlations were weaker or non-significant when SDM46 was excluded from the regression analyses (Fig. 9, Table 6). In contrast, recovery of reaching skill was only weakly negatively correlated with white matter lesion volume (and only when SDM46 was included - Fig. 9D, p = 0.01) and was not correlated with gray matter lesion volume (p > 0.05). Notably, many monkeys showed good to excellent recovery with ratios of post-lesion/pre-lesion manipulation skill levels for the best well exceeding 0.4 (i.e., recovery to at least 40% of pre-lesion skill over 5 consecutive test session) and up to 1.41 (i.e., 41% better than pre-lesion skill) on the mDB task (Table 2, Fig. 9). Recovery was usually similar for the smaller 2nd well on the mDB task (Table 2) but was generally better in the mMAP curved-rod task, ranging from 0.5 to 2.3 (130% better than pre-lesion skill) (Fig. 9, Table 2). Recovery to 66% and 130% better than pre-lesion levels in two monkeys on the mMAP task was due to higher average performance scores (i.e., lower forces exerted, shorter duration) with lower variability in one (SDM45) and similar average performance scores but substantially lower variability in the other (SDM67) as shown by plots of 3-dimensional forces exerted during this task (e.g., Fig. 10). However, among the 4 monkeys with M1 + LPMC lesions that had similar gray and white matter lesions in terms of location and volume (total lesion volumes ranging from 225 to 261 mm3 – Table 1 – excludes SDM70 only), the ratio of post/pre-lesion manipulation skill varied greatly (from 0.4 to 1.66 – Table 2). Moreover, some monkeys with M1+LPMC lesions had poorer skill recovery in one or both tasks than monkeys with larger M1 + LPMC + M2 lesions and multi-focal lesions (Fig. 9, Table 2 – compare SDM48 and SDM64 to SDM50 and SDM67).
Figure 9.
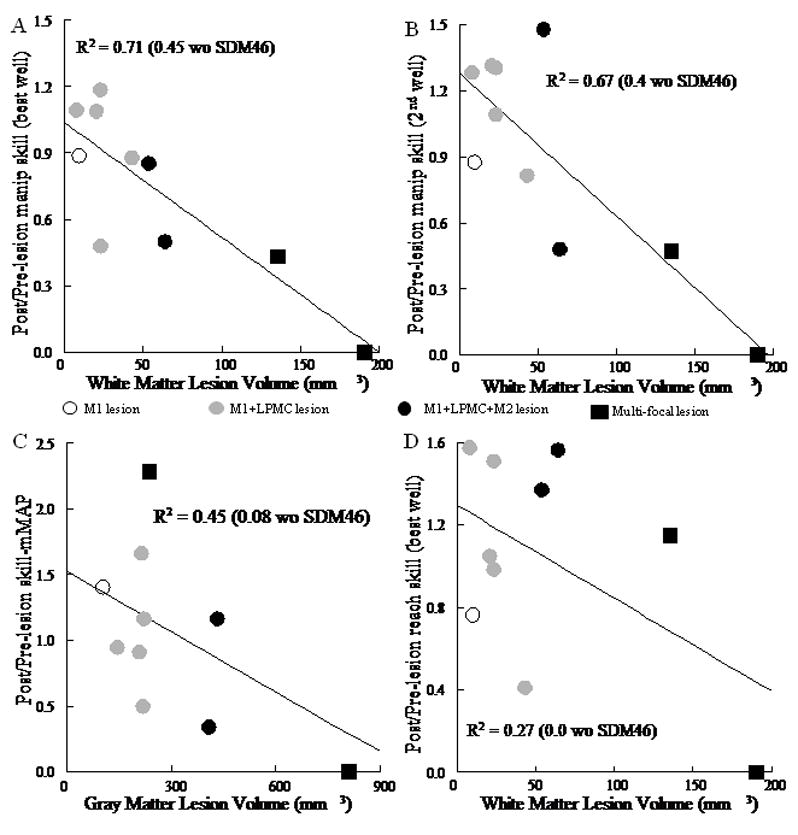
Recovery of skill on the mDB and mMAP tasks. Each plotted point shows the ratio of post-lesion to pre-lesion manipulation skill for a single monkey plotted against white matter lesion volume for the mDB (best well) task (A) and mDB (2nd well) task (B). Recovery of skill on the mMAP curved rod task is plotted against gray matter lesion volume in C. In D the ratio of post-lesion to pre-lesion reach skill is plotted against white matter lesion volume. Each plotted point is data from a single monkey with type of lesion indicated by the symbol (see legend on figure). Coefficients of determination for the plotted relationships are shown (p < 0.05).
Table 6.
Coefficients of determination for multiple and single linear regression for dependence of recovery of manipulation skill (i.e., ratio of post-lesion best skill to pre-lesion skill over 5 testing sessions) on gray and white matter lesion volumes.
| mDB (best well) | mDB (2nd well) | mMAP (curved rod) | ||||
|---|---|---|---|---|---|---|
| wSDM46 | woSDM46 | wSDM46 | woSDM46 | wSDM46 | woSDM46 | |
| MLR1 | 0.714* | 0.450* | 0.680* | 0.455* | 0.575* | 0.451* |
| SLR-GM2 | 0.543* | 0.144 | 0.370* | 0.004 | 0.351* | 0.008 |
| SLR-WM3 | 0.704* | 0.438* | 0.671* | 0.402* | 0.040 | 0.186 |
| SLR-GM+WM4 | 0.619* | 0.267* | 0.461* | 0.060 | 0.273* | 0.011 |
multiple linear regression with gray and white matter lesion volumes as independent (predictor) variables
single linear regression with gray matter lesion volume as the independent variable
single linear regression with white matter lesion volume as the independent variable
single linear regression with sum of gray and white matter lesion volumes as the independent variable
p < 0.05
Figure 10.
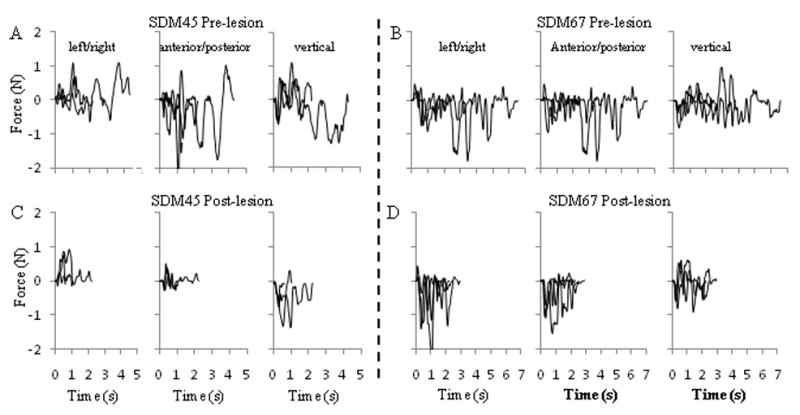
Force vs. time records for SDM45 (A, C) and SDM67 (B, D) in the mMAP curved rod task. Each graph contains force records in X (left/right), Y (anterior-posterior) or Z (vertical) directions from 3 trials (with short, medium, long durations) within a single testing session pre-lesion (one of the final 5 pre-lesion testing sessions – top row) and post-lesion (10 weeks post-lesion for SDM45, 20 weeks post-lesion for SDM67 – bottom row). Records were aligned to time of first contact for each trial. Note the generally shorter durations and lower forces (especially anterior-posterior forces for SDM45 and vertical forces for SDM67) in the post-lesion trials.
Qualitative analysis of hand/digit movements showed that most monkeys developed the same strategy for acquiring food targets during post-lesion recovery as exhibited prior to the lesion, but a variety of strategies were observed among the monkeys. In the mMAP task, most used precision or key-type grasp of the carrot chip (sometimes with the 3rd digit under the 2nd) with the hand initially semi-pronated and then lifted the carrot chip while manipulating hand orientation to orient the carrot over the curved rod (e.g., SDM45, SDM50, SDM56). Others would spin the carrot chip to help move it up/along the curved rod and then would spin it off the end of the rod (e.g., SDM38) or would use precision grasp initially to lift the carrot, then place the index and 3rd digit under the carrot to flip it off the rod and then pick it up off the flat surface (e.g., SDM46 in pre-lesion testing and in post-lesion testing for the straight-rod task, SDM48, SDM50). Strategies used in the mDB task were generally similar among all monkeys as they usually reached with the hand pronated, touched the pellet first with the tip of the index finger and then used the index to press on the side of the pellet and drag it towards the thumb for grasping with a precision or key-type grasp (Murata, et al., 2008). However, some monkeys (e.g., SDM45, SDM55) successfully used a single finger (index) strategy on some trials in which they simply pressed hard on the pellet such that it stuck to the finger. Other monkeys (e.g., SDM55) used a different technique for well E (a dimple to hold the food pellet in place) in which the thumb-tip first contacted the pellet with the hand fully pronated and then the index-tip contacted the pellet to form a precision grip on most pre-lesion trials. During recovery after the lesion, all but three monkeys eventually developed the same strategy. One exception was SDM55 who consistently first contacted the pellet with the index-tip instead of the thumb-tip on well E (the well on which he performed with the highest skill) throughout the post-lesion period. A second exception was SDM67, who used the thumbtip to manipulate the pellet in wells A–D during pre-lesion trials but used the index and long finger to manipulate the pellet in wells A–D during post-lesion trials. This monkey always used the index finger-tip to initially contact and manipulate the pellet for well E. The third exception was SDM46, who was unable to successfully perform this task after the lesion, but showed some grasping ability in a similar task (see Discussion).
Overall, there was no evidence that monkeys used a clearly different strategy, wrist/hand posture or additional digits to acquire the food targets after recovering from the lesion. Indeed, the primary reason for lower post-lesion performance scores appeared to be slowness of movements, higher forces applied in the mMAP task and some clumsiness leading to dropping the carrot more often in the mMAP task or additional losses of fingertip-pellet contact in the mDB task. This led to higher variability of post-lesion scores in many monkeys, which caused lower skill. It should also be noted that the higher skill and post-lesion performance scores observed in some monkeys on the mMAP task were due to lower exerted force/impulse and duration (e.g., Fig. 10). The lower forces may indicate some loss of strength or a desire to use lower force when performing this task after the lesion. For example, SDM45 and SDM67 decreased both duration and forces exerted in the mMAP curved rod task to improve performance scores post-lesion (Fig. 10). However, SDM45 rarely dropped the carrot during successful acquisitions pre or post lesion and exhibited higher skill after, than before, the lesion (Table 2). In contrast, SDM67’s mean scores were lower post-lesion despite lower forces and duration because she almost always dropped the carrot chip whereas during pre-lesion testing she rarely dropped it (i.e., lower outcomes on post-lesion trials). Indeed, consistently dropping the carrot chip actually produced lower variability in post-lesion performance scores, such that this monkey’s measured skill on the mMAP curved rod task was much higher post-lesion than pre-lesion (Table 2). This seems paradoxical, but forces and durations were also lower and less variable after the lesion, which do indicate better skill. Notably, all monkeys except SDM46 had individual trials during post-lesion testing with performance scores that were as high as average pre-lesion performance scores on both the mDB and mMAP tasks.
Discussion
One of our most salient findings illustrates that duration of severe impairment of hand motor function depended primarily on volume of damage to white matter located subadjacent to frontal motor cortex. For example, injury limited to lateral motor cortex that also affected a relatively large volume of subjacent white matter caused much longer duration of deficit than lateral motor cortex lesions affecting less white matter but similar or even larger volumes of gray matter. Even when gray matter injury extended to selectively involve the medial motor cortex, duration of impairment continued to be related to extent of white matter involvement. For instance, lesions that removed most of the arm areas of M1, LPMC and M2 did not preclude use of the contralateral hand for more than a few weeks following injury. Importantly, this lesion was also accompanied by remarkable recovery of fine hand motor function (i.e., ability reach accurately to and grasp small food objects) and the duration of this impairment also depended primarily on volume of white matter damage. In contrast, recovery of skill of hand/digit movements (i.e., consistent high performance scores in the mMAP and mDB tasks) depended on both gray and white matter lesion volume. Only a very large lesion that affected several major corticocortical white matter tracts in addition to dorsomedial prefrontal cortex and frontal lobe motor areas induced a lasting major deficit of hand motor function that persisted for 3 months. We also found that gray and white matter lesion volumes were not well correlated with recovery of skill in gross upper limb function (i.e., reaching). These results have important neurological and neurosurgical implications as they suggest that even a fairly large volume of gray matter damage affecting frontal lobe motor areas is unlikely to produce lasting severe impairment of fine motor function, but is likely to cause some reduction in the skill of dexterous movements. However, a large volume of white matter damage subjacent to motor cortex may produce lasting impairment of fine motor function.
Our findings are consistent with early work showing that apes and monkeys recover fine digit motor function after lesions to the forelimb or hand/digit cortical motor areas (Denny-Brown, 1950; Glees and Cole, 1950; Travis, 1955a,b). Progressively larger gray matter lesions of frontal lobe motor areas generally slowed the pace of recovery, but good recovery occurred within 5 weeks in animals that had minimal white matter damage. Recent findings demonstrating precision grasp and manipulation can be restored with intensive training following rather large ibotenic acid lesions of M1 digit areas (similar in volume to the lesion of SDM38 in this study) are also consistent with this conclusion (Murata, et al., 2008). These results contrast sharply with findings of studies indicating that even small lesions of hand area of the primary motor and primary somatosensory cortices (M1 + S1) are sufficient to produce more significant, and longer lasting deficits in fine hand motor function (Liu and Rouiller, 1999; Passingham, et al., 1983). Clinically, taken together these observations suggest that rapid and favorable recovery would be predicted in patients with injury that is localized to frontal motor cortex. In contrast, the involvement of a small portion of motor cortex combined with injury extending into the postcentral parietal region would likely result in a comparatively poorer recovery profile requiring more aggressive post-injury therapeutic intervention.
Our observations showing recovery of precision grip are consistent with reports showing that recovery of fine hand/digit motor function is possible in macaques even after unilateral section of the mid-cervical spinal cord which interrupt the corticospinal tract (Darian-Smith, et al., 1999; Galea and Darian-Smith, 1997; Nishimura, et al., 2007). Similarly, the classic work of Lawrence and Kuypers (1968) showed that bilateral section of the medullary pyramids impaired independent finger movements such that monkeys could not pick up food from small diameter wells (less than 3.9 cm) of a dexterity board, but successful removal of the food was possible in larger diameter wells. In contrast, dorsal root section at cervical spinal levels, which abolishes sensory input to the CNS from digits or forelimb, produce greater impairments and poorer recovery of fine motor function (Darian-Smith and Ciferri, 2005; Taub, et al., 1977) again supporting the idea that sensory input is critical for control of independent digit motion for grasp in nonhuman primates and an important factor for attaining favorable recovery of movement following CNS injury.
As noted in our histological results, the surgically induced lesions generally spared the deep regions of M1 located the anterior bank of the central sulcus. Thus, sparing this gray matter may be a contributing factor in recovery of independent digit motor function for precision grasp because this region contains neurons that evoke intrinsic digit movements when stimulated with low physiological currents (Lemon, et al., 1986; Sato and Tanji, 1989). Indeed, all monkeys in the present study recovered some ability to use precision grip to grasp a small food pellet in the mDB task. It is noteworthy that even SDM46 showed rudimentary precision grip and could acquire food pellets from a flat surface, but only in testing for spontaneous use of the impaired hand in a less constrained task in which either hand could be used - (Darling, et al., 2008). However, a recent report indicates that precision grasp can recover with daily training and constraint of the unaffected hand, if the digit areas of M1 including those in the anterior bank of the central sulcus are damaged (Murata, et al., 2008). Thus, the good to excellent recovery of digit motor function observed in our experiments may, in fact, be due to the minimal forced use of the impaired hand in weekly post-lesion testing along with some sparing of digit areas of M1 (in the anterior bank of the central sulcus) and possibly contributions from other spared cortical areas. Nevertheless, it is remarkable that excellent recovery of precision grasp can occur in the absence of a significant portion of frontal motor areas contributing to the execution of arm/hand movements.
Although lesion volume was clearly correlated with magnitude and duration of impairment in the first several weeks post-lesion and with recovery of skilled hand/digit motor function, it is apparent that there was some variation among monkeys that could not be attributed solely to lesion volume. For example, although duration of severe and moderate impairment was strongly correlated with white matter lesion volume, most of the monkeys with focal lesions (Table 1) made their first post-lesion attempts and successes on the mDB and/or mMAP curved rod tasks at either 1 or 2 weeks post-lesion. Indeed, it is possible that daily testing may have provided more detailed insight to the precise recovery rates in these monkeys related to their specific volumetric level of injury. However, in the interest of tracking motor recovery in the absence of any rehabilitation-like intervention, we tested only once per week for the first 2 months post-injury. In fact, daily testing of the contralesional limb would have resembled the experimental implementation of forced-use therapy and would have the potential to alter the normal course of recovery. On this note, it is important to mention that our weekly testing sessions provided the monkey with a maximum of only 40 opportunities per week to retrieve food with the affected hand (25 mDB trials, 15 mMAP trials), which contrasts greatly with a recent study using daily post-lesion training on a task similar to our mDB task but with over 1000 trials/day (Murata, et al., 2008). In fact, fewer than 40 actual movements occurred in most testing sessions because on many trials following brain injury, there were no attempts during the first or second post-lesion weeks (longer, in some cases). Thus, we considered this testing to be a minimal intervention necessary to track natural recovery of hand motor ability following isolated motor cortex injury. Nevertheless, it is probable that even this minimal forced-use of the contralesional hand without any constraint of the ipsilesional hand facilitated, to some degree, the recovery process of fine hand motor function in our cases. Indeed, early work on monkeys rendered hemiparetic by removal of the entire motor cortex showed qualitatively complete recovery of upper and lower limb function within 1–2 months if the ipsilesional hand was constrained and attempts were made to force the monkey to use the contralesional hand (Ogden and Franz, 1917).
As emphasized in this report, a large contributor to inter-subject variation in recovery was the volume of white matter damage. Specifically the cases with the largest white matter lesion volumes (SDM64, SDM50, SDM56, SDM67, SDM46) (Table 1) had the slowest recoveries. In these monkeys, consistent successful acquisitions on one or both of the mMAP curved rod task and mDB task did not occur until six or more weeks post-lesion. SDM46 did not have any successful acquisitions on either task after the lesion. In addition to motor cortex injury, cases SDM46 and SDM67 also had larger than intended gray matter involvement affecting prefrontal areas mediating cognitive aspects of motor behavior (Shima and Tanji, 2006) providing an added, and distinctive, contribution to the notable delay in recovery that can only be pinpointed with further stages of experimentation. However, the slow recovery of SDM64 is particularly notable as the gray matter lesion was limited to lateral motor cortex but the white matter lesion was much larger than other monkeys with a similar lesion. Other monkeys with gray matter lesions limited to frontal lobe motor areas and small white matter lesions (e.g., SDM38, SDM45, SDM48, SDM70, SDM56) showed much faster recovery than SDM64. Interrupting corticocortical projection fibers disconnects cortical areas that normally coordinate sensorimotor activities during control of hand/digit function and may create greater lasting impairments of function than lesions limited primarily to gray matter (Haaxma and Kuypers, 1975). Indeed, monkeys with surgically induced lesions of posterior parietal cortex that were limited primarily to gray matter have reported recovery of visually guided hand function occurring 2–3 weeks after injury (Battaglini, et al., 2002; Faughier-Grimaud, et al., 1985; Faughier-Grimaud, et al., 1978). Moreover, some studies of surgical lesions to motor areas of the frontal lobe in humans for treatment of cancer, epilepsy and arteriovenous malformations have found relatively minor lasting motor function deficits in most cases (Chamoun, et al., 2007; Da Pian, et al., 1980; Laplane, et al., 1977; Mikuni, et al., 2005; Rostomily, et al., 1991). As mentioned earlier, such results contrast with the more lasting deficits caused when a small lateral frontal cortical lesion includes the primary somatosensory cortex of the parietal lobe (Liu and Rouiller, 1999)..
Duration for upper limb functional recovery following frontal lobe lesions may also be an important factor underlying the clinical outcome of motor recovery. Studies in humans have shown that increased time for recovery may explain 16% – 26% of spontaneous arm motor recovery during the first 16 weeks post-stroke (Kwakkel, et al., 2006). Duration of recovery was varied in our studies because an important focus of this work, in addition to motor recovery characterization, was on reorganization of neural connections and cellular and molecular responses (McNeal, et al., accepted with review; McNeal, et al., 2007) at different time periods following brain injury (Nagamoto-Combs, et al., 2007; Nagamoto-Combs, et al., in revision). However, recovery of hand motor skill by the monkeys that recovered for 6 versus 12 months following the lesion were similar (see Tables 1, 2), suggesting that most recovery occurred in the first 6 months (indeed usually within the first 2–3 months – Fig. 4, 6) following the lesion. Only one monkey (SDM46), who showed the poorest recovery, had less than 6 months to recover. However, this poor recovery was clearly due primarily to the very large lesion that was approximately double the volume of the M1+LPMC+M2 lesions in SDM 50 and SDM 56.
We were surprised by the finding that performance of a previously learned motor task improved to greater than pre-lesion skill levels in some monkeys even with substantial damage to the major frontal motor areas (Fig. 9, 10, Table 2). Thus, improvements in performance with minimal task practice not only reappear after isolated frontal motor cortex injury, but motor performance can improve beyond pre-lesion levels, at least when reaching for food targets in a highly motivated state and on simple but specific tasks without high force requirements. We attribute this unexpected finding to the lack of intense training before the lesion, such that the monkeys had not achieved their highest levels of skill, in terms of high mean and low variability of performance scores, before the lesion was made. This would leave some potential for recovery to higher skill levels after the lesion. It is also possible that when recovering from the lesion the monkeys become more careful and exert lower forces when performing hand/digit movements to minimize the possibility of not acquiring the food. This could lead to low variability of performance and, thus, higher skill in terms of our skill measure. One could also argue that use of the best 5 consecutive post-lesion performances to determine post-lesion skill caused the high post/pre-lesion ratios we observed in some monkeys. However, we were interested in defining the best observed skill recovery over the same number of consecutive test sessions as in the pre-lesion tests because we believe this is most indicative of the potential for recovery of fine motor skill following brain lesions.
In conclusion, we have demonstrated that duration of fine hand motor impairment is closely correlated with volume of white matter damage that accompanies lesions of the frontal motor areas and medial prefrontal region. The volume of gray matter damage in these areas did not correlate strongly with duration of fine motor impairment. However, volume of gray matter and white matter damage was strongly correlated with recovery of skill in manipulation of small food targets (Fig. 9A,B,C) but not with recovery of skill in gross upper limb function (i.e., reaching – Fig. 9D). Finally, it is notable that recovery of function can proceed to reach higher levels of both gross and fine motor skills than observed in the pre-lesion state with isolated frontal lobe injury. These findings strongly suggests that the central nervous system of subhuman primates can significantly reorganize to compensate for substantial damage to frontal cortical arm areas when given brief weekly practice sessions on hand motor tasks.
Supplementary Material
Acknowledgments
We would like to thank the animal care staff at the University of South Dakota for their assistance and Grant Headley and Nicole Helle at the University of Iowa for their assistance in digitizing video data.
Grants: This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-046367 and The South Dakota Spinal Cord and Traumatic Brain Injury Research Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alaverdashvili M, Moon SK, Beckman CD, Virag A, Whishaw IQ. Acute but not chronic differences in skilled reaching for food following motor cortex devascularization vs. photothrombotic stroke in the rat. Neuroscience. 2008;157:297–308. doi: 10.1016/j.neuroscience.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Battaglini PP, Muzur A, Galletti C, Skrap M, Brovelli A, Fattori P. Effects of lesions to area V6A in monkeys. Exp Brain Res. 2002;144:419–422. doi: 10.1007/s00221-002-1099-4. [DOI] [PubMed] [Google Scholar]
- 3.Binkofski F, Seitz RJ, Hacklander T, Pawelec D, Mau J, Freund HJ. Recovery of motor functions following hemiparetic stroke: a clinical and magnetic resonance-morphometric study. Cerebrovasc Dis. 2001;11:273–281. doi: 10.1159/000047650. [DOI] [PubMed] [Google Scholar]
- 4.Black P, Cianci SN, Markowitz RS. Differential recovery of proximal and distal motor power after cortical lesions. Trans Am Neurol Assoc. 1971;96:173–177. [PubMed] [Google Scholar]
- 5.Bucy PC. Organization of the central nervous control of muscular activity. Bull Chic Med Soc. 1949;51:863–866. [PubMed] [Google Scholar]
- 6.Bucy PC. The central neural mechanism controlling movement, with special reference to the pyramidal tract. Acta Neurochir (Wien) 1964;11:731–738. doi: 10.1007/BF01402144. [DOI] [PubMed] [Google Scholar]
- 7.Chamoun RB, Mikati MA, Comair YG. Functional recovery following resection of an epileptogenic focus in the motor hand area. Epilepsy Behav. 2007;11:384–388. doi: 10.1016/j.yebeh.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Da Pian R, Pasqualin A, Scienza R, Vivenza C. Microsurgical treatment of ten arteriovenous malformations in critical areas of the cerebrum. J Microsurg. 1980;1:305–320. doi: 10.1002/micr.1920010409. [DOI] [PubMed] [Google Scholar]
- 9.Darian-Smith C, Ciferri MM. Loss and recovery of voluntary hand movements in the macaque following a cervical dorsal rhizotomy. The Journal of Comparative Neurology. 2005;491:27–45. doi: 10.1002/cne.20686. [DOI] [PubMed] [Google Scholar]
- 10.Darian-Smith I, Burman K, Darian-Smith C. Parallel pathways mediating manual dexterity in the macaque. Exp Brain Res. 1999;128:101–108. doi: 10.1007/s002210050824. [DOI] [PubMed] [Google Scholar]
- 11.Darling WG, Peterson CR, Herrick JL, McNeal DW, Stilwell-Morecraft KS, Morecraft RJ. Measurement of coordination of object manipulation in non-human primates. Journal of Neuroscience Methods. 2006;154:38–44. doi: 10.1016/j.jneumeth.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Darling WG, Pizzimenti MA, Rotella DL, Hynes SM, Ge J, KC, KSs, S-M, DWM, RJM Minimal forced use can overcome nonuse following isolated frontal motor cortex lesions in subhuman primates. Proceedings of the 2008 Society for Neural Control of Movement meeting; 2008. [Google Scholar]
- 13.Denny-Brown D. Disintegration of motor function resulting from cerebral lesions. J Nerv Ment Dis. 1950;112:1–45. [PubMed] [Google Scholar]
- 14.Denny-Brown D, Yanagisawa N, Kirk E. The localization of hemispheric mechanisms of visually directed reaching and grasping. In: Zulch KJ, Kruetzfeldt O, Galbraith GC, editors. Cerebral localization. Springer; Berlin: 1975. pp. 62–65. [Google Scholar]
- 15.Faughier-Grimaud S, Frenois C, Peronnet F. Effects of posterior parietal lesions on visually guided movements in monkeys. Experimental Brain Research. 1985;59:125–138. doi: 10.1007/BF00237673. [DOI] [PubMed] [Google Scholar]
- 16.Faughier-Grimaud S, Frenois C, Stein DG. Effects of posterior parietal lesions on visually guided behavior in monkeys. Neuropsychologia. 1978;16:151–168. doi: 10.1016/0028-3932(78)90103-3. [DOI] [PubMed] [Google Scholar]
- 17.Friel KM, Barbay S, Frost SB, Plautz EJ, Hutchinson DM, Stowe AM, Dancause N, Zoubina EV, Quaney BM, Nudo RJ. Dissociation of sensorimotor deficits after rostral versus caudal lesions in the primary motor cortex hand representation. Journal of Neurophysiology. 2005;94:1312–1324. doi: 10.1152/jn.01251.2004. [DOI] [PubMed] [Google Scholar]
- 18.Galea MP, Darian-Smith I. Manual dexterity and corticospinal connectivity following unilateral section of the cervical spinal cord in the macaque monkey. Journal of Comparative Neurology. 1997;381:307–319. [PubMed] [Google Scholar]
- 19.Gash DM, Zhang Z, Umberger G, Mahood K, Smith M, Smith C, Gerhardt GA. An automated movement assessment panel for upper limb motor functions in rhesus monkeys and humans. J Neurosci Methods. 1999;89:111–117. doi: 10.1016/s0165-0270(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 20.Geyer S, Matelli M, Luppino G, Zilles K. Functional neuroanatomy of the primate isocortical motor system. Anatomy & Embryology. 2000;202:443–474. doi: 10.1007/s004290000127. [DOI] [PubMed] [Google Scholar]
- 21.Glees P, Cole J. Recovery of skilled motor funcrtions after small repeated lesions of motor cortex in macaque. J Neurophysiol. 1950;13:137–148. [Google Scholar]
- 22.Gonzalez CL, Kolb B. A comparison of different models of stroke on behaviour and brain morphology. European Journal of Neuroscience. 2003;18:1950–1962. doi: 10.1046/j.1460-9568.2003.02928.x. [DOI] [PubMed] [Google Scholar]
- 23.Graham DI, Landtos PL. Greenfield’s Neuropathology. Oxford University Press; New York: 2002. [Google Scholar]
- 24.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 25.Haaxma R, Kuypers HG. Intrahemispheric cortical connexions and visual guidance of hand and finger movements in the rhusus monkey. Brain. 1975;98:239–260. doi: 10.1093/brain/98.2.239. [DOI] [PubMed] [Google Scholar]
- 26.Heffner R, Masterton B. Variation in form of the pyramidal tract and its relationship to digital dexterity. Brain Behav Evol. 1975;12:161–200. doi: 10.1159/000124401. [DOI] [PubMed] [Google Scholar]
- 27.Heffner RS, Masterton RB. The role of the corticospinal tract in the evolution of human digital dexterity. Brain Behav Evol. 1983;23:165–183. doi: 10.1159/000121494. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman DS, Strick PL. Effects of a primary motor cortex lesion on step-tracking movements of the wrist. J Neurophysiol. 1995;73:891–895. doi: 10.1152/jn.1995.73.2.891. [DOI] [PubMed] [Google Scholar]
- 29.Jelsing J, Rostrup E, Markenroth K, Paulson OB, Gundersen HJ, Hemmingsen R, Pakkenberg B. Assessment of in vivo MR imaging compared to physical sections in vitro--a quantitative study of brain volumes using stereology. Neuroimage. 2005;26:57–65. doi: 10.1016/j.neuroimage.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Kermadi I, Liu Y, Tempini A, Rouiller EM. Effects of reversible inactivation of the supplementary motor area (SMA) on unimanual grasp and bimanual pull and grasp performance in monkeys. Somatosens Mot Res. 1997;14:268–280. doi: 10.1080/08990229770980. [DOI] [PubMed] [Google Scholar]
- 31.Kuypers H. Anatomy of the descending motor pathways. In: Brooks VB, editor. Handbook of Physiology, Section I, The Nervous system, Vol II, Motor Control. Pt I. 1981. pp. 567–666. [Google Scholar]
- 32.Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke. 2006;37:2348–2353. doi: 10.1161/01.STR.0000238594.91938.1e. [DOI] [PubMed] [Google Scholar]
- 33.Laplane D, Talairach J, Meininger V, Bancaud J, Bouchareine A. Motor consequences of motor area ablations in man. J Neurol Sci. 1977;31:29–49. doi: 10.1016/0022-510x(77)90004-1. [DOI] [PubMed] [Google Scholar]
- 34.Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- 35.Lemon RN, Mantel GW, Muir RB. Corticospinal facilitation of hand muscles during voluntary movement in the conscious monkey. J Physiol. 1986;381:497–527. doi: 10.1113/jphysiol.1986.sp016341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res. 1999;128:149–159. doi: 10.1007/s002210050830. [DOI] [PubMed] [Google Scholar]
- 37.Mason CR, Miller LE, Baker JF, Houk JC. Organization of reaching and grasping movements in the primate cerebellar nuclei as revealed by focal muscimol inactivations. Journal of Neurophysiology. 1998;79:537–554. doi: 10.1152/jn.1998.79.2.537. [DOI] [PubMed] [Google Scholar]
- 38.McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon K, Hynes SM, Pizziment MA, Rotella D, Vanadurongvan T, Morecraft RJ. Selective Long-term Reorganization of the Corticospinal Projection from the Supplementary Motor Cortex following Recovery from Lateral Motor Cortex Injury. Journal of Comparative Neurology. doi: 10.1002/cne.22218. accepted with review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNeal DW, Darling WG, Pizzimenti MA, Combs CK, Nagamoto-Combs K, Rotella D, Peterson C, Stilwell-Morecraft KS, Morecraft RJ. Long-term reorganization of the corticospinal projection from the supplementary motor cortex is accompanied by motor recovery and prolonged microgliosis in the Rhesus Monkey. Society for Neuroscience 2007. 2007 Abstract Viewer #77.4. [Google Scholar]
- 40.Metz GA, Antonow-Schlorke I, Witte OW. Motor improvements after focal cortical ischemia in adult rats are mediated by compensatory mechanisms. Behav Brain Res. 2005;162:71–82. doi: 10.1016/j.bbr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Mikuni N, Ikeda A, Yoneko H, Amano S, Hanakawa T, Fukuyama H, Hashimoto N. Surgical resection of an epileptogenic cortical dysplasia in the deep foot sensorimotor area. Epilepsy Behav. 2005;7:559–562. doi: 10.1016/j.yebeh.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Miyai I, Suzuki T, Kang J, Kubota K, Volpe BT. Middle cerebral artery stroke that includes the premotor cortex reduces mobility outcome. Stroke. 1999;30:1380–1383. doi: 10.1161/01.str.30.7.1380. [DOI] [PubMed] [Google Scholar]
- 43.Mohr JP, Foulkes MA, Polis AT, Hier DB, Kase CS, Price TR, Tatemichi TK, Wolf PA. Infarct topography and hemiparesis profiles with cerebral convexity infarction: the Stroke Data Bank. J Neurol Neurosurg Psychiatry. 1993;56:344–351. doi: 10.1136/jnnp.56.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morecraft RJ, Cipolloni PB, Stilwell-Morecraft KS, Gedney MT, Pandya DN. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. Journal of Comparative Neurology. 2004;469:37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- 45.Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- 46.Morecraft RJ, Herrick JL, Stilwell-Morecraft KS, Louie JL, Schroeder CM, Ottenbacher JG, Schoolfield MW. Localization of arm representation in the corona radiata and internal capsule in the non-human primate. Brain. 2002;125:176–198. doi: 10.1093/brain/awf011. [DOI] [PubMed] [Google Scholar]
- 47.Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124:176–208. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- 48.Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Dvanajscak Z, Ge J, Schneider P. Localization of arm representation in the cerebral peduncle of the non-human primate. J Comp Neurol. 2007;504:149–167. doi: 10.1002/cne.21438. [DOI] [PubMed] [Google Scholar]
- 49.Murata Y, Higo N, Oishi T, Yamashita A, Matsuda K, Hayashi M, Yamane S. Effects of motor training on the recovery of manual dexterity after primary motor cortex lesion in macaque monkeys. J Neurophysiol. 2008;99:773–786. doi: 10.1152/jn.01001.2007. [DOI] [PubMed] [Google Scholar]
- 50.Nagamoto-Combs K, McNeal DW, Morecraft RJ, Combs CK. Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J Neurotrauma. 2007;24:1719–1742. doi: 10.1089/neu.2007.0377. [DOI] [PubMed] [Google Scholar]
- 51.Nagamoto-Combs K, Morecraft RJ, Darling WG, Combs CK. Long-Term Gliosis and Molecular Changes in the Cervical Spinal Cord of the Rhesus Monkey after Traumatic Brain Injury. doi: 10.1089/neu.2009.0966. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- 53.Nudo R, Jenkins W, Merzenich M, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. Journal of Neurophysiology. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 55.Ogden R, Franz SI. On cerebral motor control: the recovery from experimentally produced hemiplegia. Psychobiology. 1917;1:33–47. [Google Scholar]
- 56.Passingham RE, Perry VH, Wilkinson F. The long-term effects of removal of sensorimotor cortex in infant and adult rhesus monkeys. Brain. 1983;106 (Pt 3):675–705. doi: 10.1093/brain/106.3.675. [DOI] [PubMed] [Google Scholar]
- 57.Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 58.Pineiro R, Pendlebury ST, Smith S, Flitney D, Blamire AM, Styles P, Matthews PM. Relating MRI changes to motor deficit after ischemic stroke by segmentation of functional motor pathways. Stroke. 2000;31:672–679. doi: 10.1161/01.str.31.3.672. [DOI] [PubMed] [Google Scholar]
- 59.Pizzimenti MA, Darling WG, Rotella DL, McNeal DW, Herrick JL, Ge J, Stilwell-Morecraft KS, Morecraft RJ. Measurement of reaching kinematics and prehensile dexterity in nonhuman primates. J Neurophysiol. 2007;98:1015–1029. doi: 10.1152/jn.00354.2007. [DOI] [PubMed] [Google Scholar]
- 60.Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, Marshall RS, Krakauer JW. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 61.Roland PE, Zilles K. Functions and structures of the motor cortices in humans. Curr Opin Neurobiol. 1996;6:773–781. doi: 10.1016/s0959-4388(96)80027-4. [DOI] [PubMed] [Google Scholar]
- 62.Rostomily RC, Berger MS, Ojemann GA, Lettich E. Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg. 1991;75:62–68. doi: 10.3171/jns.1991.75.1.0062. [DOI] [PubMed] [Google Scholar]
- 63.Sato KC, Tanji J. Digit-muscle responses evoked from multiple intracortical foci in monkey precentral motor cortex. J Neurophysiol. 1989;62:959–970. doi: 10.1152/jn.1989.62.4.959. [DOI] [PubMed] [Google Scholar]
- 64.Schieber MH. Chapter 2 Comparative anatomy and physiology of the corticospinal system. Handb Clin Neurol. 2007;82:15–37. doi: 10.1016/S0072-9752(07)80005-4. [DOI] [PubMed] [Google Scholar]
- 65.Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford University Press; New York: 2006. [Google Scholar]
- 66.Shima K, Tanji J. Binary-coded monitoring of a behavioral sequence by cells in the pre-supplementary motor area. J Neurosci. 2006;26:2579–2582. doi: 10.1523/JNEUROSCI.4161-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taub E, Heitmann RD, Barro G. Alertness, level of activity, and purposive movement following somatosensory deafferentation in monkeys. Ann N Y Acad Sci. 1977;290:348–365. doi: 10.1111/j.1749-6632.1977.tb39737.x. [DOI] [PubMed] [Google Scholar]
- 68.Travis AM. Neurological deficiencies after ablation of the precentral motor area in Macaca mulatta. Brain. 1955a;78:155–173. doi: 10.1093/brain/78.2.155. [DOI] [PubMed] [Google Scholar]
- 69.Travis AM. Neurological deficiencies following supplementary motor area lesions in Macaca mulatta. Brain. 1955b;78:174–198. doi: 10.1093/brain/78.2.174. [DOI] [PubMed] [Google Scholar]
- 70.van der Worp HB, Claus SP, Bar PR, Ramos LM, Algra A, van Gijn J, Kappelle LJ. Reproducibility of measurements of cerebral infarct volume on CT scans. Stroke. 2001;32:424–430. doi: 10.1161/01.str.32.2.424. [DOI] [PubMed] [Google Scholar]
- 71.Vilensky JA, Gilman S. Lesions of the precentral gyrus in nonhuman primates: A Pre-Mediline Bibliography. International Journal of Primatology. 2002;23:1319–1333. [Google Scholar]
- 72.Warabi T, Inoue K, Noda H, Murakami S. Recovery of voluntary movement in hemiplegic patients. Correlation with degenerative shrinkage of the cerebral peduncles in CT images. Brain. 1990;113 (Pt 1):177–189. doi: 10.1093/brain/113.1.177. [DOI] [PubMed] [Google Scholar]
- 73.Weber R, Ramos-Cabrer P, Justicia C, Wiedermann D, Strecker C, Sprenger C, Hoehn M. Early Prediction of Functional Recovery after Experimental Stroke: Functional Magnetic Resonance Imaging, Electrophysiology, and Behavioral Testing in Rats. J Neurosci. 2008;28:1022–1029. doi: 10.1523/JNEUROSCI.4147-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whishaw IQ. Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology. 2000;39:788–805. doi: 10.1016/s0028-3908(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 75.Zilles K, Schlaug G, Matelli M, Luppino G, Schleicher A, Qu M, Dabringhaus A, Seitz R, Roland PE. Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. J Anat. 1995;187 (Pt 3):515–537. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


