Abstract
Background
Lectins are a diverse group of carbohydrate-binding proteins exhibiting numerous biological activities and functions.
Methods
Two-step serial carbohydrate affinity chromatography was used to isolate a lectin from the edible mushroom clouded agaric (Clitocybe nebularis). It was characterized biochemically, its gene and cDNA cloned and the deduced amino acid sequence analyzed. Its activity was tested by hemagglutination assay and carbohydrate-binding specificity determined by glycan microarray analysis. Its effect on proliferation of several human cell lines was determined by MTS assay.
Results
A homodimeric lectin with 15.9-kDa subunits agglutinates human group A, followed by B, O, and bovine erythrocytes. Hemagglutination was inhibited by glycoprotein asialofetuin and lactose. Glycan microarray analysis revealed that the lectin recognizes human blood group A determinant GalNAcα1–3(Fucα1–2)Galβ-containing carbohydrates, and GalNAcβ1–4GlcNAc (N,N’-diacetyllactosediamine). The lectin exerts antiproliferative activity specific to human leukemic T cells.
Conclusions
The protein belongs to the ricin B-like lectin superfamily, and has been designated as Clitocybe nebularis lectin (CNL). Its antiproliferative effect appears to be elicited by binding to carbohydrate receptors on human leukemic T cells.
General Significance
CNL is one of the few mushroom ricin B-like lectins that have been identified and the only one so far shown to possess immunomodulatory properties.
Keywords: Mushroom, Clitocybe nebularis, Ricin B-like lectin, Antiproliferative effect, Leukemic T cells
1. Introduction
Fungi are well-known for their nutritional and medicinal value due to their content of a variety of bioactive substances with pharmacological properties [1]. For this reason, mushrooms and their derivatives are increasingly used worldwide in traditional medicine and as dietary supplements [2]. Numerous mushroom-derived compounds with antitumor, immunomodulatory, antiviral, antimicrobial and other activities have been isolated and identified, including polysaccharides, polysaccharide-protein complexes, triterpenes, phenols, peptides, and proteins such as lectins [3].
Lectins are biologically active proteins that have been isolated from humans, animals, plants and microorganisms, and also from fungi. They constitute a diverse group of proteins that specifically bind different types of carbohydrates. The widespread occurrence of lectins suggests their role in basic biological functions. Most lectins contain more than one carbohydrate-binding site and can therefore agglutinate cells or cross-link cell surface carbohydrates [4]. Their biological activity can be exerted through binding and clustering glycosylated receptors on cell surfaces, leading to signal transduction pathway activation, and by binding to extracellular matrix glycoconjugates, cytoplasmic and nuclear glycoproteins. In this manner, lectins can be involved in various cellular processes, including cell adhesion, migration, differentiation, apoptosis and proliferation, and thus possess immunomodulatory properties and have potential roles in cancer [5].
Many lectins have been isolated and characterized from higher fungi [6,7], and six different folds have been identified for mushroom lectins [8,9]. One of them is a β-trefoil fold typical of the ricin B domain in the crystal structures of Laetiporus sulphureus lectin [10] and Marasmius oreades agglutinin [11]. In addition, other fungal lectins that contain ricin B-like lectin domains have been isolated from Rhizoctonia solani [12] and Polyporus squamosus [13]. A lectin from a basidiomycete, clouded agaric (Clitocybe nebularis), has been reported [14] but has not been characterized at the molecular level. In previous work, our group isolated a novel inhibitor of cysteine proteinases, clitocypin, from this mushroom [15] and also several distinct lectins with specificities for different carbohydrates which have been examined for biological activities. In this study, a ricin B-like lectin designated Clitocybe nebularis lectin (CNL) has been isolated, characterized biochemically and its gene, cDNA and deduced amino acid sequences determined and analyzed. Moreover, the effect on proliferation of several human cell lines was assayed in order to evaluate the antitumor and immunomodulatory potential of CNL.
2. Materials and methods
2.1. Fungal material
Basidiocarps of the basidiomycete Clitocybe nebularis were collected in their natural habitat in Kras forest, Slovenia in October 2004 and frozen at −30°C until use. A specimen is deposited at the Department of Biotechnology, Jožef Stefan Institute, Ljubljana, Slovenia. The cultured mycelium isolated from the same specimen was confirmed by ribosomal DNA spacer sequencing to belong to the C. nebularis species [16]. It is kept in the collection of fungi, lichens and higher plants at the Slovenian Forestry Institute, Ljubljana, Slovenia.
2.2. Purification of CNL
After defrosting, C. nebularis fruiting bodies (1000 g fresh weight) were homogenized and extracted in 1000 ml of 20 mM Tris/HCl buffer, pH 7.5, containing 0.4 M NaCl (Buffer A). The homogenate was centrifuged for 15 min at 11,000 × g and 4°C. The resulting supernatant was filtered and subjected to two-step serial carbohydrate affinity chromatography, using lactosyl- and glucosyl-Sepharose 4B columns (Pharmacia Fine Chemicals, Uppsala, Sweden). The columns were prepared as described [17], and equilibrated with Buffer A. The extract was loaded on a lactosyl-Sepharose column, which was then washed with Buffer A to remove unbound material. Adsorbed proteins were eluted with either 0.2 M lactose or 0.01 M NaOH in the same buffer. In the latter case, fractions were immediately neutralized with 2 M Tris/HCl buffer, pH 6.5. The eluted lactose-binding proteins were then applied to a glucosyl-Sepharose column and the unbound fractions (containing CNL) were collected, pooled and concentrated using an Amicon UM-10 ultrafiltration membrane (Amicon Corp., Lexington, MA). The purity of isolated CNL was assessed by a reversed-phase high-performance liquid chromatography (RP-HPLC; Hewlett-Packard Series 1100 system, Germany) on a Chromsep HPLC column (ChromSpher C8, 100 × 3 mm; Chrompack international, The Netherlands). The initial solvent was water containing 0.1% trifluoroacetic acid (TFA) as ion-pairing agent. Proteins were eluted with a linear gradient of acetonitrile/water (90:10, v/v) solution containing 0.1% TFA. The eluting solution was increased linearly from 0 to 45% over 5 min, 45–65% over 30 min, and 65–100% over 5 min. The lectin was then dried in a SpeedVac concentrator (Savant Instruments, inc., Hicksville, NY).
2.3. Polyacrylamide gel electrophoresis and isoelectric focusing
The purity and molecular mass of CNL were estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using homogeneous 15% (w/v) acrylamide gel on a mini-Protean II apparatus from Bio-Rad Laboratories (Hercules, CA). Samples were dissolved in electrophoresis buffer, pH 6.8, containing 5% SDS and heated at 100°C in either the presence or absence of 5% (v/v) 2-mercaptoethanol for 10 min. Gels were stained with 0.1% (w/v) Coomassie Brilliant Blue R-250. Molecular mass was estimated using low molecular weight standard proteins of 14.4–97 kDa (LMW Calibration Kit for SDS Electrophoresis, Amersham Pharmacia Biotech). Non-denatured CNL was subjected to native PAGE on PhastGel Gradient 8–25 with PhastGel Native Buffer Strips, using the PhastSystem (Pharmacia LKB Biotechnology), according to the instructions provided. Isoelectric focusing was carried out with a Pharmacia PhastSystem, using a polyacrylamide gel with a pH gradient of 3–9 (PhastGel™ IEF 3–9, Pharmacia) following the manufacturer’s protocol. The isoelectric point was estimated using a Pharmacia Broad pI Calibration Kit for a pH range of 3–10.
2.4. Size exclusion chromatography
The approximate molecular mass of native CNL was estimated by analytical size exclusion fast protein liquid chromatography (FPLC; ÄKTA FPLC System, Amersham Pharmacia Biotech, Sweden). The column (Superdex™ 75 HR/10/30, Pharmacia Biotech Inc.) was equilibrated with 50 mM Tris/HCl buffer, pH 7.5, containing 0.3 M NaCl, with 0.2 M lactose to prevent interactions between the lectin and column matrix. The column was calibrated under the same conditions with Dalton standards MS II (Serva, Heidelberg).
2.5. Electrospray ionization-mass spectrometry
The molecular mass of the HPLC-purified CNL was determined by electrospray ionization (ESI) mass spectrometry. The protein was dissolved in water/methanol (60:40, v/v) solution containing 0.1% formic acid, and analyzed on an AutoSpecQ (Micromass, Manchester, UK), and on a Q-Tof Premier (Micromass MS Technologies/Waters, Manchester, UK) mass spectrometer. The protein sample was introduced into the ion source (needle voltage of 4 kV) at a flow rate of 5 µl/min using a syringe pump. The mass spectrum, with multiply charged ion series, was deconvoluted to obtain the molecular mass of the CNL.
2.6. Amino acid sequencing
N-terminal and peptide sequences were determined by automated Edman degradation using a Procise Protein Sequencing System 492 (PE Applied Biosystems, Foster City, CA). HPLC-purified CNL was denatured by heating at 100°C for 30 min and digested with trypsin or α-chymotrypsin (enzyme/substrate ratio of 1:100 (w/w)) in 0.1 M N-methyl morpholine acetate buffer, pH 8.1, for 4 h at 37°C. Cyanogen bromide cleavage was carried out in 80% formic acid at room temperature in the dark for 36 h. Resulting peptide fragments were separated by RP-HPLC and sequenced.
2.7. Molecular cloning of the gene and cDNA encoding CNL
High molecular weight genomic DNA was isolated from frozen powdered C. nebularis fruiting bodies as described [18]. Total RNA was extracted using RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol for isolation from plant tissues and filamentous fungi. First strand complementary DNA (cDNA) was synthesized from the total RNA by reverse transcription-polymerase chain reaction (PCR) using a GeneAmp RNA PCR Core Kit (Applied Biosystems, Foster city, CA) with anchored oligo(dT)-adapter primer dT(17)3’RACE (Table S1, Supplementary data). For PCR amplification of the partial cnl gene and cDNA sequences, genomic DNA and reverse transcript were used as templates. Forward (CNL-D-1f) and reverse (CNL-D-1r) degenerate primers (Table S1) were designed based on the partial amino acid sequence in regions with the lowest degeneracy. On the basis of the resulting partial nucleic acid sequences, specific primers were designed to amplify the complete gene and cDNA sequences.
3’ rapid amplification of cDNA ends (3’ RACE) was carried out using the 3’RACE adapter primer paired with a forward primer CNL-RACE-1f (Table S1) specific for the cDNA fragment. cDNA synthesized from total RNA was used as a template. The resulting PCR product (100 times diluted) served as a template for secondary PCR using a specific nested primer CNL-RACE-2f (Table S1).
Genome walking PCR was used to amplify the complete cnl gene with its promoter and terminator regions. Genome walking libraries were constructed using GenomeWalker™ Universal Kit (BD Biosciences Clontech, Palo Alto, CA) according to the manufacturer’s instructions. High molecular weight genomic DNA (2.5 µg) was digested separately with four restriction enzymes (DraI, EcoRV, PvuII, StuI) at 37°C overnight. After purification by ethanol precipitation, GenomeWalker™ Adaptors were ligated to the digested DNA at 16°C overnight. The resulting ligation mixes were used as templates in genome walking PCR amplifications, using adapter primer AP1 and a nested primer AP2 provided by the manufacturer, paired with nested forward gene-specific primers (CNL-term-1 and CNL-term-2; Table S1) for downstream amplification or nested reverse gene-specific primers (CNL-prom-1 and CNL-prom-2; Table S1) for upstream amplification. PCR amplification conditions were used as suggested by the manufacturer.
2.8. Sequence analysis
All PCR products were cloned into pGEM-T Easy Vector System I (Promega, Madison, WI) and sequenced using the Automated DNA Sequencing Service at MWG Biotech (Ebersberg, Germany). Overlapping fragments were assembled using the BioEdit program (http://www.mbio.ncsu.edu/BioEdit/bioedit.html); complete putative cnl gene and cDNA sequences were obtained. Both were confirmed by amplifying the full-length sequences using forward (CNL-1f) and reverse (CNL-1r) primers (Table S1).
Gene promoter analysis was performed using TESS (transcription element search software at http://www.cbil.upenn.edu/cgi-bin/tess/tess). The cDNA-deduced amino acid sequence of CNL (Swiss-Prot accession no. B2ZRS9) was analyzed using online proteomics tools at the ExPASy server (http://www.expasy.org/tools/). Similarity searches were performed using BLASTn and BLASTp algorithms at the NCBI server (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi), as well as FASTA3 and PSI-BLAST [19] programs at the EBI server (http://www.ebi.ac.uk/Tools/similarity.html). In addition, the CNL amino acid sequence was analyzed for protein domain and family identification using SMART (http://smart.embl-heidelberg.de/), and the SUPERFAMILY program [20] (http://supfam.cs.bris.ac.uk/SUPERFAMILY/). Using SMART, a multiple alignment of the CNL amino acid sequence with ricin B domain sequences was performed and manually adjusted in the BioEdit program.
2.9. Molecular modeling of CNL
The secondary structure of CNL was predicted using PSIPRED V2.6 (http://bioinf.cs.ucl.ac.uk/psipred/psiform.html). Structurally conserved regions of the lectin were identified by hydrophobic cluster analysis using the online Drawhca program (http://mobyle.rpbs.univ-paris-diderot.fr/cgi-bin/portal.py?form=HCA). Fully automated fold recognition was carried out using the LOOPP program (http://cbsuapps.tc.cornell.edu/loopp.aspx) to predict the three-dimensional structure of CNL. The predicted model was visualized using the PyMOL Molecular Graphics System (DeLano Scientific, San Carlos, CA).
2.10. Hemagglutination assay
The specificity of CNL for different types of erythrocytes was determined by hemagglutination using human groups A, AB, B, O, and bovine erythrocytes. Red blood cells were washed three times with 0.9% saline and twice with 20 mM Tris/HCl buffer, pH 7.5, containing 130 mM NaCl (erythrocyte buffer). Erythrocyte suspension (2%), corresponding to a final concentration of 4.3 × 108 erythrocytes/ml, was pipetted into a microtiter plate (Nunclon Delta SI, Nunc, Denmark), 100 µl per well, and 1–20 µl of CNL were added to give final concentrations of 1–325 µg/ml. Agglutination was examined visually after 1 hour incubation at room temperature.
A preliminary study of carbohydrate-binding specificity of CNL was performed by hemagglutination inhibition assay using mono- and disaccharides (D-glucose (Kemika, Zagreb, Croatia), D-galactose, D-sucrose, D-lactose (Fluka, Buchs, Switzerland), β-D-fructose, D-mannose (Sigma, St. Louis, Missouri)), and a glycoprotein asialofetuin (Sigma, St. Louis, Missouri). Prior to the test, microtiter plate wells were blocked with 0.1% (w/v) bovine serum albumin. CNL, at a final agglutinating concentration of 18.6 µg/ml, was incubated with serial dilutions of carbohydrate inhibitors in a microtiter plate for 1 h. Human group A erythrocytes suspended in erythrocyte buffer, pH 7.5, were then added.
The thermal stability of CNL was assessed by incubation at different concentrations for 30 min at 4 to 100°C, and storing overnight at 4°C. Samples were assayed by hemagglutination using human group A erythrocytes in erythrocyte buffer, pH 7.5. To study the activity of CNL in the pH range of 5–9, the lectin was diluted to different concentrations with 20 mM Mes/NaOH buffer, pH 5, 5.5, 6 or 6.5, containing 130 mM NaCl, or with erythrocyte buffer, pH 7, 7.5, 8, 8.5 or 9. Human group A erythrocytes were suspended in the same buffers and hemagglutinating activity assayed. Activity of the lectin at lower and higher pH values could not be tested due to the spontaneous lysis of erythrocytes.
2.11. Glycan microarray analysis
To determine fine carbohydrate-binding specificity of CNL, glycan microarray analysis was performed by The Consortium for Functional Glycomics (Core H; http://www.functionalglycomics.org/static/consortium/resources/resourcecoreh.shtml). Microarrays (Mammalian Printed Array version 3.2) containing ~400 different glycan structures in replicates of 6 were printed as described [21]. The lectin was biotinylated using No-Weight™ NHS-PEO4-Biotin (Pierce, Rockford, IL) according to the manufacturer’s instructions. Streptavidin-conjugated horseradish peroxidase was used to confirm biotinylation of CNL transferred to a PVDF membrane (Immobilon, Millipore, Bedford, MA) and the activity of the biotinylated lectin was examined by hemagglutination assay. In a binding assay, microarray slides were incubated with various concentrations of biotinylated CNL (200, 1 and 0.1 µg/ml). Slides were washed and bound lectin detected using fluorescently labeled streptavidin. Microarray slides were subjected to imaging, fluorescence was measured and images analyzed [21].
2.12. Cell proliferation assay
U-937 human pro-monocytic lymphoma cell line (CRL-1593.2; ATCC, Manassas, VA), and Mo-T and Jurkat human leukemic T cell lines (CRL-8066 and TIB-152; ATCC, Manassas, VA) were used and maintained according to the supplier’s instructions. In addition, MCF-10A neoT cell line originating from the MCF-10 human fibrocystic breast epithelial cell line (provided by Prof. Bonnie F. Sloane from Wayne State University, Detroit, MI) was used and cultured as described previously [22]. U-937, Mo-T, Jurkat and MCF-10A neoT cells were resuspended in the appropriate growth media to final concentrations of 4 × 105 cells/ml, and samples (100 µl/well) added to a 96-well microtiter polystyrene plate (Costar, Schiphol-Rijk, The Netherlands). Differentiated U-937 cells with macrophage-like properties were obtained by treating U-937 cells with phorbol 12-myristate 13-acetate (PMA; Sigma, St. Louis, MO) for 24 h at 37°C under 5% CO2. Non-adherent cells were washed away with warm (37°C) medium. CNL dissolved in phosphate buffer saline (PBS), pH 7.4, was added to the growth medium to different final concentrations. The appropriate volume of PBS was added to control samples of cells devoid of the lectin and incubated for 4 days at 37°C. Cells were then washed and their viability assessed using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS colorimetric assay; Promega, Madison, WI) according to the instructions provided by the manufacturer. Percentages of cell proliferation were determined as (Atest cells/Acontrol cells) × 100, where Atest cells and Acontrol cells are the absorbances of formazan measured for cells treated with different concentrations of CNL, and for non-treated control cells, respectively. In order to see if the effect was elicited due to carbohydrate-binding properties of CNL another control experiment was performed, where the lectin was preincubated with 0.1 M lactose diluted in PBS and added to cells. Two independent experiments were performed in triplicate for each cell type. A comparative analysis of proliferation of non-treated versus treated cells was performed with two-sample t-tests, using the Origin software (Origin 7.5 SR4, OriginLab Corporation, Northampton, MA).
3. Results
3.1. Purification of CNL
Purified CNL (~8 mg) was isolated from Clitocybe nebularis fruiting bodies (1000 g) by two-step serial carbohydrate affinity chromatography. In the first step, a crude extract was loaded onto a lactosyl-Sepharose column. Lactose-binding proteins were obtained by elution with 0.2 M lactose or 0.01 M NaOH, with respective yields of ~7 mg and ~18 mg. In the second step, CNL was separated from other lactose-binding proteins by application to a glucosyl-Sepharose column. The unbound CNL was collected and concentrated, and its purity assessed using RP-HPLC, revealing a single major peak (not shown).
3.2. CNL molecular mass and isoelectric point
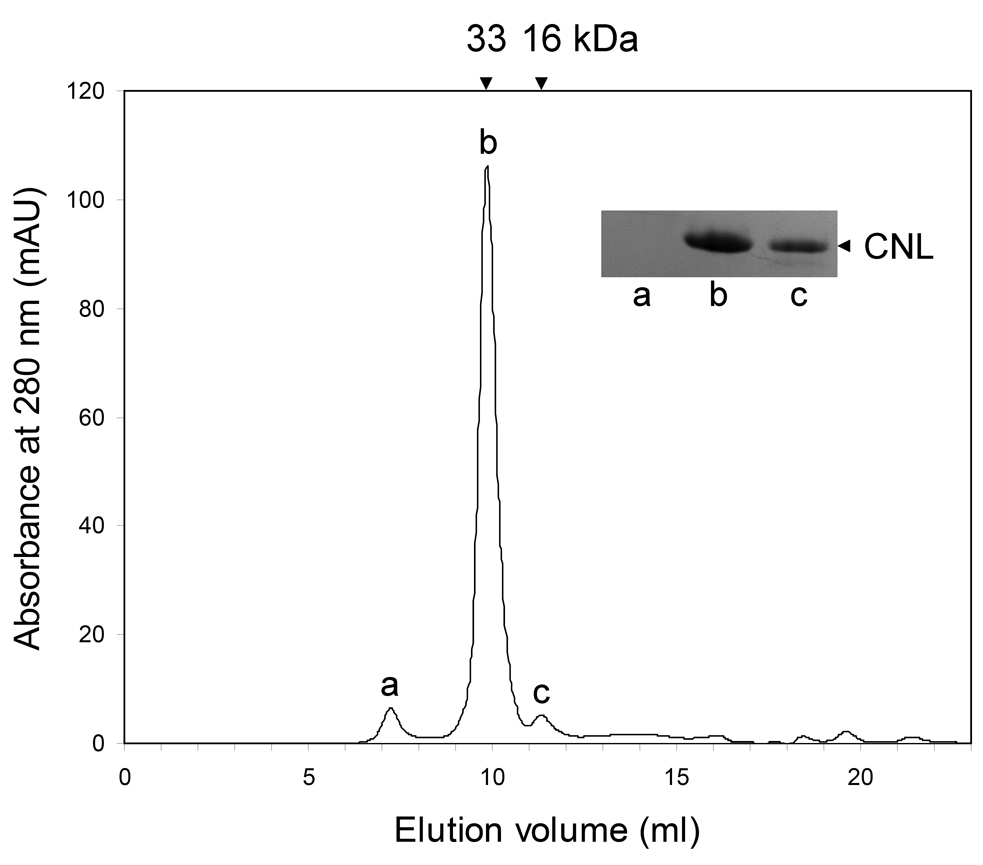
After lactosyl-Sepharose affinity chromatography and elution with either lactose or NaOH, three bands were obtained by SDS-PAGE analysis, corresponding to apparent masses of 19 kDa, 17.5 kDa, and 15.5 kDa (Fig. S1A, lane 2, Supplementary data). The glucosyl-Sepharose unbound CNL migrated as a single band and corresponded to a ~19-kDa protein (Fig. S1A, lane 3), irrespective of prior treatment with reducing agent (not shown). Native CNL was analyzed by size exclusion FPLC. It eluted as two peaks with approximate molecular masses of 33 kDa and 16 kDa, corresponding to about 97% and 3% of total protein and indicating a dimer and a monomer in slow equilibrium (Fig. 1). These results were also confirmed by native PAGE (data not shown). ESI-mass spectrometry analysis of HPLC-purified CNL revealed one major peak with a molecular mass of 15,903.5 Da. There was also a minor peak observed which is most probably an ionization artifact, since its 22 Da higher molecular mass is consistent with the sodium adduct (M+Na)+ of CNL (not shown).
Fig. 1.
Size exclusion chromatography elution profile of CNL using FPLC. Inset, SDS-PAGE analysis of peaks a, b, and c. Approximate molecular masses of eluted CNL are indicated at the top of the chromatogram.
Isoelectric focusing of CNL revealed one major and two minor bands which indicate heterogeneity at the primary structure level. The isoelectric point of CNL was determined to be approximately 4.3 (Fig. S1B, lane 2, Supplementary data).
3.3. Sequence analysis of purified CNL
The fact that CNL was blocked to Edman degradation at the amino terminus suggested its modification. Efforts to deblock the lectin using TFA in methanol (1:1, v/v) at 47°C for 48 h [23] were unsuccessful. However, a small fraction of the total protein enabled its sequence to be determined. Treatment with proteases yielded peptide fragments only when the lectin was previously denatured by heating at 100°C for 30 min, indicating that native CNL is resistant to proteolytic cleavage. The peptides were sequenced and around 85% of the complete sequence was determined (Fig. S2, Supplementary data). Two unidentified amino acid residues were detected, namely Trp50 which cannot be identified by Edman degradation and Tyr61 which was probably modified (Fig. S2).
3.4. Characterization of a cnl gene and CNL-encoding cDNA sequence
PCR amplification using degenerate primers with genomic DNA and reverse transcript as templates yielded fragments of 386 and 275 bp, respectively. Specific nested primers were designed and the complete cnl gene with its promoter and terminator regions, as well as the downstream region of the cDNA sequence, were obtained by genome walking and 3’ RACE methods, respectively. The overlapping segments were assembled and the complete cnl gene and cDNA sequences were amplified to confirm the determined nucleotide sequences containing the complete open reading frame encoding the full-length CNL. Alignment of the cnl gene and cDNA sequences showed no differences (Fig. S2). The 669-bp gene is composed of five exons (147, 96, 74, 61, 72 bp) and four introns (63, 48, 52, 56 bp) (Fig. S2). The intron-exon boundaries closely match the consensus splice sites predicted for eukaryotic genes, i.e. GTRNGY for the 5’-splice site and YNYAG for the 3’-splice site [24]. The 264-bp promoter sequence contains a consensus TATA box sequence (TATAAAA) at position −76, and a predicted transcription initiation site (TCACCCTC) at position −39 (Fig. S2). A putative TATA box, located 37 nt upstream from the putative transcription initiation site, is consistent with the locations found in several other fungal genes, which are generally 30–60 nt upstream from the transcriptional start site [24]. The 620-bp terminator sequence of cnl does not contain the consensus eukaryotic polyadenylation signal sequence AATAAA, however a putative variant signal, AATCAA, was identified 21 nt upstream of the poly(A) tail. The same polyadenylation signal sequence was reported for the Onchocerca volvulus galectin (Ov-gbp-2) gene (32 nt upstream) [25], the lignin peroxidase (LG2) gene from basidiomycete Phanerochaete chrysosporium (24 nt upstream) [26], the calpain-like protease (palBory) gene from Aspergillus oryzae [27] and the Gossypium hirsutum Δ-12 fatty acid desaturase (FAD2–3) gene (34 nt upstream) [28].
BLASTn and FASTA3 similarity searches against public sequence databases using the complete cnl gene, its coding region, or cDNA sequence as queries revealed no significant similarities (cut-off e < 10).
3.5. Analysis of the cDNA-deduced CNL amino acid sequence
The amino acid sequence was translated from the open reading frame and aligned with the cnl gene and cDNA sequences in Fig. S2. The deduced amino acid sequence indicates that the unprocessed polypeptide is composed of 149 amino acids that match the residues determined by amino acid sequencing of the purified lectin. However, two alternative amino acid residues are observed, Thr31 and Leu32 instead of Val31 and Ile32 (Fig. S2).
Using online proteomics tools, the N-terminus of the primary translation product was predicted with 85% likelihood to be post-translationally processed by N-terminal methionine excision and further modified by N-acetylation of the following serine residue. The predicted N-acetyl group is consistent with the blocked N-terminus detected by N-terminal amino acid sequencing of purified CNL. This was also confirmed by the calculated molecular mass of the 149 amino acid-long unprocessed protein (15,992.8 Da), and the molecular mass calculated for the predicted mature CNL lacking the initiating methionine (15,861.6 Da) but acetylated (+42.0372 Da), which totals 15,903.6 Da. This calculated molecular mass of the predicted mature CNL corresponds to the 15,903.5-Da molecular mass of the purified protein determined by mass spectrometry, indicating that the protein is modified post-translationally. Mature CNL contains no cysteine residues and only one methionine. The calculated isoelectric point of the protein is 4.87, that differs slightly from the isoelectric point determined experimentally (4.3; Fig. S1B, lane 2).
3.6. CNL is a member of the ricin B-like lectin superfamily
The CNL amino acid sequence similarity searches against protein databases, using BLASTp and FASTA3, and PSI-BLAST which is more sensitive to weak but biologically relevant sequence similarities [19], returned numerous fungal and bacterial proteins, including proteases and proteins involved in carbohydrate metabolism. These proteins all contain ricin B-type lectin domains that generally exhibit low sequence similarity to CNL. However, some proteins containing ricin B-like lectin domains with around 20% amino acid sequence identity to CNL were identified. These include the zinc metalloendopeptidase flavastacin precursor from Flavobacterium meningosepticum (Swiss-Prot Q47899), predicted proteins from mushrooms Coprinopsis cinerea (A8PCJ2) and Laccaria bicolor (B0DSD3), ricin B-related lectin from fungus Polyporus squamosus (Q75WT8), mushroom Marasmius oreades agglutinin (Q8X123) and Aspergillus oryzae predicted protein (Q2U1N6). A profile hidden Markov model-based searches for detecting distant family members [29], using SMART (with included Pfam domains), revealed that CNL consists of a ricin B lectin domain ranging from residues 6 to 141. It lacks a signal peptide, suggesting that the molecule is not secreted via the endoplasmic reticulum–Golgi apparatus pathway. Additionally, a SUPERFAMILY sequence search predicted that CNL is a member of the all-β class of proteins with a β-trefoil fold and a member of the ricin B-like lectins superfamily.
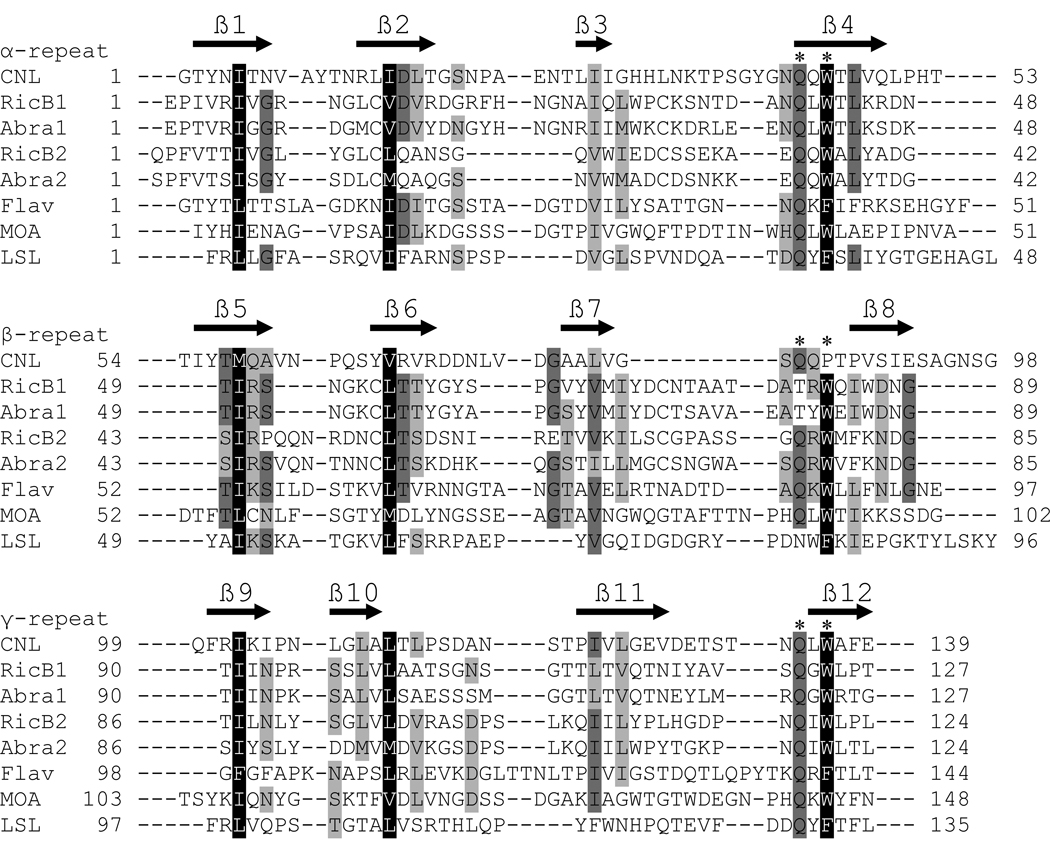
The ricin B-like lectin domain of CNL was aligned with the representative ricin B-type lectin domains of proteins with known structures [10,11,30,31] (Fig. 2). Closer examination of the multiple amino acid sequence alignment revealed extensive divergence between the ricin B-type domains, which was also noted by Hazes [32]. Despite low sequence identity, the domains share common conserved residues and internal homology of amino acid sequences. The three distinct homologous subdomains, designated as α-, β- and γ-repeats [33], are also apparent in CNL and contain glutamine – any residue – tryptophan, QxW, motifs typical of ricin B-like lectin (QxW)3 domains [32] (Fig. 2). The three CNL repeats display divergence that is most evident in the less conserved β-repeat that does not contain a genuine QxW motif. The β-repeats are also most divergent in other (QxW)3 domains, including RicB1, Abra1 and LSL (Fig. 2). Besides more or less conserved QxW motifs, CNL contains conserved hydrophobic residues important for the hydrophobic core of the fold (Fig. 2). CNL presumably contains putative carbohydrate-binding sites in the α- and γ-repeats, though the residues involved in sugar binding are hard to predict on the basis of variant carbohydrate-binding site residues determined from the crystallographic analysis of ricin B-type lectin-sugar complex structures [10,11,30,31,34].
Fig. 2.
Sequence alignment of the CNL ricin B-like lectin domain with representative ricin B-type lectin domains of proteins with known structures. RicB1, ricin B chain lectin domain 1, and RicB2, ricin B chain lectin domain 2 (Swiss-Prot P02879); Abra1, abrin-a B chain lectin domain 1, and Abra2, abrin-a B chain lectin domain 2 (P11140); Flav, flavastacin (Q47899); MOA, Marasmius oreades agglutinin (Q8X123); LSL, Laetiporus sulphureus lectin (Q7Z8V1). Identical amino acid residues are shaded dark gray, conserved hydrophobic residues forming a hydrophobic core are shaded black and other similar residues are shaded light gray. The ricin B-type domains consist of three homologous repeats (α, β, and γ; denoted above sequence names), each containing the QxW motif indicated by two asterisks above the sequence (* *). The predicted β-strands of CNL secondary structure are indicated by black arrows above the amino acid sequence.
3.7. Molecular modeling of CNL predicts a β-trefoil fold typical of ricin B-like lectins
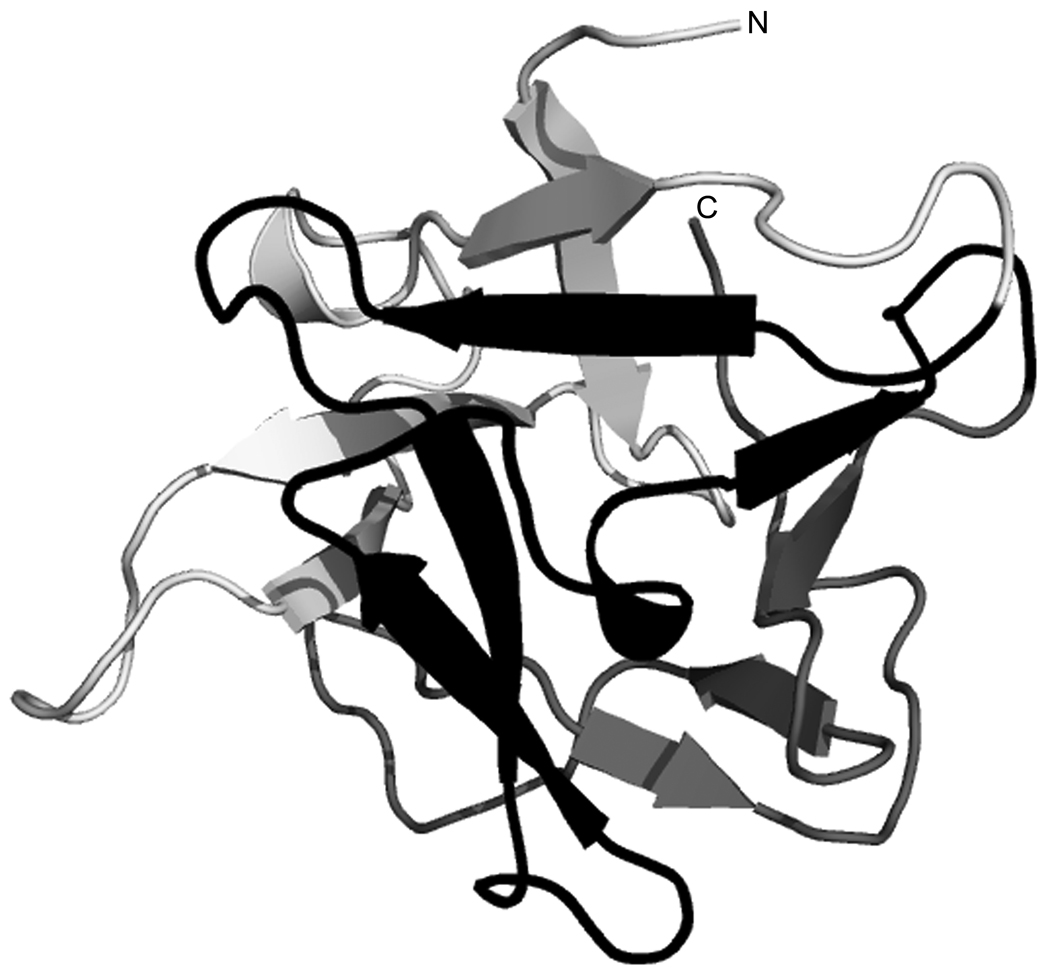
The predicted secondary structure of CNL indicates that the protein contains twelve β-strands (four per repeat), which is consistent with the all-β class of proteins. The number and positions of CNL β-strands (Fig. 2) are consistent with secondary structures of other mushroom ricin B-like lectins [10,11]. To further ascertain a possible structural relationship between CNL and ricin B-type lectins, hydrophobic cluster analysis and three-dimensional structure prediction were performed. Structural similarities were readily recognized on comparing the HCA plots of CNL and ricin B-type lectins (not shown), suggesting that these proteins have similar three-dimensional structures. Finally, fully automated fold recognition was carried out using the LOOPP program. The predicted three-dimensional structure of CNL revealed that it adopts a β-trefoil fold (Fig. 3) typical of ricin B-type lectins [10,11,30,31]. It consists of three distinct repetitive homologous subdomains (α-, β-, and γ-repeats), which form a pseudo-threefold symmetry around the hydrophobic core of the fold.
Fig. 3.
Ribbon diagram of the predicted three-dimensional structure of CNL. The lectin adopts a β-trefoil fold typical of ricin B-type lectins, consisting of three subdomains depicted in the following colors: α-repeat, light gray; β-repeat, black; γ-repeat, dark gray. N and C mark the N-and C-terminal ends of the domain.
3.8. CNL specificity for different types of erythrocytes and for carbohydrates
Hemagglutination assay showed that the lectin agglutinated all types of red blood cells tested. The lowest minimum concentrations of CNL exhibiting agglutination were observed for human groups A, AB and B (8.9, 12.8, and 14.1 µg/ml, respectively), followed by group O (47.6 µg/ml), and bovine (148.1 µg/ml ) erythrocytes.
The preliminary study of carbohydrate specificity by hemagglutination inhibition assay showed that, of all the carbohydrate inhibitors tested, CNL agglutination was inhibited by the glycoprotein asialofetuin and lactose. The minimum inhibitory concentrations were 0.955 mg/ml for the glycoprotein and 1.676 mg/ml (4.65 mM) for lactose. Furthermore, the fine carbohydrate-binding specificity of CNL was studied by glycan microarray analysis which revealed that the lectin recognizes N,N’-diacetyllactosediamine (GalNAcβ1–4GlcNAc) and human blood group A determinant-containing sugar epitopes. These include human blood group A-specific tetrasaccharide A tetra type II (GalNAcα1–3(Fucα1–2)Galβ1–4GlcNAc), followed by A tetra Lac (GalNAcα1–3(Fucα1–2)Galβ1–4Glc), A tetra type I (GalNAcα1–3(Fucα1–2)Galβ1–3GlcNAc), and human blood group A-specific trisaccharide A tri (GalNAcα1–3(Fucα1–2)Galβ). The list of glycans with the highest affinities for CNL is presented in Table 1. The glycan microarray results indicate CNL recognition of terminal non-reducing N-acetylgalactosamine (GalNAcα/β-) and recognition of N-acetylglucosamine (-GlcNAc) or N-acetyllactosamine (-Galβ1–4GlcNAc) at the reducing end.
Table 1. Carbohydrate-binding specificity of CNL determined by glycan microarray analysis.
Glycans with the highest affinities for CNL are listed. Human blood group A-specific tetrasaccharide A tetra type II is shaded black, A tetra Lac dark gray, A tetra type I light gray, trisaccharide A tri printed bold, and N,N’-diacetyllactosediamine is underlined. Microarray slides were incubated with a lectin concentration of 1 µg/ml.
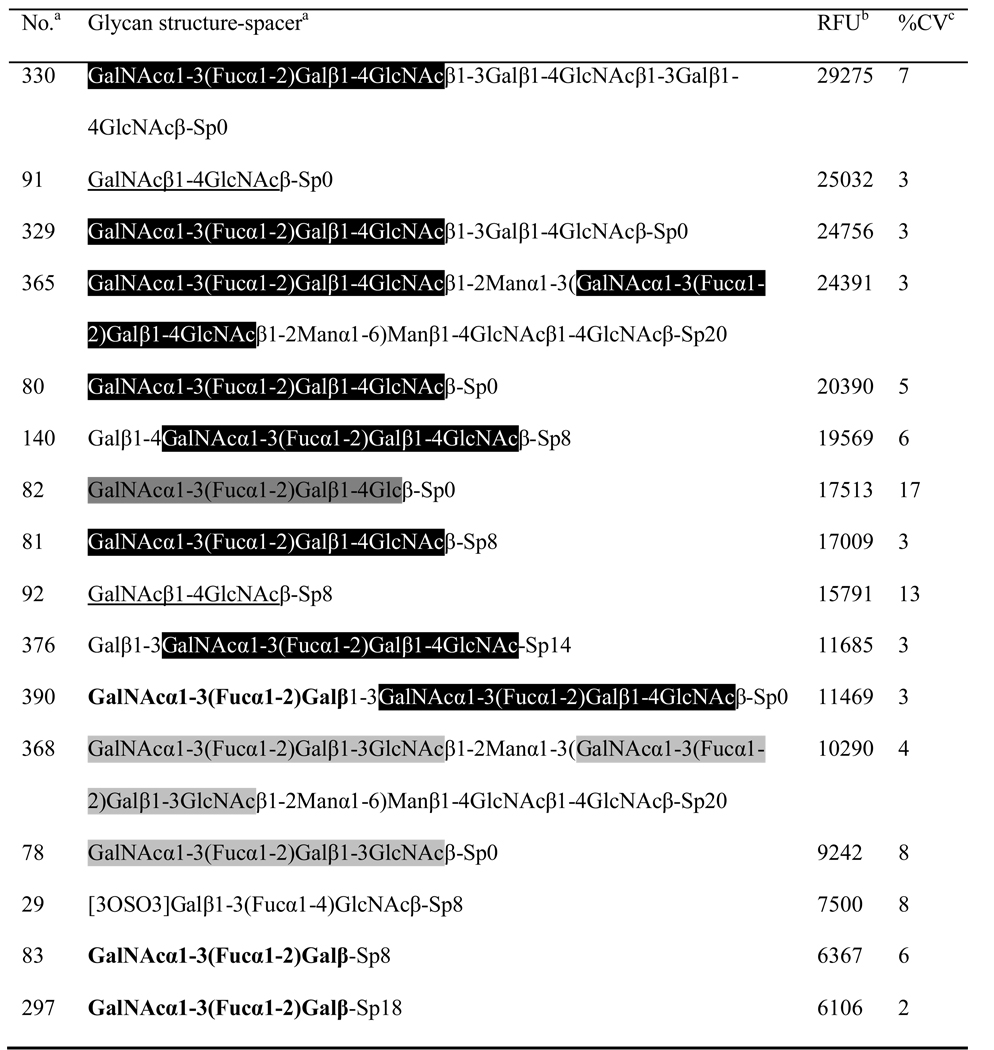 |
Mammalian Printed Array Version 3.2 consisting of 406 glycans in replicates of six was used. A list of glycans and designation of spacer arms are available at http://www.functionalglycomics.org/static/consortium/resources/resourcecoreh8.shtml.
Relative fluorescence units represent the degree of binding of fluorescently labeled streptavidin to biotin-labeled CNL, which indicates the specificity of CNL for individual glycans. The value represents the mean of four replicates (the highest and lowest point from six replicates has been removed).
Percentage of coefficient of variation determined as standard deviation/mean × 100.
3.9. Thermal and pH stability of CNL
CNL exhibited unchanged hemagglutinating activity after 30-min incubation at temperatures up to 50°C, while at higher temperatures it decreased. Under the conditions used 14% of the initial activity was retained even after heating at 100°C. CNL retained more than 70% of its maximum agglutinating activity over the tested pH range (5–9), with the optimum at 6–6.5.
3.10. Antiproliferative effect of CNL on human leukemic T cell lines
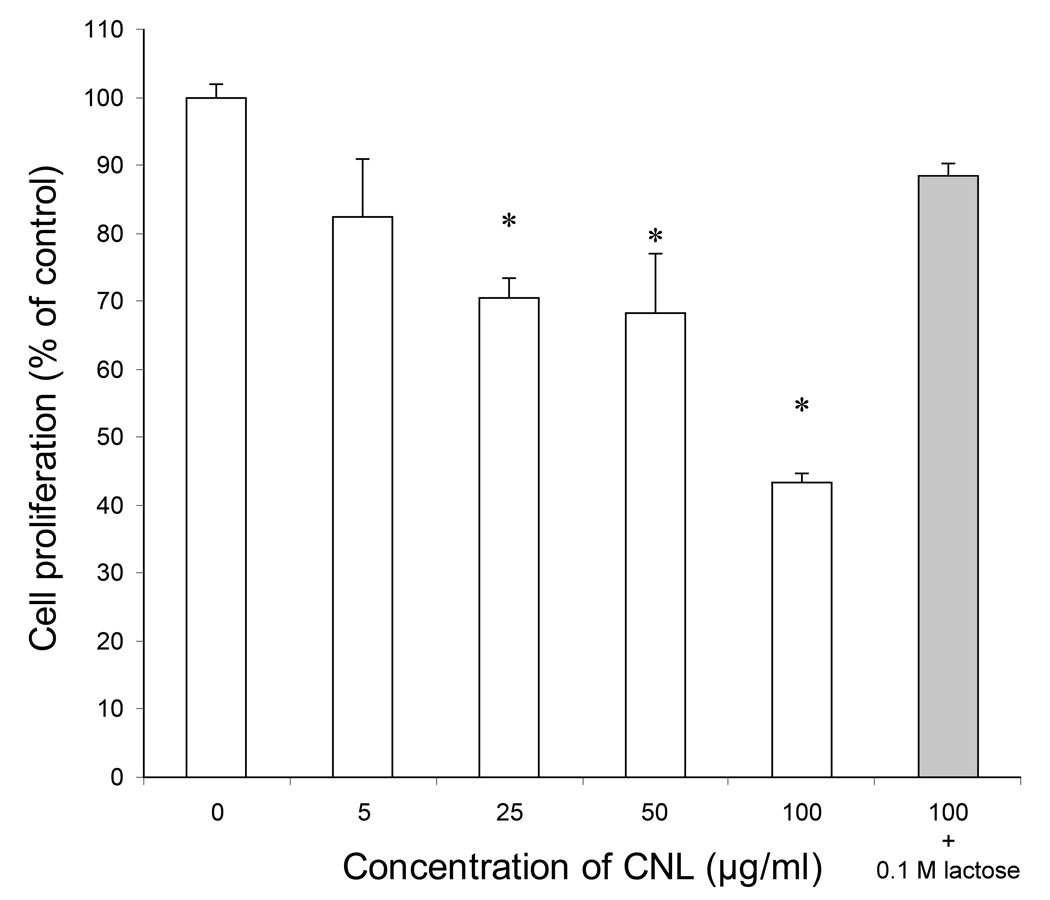
The effect of CNL on proliferation of several human cell lines was assessed by the MTS assay. No effect was observed on pro-monocytic lymphoma and fibrocystic breast epithelial cells. However, a dose-dependent antiproliferative effect of CNL was observed on both leukemic T cell lines, i.e. Mo-T (Fig. 4) and Jurkat. At concentrations of 25 µg/ml and above, the number of viable leukemic cells was significantly reduced (p < 0.05) with a ~60% decrease at the highest concentration (100 µg/ml) of CNL. In a control experiment, in which CNL was preincubated with 0.1 M lactose, the antiproliferative effect was abolished (Fig. 4), the carbohydrate-binding sites of CNL being blocked by the sugar.
Fig. 4.
Antiproliferative effect of CNL on leukemic Mo-T cells. Bars represent means ± SD of percentage of control cell proliferation, each assessed in three independent experiments. The gray bar represents the control experiment, in which CNL was preincubated with 0.1 M lactose. Asterisks above bars indicate statistically significant differences (p < 0.05) between the proliferation of non-treated and treated cells.
4. Discussion
A novel ricin B-like lectin, designated Clitocybe nebularis lectin (CNL), was purified from mushroom C. nebularis. The lectin consists of a single ricin B domain, has a molecular mass of 15.9 kDa and isoelectric point around 4.3. Under non-denaturing conditions, CNL forms a homodimeric structure by noncovalent self-association.
Two-step serial carbohydrate affinity chromatography proved to be the simplest and most rapid method for separating CNL from other lectins and impurities. Besides commonly used carbohydrates (lactose in our case) for lectin elution from the affinity column, NaOH solution was also used and proved to be more efficient. In addition, subsequent removal of the lectin-bound lactose after elution with the sugar was also avoided by NaOH elution.
The carbohydrate-binding specificity of CNL was determined by glycan microarray analysis. It revealed specificity for N,N’-diacetyllactosediamine and human blood group A determinant-containing carbohydrates, which is in agreement with the agglutination of human group A erythrocytes. These results indicate CNL recognition of terminal non-reducing GalNAcα/β- and recognition of -GlcNAc or -Galβ1–4GlcNAc at the reducing end. However, the method of purification using lactose and hemagglutination inhibition assay suggest some recognition of terminal non-reducing β-galactose, which was also shown by the microarray analysis.
The complete cnl gene, consisting of five exons and four introns, and CNL-encoding cDNA were obtained by molecular cloning. A putative TATA box was found in the promoter region at a position that suggests transcriptional activity of the gene. Moreover, the deduced amino acid sequence matched almost exactly the residues determined for purified CNL, indicating that the obtained gene encodes a very similar isoform of the lectin. Heterogeneity of purified CNL was also observed by isoelectric focusing (Fig. S1B, lane 2). The two variant amino acid residues observed in CNL (Fig. S2) are probably not important for the structure or significantly involved in carbohydrate binding, as concluded from the multiple sequence alignment of ricin B-type lectin domains. Heterogeneity is common for mushroom lectins including ricin B-like lectins [13,35,36] and could be the result of gene polymorphism, or the presence of highly conserved multiple gene families encoding the isolectins [37].
The in silico prediction analyses of the deduced amino acid sequence suggest that the primary translation product undergoes post-translational modification by removal of the initiating methionine and acetylation of the following serine. This was consistent with the blocked N-terminus detected by amino acid sequencing of purified CNL and confirmed by comparing the calculated molecular mass of the putative mature lectin and mass spectrometry analysis of purified CNL, revealing masses of 15,903.6 Da and 15,903.5 Da, respectively. The molecular mass analysis, together with the fact that the lectin lacks signal peptide and thus is synthesized on free ribosomes, indicate that CNL is not glycosylated.
Similarity searches for homologous proteins in databases revealed numerous fungal and bacterial proteins containing ricin B-like lectin domains that show low sequence identity to CNL. Ricin B-like lectins constitute a family of carbohydrate-binding proteins with structures known from the lectin domains of plant toxins ricin and abrin [30,31]. Although a high degree of divergence between members of the ricin B-like lectin family is its characteristic feature [32], further investigation of the uncertain relationship between CNL and ricin B-like lectins was conducted. A profile hidden Markov model-based searches, that provide greater sensitivity when searching for evolutionarily distant proteins [29], identified a ricin B lectin domain in CNL. In addition, the lectin was classified as a member of the ricin B-like lectin superfamily, which groups together proteins with structural, functional and sequence evidence for a common evolutionary ancestor [20]. Multiple sequence alignment of CNL with representative ricin B-type lectin domains revealed that, despite low sequence identity, CNL contains the internal homology displayed in the three repeats with QxW motifs and conserved hydrophobic residues structurally important for the hydrophobic core of the fold, all typical of ricin B-type lectins [32]. The secondary structure prediction, hydrophobic clustering analysis and predicted three-dimensional structure together lead to the conclusion that CNL adopts the β-trefoil fold typical of ricin B-type lectins. Besides amino acid sequence analysis, there is biochemical evidence supporting CNL inclusion in the ricin B-like lectin superfamily. The agglutinating and carbohydrate-binding activities of CNL, recognizing asialofetuin and lactose, corresponds to the properties of ricin B chain [38] and mushroom ricin B-like lectins [13,36,39]. However, fine carbohydrate-binding specificities of mushroom ricin B-like lectins show no particular similarities, except for higher or lower affinities for lactose and N-acetyllactosamine-containing carbohydrates.
CNL could be distantly related to a ricin B-like lectin with known structure, designated as Marasmius oreades agglutinin (MOA; Swiss-Prot Q8X123). This agglutinin was isolated from the mushroom M. oreades which is related to C. nebularis, both belonging to the basidiomycete order Agaricales and to the same family of Tricholomataceae. Of the proteins with known folds, MOA displays the highest similarity score with CNL. Further, both proteins are acidic and acetylated at their N-terminus [35]. MOA apparently lacks sequence homology to ricin B-type lectin domains but still adopts the highly conserved β-trefoil fold (PDB code 2iho) [11], similar to that in the predicted three-dimensional structure of CNL (Fig. 3). We therefore conclude that, despite low amino acid sequence identity, there are strong grounds for CNL being structurally related to ricin B-type lectins, sharing similar β-trefoil folds and being classified as a member of the ricin B-like lectin superfamily.
Finally, CNL was shown to possess antiproliferative activity against leukemic T lymphocytes. The effect is comparable to those described for other mushroom lectins [40,41] but, to our knowledge, no antiproliferative activity has been reported for ricin B-like lectins that do not contain a toxic domain. The inhibition of proliferation of leukemic T cells by CNL was abolished by lactose, which binds to and blocks the carbohydrate-binding sites of CNL. Furthermore, other lectins isolated from C. nebularis with sugar specificities different from those of CNL did not inhibit the proliferation of leukemic T cells (results not shown). Therefore, the antiproliferative effect appears to be at least partially elicited by CNL recognizing and binding to cell surface glycoconjugates specific for this particular lectin. The fact that the effect was not exhibited on pro-monocytic lymphoma and fibrocystic breast epithelial cell lines suggests that the antiproliferative effect of CNL is specific to leukemic T lymphocytes which presumably possess appropriate receptors. Similarly, the ricin B chain of the plant toxin ricin has been shown to bind cell surface glycoconjugates and thus facilitate internalization of the protein by endocytosis, which is the first step in mediating cytotoxicity by the toxic domain [42]. Moreover, studies on human galectin-1 have shown that the galectin induces apoptosis of T cells by cross-linking the specific T cell surface glycoprotein receptors [43].
In conclusion, CNL is a mushroom-derived, biologically active carbohydrate-binding protein. It is one of the few mushroom ricin B-like lectins that have been identified and the only one so far shown to possess immunomodulatory properties. It exhibits antiproliferative effects specific to human leukemic T cells. Therefore, the lectin has potential therapeutic applications in treating T cell mediated autoimmune and inflammatory disorders and hematopoietic malignancies, and could be used for targeting leukemic T cells.
Supplementary Material
Fig. S1. SDS-PAGE (A) and isoelectric focusing (B) analysis of CNL. (A) Lane 1, Pharmacia low molecular weight standard proteins (14.4 to 97 kDa); lane 2, lactose-binding proteins isolated by lactosyl-Sepharose affinity chromatography with elution by 0.01 M NaOH solution; lane 3, purified CNL. (B) Lane 1, Pharmacia Broad pI Calibration Kit for a pH range of 3–10; lane 2, RP-HPLC-purified CNL.
Fig. S2. Alignment of the complete cnl gene and CNL-encoding cDNA sequences with deduced amino acid sequence. The genomic sequence is denoted by gDNA and introns are marked with hyphens in the cDNA sequence. The amino acid sequence translated from the open reading frame is given below the nucleotide sequences. ‡, amino acid residues determined by amino acid sequencing; two variant residues are shaded gray and unidentified residues are denoted by X. Nucleotides and amino acids are numbered, starting with the start codon (ATG) printed in boldface. The stop codon (TGA) is indicated by an asterisk. The predicted TATA box is shaded black, the predicted transcription initiation site is shaded gray, and the putative polyadenylation signal in the 3’-UTR is underlined.
Acknowledgements
The authors thank Prof. Dr. Roger Pain for critical review of the manuscript and Adrijana Leonardi for amino acid sequencing analysis. The authors would also like to acknowledge The Consortium for Functional Glycomics and the Protein-Carbohydrate Interaction Core H at Emory University School of Medicine, Atlanta, GA, supported by NIGMS Grant GM62116, for glycan array analysis. The work was supported by the Ministry of Higher Education, Science and Technology of the Republic of Slovenia under Grants No. J4-9425-0106-06 and P4-0127.
List of abbreviations
- CNL
Clitocybe nebularis lectin
- RP-HPLC
reversed-phase high-performance liquid chromatography
- TFA
trifluoroacetic acid
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamidegel electrophoresis
- FPLC
fast protein liquid chromatography
- PCR
polymerase chain reaction
- ESI
electrospray ionization
- 3’ RACE
3’ rapid amplification of cDNA ends
- RicB1
ricin B chain lectin domain 1
- Abra1
abrin-a B chain lectin domain 1
- RicB2
ricin B chain lectin domain 2
- Abra2
abrin-a B chain lectin domain 2
- Flav
flavastacin
- MOA
Marasmius oreades agglutinin
- LSL
Laetiporus sulphureus lectin.
Footnotes
The nucleotide sequence reported in this paper has been submitted to the GenBank™/EMBL Data Bank with accession number EU682006.
References
- 1.Chang R. Functional properties of edible mushrooms. Nutr. Rev. 1996;54:S91–S93. doi: 10.1111/j.1753-4887.1996.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 2.Wasser SP, Sokolov D, Reshetnikov SV, Timor-Tismenetsky M. Dietary supplements from medicinal mushrooms: Diversity of types and variety of regulations. Int. J. Med. Mushr. 2000;2:1–19. [Google Scholar]
- 3.Lindequist U, Niedermeyer THJ, Julich W-D. The pharmacological potential of mushrooms. Evid. Based Complement. Alternat. Med. 2005;2:285–299. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varki A, Cummings R, Esko J, Freeze H, Hart G, Marth J. Essentials of glycobiology. New York: Cold Spring Harbor Laboratory Press; 1999. [PubMed] [Google Scholar]
- 5.Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J. Mol. Med. 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 6.Guillot J, Konska G. Lectins in higher fungi. Biochem. Syst. Ecol. 1997;25:203–230. [Google Scholar]
- 7.Wang H, Ng TB, Ooi VEC. Lectins from mushrooms. Mycol. Res. 1998;102:897–906. [Google Scholar]
- 8.Imberty A, Mitchell EP, Wimmerová M. Structural basis of high-affinity glycan recognition by bacterial and fungal lectins. Curr. Opin. Struct. Biol. 2005;15:525–534. doi: 10.1016/j.sbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Cioci G, Mitchell EP, Chazalet V, et al. β-propeller crystal structure of Psathyrella velutina lectin: An integrin-like fungal protein interacting with monosaccharides and calcium. J. Mol. Biol. 2006;357:1575–1591. doi: 10.1016/j.jmb.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 10.Mancheño JM, Tateno H, Goldstein IJ, Martínez-Ripoll M, Hermoso JA. Structural analysis of the Laetiporus sulphureus hemolytic pore-forming lectin in complex with sugars. J. Biol. Chem. 2005;280:17251–17259. doi: 10.1074/jbc.M413933200. [DOI] [PubMed] [Google Scholar]
- 11.Grahn E, Askarieh G, Holmner Ǻ, et al. Crystal structure of the Marasmius oreades mushroom lectin in complex with a xenotransplantation epitope. J. Mol. Biol. 2007;369:710–721. doi: 10.1016/j.jmb.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Candy L, Peumans WJ, Menu-Bouaouiche L, et al. The Gal/GalNAc-specific lectin from the plant pathogenic basidiomycete Rhizoctonia solani is a member of the ricin-B family. Biochem. Biophys. Res. Commun. 2001;282:655–661. doi: 10.1006/bbrc.2001.4626. [DOI] [PubMed] [Google Scholar]
- 13.Tateno H, Winter HarryC, Goldstein IrwinJ. Cloning, expression in Escherichia coli and characterization of the recombinant Neu5Acα2,6Galβ1,4GlcNAc-specific high-affinity lectin and its mutants from the mushroom Polyporus squamosus. Biochem. J. 2004;382:667–675. doi: 10.1042/BJ20040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horejsí V, Kocourek J. Studies on lectins. XXXVI. Properties of some lectins prepared by affinity chromatography on O-glycosyl polyacrylamide gels. Biochim. Biophys. Acta. 1978;538:299–315. doi: 10.1016/0304-4165(78)90358-6. [DOI] [PubMed] [Google Scholar]
- 15.Brzin J, Rogelj B, Popovič T, Štrukelj B, Ritonja A. Clitocypin, a new type of cysteine proteinase inhibitor from fruit bodies of mushroom Clitocybe nebularis. J. Biol. Chem. 2000;275:20104–20109. doi: 10.1074/jbc.M001392200. [DOI] [PubMed] [Google Scholar]
- 16.Sabotič J, Gaser D, Rogelj B, Gruden K, Štrukelj B, Brzin J. Heterogeneity in the cysteine protease inhibitor clitocypin gene family. Biol. Chem. 2006;387:1559–1566. doi: 10.1515/BC.2006.194. [DOI] [PubMed] [Google Scholar]
- 17.Levi G, Teichberg VI. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J. Biol. Chem. 1981;256:5735–5740. [PubMed] [Google Scholar]
- 18.Möller EM, Bahnweg G, Sandermann H, Geiger HH. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992;20:6115–6116. doi: 10.1093/nar/20.22.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altschul SF, Madden TL, Schäffer AA, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gough J, Chothia C. SUPERFAMILY: HMMs representing all proteins of known structure. SCOP sequence searches, alignments and genome assignments. Nucleic Acids Res. 2002;30:268–272. doi: 10.1093/nar/30.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blixt O, Head S, Mondala T, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obermajer N, Premzl A, Zavašnik-Bergant T, Turk B, Kos J. Carboxypeptidase cathepsin X mediates β2-integrin-dependent adhesion of differentiated U-937 cells. Exp. Cell Res. 2006;312:2515–2527. doi: 10.1016/j.yexcr.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Gheorghe MT, Jörnvall H, Bergman T. Optimized alcoholytic deacetylation of N-acetyl-blocked polypeptides for subsequent Edman degradation. Anal. Biochem. 1997;254:119–125. doi: 10.1006/abio.1997.2380. [DOI] [PubMed] [Google Scholar]
- 24.Gurr SJ, Unkles SE, Kinghorn JR. The structure and organization of nuclear genes of filamentous fungi. Oxford: IRL Press; 1987. [Google Scholar]
- 25.Joseph GT, Huima T, Klion A, Lustigman S. A novel developmentally regulated galectin of Onchocerca volvulus. Mol. Biochem. Parasitol. 2000;106:187–195. doi: 10.1016/s0166-6851(99)00208-x. [DOI] [PubMed] [Google Scholar]
- 26.Gold MH, Alic M. Molecular biology of the lignin-degrading basidiomycete Phanerochaete chrysosporium. Microbiol. Rev. 1993;57:605–622. doi: 10.1128/mr.57.3.605-622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Futai E, Sorimachi H, Jeong S-Y, Kitamoto K, Ishiura S, Suzuki K. Aspergillus oryzae palBory encodes a calpain-like protease: Homology to Emericella nidulans PalB and conservation of functional regions. J. Biosci. Bioeng. 1999;88:438–440. doi: 10.1016/s1389-1723(99)80223-0. [DOI] [PubMed] [Google Scholar]
- 28.Pirtle IL, Kongcharoensuntorn W, Nampaisansuk M, Knesek JE, Chapman KD, Pirtle RM. Molecular cloning and functional expression of the gene for a cotton Δ-12 fatty acid desaturase (FAD2) Biochim. Biophys. Acta. 2001;1522:122–129. doi: 10.1016/s0167-4781(01)00312-8. [DOI] [PubMed] [Google Scholar]
- 29.Eddy SR. Profile hidden Markov models. Bioinformatics. 1998;14:755–763. doi: 10.1093/bioinformatics/14.9.755. [DOI] [PubMed] [Google Scholar]
- 30.Montfort W, Villafranca JE, Monzingo AF, et al. The three-dimensional structure of ricin at 2.8 Ǻ. J. Biol. Chem. 1987;262:5398–5403. [PubMed] [Google Scholar]
- 31.Tahirov TH, Lu T-H, Liaw Y-C, Chen Y-L, Lin J-Y. Crystal structure of abrin-a at 2.14 Ǻ. J. Mol. Biol. 1995;250:354–367. doi: 10.1006/jmbi.1995.0382. [DOI] [PubMed] [Google Scholar]
- 32.Hazes B. The (QxW)(3) domain: A flexible lectin scaffold. Protein Sci. 1996;5:1490–1501. doi: 10.1002/pro.5560050805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutenber E, Ready M, Robertus JD. Structure and evolution of ricin B chain. Nature. 1987;326:624–626. doi: 10.1038/326624a0. [DOI] [PubMed] [Google Scholar]
- 34.Tateno H, Goldstein IJ. Partial identification of carbohydrate-binding sites of a Galα1,3Galβ1,4GlcNAc-specific lectin from the mushroom Marasmius oreades by site-directed mutagenesis. Arch. Biochem. Biophys. 2004;427:101–109. doi: 10.1016/j.abb.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Kruger RP, Winter HC, Simonson-Leff N, Stuckey JA, Goldstein IJ, Dixon JE. Cloning, expression, and characterization of the Galα1,3Gal high affinity lectin from the mushroom Marasmius oreades. J. Biol. Chem. 2002;277:15002–15005. doi: 10.1074/jbc.M200165200. [DOI] [PubMed] [Google Scholar]
- 36.Tateno H, Goldstein IJ. Molecular cloning, expression, and characterization of novel hemolytic lectins from the mushroom Laetiporus sulphureus, which show homology to bacterial toxins. J. Biol. Chem. 2003;278:40455–40463. doi: 10.1074/jbc.M306836200. [DOI] [PubMed] [Google Scholar]
- 37.Van Damme EJM, Peumans WJ, Pusztai A, Bardocz S. Handbook of plant lectins: Properties and biomedical applications. New York: John Wiley and Sons; 1998. [Google Scholar]
- 38.Wu JH, Singh T, Herp A, Wu AM. Carbohydrate recognition factors of the lectin domains present in the Ricinus communis toxic protein (ricin) Biochimie. 2006;88:201–217. doi: 10.1016/j.biochi.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Winter HC, Mostafapour K, Goldstein IJ. The mushroom Marasmius oreades lectin is a blood group type B agglutinin that recognizes the Galα1,3Gal and Galα1,3Galβ1,4GlcNAc porcine xenotransplantation epitopes with high affinity. J. Biol. Chem. 2002;277:14996–15001. doi: 10.1074/jbc.M200161200. [DOI] [PubMed] [Google Scholar]
- 40.Yu L, Fernig DG, Smith JA, Milton JD, Rhodes JM. Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin. Cancer Res. 1993;53:4627–4632. [PubMed] [Google Scholar]
- 41.Zhao C, Sun H, Tong X, Qi Y. An antitumour lectin from the edible mushroom Agrocybe aegerita. Biochem. J. 2003;374:321–327. doi: 10.1042/BJ20030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandvig K, van Deurs B. Endocytosis and intracellular transport of ricin: recent discoveries. FEBS Lett. 1999;452:67–70. doi: 10.1016/s0014-5793(99)00529-3. [DOI] [PubMed] [Google Scholar]
- 43.Pace KE, Lee C, Stewart PL, Baum LG. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J. Immunol. 1999;163:3801–3811. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. SDS-PAGE (A) and isoelectric focusing (B) analysis of CNL. (A) Lane 1, Pharmacia low molecular weight standard proteins (14.4 to 97 kDa); lane 2, lactose-binding proteins isolated by lactosyl-Sepharose affinity chromatography with elution by 0.01 M NaOH solution; lane 3, purified CNL. (B) Lane 1, Pharmacia Broad pI Calibration Kit for a pH range of 3–10; lane 2, RP-HPLC-purified CNL.
Fig. S2. Alignment of the complete cnl gene and CNL-encoding cDNA sequences with deduced amino acid sequence. The genomic sequence is denoted by gDNA and introns are marked with hyphens in the cDNA sequence. The amino acid sequence translated from the open reading frame is given below the nucleotide sequences. ‡, amino acid residues determined by amino acid sequencing; two variant residues are shaded gray and unidentified residues are denoted by X. Nucleotides and amino acids are numbered, starting with the start codon (ATG) printed in boldface. The stop codon (TGA) is indicated by an asterisk. The predicted TATA box is shaded black, the predicted transcription initiation site is shaded gray, and the putative polyadenylation signal in the 3’-UTR is underlined.