Introduction
Periodontal disease (PD) has been associated with human immunodeficiency virus type one (HIV-1) over the past several decades (1, 2). Initial descriptions of oral health in HIV+ patients focused on extreme forms of PD (3, 4), followed later by more conventional definitions (1, 5, 6), making the comparison of study results difficult. Without appropriate and consistent characterization of PD in at-risk populations, such as those with HIV-1 infection, the level and severity of traditionally-defined PD may be underestimated, which could lead to inadequate prevention and treatment as well as a diminished sense of concern regarding periodontal health.
Persons with HIV-1 infection are especially vulnerable to comorbidities; their immune systems are compromised, and in the era of highly active antiretroviral therapy (HAART), some metabolic factors (i.e. lipids, glucose and insulin levels) are altered (7). Utilization of dental care services may be restricted due to real or perceived stigma, confidentiality concerns, or economic and social factors even if access to a dentist is available. Substance abuse, psycho-social stressors, depression and denial may result in the neglect of health promoting behaviors and lead to lapses in medication adherence, which can further limit immune reconstitution and the efficacy of remaining HIV treatment options. In sum, persons with HIV have an increased risk for adverse oral and general health outcomes.
There have been inconsistent reports of the levels of PD in HIV+ persons (1, 2, 5, 6, 8–16), including both high (5, 16, 17), and low (8, 9, 13) levels of PD. There are a number of possible reasons for these inconsistencies. Different definitions were used to characterize PD (2) and methodologies used to collect clinical periodontal measurements also varied (e.g. partial mouth vs. full mouth probing and the number of sites probed per tooth). Many studies reporting low levels of periodontal disease have used partial mouth periodontal data collection methodologies (6, 8, 9, 13), but partial mouth analyses can underestimate the prevalence of PD (18). Further, the prevalence of periodontal disease may vary by patient characteristics in the cohort (1, 2). Such patient characteristics may include immune factors (importantly, the distribution of the cohort’s CD4+ T-cell counts) (5, 6, 16), metabolic factors (19), utilization of dental care services (20) and oral health behaviors (21). These patient characteristics are not always well described in the literature, nor are all of these characteristics routinely included in reports of PD in HIV+ cohorts.
To provide a more comprehensive view of PD in the HAART era, we clearly characterized our HIV+ cohort according to select immune and metabolic markers, the presence of subgingival bacterial pathogens, dental care utilization and oral health behaviors. We examined the level of PD in our cohort using two consensus definitions of PD (18, 22), as well as by its two distinct clinical measures (periodontal probing depth and recession) and one summary measure (clinical attachment level), as has been suggested by authors investigating the perio-systemic connection (23).
Methods
Subject Selection
Adult subjects were recruited from three outpatient HIV medical clinics in Cleveland, Ohio: University Hospitals of Cleveland (UH), The Cleveland Clinic Foundation (CCF) and MetroHealth Medical Center (Metro). The study was approved for patient recruitment by participating institutions. Most participants were self-referred. All subjects signed a UH IRB-approved informed consent document and an authorization form to use and disclose Protected Health Information (PHI) for research purposes. Exclusion criteria included evidence of cardiovascular disease, a history of Type I or II diabetes mellitus, fewer than 20 teeth, uncontrolled systemic illnesses, diagnosis or treatment of cancer in the past five years, pregnancy, and need for antibiotic prophylaxis prior to dental care as per the current American Dental Association (ADA) and other guidelines (24, 25). Inclusion criteria were medication-compliant adult (age 18 and over) subjects on HAART or about to start HAART within 2 months of their baseline visit. We accessed pre-study clinical data from the primary HIV-clinic’s longitudinal electronic database or from retrospective chart reviews. Important variables from these sources included: CD4+ T-cell count and plasma HIV RNA levels (Roche Amplicor) nearest to and before the baseline visit, complete antiretroviral medication history, nadir CD4 T-cell count and risk factors for HIV-1 infection. Study subjects were seen from May, 2005 through January, 2008.
Periodontal Disease Measurements
The periodontal probing depth (PPD)—the height of the free gingival margin to the most apical location of the periodontal pocket—was determined at six sites per tooth (mesio-facial, mid facial, disto-facial, disto-lingual, mid lingual, and mesio-lingual) using a University of North Carolina (UNC) #15 probe. The distance between the height of the free gingival margin to the cemento-enamel junction (CEJ) was termed recession (REC), and was also measured at the same six sites per tooth, with values apical to the CEJ being positive and values coronal to the CEJ being negative. PPD plus REC yielded the clinical attachment level (CAL), where a positive number indicated loss of attachment. PPD and REC values were rounded up to the next whole millimeter value. A viable tooth was defined as having at least one-half of a remaining clinical crown (i.e. at least three contiguous sites in which PPD and REC were measurable). Full-mouth periodontal probing was performed by one dentist (LV), whose measurements were previously calibrated to a trained and experienced periodontist. The percent agreement from ongoing intra-rater reliability for periodontal probing, +/− 1 mm, was 98% with an intra-class correlation coefficient of 0.88. The periodontal data collection methodology conformed to expert guidelines (26) Supragingival plaque, debris, blood or saliva was removed prior to performing precise full-mouth periodontal measurements, which typically required over 60 minutes in order to ensure accurate measurements.
Definitions of Periodontal Disease
We used three different definitions of PD to characterize our cohort. The first definition of PD was proposed by a working group with representatives from both the Centers for Disease Control and Prevention (CDC) and the American Academy of Periodontology (AAP). They defined severe periodontitis as ≥2 interproximal sites with CAL ≥6 mm (not on same tooth) and ≥1 interproximal site with PPD ≥5 mm; moderate periodontitis as ≥2 interproximal sites with CAL ≥4 mm (not on same tooth) or ≥2 interproximal sites with PPD ≥5 mm (not on same tooth); and no or mild periodontitis as neither “moderate” nor “severe” periodontitis (18). The second definition of PD was proposed by Tonetti and Claffey for the Group C Consensus Report of the 5th European Workshop in Periodontology (EWP), 2005. This two-level definition included the presence of proximal attachment loss of ≥3 mm at ≥2 non-adjacent teeth and the presence of proximal attachment loss of ≥5 mm at ≥30% of teeth present (22); we adapted this definition into three categories (see legend on Figure two). To minimize possible misclassification due to categorizing data, we defined and analyzed the PD data as a continuous variable. Thus, we evaluated PD by its component parts, PPD, REC and the summary measure, CAL, as previously described (23, 27). These components may represent different processes contributing to periodontal disease; while PPD more closely reflects acute PD, REC represents the cumulative results of long-term destruction of periodontal tissue and bone (23, 28). Each component of periodontal disease may be viewed as a potential independent contributor to longitudinal systemic outcomes (23, 27) and may help illuminate how HIV-1 infection and/or systemic factors influence or interact with the progression of PD. Guided by expert reports (18, 22), we selected the following three separate thresholds to define periodontal disease: PPD ≥5 mm, REC >0 mm and CAL ≥4mm. For each component we calculated the percent of teeth with at least one site per tooth that met the threshold and analyzed these outcome measures as continuous variables.
Demographic/Behavioral Data
One researcher/dentist (LV) administered questionnaires to obtain demographic, medical and oral behavioral data were completed on each study subject. Patients reported the number of times per week that they brushed and flossed. Smoking was recorded as ever having smoked more than 100 cigarettes, a calculation of pack per day years (ppdyrs) of cigarette smoking, and the total number of years of smoking. Drug use was coded as yes or no, and included past or present use of illegal substances, including marijuana (more than experimental use), cocaine (any type) and other “street drugs”.
Definition of HAART
HAART was defined as a treatment regimen that included at least three different antiretroviral drugs from at least two different classes, i.e., nucleoside/nucleotide analogues (NUC), protease inhibitors (PI), non-nucleotide reverse transcriptase inhibitors (NNRTI) and entry or fusion inhibitors (EI). Most subjects were taking a backbone of two NUCs with either a PI or an NNRTI.
Metabolic Measurements
All metabolic measurements, including a lipid panel (i.e. HDL-cholesterol, LDL cholesterol, VLDL cholesterol and triglycerides), insulin, glucose and high sensitivity C-reactive protein (hs-CRP), were determined using standard laboratory serum assays after at least 12 hours of fasting. For complete information regarding these assays, see Appendix A. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting insulin (µIU/ml) × glucose (mg/dL)/405 (29). Patients were considered to be insulin resistant when HOMA-IR was ≥2.6 (30).
Collection of Dental Plaque for Periodontal Pathogens
Using separate sterile 13/14 Gracey curettes for each tooth, subgingival dental plaque was collected from the most apical portion of the accessible probing depth and alongside the root of the tooth at two different interproximal sites (usually the distolingual and distobuccal) of the first molar in each quadrant, and transferred into a microcentrifuge tube containing 0.5 ml Tri-reagent (Molecular Research Center Inc., Cincinnati, Ohio). For each patient, eight sites were harvested from molar teeth and pooled into one plaque sample. If the first molar tooth was missing, then the next most distal tooth was accessed. If all molar teeth were missing, then the most distal tooth in that quadrant was sampled, as previously described (31). After temporary storage on ice, microcentrifuge tubes were transferred to a −70°C freezer for storage until accessed for analysis.
Isolation and quantification of DNA
DNA was extracted from each plaque sample according to the manufacturer’s instructions as previously described by Toossi et al., 2005 (32). Bacterial DNA was assessed by real-time PCR (Taqman 7700, Applied Biosystems, Foster City, CA). Oral bacteria examined included P. gingivalis (Pg), T. forsythia (Tf), and T. denticola (Td). In addition, DNA encoding total bacterial 23s ribosomal RNA was assessed in each sample. Taqman primer and probes for Pg, Td, and bacterial 23s ribosomal RNA were designed using Primer Express software (Applied Biosystems) Primers and probe for Tf were as previously reported by Morillo et al., 2004 (33). For details on probes and primers, see Appendix B. Bacterial 23 S ribosomal DNA products were detected by Sybr green (Applied Biosystems). Quantities of each bacterial DNA were determined by using a dilution series of oligonucleotide sequences (Invitrogen, Carlsbad, CA) synthesized for the target DNA in each assay. The concentration and purity of plaque DNA was determined by spectrophotometery (BECKMAN DU640). DNA copies for each pathogen were calculated by correction to the total DNA in each plaque sample and expressed as copies of bacterial DNA per microgram of DNA.
Data Analysis
Data analyses were conducted using SPSS v. 13 (SPSS Corporation, Chicago, Illinois, USA). Bivariate relationships between continuous variables were examined by Pearson and Spearman correlations, as appropriate. Logarithmic transformation was used to normalize the distribution of pathogen DNAs. For dichotomous independent variables, differences in periodontal measures between the groups were tested using t-tests. HOMA-IR was dichotomized into values < and ≥ 2.6. CD4+ T-cell counts were dichotomized into <200 cells/mm3 and ≥ 200 cells/mm3; smoking and flossing were categorized as ever (yes) or never (no). The number of dental visits in the past 5 years was averaged and dichotomized to two categories, those with at least one dental visit per year versus those with less than one visit per year. We set a significance level of p<.05 for all statistical tests. Backward stepwise multiple regression (p=.10 to enter and p=.05 to leave) was performed to identify factors associated with the periodontal outcome measure, clinical attachment loss (CAL). Predictor variables significant at p<.05 in the bivariate analysis with CAL that were entered into the model included flossing, annual dental visits, levels of Pg. DNA, total plaque DNA, smoking status, CD4+ T-cell counts, LDL-cholesterol, BMI, HOMA-IR and insulin. Although not significant in the backward strategy, sex, race, primary insurance and viral load were retained in the final model for adjustment.
Results
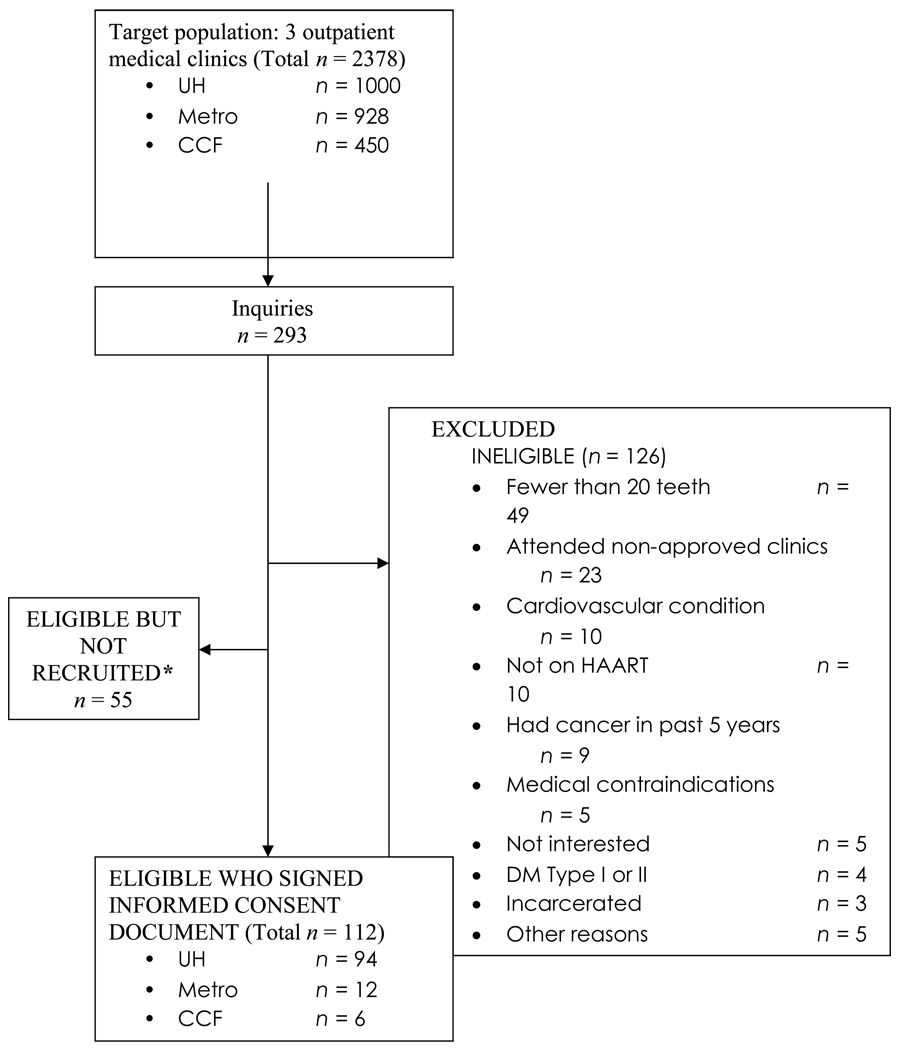
A total of 112 subjects completed all baseline measures and were included in the cross-sectional analysis (Table 1). Patients in this cohort were predominantly male (74%), African-American (64%), had more than a high school education (59%), received Medicaid (56%), and had a median age of 42 years (range 21–57). Among the 78 subjects (69%) who self-reported cigarette smoking, the mean ppdyrs was 11.0 (+/− 10.6), with a median of 8.8 ppdyrs. Drug use history was reported by 61% of study participants. Oral hygiene behaviors were poor and less than half averaged an annual dental visit over the past 5 years. Patients had an average of 26 teeth (range, 20–32). Of the 123 ineligible volunteers, 49 people (40%) were excluded because they had fewer than 20 teeth (Figure 1).
Table 1.
Cohort characteristics including demographics and oral health behaviors of 112 HIV+ adults.
| Variable | N (%) |
|---|---|
| Demographics | 112 (100) |
| Age (median, range in yrs) | 42 (21–57) |
| Gender | |
| Male | 83 (74) |
| Female | 29 (26) |
| Race | |
| Black | 72 (64) |
| White | 33 (30) |
| Other | 7 (6) |
| Education | |
| <HS Graduate/GED | 19 (17) |
| HS Graduate/GED | 27 (24) |
| >HS Graduate/GED | 65 (59) |
| Primary Insurance | |
| Medicaid: | 62 (56) |
| Ryan White: | 25 (22) |
| Private: | 25 (22) |
| Oral Health Behaviors | |
| Brushing | |
| Twice daily | 64 (57) |
| Less than twice daily | 48(43) |
| Flossing | |
| Yes | 44 (39) |
| No | 68 (61) |
| Dental visits in past 5 yrs | |
| < 5 visits | 58 (52) |
| ≥5 visits | 54 (48) |
| Smoking status | |
| Ever | 77 (69) |
| Never | 35 (31) |
| Pack Per DayYears (median, range) | 3 (0–56) |
Demographics: High School (HS), General Educational Development (GED).
Figure 1.
Subject recruitment flow diagram.
* Reasons included: Could not be reached by phone, subject lost interest, scheduling conflicts.
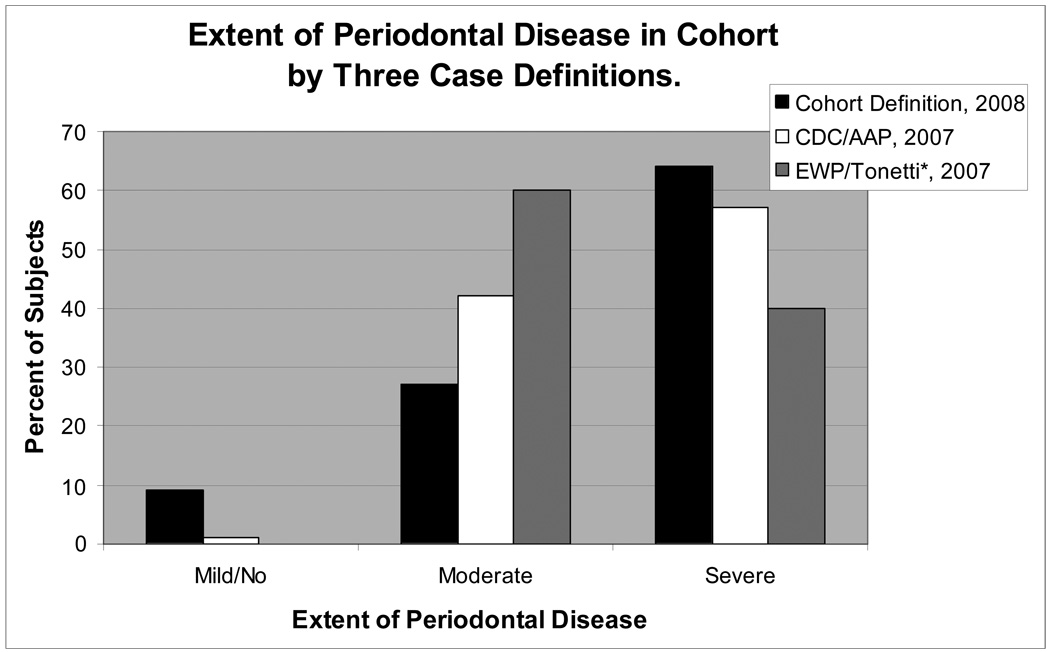
Figure 2 compares the distribution of our cohort by different case definitions of periodontal disease (18, 22), demonstrating that over 90% of persons in this cohort had traditionally-defined periodontal disease that was moderate or severe. Note in Figure 2 that the CDC/AAP 2007 definition classifies more subjects as having severe periodontal disease, whereas the EWP/Tonetti 2007 definition classifies a greater proportion of subjects as having moderate periodontal disease. We modeled the three periodontal disease measures as continuous variables. By our definitions of periodontal disease, subjects had an average 38% (±24%) of their teeth with at least one site of PD ≥5mm, 55% (±31%) of their teeth with at least one site of REC >0mm, and 50% (±32%) of their teeth with at least one site of CAL ≥4mm.
Figure 2.
Case Definitions of PD: Cohort Definition: the percent of teeth with at least one site meeting the cut points of severe, ≥30% of teeth with CAL ≥4 mm; moderate, 11–29% of teeth with CAL ≥ 4 mm; mild/no, <10% of teeth with CAL ≥4 mm. CDC/AAP Definition (18): severe, ≥2 interproximal sites with CAL ≥6 mm (not on same tooth) and ≥1 interproximal site with PPD ≥5 mm; moderate, ≥2 interproximal sites with CAL ≥4 mm (not on same tooth) or ≥2 interproximal sites with PPD ≥5 mm (not on same tooth); and mild/no PD, neither “severe” nor “moderate”. *(Adapted) EWP/Tonetti Definition (22): severe, the presence of proximal attachment loss of ≥3 mm at ≥2 non-adjacent teeth and the presence of proximal attachment loss of ≥5 mm at ≥30% of teeth present; moderate, the presence of proximal attachment loss of ≥ 3 mm at ≥2 non-adjacent teeth; and mild/no PD, neither “severe” nor “moderate” PD. Note: No subjects met the adapted EWP/Tonetti mild/no definition of PD.
Study participants had a wide range of immune competence (see Table 2). Participants had a median CD4 + T-cell count of 410 cells/mm3; 19 patients (17%) had CD4 + T-cells counts < 200 cells/mm3. A total of 53% of subjects had previously experienced a nadir CD4+ t-cell count <200 cells/mm3. Viremia was generally well controlled. Forty-eight subjects (43%) were classified as having an undetectable viral load, and the median value was 86 copies/mL. The median time since first HIV seropositive test result was 7.2 years; the most recent diagnosis was 2 months before the baseline visit, while the most remote diagnosis was 24 years before the baseline visit. At the baseline visit, 90% of subjects were on HAART and the median time on HAART was 14 months, with a range from 0 to 137 months.
Table 2.
Characterization of periodontal, microbiological and immunological measures in 112 HIV+ adults.
| Variable | Summary Measure |
|---|---|
| Periodontal Measures (mm) | Mean (SD) |
| Total teeth | 26.2 (3.0) |
| Mean Periodontal Probing Depth (PPD) | 2.9 (0.6) |
| Mean Max PPD* | 4.2 (0.8) |
| Mean Clinical Attachment Level (CAL) | 2.5 (1.3) |
| Mean Max CAL* | 4.0 (1.7) |
| Mean % of teeth with ≥ 1 site with: | |
| PPD ≥5 mm | 38 ( 24) |
| CAL ≥4 mm | 50 ( 32) |
| Recession (REC) > 0 mm | 55 ( 31) |
| Microbiological Measures (log genome copy number/ug DNA) | |
| Porphymonas gingivalis (Pg) | 4.1 (2.1) |
| Treponema denticola (Td) | 4.7 (1.7) |
| Tannerella forsythia (Tf) | 5.3 (1.4) |
| Total Plaque DNA (µg) | 5.5 (3.37) |
| Clinical Measures | Median (Range) |
| CD4+ T-cell count (cells/mm3)** | 410 (5–1,186) |
| Viral Load (copies/mL)** | 63 ( 50–750,000) |
| Nadir CD4+ T-cell count (cells/mm3)** | 172 (0–615) |
| Months since first seropositive | 86 (2–290) |
| Months on HAART | 14 (0–137) |
| Not on HAART (number of subjects, %) | 10 (9.7%) |
| On HAART <2 months (number of subjects, %) | 12 (11.7%) |
Denotes the mean of the maximum value at each tooth.
Data closest to and before the baseline visit.
We examined the association of PPD, REC and CAL with gender, flossing, dental visits and smoking status (data not shown). Males had greater levels of REC (p<.05), but no other differences in periodontal measures by gender were evident. Patients who reported flossing and annual dental visits over the past 5 years had significantly lower levels of PPD and CAL (p<.01). Periodontal measures were worse for smokers compared to non-smokers but this association did not reach statistical significance in the bivariate analysis. No significant differences in the periodontal measures were observed by categories of race, primary insurance source, educational attainment or duration of time on HAART.
Metabolic measures (Table 3) were mostly within the normal range, except for HDL cholesterol in which 56% of subjects had values below the normal range (<40 mg/dL). Using the standard BMI thresholds, 26% of subjects were overweight and 27% were obese. Triglyceride levels were elevated (>130 mg/dL) in 28% of subjects and hs-CRP was elevated (≥3.0 mg/L) in 35% of subjects.
Table 3.
Metabolic characteristics of 112 HIV+ subjects
| Metabolic Measures | Median (Range) | Normal Range | N (%) in Normal Range |
|---|---|---|---|
| Lipids | |||
| Cholesterol (mg/dL) | 172 (93–261) | (80–200) | 89 (80.9) |
| HDL Cholesterol (mg/dL) | 37 (18–97) | >40 | 48 (43.6) |
| LDL (mg/dL) | 106 (27–181) | <130 | 83 (76.9) |
| VLDL (mg/dL) | 22 (7–74) | 0–40 | 95 (88.0) |
| Triglycerides (mg/dL) | 111 (34–652) | <150 | 79 (71.8) |
| BMI (kg/m2) | 26 (16–64) | ||
| Underweight | <18.5 | 2 (1.8) | |
| Normal | 18.5–24.9 | 49 (44.5) | |
| Overweight | 25–29.9 | 29 (26.4) | |
| Obese | >30 | 30 (27.3) | |
| Hs-CRP (mg/L) | 1.8 (0.1–64.4) | <3.0 | 71 (65.1) |
| Insulin (µIU/ml) | 4 (0–42) | ||
| Glucose (mg/dL) | 91 (73–123) | 70–110 | 105 (94.6) |
| HOMA-IR | 0.88 (0–9.32) | ≤2.6 | 99 (89.2) |
Metabolic Measures: Body mass index (BMI), high-sensitivity C-reactive protein (hs-CRP), triglycerides (TRIG), high density lipoproteins (HDL), low density lipoproteins (LDL), very low density lipoproteins (VLDL), homeostasis model assessment-insulin resistance (HOMA-IR). Note: Normal range values for insulin are most appropriately interpreted within the context of HOMA-IR.
The independent variables were not uniformly associated with the outcome variables, PPD, REC and CAL (Table 4). While all microbiological variables were associated with PPD, only the level of Pg DNA was associated with PPD, REC and CAL. Most immune and metabolic variables (i.e. CD4+ T cell count, insulin, LDL-cholesterol and HOMAIR) that were associated with REC were also associated with CAL. CD4+ T-cell count and duration of time on HAART were not correlated with PPD in this cohort. Only one variable, BMI, was correlated with CAL (Spearman’s rho= −.238, p<.05), but not with PPD or REC. Glucose levels and hs-CRP levels were not associated with any periodontal measures in this cross-sectional analysis. An unexpected finding was the correlation of Td levels in a positive direction with PPD (Spearman’s rho= .372, p<.001) and in a negative direction with REC (Spearman’s rho= −.229), resulting in no association with CAL.
Table 4.
Spearman’s Correlation Coefficient (rho) for percent of teeth exceeding the threshold of periodontal probing depth, clinical attachment level and recession with independent predictors.
| Variable | Periodontal Probing Depth (PPD) |
Recession (REC) |
Clinical Attachment Level (CAL) |
|---|---|---|---|
| Age | −.040 | .398** | .211* |
| Microbiological Measures | |||
| Tf | .420** | −.023 | .179 |
| Td | .372** | −.229* | −.005 |
| Pg | .330** | .265** | .374** |
| Total Plaque DNA | .237* | .184 | .197* |
| Immunological Measures | |||
| CD4 (cells/mm3) | −.168 | −.306** | −.424** |
| Viral Load (copies/mL) | −.003 | .036 | .143 |
| Months on HAART | .001 | .202* | .074 |
| Months sero positive | −.045 | .130 | .071 |
| Metabolic Measures | |||
| BMI | −.093 | −.172 | −.238* |
| Insulin | −.120 | −.250** | −.283** |
| Glucose | −.006 | −.087 | −.141 |
| hs-CRP | .013 | .112 | .061 |
| TRIG | −.196* | .028 | −.156 |
| HDL | .086 | −.115 | .009 |
| LDL | −.012 | −.221* | −.280** |
| VLDL | −.203* | .035 | −.141 |
| HOMA-IR | −.151 | −.203* | −.246** |
Significance: p<.05
p<.001
From the backward stepwise multiple regression analysis, eight variables were identified as significant predictors of CAL (CD4+ T-cell count, annual dental visits, smoking, levels of Pg, total plaque DNA, LDL-cholesterol, HOMA-IR and age), while adjusting for gender, race, insurance and viral load. This full model explains slightly more than half of the variability in CAL (F=9.559, adjusted R2=.509, p<.001). Coefficients for the independent variables from the regression model are shown in Table 5. In the second column, B-coefficients (B) show the effect on CAL (the percent of teeth) for one unit of change in the independent factor or factor level, when all other variables are held constant. For example, holding all other variables constant, patients with CD4+ T-cell counts less than 200 cells/mm3 are expected to have an additional 25.2% of their teeth with at least one site of CAL ≥4mm, as compared to patients with CD4+ T-cell counts greater than 200 cells/mm3. Smokers and patients without annual dental visits in the past five years are predicted to have higher CAL values (by 11.8% and 15.7% respectively) compared to non-smokers and patients who obtained annual dental care. Older age, increased levels of Pg DNA and increased total subgingival plaque DNA also predicted greater periodontal disease in this cohort. Unexpected findings were the association of higher levels of serum LDL-cholesterol and higher levels of HOMA-IR with lower levels of CAL. No other metabolic measure remained significant in the multivariable model.
Table 5.
Final prediction model for the percent of teeth with CAL ≥4mm.
| Factor | B | Standard Error |
Standardized β |
P-value |
|---|---|---|---|---|
| Constant | .257 | .186 | .172 | |
| CD4+ T-cell count (<200) | .252 | .067 | .306 | <.001 |
| P. gingivalis (log) | .047 | .012 | .290 | <.001 |
| Age (yrs) | .010 | .003 | .268 | .001 |
| < 1 Annual Dental Visit | .157 | .053 | .242 | .004 |
| Total Plaque DNA | .019 | .007 | .207 | .006 |
| Ever Smoker | .118 | .053 | .164 | .029 |
| LDL | −.002 | .001 | −.202 | .011 |
| HOMA-IR(elevated) | −.118 | .075 | −.189 | .014 |
Analysis of variance for final model: F= 9.559; P<.001; R2=0.569; adjusted R2=.509. Model adjusted for: gender, race, primary insurance and baseline viral load. Variables: Total copy number of subgingival plaque DNA (Total Plaque DNA); HOMA-IR ≥2.6 (HOMA-IR elevated).
The standardized beta-coefficients (β) from the multivariable regression model represented the effect of the independent factor on the periodontal disease outcome measurement (CAL) in standardized units, to permit comparison of the relative predictive significance of each factor. CD4+ T cell counts had the strongest effect (β=.306) on periodontal disease followed closely by the level of Pg (β=.290). Age (β=.268) and annual dental visits over the past five years (β=.240) exerted similar effects. CD4+ T-cell counts <200 cells/mm3 had approximately twice the effect on CAL compared to smoking (standardized β coefficient .306 versus .164). This same model was run using REC as the continuous outcome variable, yielding an adjusted R2 of .475, F=8.5, p< .001 (data not shown).
Discussion
Each subject had on average 38% (±24%) of their teeth with at least one site of PD ≥5mm, 55% (±31%) of their teeth with at least one site of REC >0mm, and 50% (±32%) of their teeth with at least one site of CAL ≥4mm. Factors associated with high levels of periodontal disease in this cohort, as measured by CAL, were immunosupression (i.e. CD4+ T-cell counts < 200 cells/mm3), the level of Pg DNA and total DNA in subgingival plaque, substandard utilization of dental care, cigarette smoking and older age. In this cohort, lowered CD4+ T cell counts (<200 cells/mm3) had approximately twice the effect on periodontal disease as did cigarette smoking, a known, strong risk factor for PD (22, 34). Having an annual dental visit remained an independent predictor for lower levels of PD. Cohort characteristics were precisely described.
To characterize a patient’s level of periodontal disease, we used three outcome measures (PPD, REC and CAL) as continuous variables representing the percent of teeth which met or exceeded the measure’s cut point (Table 2, Table 4 and Table 5). Modeling periodontal disease as a continuous variable allows us to examine the entire spectrum of periodontal disease and minimizes the chance of misclassification based on categorical thresholds (i.e. mild, moderate and severe).
We examined the correlation of independent variables with the dependent outcome variables: PPD, REC and CAL. In the bivariate analysis (Table 4), the independent variables correlated differently across the three outcome measures of periodontal disease. For example, only the level of Pg DNA demonstrated a comparable and significant effect on PPD, REC and CAL. However, a limited set of immune (CD4+ T cell count) and metabolic (insulin, LDL-cholesterol and HOMA-IR) host factors appeared to correlate better with the chronic or historic processes (23) (REC) than with the more acute component (23) (PPD) of periodontal disease. These observations support the previous premise by Beck et al, 2002 that PPD and REC capture independent processes (23). Further, these same immune and metabolic factors that were significantly correlated with REC were also significantly correlated with CAL, suggesting that HIV and/or immune reconstitution (i.e. while on HAART) influences PD through a systemic mechanism associated with REC. This observation is supported by previous cross-sectional findings (5, 35).
Our findings concerning the impact of CD4+ T-cell count on periodontal disease are in agreement with several previous studies (1, 5, 10, 15, 16). Our data most closely agree with a report by Robinson et al, 1996, which found, prior to the widespread use of HAART, that decreased CD4+ T-cell count predicted the presence, extent and severity of CAL, but not PPD (5). In terms of immune status, their cohort was very similar to ours; over half (54%) of their subjects met the 1993 case definition for AIDS (5) and 53% of our subjects had a CD4+ T-cell count <200 cells/mm3. McKaig et al, 1998, whose cohort had a similar racial composition to ours, also found severe and extensive periodontal disease in their cohort (16). They found that the extent of recession was significantly greater (p<.01) in persons with CD4+ T-cell count <200 cells/mm3 than in two groups with higher CD4+ T-cell counts (16). In their study, 58% of their cohort had experienced an AIDS-defining event, and 48% had a baseline CD4+ T-cell count <200 cells/ mm3 (i.e. some subjects had experienced immune reconstitution on combination antiviral medication) (16).
Our results, concerning the relationship between PD and CD4 + T-cell count, differ from other studies. For example, Tomar et al., 1995, did not find a cross-sectional association between PD and CD4+ T-cell count using multiple logistic regression; however, they used <300 cells/mm3 as their cut point for CD4+ T-cell counts and their cohort was characterized using Walter Reed staging classification system (instead of CD4+ T-cell count and viral load), making comparison to our study difficult (14). As well, Gonclaves (36) examined 64 HIV+ Brazilian persons and found no statistically significant associations between CD4+ T-cell count and periodontal measures; however, they excluded persons with severe periodontal conditions and all subjects were taking trimethoprim and sulfamethoxazole (Bactrim®) prophylaxis to prevent opportunistic infections, a regimen which could limit the level and severity of PD (10, 12, 37). Further, 34% of their study subjects had a baseline CD4+ T count <200 cells/ mm3 and nadir CD4 + T-cell counts were not reported. The above analysis highlights some of the complexities of comparing previous HIV-related epidemiological research, and underscores the importance of thorough reporting of cohort characteristics, especially in those with HIV-1 infection during the era of HAART.
Older studies (8, 9, 13) as well as more recent studies (6, 36, 38) have reported lower levels of periodontal disease in HIV+ patients, compared to the level of PD in our cohort. Reasons for this may be due to: 1) how authors defined periodontal disease, 2) the methodology used to collect periodontal data, 3) the distribution of patient characteristics within cohorts (especially immune and medication-related), and 4) the limited number of post-HAART era investigations on this topic. Some studies that found low levels of periodontal disease (6, 8, 9, 13, 38) used partial mouth examination techniques that are known to potentially underestimate PD (18) and have not, to our knowledge, been validated in an HIV-1 infected cohort.
As previously reported (39–41), the levels of Pg DNA in our study were highly correlated with periodontal disease. We found that traditional periodontal “red” complex (42) bacteria, Pg, Td and Tf, were highly correlated with PPD. While levels of Pg DNA were correlated with all individual measures of periodontal disease (PPD, REC and CAL), Td was negatively correlated with REC, perhaps suggesting that it is displaced by other species in chronic periodontal disease. This negative association of Td with REC may agree in part with findings by Aas et al, 2007. They propose that there is a shift in the biological ecology away from some of the traditional red complex species (i.e. Pg, Td and Tf) (43). However, our data suggest that Pg played a role in chronic periodontal disease, as measured by REC and CAL (23).
While HAART greatly reduces the prevalence of HIV-related oral manifestations (44), this report demonstrated that traditionally defined periodontal disease is clearly still present and represents a potential challenge to the long-term oral health of persons with HIV-1 infection. CD4+ T-cell count and duration of time on HAART were not correlated with PPD, suggesting that these immune factors have less impact on acute periodontal disease (23). Since increases in LDL cholesterol can occur while taking HAART (45), and insulin resistance can increase due to PI use (46), the association of higher levels of serum LDL cholesterol and higher levels of HOMA-IR with lower levels of CAL could indicate a protective effect of HAART in this cross-sectional analysis. Analysis of our longitudinal data will better address this finding.
Limitations of this study include the cross-sectional design, from which only associations can be made, not causal inferences. We could have slightly overestimated the extent and severity of periodontal disease by rounding up to the next whole millimeter in our periodontal probing measurements. Also, our participants were self-referred, which may have resulted in a convenient sample with poorer periodontal health and/or those interested in obtaining dental care. However, of the 123 ineligible subjects, 48 subjects were excluded because they had fewer than 20 teeth, suggesting that caries and/or periodontal disease have already had a significant effect on many HIV+ persons from this geographic location.
The strength of this study is in its detailed and extensive characterization of periodontal disease and associated factors. The previously applied CDC definition is more sensitive to detect localized areas of severe periodontal disease, while the EWP definition detects a more generalized loss of attachment (i.e. ≥30% of teeth present with CAL ≥5 mm). To minimize the potential misclassification from using categorical threshold cut points (i.e. mild, moderate and severe), we separated and modeled the three periodontal disease measures as continuous variables. This methodology allowed us to explore the link of HIV to PD more comprehensively. Our adjusted multivariable model accounted for slightly over 50% (adjusted R2=.509) of the variability in CAL, which suggests that we identified many of the major determinants of CAL in this cohort.
Periodontal disease remains a significant complication of HIV infection and AIDS. Earlier and more effective referral to dental care with ongoing monitoring of compliance is clearly indicated for those with a CD4 + T-cell count <200 cells/mm3, those who do not see the dentist regularly, and those who smoke or who are older. Earlier intervention with HAART could help limit exposure to immunosuprression (i.e. CD4+ T-cell Count < 200 cells/mm3) and reduce the morbidity of PD in this setting. As persons are living longer with HIV-1 infection, neglecting the importance of oral health may adversely affect such individuals’ general health and quality of life (47). Ongoing dental care is an important component of comprehensive care, especially for those with HIV-1 infection. A greater emphasis on treatment and prevention of periodontal disease is indicated for persons in this cohort and in other populations with similar risk factors.
Acknowledgements
We thank all our subjects who volunteered their time, the physicians, nurses and staff at the following research units as well as Drs. Wendy Armstrong, Robert Asaad, Nabil Bissada, Barabara Gripshover, Robert Kalayjian, Benigno Rodriquez, Stephen Wotman and Mr. Allan Chiunda. Supported by NIDCR, Grant #1 K23 DE15746-01A1, The Center for AIDS Research (CFAR), AI36219, The Dahms Clinical Research Unit (CRU) of the CTSC, UL1 RR024989, NIH, M01 RR000080, The General Clinical Research Center (GCRC), and Oral HIV/AIDS Research Alliance (OHARA), BRS-ACURE-Q0600136. The authors report that they do not have any conflict of interest.
Appendix A
Metabolic measures:
Lipids were determined by a clinical chemistry system using photometric absorbance (Dimension RXL, Siemens Health Care Diagnostics, Incorporated, Deerfield, Illinois, USA.). Insulin was measured by radioimmunoassay, (Coat-A-Count, Siemens Health Care Diagnostics, Incorporated, Deerfield, Illinois, USA.), glucose was measured by immobilized enzyme biosensor with an enzymatic method using glucose oxidase (YSI 2300 STAT Plus, YSI Incorporated, Yellow Springs, Ohio, USA.), and high sensitivity C-Reactive Protein was measured by an enzyme-labeled immunometric sandwich assay (IMMULITE 2500, Siemens Health Care Diagnostics, Incorporated, Deerfield, Illinois, USA).
Appendix B
Probes and Primers for select periodontal bacteria:
| Pg Probe: | 5’-TTGCCCATTCTTTCCCGTTCTCTTGC-3’ |
| Forward primer: | 5’-TCTCGGAGAAAGGTACGCCTAT-3’ |
| Reverse primer: | 5’-TCATCGCACGTGTTTCAGAAA-3’ |
| Td Probe: | 5’-CCGGATTTGATCCTGCTGCAACATCT-3’ |
| Forward primer: | 5’-GGAAAGGCCGGTGTTCATG-3’ |
| Reverse primer: | 5’-CAATCCCATACCTAAATACGGCTTA-3’ |
| 23 S ribosome: | |
| Forward primer: | 5’-AGCCCCAGTAAACGGCG-3’ |
| Reverse primer: | 5’-AATTTCGCTACCTTAGGACCGTTA-3’ |
| Tf Probe: | 5’-VIC-CCGCGACGT-GAAATGGTATTCCTC-3’ |
| Forward primer | 5’-TCCCAAAGACGCG-GATATCA-3’ |
| Reverse primer | 5’-ACGGTCGCGATGT-CATTGT-3’ |
References
- 1.Barr C, Lopez MR, Rua-Dobles A. Periodontal changes by HIV serostatus in a cohort of homosexual and bisexual men. J Clin Periodontol. 1992 Nov;19(10):794–801. doi: 10.1111/j.1600-051x.1992.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 2.Holmstrup P, Westergaard J. Periodontal diseases in HIV-infected patients. J Clin Periodontol. 1994 Apr;21(4):270–280. doi: 10.1111/j.1600-051x.1994.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 3.Greenspan JS, Barr CE, Sciubba JJ, Winkler JR The U.S.A. Oral AIDS Collaborative Group. Oral manifestations of HIV infection. Definitions, diagnostic criteria, and principles of therapy. Oral Surg Oral Med Oral Pathol. 1992 Feb;73(2):142–144. doi: 10.1016/0030-4220(92)90185-s. [DOI] [PubMed] [Google Scholar]
- 4.Winkler JR, Murray PA. Periodontal disease. A potential intraoral expression of AIDS may be rapidly progressive periodontitis. CDA J. 1987 Jan;15(1):20–24. [PubMed] [Google Scholar]
- 5.Robinson PG, Sheiham A, Challacombe SJ, Zakrzewska JM. The periodontal health of homosexual men with HIV infection: a controlled study. Oral Dis. 1996 Mar;2(1):45–52. doi: 10.1111/j.1601-0825.1996.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 6.Alves M, Mulligan R, Passaro D, Gawell S, Navazesh M, Phelan J, et al. Longitudinal evaluation of loss of attachment in HIV-infected women compared to HIV-uninfected women. J Periodontol. 2006 May;77(5):773–779. doi: 10.1902/jop.2006.P04039. [DOI] [PubMed] [Google Scholar]
- 7.Lekakis J, Tsiodras S, Ikonomidis I, Palios J, Poulakou G, Rallidis L, et al. HIV-positive patients treated with protease inhibitors have vascular changes resembling those observed in atherosclerotic cardiovascular disease. Clin Sci (Lond) 2008 Sep;115(6):189–196. doi: 10.1042/CS20070353. [DOI] [PubMed] [Google Scholar]
- 8.Drinkard CR, Decher L, Little JW, Rhame FS, Balfour HH, Jr, Rhodus NL, et al. Periodontal status of individuals in early stages of human immunodeficiency virus infection. Community Dent Oral Epidemiol. 1991 Oct;19(5):281–285. doi: 10.1111/j.1600-0528.1991.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 9.Friedman RB, Gunsolley J, Gentry A, Dinius A, Kaplowitz L, Settle J. Periodontal status of HIV-seropositive and AIDS patients. J Periodontol. 1991 Oct;62(10):623–627. doi: 10.1902/jop.1991.62.10.623. [DOI] [PubMed] [Google Scholar]
- 10.Glick M, Muzyka BC, Salkin LM, Lurie D. Necrotizing ulcerative periodontitis: a marker for immune deterioration and a predictor for the diagnosis of AIDS. J Periodontol. 1994 May;65(5):393–397. doi: 10.1902/jop.1994.65.5.393. [DOI] [PubMed] [Google Scholar]
- 11.Robinson P. Periodontal diseases and HIV infection. A review of the literature. J Clin Periodontol. 1992 Oct;19(9 Pt 1):609–614. doi: 10.1111/j.1600-051x.1992.tb01707.x. [DOI] [PubMed] [Google Scholar]
- 12.Robinson PG, Sheiham A, Challacombe SJ, Zakrzewska JM. Periodontal health and HIV infection. Oral Dis. 1997 May;3 Suppl 1:S149–S152. doi: 10.1111/j.1601-0825.1997.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 13.Scheutz F, Matee MI, Andsager L, Holm AM, Moshi J, Kagoma C, et al. Is there an association between periodontal condition and HIV infection? J Clin Periodontol. 1997 Aug;24(8):580–587. doi: 10.1111/j.1600-051x.1997.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 14.Tomar SL, Swango PA, Kleinman DV, Burt BA. Loss of periodontal attachment in HIV-seropositive military personnel. J Periodontol. 1995 Jun;66(6):421–428. doi: 10.1902/jop.1995.66.6.421. [DOI] [PubMed] [Google Scholar]
- 15.Yeung SC, Stewart GJ, Cooper DA, Sindhusake D. Progression of periodontal disease in HIV seropositive patients. J Periodontol. 1993 Jul;64(7):651–657. doi: 10.1902/jop.1993.64.7.651. [DOI] [PubMed] [Google Scholar]
- 16.McKaig RG, Thomas JC, Patton LL, Strauss RP, Slade GD, Beck JD. Prevalence of HIV-associated periodontitis and chronic periodontitis in a southeastern US study group. J Public Health Dent. 1998 Fall;58(4):294–300. doi: 10.1111/j.1752-7325.1998.tb03012.x. [DOI] [PubMed] [Google Scholar]
- 17.Ndiaye CF, Critchlow CW, Leggott PJ, Kiviat NB, Ndoye I, Robertson PB, et al. Periodontal status of HIV-1 and HIV-2 seropositive and HIV seronegative female commercial sex workers in Senegal. J Periodontol. 1997 Sep;68(9):827–831. doi: 10.1902/jop.1997.68.9.827. [DOI] [PubMed] [Google Scholar]
- 18.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007 Jul;78(7 Suppl):1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 19.McComsey GA, O'Riordan M, Hazen SL, El-Bejjani D, Bhatt S, Brennan ML, et al. Increased carotid intima media thickness and cardiac biomarkers in HIV infected children. AIDS. 2007 May 11;21(8):921–927. doi: 10.1097/QAD.0b013e328133f29c. [DOI] [PubMed] [Google Scholar]
- 20.Shiboski CH, Cohen M, Weber K, Shansky A, Malvin K, Greenblatt RM. Factors associated with use of dental services among HIV-infected and high-risk uninfected women. J Am Dent Assoc. 2005 Sep;136(9):1242–1255. doi: 10.14219/jada.archive.2005.0340. [DOI] [PubMed] [Google Scholar]
- 21.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Jr, Papapanou PN, et al. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Stroke. 2003 Sep;34(9):2120–2125. doi: 10.1161/01.STR.0000085086.50957.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tonetti MS, Claffey N. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol. 2005;32 Suppl 6:210–213. doi: 10.1111/j.1600-051X.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 23.Beck JD, Offenbacher S. Relationships among clinical measures of periodontal disease and their associations with systemic markers. Ann Periodontol. 2002 Dec;7(1):79–89. doi: 10.1902/annals.2002.7.1.79. [DOI] [PubMed] [Google Scholar]
- 24.Seymour RA, Whitworth JM, Martin M. Antibiotic prophylaxis for patients with joint prostheses - still a dilemma for dental practitioners. Br Dent J. 2003 Jun 28;194(12):649–653. doi: 10.1038/sj.bdj.4810352. [DOI] [PubMed] [Google Scholar]
- 25.Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007 Oct 9;116(15):1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 26.Lenton PA. Examiner Guide to Measuring Periodontal Parameters and Indices. Minneapolis: Minnesota: University of Minnesota; 1997. [Google Scholar]
- 27.Beck JD, Eke P, Heiss G, Madianos P, Couper D, Lin D, et al. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation. 2005 Jul 5;112(1):19–24. doi: 10.1161/CIRCULATIONAHA.104.511998. [DOI] [PubMed] [Google Scholar]
- 28.Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005 Nov;76(11 Suppl):2089–2100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, et al. Diagnosing insulin resistance in the general population. Diabetes Care. 2001 Mar;24(3):460–464. doi: 10.2337/diacare.24.3.460. [DOI] [PubMed] [Google Scholar]
- 31.Fleiss JL, Park MH, Chilton NW, Alman JE, Feldman RS, Chauncey HH. Representativeness of the "Ramfjord teeth" for epidemiologic studies of gingivitis and periodontitis. Community Dent Oral Epidemiol. 1987 Aug;15(4):221–224. doi: 10.1111/j.1600-0528.1987.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 32.Toossi Z, Mayanja-Kizza H, Baseke J, Peters P, Wu M, Abraha A, et al. Inhibition of human immunodeficiency virus-1 (HIV-1) by beta-chemokine analogues in mononuclear cells from HIV-1-infected patients with active tuberculosis. Clin Exp Immunol. 2005 Nov;142(2):327–332. doi: 10.1111/j.1365-2249.2005.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morillo JM, Lau L, Sanz M, Herrera D, Martin C, Silva A. Quantitative real-time polymerase chain reaction based on single copy gene sequence for detection of periodontal pathogens. J Clin Periodontol. 2004 Dec;31(12):1054–1060. doi: 10.1111/j.1600-051x.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 34.Ryder MI. The influence of smoking on host responses in periodontal infections. Periodontol 2000. 2007;43:267–277. doi: 10.1111/j.1600-0757.2006.00163.x. [DOI] [PubMed] [Google Scholar]
- 35.Patton LL, Shugars DC. Immunologic and viral markers of HIV-1 disease progression: implications for dentistry. J Am Dent Assoc. 1999 Sep;130(9):1313–1322. doi: 10.14219/jada.archive.1999.0401. [DOI] [PubMed] [Google Scholar]
- 36.Goncalves Lde S, Ferreira SM, Silva A, Jr, Villoria GE, Costinha LH, Colombo AP. Association of T CD4 lymphocyte levels and chronic periodontitis in HIV-infected brazilian patients undergoing highly active anti-retroviral therapy: clinical results. J Periodontol. 2005 Jun;76(6):915–922. doi: 10.1902/jop.2005.76.6.915. [DOI] [PubMed] [Google Scholar]
- 37.Kucers A, Bennet NM. The Use of Antibiotics. London: William Heinemann; 1987. [Google Scholar]
- 38.Mulligan R, Phelan JA, Brunelle J, Redford M, Pogoda JM, Nelson E, et al. Baseline characteristics of participants in the oral health component of the Women's Interagency HIV Study. Community Dent Oral Epidemiol. 2004 Apr;32(2):86–98. doi: 10.1111/j.0301-5661.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 39.Cross DL, Smith GL. Comparison of periodontal disease in HIV seropositive subjects and controls (II). Microbiology, immunology and predictors of disease progression. J Clin Periodontol. 1995 Jul;22(7):569–577. doi: 10.1111/j.1600-051x.1995.tb00806.x. [DOI] [PubMed] [Google Scholar]
- 40.Murray PA, Grassi M, Winkler JR. The microbiology of HIV-associated periodontal lesions. J Clin Periodontol. 1989 Nov;16(10):636–642. doi: 10.1111/j.1600-051x.1989.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 41.Zambon JJ, Reynolds HS, Genco RJ. Studies of the subgingival microflora in patients with acquired immunodeficiency syndrome. J Periodontol. 1990 Nov;61(11):699–704. doi: 10.1902/jop.1990.61.11.699. [DOI] [PubMed] [Google Scholar]
- 42.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998 Feb;25(2):134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 43.Aas JA, Barbuto SM, Alpagot T, Olsen I, Dewhirst FE, Paster BJ. Subgingival plaque microbiota in HIV positive patients. J Clin Periodontol. 2007 Mar;34(3):189–195. doi: 10.1111/j.1600-051X.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 44.Patton LL, McKaig R, Strauss R, Rogers D, Eron JJ., Jr Changing prevalence of oral manifestations of human immuno-deficiency virus in the era of protease inhibitor therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Mar;89(3):299–304. doi: 10.1016/s1079-2104(00)70092-8. [DOI] [PubMed] [Google Scholar]
- 45.Riddler SA, Li X, Chu H, Kingsley LA, Dobs A, Evans R, et al. Longitudinal changes in serum lipids among HIV-infected men on highly active antiretroviral therapy. HIV Med. 2007 Jul;8(5):280–287. doi: 10.1111/j.1468-1293.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 46.Grinspoon SK. Metabolic syndrome and cardiovascular disease in patients with human immunodeficiency virus. Am J Med. 2005 Apr;118 Suppl 2:23S–28S. doi: 10.1016/j.amjmed.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 47.Oral health in America: a report of the Surgeon General. J Calif Dent Assoc. 2000 Sep;28(9):685–695. [PubMed] [Google Scholar]