Abstract
The α4 integrins, α4β7 and α4β1, and their ligands, mucosal vascular addressin cell adhesion molecule (MAdCAM-1) and vascular cell adhesion molecule (VCAM-1), have diverse functions, including roles in the formation of secondary lymphoid tissues at early time points during the colonization and clustering of the fetal lymphoid tissue inducer (LTi) cells, and at later time points during the recruitment of lymphocytes. In this study, we evaluated the role of α4 integrins in the development of a recently appreciated class of intestinal lymphoid tissues, isolated lymphoid follicles (ILFs). We observed that diverse ILF cellular populations express α4β7 and α4β1, including the LTi-like cells and lymphocytes, while ILF stromal cells and vessels within ILFs express VCAM-1 and MAdCAM-1 respectively. Evaluation of adult and neonatal β7−/− mice and adult and neonatal mice given blocking antibodies to α4β7, MAdCAM-1, or VCAM-1 did not identify a role for α4 integrins in cryptopatch (CP) development, however, these studies demonstrated that α4β7 and MAdCAM-1 are required for the transitioning CP into lymphoid tissues containing lymphocytes, or ILFs. Competitive bone marrow transfers demonstrated that β7−/− LTi-like cells had a reduced but not significantly impaired ability to localize to CP. Bone marrow transfers and adoptive transfers of B-lymphocytes revealed that β7 expression by B-lymphocytes was essential for their entry into the developing ILF. These findings demonstrate an essential role for α4β7/MAdCAM-1 in ILF development corresponding to the influx of β7 expressing lymphocytes, and a non-essential role for β7 localizing LTi-like cells to the small intestine.
Keywords: Rodent, B-cells, mucosa
Introduction
Integrins are heterodimeric proteins composed of α and β subunits that promote cell-cell interactions and consequently perform diverse roles in the immune system. Only two integrins are known to contain a α 4 subunit, α4β7 and α4β1. These integrins and their ligands, mucosal vascular addressin cell adhesion molecule (MAdCAM-1) and vascular cell adhesion molecule (VCAM-1), are expressed in restricted manners on hematopoietic cells, high endothelial venules (HEVs), and stromal cells (3, 12, 13, 18, 24, 28) respectively, and have critical roles in secondary lymphoid structure formation and inflammatory responses(1–7).
One of the earliest events in secondary lymphoid structure organogenesis is the clustering of fetal CD3−CD4+CD45+ lymphoid tissue inducer (LTi)3 cells, which express lymphotoxin (LT), and stromal organizer cells, which express the lymphotoxin beta receptor (LTβR)(8–10). Interactions of the α4 integrins expressed on the fetal LTi and VCAM-1 expressed by the LTβR+ stromal cells are felt to be instrumental in maintaining the early cellular clusters, thus sustaining LT/LTβR interactions and leading to a cascade of events resulting in the formation of secondary lymphoid tissues (2, 10–12). Likewise the expression of MAdCAM-1 on lymph node HEVs during embryogenesis contributes to the colonization of α4β7 expressing fetal LTi cells(12). However this interaction is not solely responsible for fetal LTi cell retention, as antibodies that block this interaction only partially inhibit fetal LTi cell colonization(12). Fetal LTi cells also express α4β1 and therefore interactions with VCAM-1 expressing stromal cells may also contribute to stabilizing this cellular interaction. Collectively these observations suggest that both α4β7 and α4β1 expression by fetal LTi cells and VCAM-1 expression by organizer cells are important in the early steps of secondary lymphoid tissue formation.
Following the formation of a self sustaining cluster of fetal LTi cells and organizer cells, mature hematopoietic cells are recruited to the forming the lymph node or PP; α4 integrins are crucial to this process. Studies using knockout mice and antibody blockade demonstrate a critical role for α4β7 and its ligand, MAdCAM-1, in mature lymphocyte homing to the gut (4, 13–15). This role also extends to lymphocyte trafficking in intestinal inflammation where MAdCAM-1 expression is aberrantly upregulated in chronically inflamed intestines of patients with IBD(16–20), and accordingly α4β7/MAdCAM-1 blockade has been considered as a novel organ-specific therapeutic target for the treatment of IBD (21–26). Collectively these observations suggest that α4β7 interactions with MAdCAM-1 play critical roles in later stages of secondary lymphoid tissue development and in lymphocyte trafficking in the intestine during inflammation.
Isolated lymphoid follicles (ILFs) are intestinal lymphoid aggregates that can resemble a single-domed PP. Recently these aggregates have become appreciated as distinct members of the gastrointestinal associated lymphoid tissues (27–32). In contrast to PP, ILFs are part of a spectrum of lymphoid aggregates in various stages of development. Cryptopatches (CP) are collections of unique bone marrow derived cells clustered at the base of the villi, and are believed to be the precursor cellular aggregate giving rise to ILFs. These unique cells lack the expression of mature lineage markers (lin−), but express c-kit, and share many phenotypic and developmental features with the fetal LTi cells. Accordingly the lin− c-kit+ CP cells are believed to carry out an analogous function as organizing cells delivering the early LT signals resulting in the formation of CP, which subsequently progress to become ILFs(33). ILF and PP development share many characteristics, however a primary distinction is that PP formation is developmentally driven, with critical events occurring during embryogenesis, conversely ILF development initiates after birth and its progression is augmented by exogenous stimuli including normal intestinal microbiota (27, 28, 34, 35). While the function of α4 integrins in ILF development is previously uninvestigated, a role for α4 integrins in this process is suggested by observations of VCAM-1 expression by stromal cells in CP and the role of inflammatory stimuli in augmenting ILF development(36–39). Paralleling the events in PP formation, α4 integrins could be important at multiple points in CP and ILF formation, including early events required for the clustering of lin− c-kit+ cells to form CP and later events related to the recruitment of mature lymphocyte lineages to form the mature ILF.
In this study, we evaluated the role of α4 integrins in the development of ILFs. We observed that a significant population of the lin− c-kit+ cells, ILF B lymphocytes, and ILF T lymphocytes express α4β7 and α4β1. In a related manner, we found that stromal cells within the ILFs express VCAM-1, while MAdCAM-1 expression was restricted to non-lymphatic vascular structures within ILFs. A functional role for α4 integrins in CP and ILF development was defined by knockout mice, antibody blockade, and bone marrow reconstitution. Surprisingly, we observed that β7 is dispensable for the formation of CP and the recruitment of dendritic cells to CP, however β7 is essential for the development of ILFs. Parallel studies using antibody blockade in adult and neonatal mice demonstrated that antibodies specific for murine α4β7 or MAdCAM-1, but not VCAM-1, significantly decreased the numbers of ILFs in adult mice, while the number of CP remained unaffected in all treatment groups of adult and neonatal mice. Consistent with this block corresponding to the influx of B-lymphocytes into the developing ILF, bone marrow reconstitution and adoptive transfer of lymphocytes demonstrated an absolute requirement for β7 expression by lymphocytes for their localization to developing ILFs and a redundant role for β7 in localizing the LTi-like cells to the small intestine. Collectively these findings demonstrate an absolute role for α4β7/MAdCAM-1 interactions at late stages in ILF development corresponding to the influx of mature β7 expressing lymphocytes into the developing ILF, and a redundant role for β7 in the localization of the LTi-like cells to the small intestine and CP development.
Materials and Methods
Mice
BALB/c mice, C57BL/6 mice, β7−/− mice on the C57BL/6 background (stock# 002965), C57BL/6 congenic mice expressing the CD90.1 allele (stock# 001317), C57BL/6 congenic mice expressing the CD45.1 allele (stock# 002014), and RAG−/− mice on the C57BL/6 background (stock# 002216) were purchased from The Jackson Laboratory (Bar Harbor, MN). Animals were housed in a specific pathogen free facility and fed routine chow diet. Animals were 8 to 16 weeks of age at the time of analysis except where noted otherwise. Animal procedures and protocols were carried out in accordance with the institutional review board at Washington University School of Medicine.
Isolation of cellular populations from spleen, PP, and ILFs
Spleens and PP were removed from mice and disrupted by mechanical dissociation. Small intestines were removed from mice, flushed with cold PBS, opened along the mesenteric border, and mounted with the lumen facing up in cold PBS. Utilizing the dissecting microscope, and a 26-gauge needle and syringe, the contents of multiple mature ILFs were aspirated, placed in cold PBS, and mechanically disrupted. Red blood cells were lysed from cellular suspensions and then utilized for flow cytometric analysis as described below. Average yield of viable mononuclear ILFs cells ranged from 3–7 × 105 cells/ small intestine.
Flow cytometric analysis
Single cell suspensions from spleen, PP and ILFs obtained as above were used for flow cytometric analysis. Antibodies used for analysis were anti-mouse β1(BD Biosciences, San Diego, CA), anti-mouse β7, anti-mouse α4, anti-mouse CD3, anti-mouse CD19, anti-mouse c-kit, anti mouse linage marker cocktail (anti mouse CD3, CD11b, B220, Gr-1, TER119, CD11c ) and appropriate isotype control antibodies (all from eBioscience, San Diego, CA). Data acquisition was performed on a FACScan cytometer (BDbiosciences) retrofitted with a second laser using Cellquest (BD Biosciences) and Rainbow (Cytek, Fremont, CA) software. Data analysis was performed on a Macintosh G4 computer running FlowJo software (Tree Star Inc., Ashland, OR) or CellQuest software (BD Biosciences). Dead cells were excluded based on forward and side light scatter. Gates for positive staining was defined such that ~1% of the analyzed population stained positive with the appropriate isotype control antibodies.
Immunohistochemistry
Small intestines were opened and 1.5cm sections were snap-frozen in OCT medium (Sakura Finetek, Torrence, CA). For the purpose of evaluating MAdCAM-1 and VCAM-1 expression, 7µm sections were cut parallel to the axis of the villi (longitudinal sections). For the purpose of evaluating cryptopatches, 7µm sections were cut perpendicular to the axis of the villi (horizontal sections). Endogenous peroxidase activity was quenched with 3% H2O2 in PBS for 10 min at room temperature, endogenous biotin was blocked with Avidin/Biotin blocking kit (Vector Laboratories, Burlingame, CA). Sections were washed in PBS 3 times, blocked with PBS plus 1% BSA for 30 minutes at room temperature and incubated with the primary antibody for 1 hour at room temperature. Sections were washed in PBS 3 times. Sections incubated with unconjugated primary antibodies, were subsequently incubated with biotinylated secondary antibodies for 1 hour at room temperature and washed 3 times in PBS. Tyramide signal amplification (PerkinElmer LAS, Inc., Boston, MA) was used for the detection of MAdCAM-1 and VCAM-1 per the manufacturers recommendations. Detection of other antibodies utilized streptavidin conjugated Cy2 or streptavidin conjugated Cy3 (Jackson Immuno Research). In experiments using multiple fluorophores, sections were treated with Avidin/Biotin blocking kit (Vector Laboratories Inc., Burlingame, CA), and the above protocol was repeated using a second flourophore for detection. Sections were counterstained with Hoechst dye (Sigma-Aldrich, St. Louis, MO) to visualize nuclei (blue staining in photomicrographs in figure 2,figure 3,figure 5, and figure 6).
Figure 2.
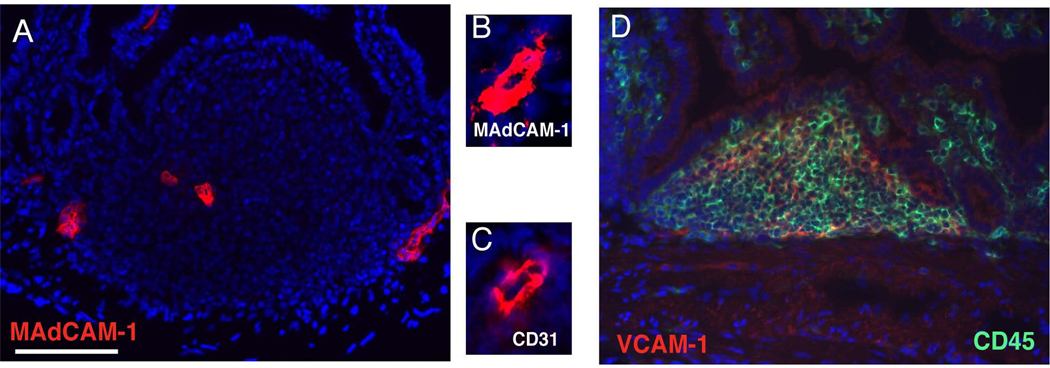
Vessels within ILFs express MAdCAM-1 and stromal cells within ILFs cells express VCAM-1. To evaluate the expression of the ligands for α4β1 and α4β7, frozen sections of ILFs from BALB/c were stained with anti mouse MAdCAM-1 and VCAM-1 as described in material and methods. The expression of MAdCAM-1 localized to structures that had the appearance of vessels (panel A), which was confirmed by positive staining for CD31 (panels B and C: serial sections of a vessel at higher magnification stained with anti-MAdCAM-1 and CD31 respectively). These vascular structures displayed low or no PNAd expression (not shown). VCAM-1 expression was diffusely distributed in the ILF, and primarily restricted to CD45− (non-hematopoietic) stromal cells (panel D). Scale bar in panel A = 100µm.
Figure 3.
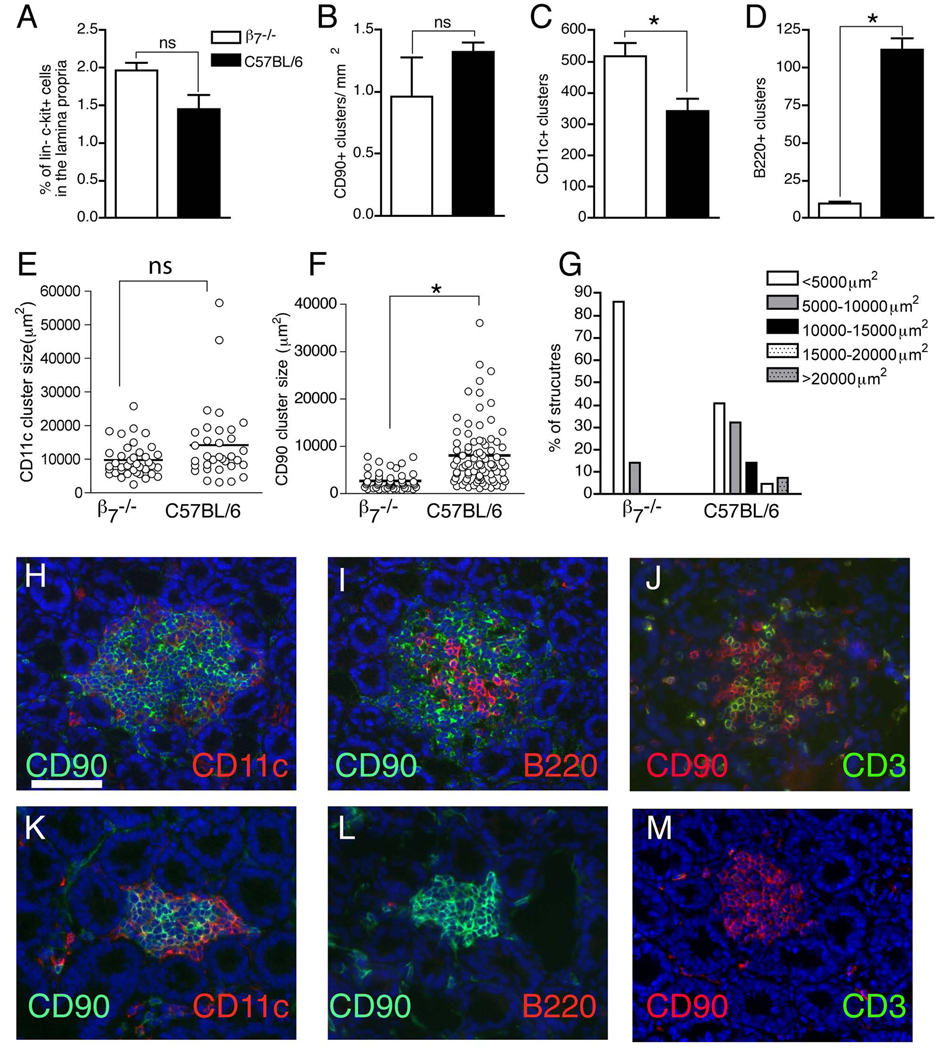
ILF formation in β7−/− mice is arrested at a stage corresponding to the influx of B-lymphocytes. To assess a role for β7 in CP and ILF development we evaluated β7−/− mice for the presence of intestinal lin− c-kit+ cells and the presence of cellular aggregates within the CP/ILF continuum. Photomicrographs in panels H, I, and J are from intestinal sections from wildtype mice, photomicrographs from panes K, L, and M are from intestinal sections from β7−/− mice. There was no difference in the percentage of lamina propria cells that were lin− c-kit+ between wildtype and β7 deficient mice (panel A). The lin− c-kit+ CP cells also express CD90, and due to its intensity, anti-CD90 staining is useful to identify cellular clusters that encompass all the cellular aggregates in the CP/ILF spectrum. There was no difference in the density of CD90+ clusters when comparing wildtype and β7−/− mice (panel B). β7−/− mice had an increased number of intestinal CD11c+ clusters, corresponding to an increase in the number of CD90+ clusters that are infiltrated with a large population of CD11c+ cells (panels C, H, and K). The size of the CD11c+ clusters in β7−/− mice was not significantly different from those in wildtype mice (panel E). In contrast β7−/− mice had dramatically reduced number of intestinal B220+ clusters (panels D, I, and L) and a reduced population of T-lymphocytes infiltrating the CD90+ clusters (panels J and M). CD90 clusters in β7−/− mice were significantly smaller (panel F) and few clusters were larger than 10,000µm2 (panel G), corresponding to the absence of structures with a higher cellular complexity. The data in panels A–D are generated from 4 or mice from each group, and presented as the mean±one standard deviation. The data in panels E, F, and G are generated from 3 mice in each group. The photos in panels H–M are representative of one of 4 or more mice from each group. The scale bar in panel H = 100µm. * = p<0.05, ns = not significant.
Figure 5.
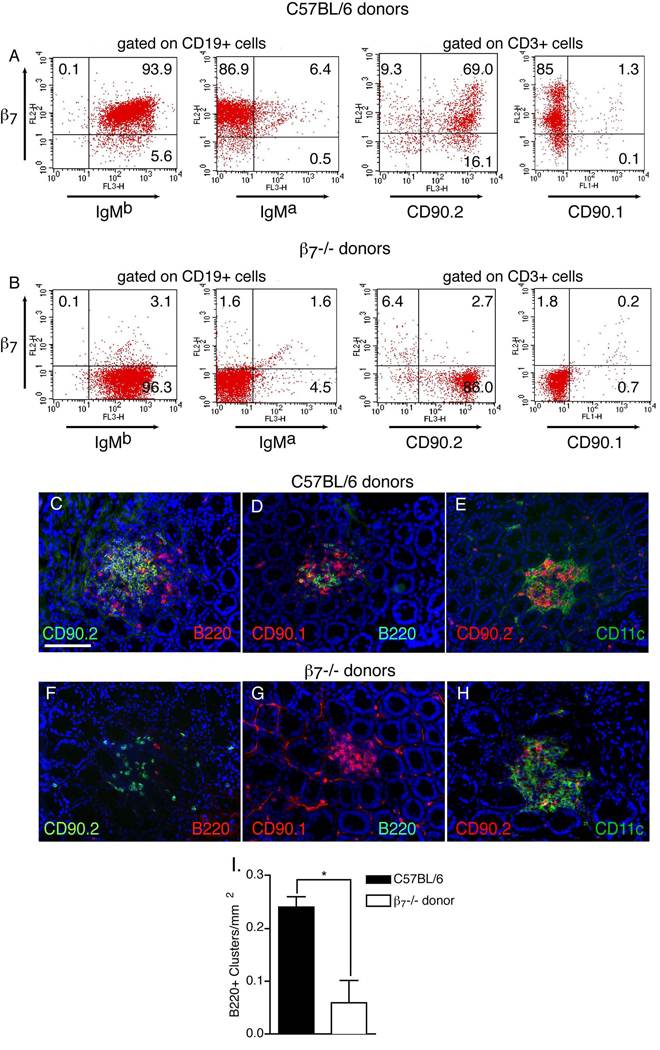
β7 sufficient bone marrow derived cells are essential for the formation of ILFs. Lethally irradiated C57BL/6 congenic (IgMa, CD90.1) mice were given bone marrow from gender-matched β7−/− (IgMb, CD90.2) or C57BL/6 (IgMb, CD90.2) donors and examined for the presence of donor lymphocytes in the spleen, intestinal CD90 clusters, and intestinal B220 clusters. Flow cytometric analysis of splenocytes demonstrated effective engraftment of donor B-lymphocytes (IgMb+) and T-lymphocytes (CD90.2+), and the absence of β7 expression in splenocytes from recipients of β7−/− bone marrow (panels A and B). There were no differences in the infiltration of the CD90+ clusters with CD11c+ cells (panels E and H). Few CD90+ clusters from recipients receiving β7−/− mice bone marrow contained B-lymphocytes (panels C,D,F, and G), and quantitatively this was associated with a significant decrease in the number of B220+ clusters in these recipients (panel H). All CD90 clusters in both groups contained a population of CD90.1+ (recipient derived, wildtype) CP cells. However the CD90+ clusters in the recipients of β7−/− bone marrow contained few CD90.2+ (donor derived) CP cells (compare panels C and F), suggesting a relative defect in the ability of these cells to localize to the CP in the absence of β7. Data in panel I is presented as the mean ± the standard error of the mean of data generated from 3 mice in each group. The scale bar in panel C = 100µm. * = p<0.05.
Figure 6.
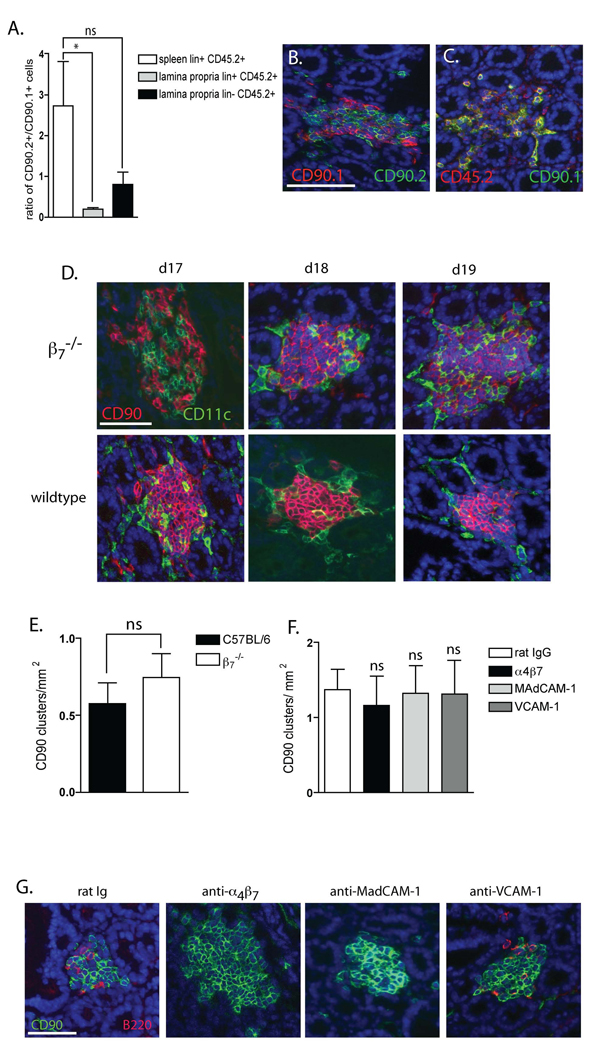
β7 plays a redundant role in localizing LTi-like cells to the small intestine and a non-essential role in CP development in neonatal mice. To more definitively address the role for β7 in localizing the CD90+ cells to the small intestine and CP we performed competitive transfers of wildtype (CD90.1, CD45.2) and β7−/− (CD90.2, CD45.2) bone marrow into wildtype (CD90.2, CD45.1) recipients. Recipients were analyzed to identify the origin of CD45.2 (donor) lin+ CD90+ and lin− CD90+ cells with flow cytometry and with immunohistochemistry to evaluate the localization of wildtype and β7−/− CD90+ cells to the CP. Flow cytometric analysis of gated on the CD45.2+ (donor) population revealed an enrichment of lin+ CD90+ cells of β7−/− origin (CD90.2) in the spleen while conversely the lamina propria contained significantly fewer CD45.2+ (donor) lin+ CD90+ cells of β7−/− origin (panel A). There were too few lin− CD90+ splenocytes for analysis, however the donor (CD45.2+) lin− CD90+ cells in the lamina propria were relatively lacking in cells from β7−/− donors (CD90.2) in comparison to cells from wildtype donors (CD90.1) (panel A). This did not reach statistical significance when compared with the origins of lin+ donor splenocytes. Immunohistochemistry revealed equal populations of CD90.1+ (wildtype donor origin) and CD90.2+ (β7−/− donor origin or recipient origin) cells in the CP (panel B). The donor (CD45.2+) CP cells were largely from wildtype donors (CD90.1+) (yellow co-staining panel C), thus demonstrating a relative deficiency of β7−/− cells to compete for this niche. To evaluate a role for β7 in the development of CP in the neonatal period, we examined small intestines from neonatal wildtype and β7−/− mice and wildtype mice given blocking antibodies to α4β7, MAdCAM-1, VCAM-1 or control IgG from day 17 of gestation until analysis at day 19 of neonatal life. CP are detectable in both wildtype and β7−/− mice on day 17 of neonatal life (panel D), and there was no difference in the density of CP on day 19 of neonatal life when CP formation is rapidly increasing in β7−/− mice (panel E) or mice given blocking antibodies to α4β7, MAdCAM-1, or VCAM-1 (panel F). Consistent with the observation of antibody blockade in adult mice, we observed that anti-α4β7 and anti-MadCAM-1 inhibited the influx of B-lymphocytes into the cellular clusters and anti-VCAM-1 had no effect on B-lymphocyte influx into the cellular clusters at day 19 of neonatal life (panel G). Data in panels A and E is generated from 4 mice in each group. Data in panel F is generated from 3 mice in each group. * = p<0.05, ns= not significant. The scale bar panel B = 100µm, panel D = 50µm, and panel G = 50µm.
Determination of cluster density and size
To enumerate and determine the density of clusters, sections corresponding to identical regions of the small intestine were obtained from experimental and control mice. The entire small intestine was mounted in four equivalent pieces, and a one cm long segment from each end of the piece, totaling eight small intestine sections from each animal, was embedded in OCT compound and frozen. Immunohistochemistry on sections cut perpendicular to the villi was performed as described above. Under 100X magnification, clusters located in the crypt area were counted using immunofluoresence microscope. The same section was then stained with H&E staining and the total crypt surface area was determined using MetaVue software (Molecular Device Corporation, Downingtown, PA). The density of clusters was calculated by dividing the total number of clusters by the total crypt area for each animal. The area clusters were examined using Image J software (ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/).
Enumeration of CD11c and B220 clusters
Enumeration of B220+ and CD11c+ cellular clusters was performed using anti-B220 or anti-CD11c stained whole mounts as previously described(30). The numbers of B220+ and CD11c+ clusters were determined using a dissecting microscope at a magnification of 25X or greater.
In vivo antibody blockade
The hybridoma cell line producing rat anti mouse α4β7 (clone DATK 32) (ATCC) was cultured in CD hybridoma serum free medium(Gibco Life Technologies) and antibody was purified from the culture supernatant by protein G chromatography (Pierce, Rockford, IL) under endotoxin-free conditions. The endotoxin level was determined with QCL-1000 kit (BioWhittaker) using limulus amebocyte lysate method. The concentration of purified antibody was determined using ELISA specific for rat IgG2a, and the activity of the purified antibody was assessed by flow cytometry.
To evaluate CP and ILF development in adults, 7–8 week old mice were injected i.p. with 200µg of rat anti-mouse MAdCAM-1 (BioExpress, West Lebanon, NH), rat anti-mouse VCAM-1(BioExpress), rat anti-mouse α4β7, purified as above, or rat IgG (Southern Biotechnology Associates, Birmingham, AL) every other day for 2 weeks at which time they were sacrificed for analysis. To examine CP development in the neonatal period, 200ug of anti-α4β7, MAdCAM-1, VCAM-1 or rat IgG was injected i.p. at 17 days of gestation and every other day after birth until analysis on day 19 of neonatal life.
Bone marrow transfers
Bone marrow chimeric mice were generated as previously described(28). Seven-week old bone marrow recipients received 1000Gy of γ irradiation in divided doses over two sequential days and were injected i.v. with 1 × 107 T-lymphocyte depleted bone marrow cells from gender-matched donors. In experiments using mixed chimeras, recipients received 5 × 106 cells from each donor. Mice were allowed 12 weeks for reconstitution with donor bone marrow prior to use for experiments. Appropriate reconstitution of lymphocyte compartments were examined by flow cytometry at the time of sacrifice.
Adoptive transfer of lymphocytes
To assess a role for β7 expression by B-lymphocytes in localizing to ILFs, wildtype and β7−/− deficient mature B-lymphocytes were co-transferred into RAG−/− recipients. Splenocytes were isolated from wildtype (CD45.1) and β7−/− (CD45.2) mice and the number of B-lymphocytes in each population was determined by flow cytometry. 1.9 × 107 splenic B-lymphocytes from each donor genotype were co-injected intravenously into RAG−/− recipients. Recipients were sacrificed 1 week later and evaluated for the presence of transferred cellular populations by flow cytometry and immunohistochemistry. The number of total B-lymphocytes (B220+ cells) and the number of wildtype B-lymphocytes (B220+ CD45.1+) in the ILFs was determined by examining intestinal sections stained for B220 and CD45.1 at 200X or greater magnification. Flow cytometric analysis was performed by gating on live B-lymphocytes (B220+ CD19+) and evaluating the ratio of CD45.1+ (wildtype) vs. CD45.2+ (β7−/−) cells.
Statistical analysis
Data analysis using Student’s t test and one-way ANOVA followed by Tukey’s multiple comparison post-test was performed using GraphPad Prism (GraphPad Software). A value of p < 0.05 was used as a cutoff for statistical significance.
Results
α4 integrins and their ligands are expressed within ILFs
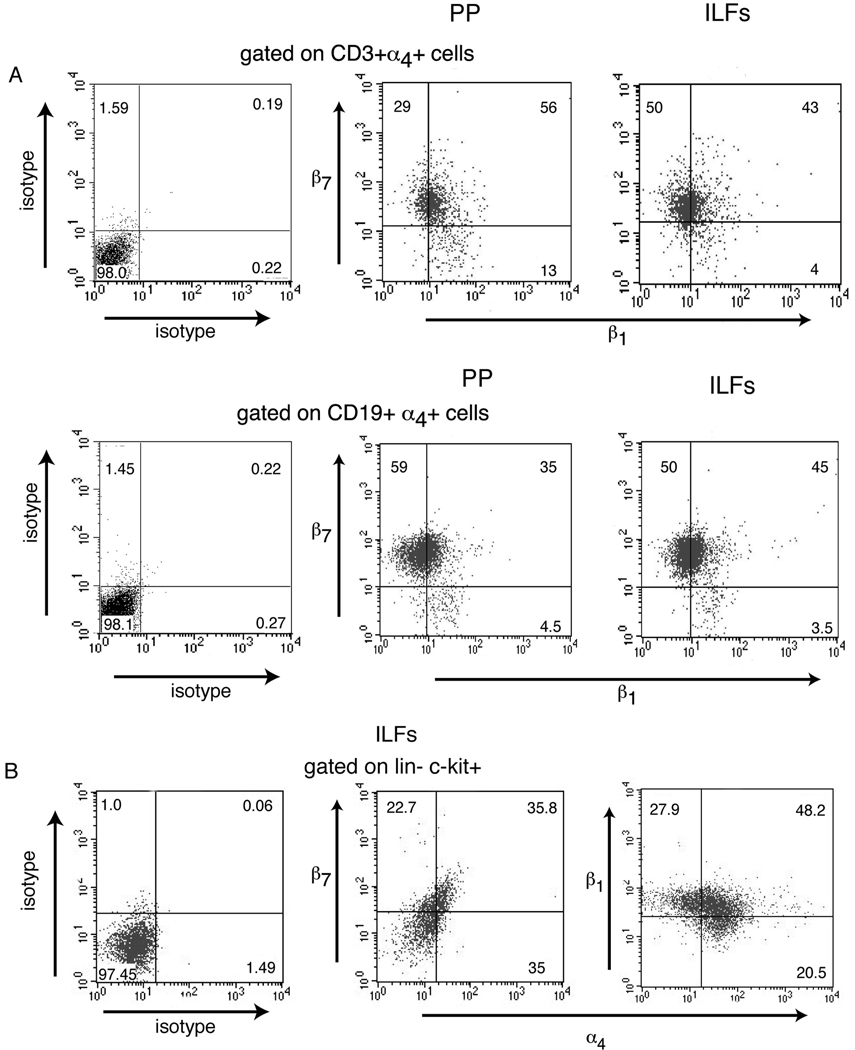
To assess a role for α4 integrins in ILF formation, we examined the expression of α4β7 and α4β1 by ILF cell types by flow cytometry. Similar to PP, the majority of ILF B-lymphocytes (CD19+) were α4β7+, while in comparison fewer ILF T-lymphocytes (CD3+) were α4β7+ (Table I). Approximately one half of the B and T-lymphocytes from ILFs and PP were α4β1+ (Table I). Approximately half of the α4β7+ B-lymphocytes and T-lymphocytes also express α4β1 (figure 1A). To assess a potential role for α4 integrins in the early steps of ILF development, we evaluated α4β7 and α4β1 expression by lin− c-kit+ cells in ILFs and found that 32% of these cells expressed α4β7 and 44% of these cells expressed α4β1 (Table I), and a significant proportion express both α4β7 and α4β1 (Figure 1B).
Table I.
α4 integrin expression by PP and ILF cellular populations
| PP | ILF | ||||
|---|---|---|---|---|---|
| CD3 | CD19 | CD3 | CD19 | Lin−c-kit+ | |
| α4β7 | 66.5±16.91 | 79.1±3.5 | 27.3±5.3 | 66.8±6 | 32.5±4.7 |
| α4β1 | 43.3±8.6 | 48±12.7 | 36.8±7.3 | 60.8±11.9 | 44.1±5.9 |
cellular populations were pooled from 3 mice for all replicates and repeated twice
Figure 1.
α4 integrins are expressed by ILF lymphocytes and LTi-like cells. The expression of α4β7 and α4β1 on ILFs and PP cellular populations from Balb/c mice was performed using multi-color flow cytometry. Gates were set such that ~1% of the cellular population stained positive with isotype control antibodies. Isotype control staining for panel is shown for PP CD3+ α4+ cells and CD19+ α4+ cells; no difference was observed in isotype control staining between the PP and ILFs in these cellular populations. Similar to PP, the majority of ILF B-lymphocytes express α4β7, and in comparison fewer ILF T-lymphocytes express α4β7 (Table I). Approximately one half of PP and ILF T and B-lymphocytes express α4β1 (TableI), and the majority of these α4β1 expressing cells also express α4β7 (figure 1A). Lin− c-kit+ cells make up approximately 10% of the ILF cellular population, and a significant proportion of these cells express both α4β7 and α4β1 (Table I and figure 1B). These findings are consistent with a role for the α4 integrins and their ligands in ILF development and/or function. Dot plots in panel A–F are representative of one of two independent experiments using pooled cellular populations from three mice.
To further define a potential role for the α4 integrins in this process, we examined the expression of their ligands, MAdCAM-1 and VCAM-1 within ILFs using immunohistochemistry. The expression of MAdCAM-1 (Figure 2A, B) in ILFs co-localized with CD31 expression (Figure 2C), indicating that MAdCAM-1 expression in ILFs is restricted to vascular structures. These vascular structures were not lymphatics based upon the lack of staining for Lyve (not shown). The MAdCAM-1+ structures were also PNAd negative, which could be consistent with a vessel with an immature HEV phenotype(40). We did not observe the expression of MAdCAM-1 in CPs. Consistent with prior observations demonstrating VCAM-1 expression in CP stroma, we found VCAM-1 was expressed diffusely within the ILF stroma and localized to cells of non-hematopoietic origin (CD45−) (Figure 2D).
ILF formation is arrested at a stage corresponding to the influx of lymphocytes in the absence of β7
Recent observations indicate that ILFs are a spectrum of lymphoid structures ranging from CPs, small clusters of intestinal lineage marker-negative c-kit-positive cells located at the base of the villi, to larger isolated lymphoid follicles rich in B cells (33, 41). To determine whether β7 integrins play an important role in the formation of CPs and ILFs, and to determine the stage in which this role exists, we examined β7−/− mice for the presence of CPs and ILFs. An early event in CP development is the localization of the lin− c-kit+ cells to the small intestine. We examined the percentage of lin− c-kit+ cells among lamina propria cellular population by flow cytometry and found no difference between β7−/− and wild type mice (figure 3A). Once localized to the small intestine, the lin− c-kit+ cells cluster to form CP. The lin− c-kit+ CPs cells also express CD90, and due to its intensity in immunohistochemistry, anti-CD90 staining is useful to identify and to enumerate every structure in the CP/ILF continuum. We observed that β7−/− mice had an equivalent number of CD90+ clusters when compared to wild-type mice (figure 3B), indicating that β7 is not required for the early steps of CP development, and that in the absence of β7 the total number of structures in the CP/ILF continuum is unchanged.
As CPs transition into ILFs, they become infiltrated with a prominent population of CD11c+ cells, which form a distinct cluster (our unpublished observations). We observed that CD11c+ clusters were present in β7−/− mice and were increased in number in β7−/− mice when compared to wild-type mice (figure 3C, H, and K). Despite the increase in numbers of CD11c+ clusters, we did not see a significant difference between wildtype and knockout mice in the size of these clusters (figure 3E). Inflammatory stimuli, including changes in the intestinal flora resulting from a relative deficiency in intestinal IgA, are known to augment ILF development(42, 43). Related to this, the β7−/− mice are known to have diminished intestinal IgA production(4, 13–15), therefore the increase in the number of CD11c+ clusters may be due to altered intestinal flora and resultant increased inflammation in the β7−/− mice.
The progression of CPs to ILFs occurs when a subset of CPs are infiltrated by B lymphocytes, and accordingly enumerating the total number of (B220+) B-lymphocyte clusters is an effective way to evaluate the presence of ILFs. To evaluate the role of β7 integrins in the progression to ILFs, we compared the numbers B220+ clusters between β7−/− mice and wild-type mice. We observed a striking decrease in B220+ clusters in the small intestine of the β7−/− mice (figure 3D, I, and L), indicating a profound defect in the ability of CP to progress to ILFs in the absence of β7. Size has also been used to classify structures within the CP/ILF spectrum(35). While this approach does not formally assess cellular composition, increasing cellular complexity generally correlates with increasing size of the structures as they progress to become mature ILFs(35, 41). In further support of our findings above we observed that the CD90+ clusters, which encompass all the structures in the CP/ILF continuum, were significantly smaller in the β7−/− mice (figure 3F). The β7−/− mice lacked structures >10,000µm2, corresponding to structures containing B-lymphocytes (figure 3G). T-lymphocytes are not required for CP and ILF development and do not make up a large component of the cellular population of these structures(27, 30, 44). However, related to our above findings regarding B-lymphocytes, we also observed a relative absence of CD3+ cells associated with the CD90+ clusters in the β7−/− mice (figure 3J and M). Overall these findings suggest that β7 is not required for the clustering of the LTi-like cells to form CP, or for the initial step progressing to ILFs, the infiltration by a substantial population of CD11c+ cells, however β7 is essential for later steps in ILF development related to the influx of lymphocytes into the maturing ILF.
Blockade of α4 β7/MAdCAM-1 pathway inhibits ILF formation at a stage corresponding to the influx of B-lymphocytes
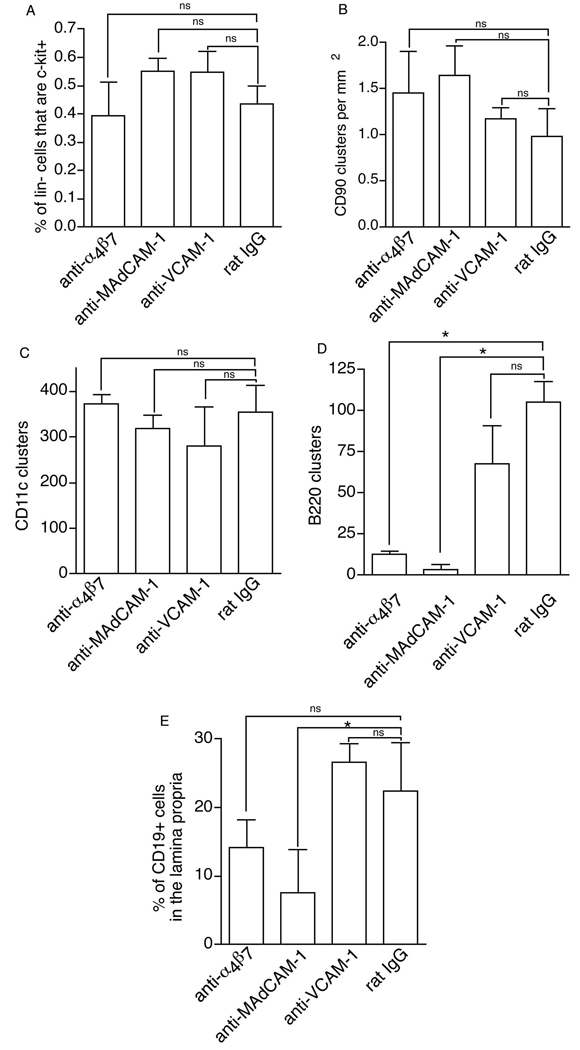
β7 associates with both the αE subunit and the α4 subunits. αEβ7 is expressed selectively on intestinal intraepithelial lymphocytes (IEL), and plays an important role in mediating the selective localization or retention of IELs by interactions with E-cadherin expressed on intestinal epithelial cells(45). We observed that less than 10% of the ILF cellular population expresses αEβ7+ (data not shown). To determine whether the deficiencies in ILF development in the β7−/− mice are mediated by the loss of α4β7 or αEβ7, we treated C57BL/6 mice with blocking antibodies specific for α4β7 and examined them for the presence of CPs and ILFs. In a correlative manner we treated mice with blocking antibodies against MAdCAM-1 and VCAM-1 to evaluate the role of these ligands in ILF development. We observed no significant differences in the percentage of lin− c-kit+ cells in the lamina propria (Figure 4A), the number of CD90+ clusters (Figure 4B), and the numbers of CD11c+ clusters (Figure 4C) between the four treatment groups. However, mice receiving antibodies to α4β7, MAdCAM-1 contained significant fewer B220 clusters, while mice receiving anti-mouse VCAM-1 antibody showed no significant difference in the numbers of B220+ clusters when compared with control Ig treated mice (Figure 4D). The defect in ILF formation in the mice receiving antibodies to MAdCAM-1 correlated with a decrease in the lamina propria B-lymphocyte population (Figure 4E). There was no significant difference in the lamina propria B-lymphocyte population in animals in the other treatment groups. This suggests that the blockade of ILF formation seen in β7−/− mice is mediated by α4β7/MAdCAM-1 pathway, and this blockade occurs at the later stage of ILF formation corresponding to the influx of B-lymphocytes.
Figure 4.
Blockade of the α4β7/MAdCAM-1 pathway in adult mice inhibits ILF formation at a stage corresponding to the influx of B-lymphocytes. C57BL/6 mice receiving blocking antibodies to mouse α4β7, mouse MAdCAM-1, mouse VCAM-1 or rat IgG (control) were evaluated for the density of CD90+ clusters, the percentage of lin− c-kit+ cells and CD19+ B-lymphocytes in the lamina propria, and the presence of CD11c clusters and B220 clusters. There were no significant differences in percentage of lamina propria lin− cells expressing c-kit (panel A), the number of CD90+ clusters (panel B), and the numbers of CD11c clusters (panel C) between the four groups. Mice receiving antibodies to α4β7 or MAdCAM-1 contained significant fewer B220+ clusters when compared with controls (panel D). Mice receiving anti-mouse VCAM-1 antibody showed no significant difference in the numbers of B220+ clusters (panel D). The defect in ILF formation in the mice receiving antibodies to MAdCAM-1 correlated with a decrease in the lamina propria B-lymphocyte population (panel E). The data is presented as the mean ± the standard error of the mean of data generated from 3 or more mice in each group. * = p <0.05, ns = not significant.
β7 sufficient bone marrow derived cells are essential for the formation of ILFs
In addition to being substantial constituents of ILFs, B-lymphocytes play an important role in ILF development by facilitating the transition of ILFs from a loose cellular cluster to an organized lymphoid tissue with an overlying FAE(30). The above findings indicate that α4β7/MAdCAM-1 pathway is important for ILF formation and imply that α4β7 expression by B-lymphocytes may be required for their localization and subsequent contribution to the developing ILF. To evaluate this, we injected bone marrow from gender-matched β7−/− (CD90.2, IgMb) mice and C57BL/6 (CD90.2, IgMb) mice into irradiated B6.Cg-IgHa Thy1a Gpi1a/J (CD90.1, IgMa) mice. This approach allowed us to distinguish donor (β7−/− or wildtype) CD90.2+ CP cells from recipient (wildtype) CD90.1+ CP cells and evaluate the ability of wildtype or β7−/− lymphocytes to localize to the wildtype CP. Analysis of splenocytes from the recipients demonstrated effective reconstitution with IgMb+ cells and CD90.2+ cells from donors, as well as a deficiency in β7 in the recipients of β7−/− bone marrow (Figure 5 A and B). We did not observe any difference in the density of total CD90+ clusters or CD11c+ clusters between mice receiving bone marrow from β7−/− and C57BL/6 donors, however the density of B220 clusters in mice receiving β7−/− bone marrow was significantly lower than that of mice receiving wildtype bone marrow (figure 5 C, D, F, G and I). Irradiation did not eliminate recipient CD90.1+ CP cells (figure 5 D and G). We found that all CD90+ clusters in both groups of animals contained a large population of CD90.1+ (recipient derived) CP cells. While the density of total CD90+ clusters was not different, we observed fewer CD90+ CD90.2+ (donor derived) cells in the CD90+ clusters in recipients of β7−/− bone marrow (figure 5 C and F). This implied a relative defect in the ability of the CD90+ CP cells from β7−/− mice to localize to the sites of CP when competing with the endogenous wildtype CP cells. The presence of wildtype (CD90.1+) CP cells in all the clusters and ability of the CD90+ clusters to recruit in CD11c+ cells (figure 5 E and H) indicates that a functional deficiency in CD90+ cells is unlikely to account for the diminished number of B220+ clusters in the β7−/− bone marrow recipients.
α4 β7 plays a redundant role in localizing LTi-like cells to the small intestine and a non-essential role in CP formation
In contrast to the above findings, we observed a normal number of CD90+ clusters in the β7−/− mice (figure 3B). CP development occurs over several days in the postnatal period. This relatively protracted period may allow time for other pathways to compensate in the absence of α4β7 function, and consequently evaluation of adult animals may not reveal subtle defects such as a delay in CP formation. To investigate this possibility we evaluated the ability of β7−/− and wildtype CP cells to compete for the CP niche following bone marrow transfer, and we evaluated the development of CP in the neonatal period in mice lacking α4β7 function.
In competitive bone marrow transfer experiments equal numbers of wildtype (CD45.2, CD90.1) and β7−/− (CD45.2, CD90.2) bone marrow cells were injected into wildtype (CD45.1, CD90.2) recipients. Recipients were analyzed by flow cytometry for the presence of donor wildtype and β7−/− lin+ splenocytes and donor wildtype and β7−/− lin+ and lin− lamina propria cells. CD45.2 marks all donor derived cells and CD90.1 and CD90.2 distinguishes between wildtype and β7−/− LTi-like cells and lymphocytes. Flow cytometry revealed that recipient lin+ splenocytes were enriched in β7−/− (CD90.2+ CD45.2+) donor cells when compared with wildtype (CD90.1+ CD45.2+) donor cells and conversely the lin+ lamina propria cells contained significantly fewer β7−/− donor cells when compared with wildtype cells (figure 6A). The spleen contained too few lin−CD90+ cells for analysis, however analysis of the lamina propria revealed a trend towards fewer β7−/− lin− donor cells localizing to the lamina propria when compared with wildtype lin− donor cells; this trend did not reach statistical significance when compared with the ratio of donor and wildtype lin+ splenocytes (figure 6A). Analysis of the composition of CP by immunohistochemistry revealed approximately equal populations of CD90+ cells were of wildtype (CD90.1+) donor origin and β7−/− (CD90.2+) donor origin or (CD90.2+) recipient origin (figure 6B). However the majority of the donor (CD45.2+) CP cells were of wildtype (CD90.1+) donor origin (figure 6C) and the vast majority of these CD90+ cells were CD3− and c-kit+ (not shown). These findings suggest a relative inefficiency of the β7−/− CD90+ cells to localize to the CP.
CP begin to form around day 14 of neonatal life (44). We observed that the clustering of CD90+ cells becomes apparent at day 17 of neonatal life with a large increase in the numbers of CD90+ clusters at neonatal day 19 (our unpublished observations). To evaluate a role for α4β7 in CP development in the neonatal period, we evaluated neonatal wildtype and β7−/− mice and mice given blocking antibodies to α4β7, MAdCAM-1, VCAM-1, or control Ig from day 17 of gestation until analysis at day 19 of neonatal life. We found that CP are detectable at day 17 of neonatal life in the absence of β7 (figure 6 D), and the absence of β7 did not alter the density of CP in day 19 neonatal mice (figure 6E). In a related manner, blocking antibodies to α4β7, MAdCAM-1, or VCAM-1 did not affect CP development (figure 6F), however, similar to our observations in adult animals, blockade with α4β7 or MAdCAM-1 did inhibit the influx of B-lymphocytes into the transitioning CP (figure 6G). These observations imply that α4β7 has a role in facilitating the localization of the LTi-like cells to the small intestine and CP, but in its absence CP formation progresses normally.
α4 β7 expression by B-lymphocytes is required for their localization to the transitioning CP
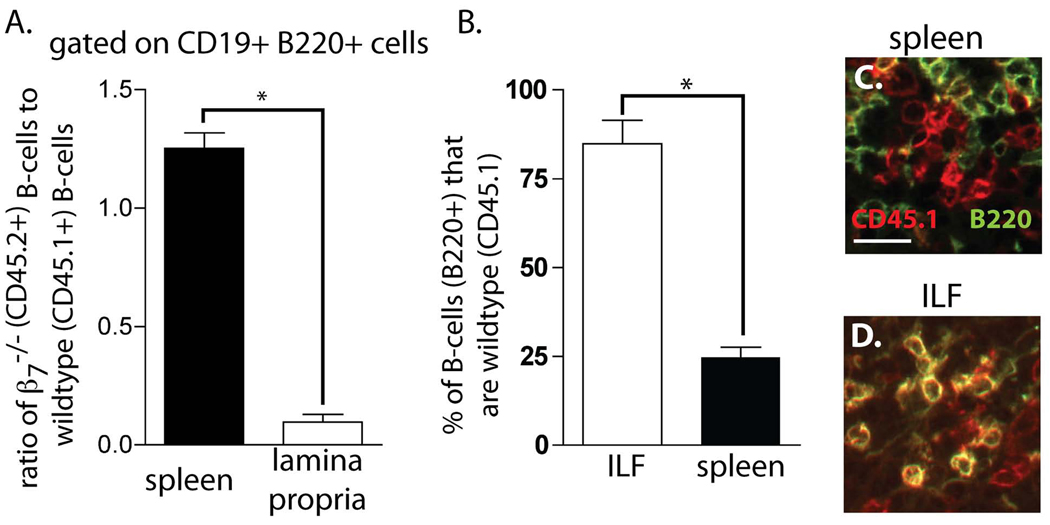
Our above observations demonstrate that α4β7 and MAdCAM-1 play important roles in localizing lymphocytes to the transitioning CP. This role could involve the expression of α4β7 directly on lymphocytes or α4β7 expression by other cell types needed to subsequently recruit lymphocytes to the transitioning CP. To evaluate the role of α4β7 expression on B-lymphocytes in their localization to ILFs we performed competitive adoptive transfers of wildtype (CD45.1) and β7−/− (CD45.2) splenic B-lymphocytes into RAG−/− mice and analyzed the ability of the B-lymphocytes to localize to the small intestine and CP by flow cytometry and immunohistochemistry. We observed that β7−/− B-lymphocytes were less efficient at localizing to the small intestine when compared to wildtype B-lymphocytes (figure 7A). Moreover analysis of the B220+ cells within the ILFs revealed that in contrast to the spleen, the majority of cells were of wildtype (CD45.1+) donor origin; the co-staining for CD45.1 (red) and B220 (green) appears yellow within the ILFs. (figure 7B, C, and D), confirming that α4β7 expression on B-lymphocytes plays a critical role in recruiting these cells into the transitioning CP and its subsequent progression into ILFs.
Figure 7.
β7 deficient B-lymphocytes have impaired localization to ILFs. To evaluate the role of β7 in localizing B-lymphocytes to ILFs, equivalent numbers of splenic B-lymphocytes from β7−/−(CD45.2) and B6SJL (CD45.1) donors were transfered into RAG−/− recipients and analyzed for the presence of donor B-lymphocyte populations in the spleen and lamina propria by flow cytometry, and in the ILFs by immunohistochemistry. Flow cytometry analysis demonstrated enrichment in β7−/− (CD45.2) B-lymphocytes in the spleen and a relative paucity of β7−/− B-lymphocytes in the lamina propria (panel A). Immunohistochemistry demonstrated that the majority of the B-lymphocytes (B220+) within the ILFs were of wildtype (CD45.1+) origin (panels B, C, and D). Data in panels A and B are displayed as mean ± standard deviation of data generated from 4 mice in each group. * = p <0.01, scale bar in panel C = 20µm.
Discussion
Organized lymphoid tissues play a crucial function by facilitating the interactions of antigen presenting cells and lymphocytes to generate protective immune responses. Within the intestinal mucosa two classes of lymphoid tissues can perform this critical function, PP and ILFs. PP are canonical secondary lymphoid tissues whose formation is developmentally driven with the crucial early events occurring during a restricted period during embryogenesis(46). While ILFs can share many morphologic and developmental features with PP, the development of ILFs is not restricted by embryonic timing and can be augmented by inflammatory stimuli encountered throughout life(28, 35). Therefore compared with the relatively uniform level of maturity among PP in adult animals ILFs are a continuum of lymphoid tissues in various stages of maturity. Recent studies indicate that CP are progenitor cellular aggregates giving rise to ILFs, and that the lin− c-kit+ cells within CP function in analogous manner to the fetal LTi cells delivering early signals required for lymphoid tissue development(33, 41). Understanding how this reservoir of immune inductive sites develops is a central issue in understanding how the mucosal immune system functions in its protection against potential pathogens.
α4 integrins play diverse roles in the immune systems including essential functions in the development and maintenance of lymphoid tissues. α4β7 and its ligand MAdCAM-1 have well described roles in lymphocyte homing to the gut(4, 13–15), and function in a related manner localizing fetal LTi cells to the intestine to deliver the early signals for PP organogenesis(47). α4β1 and its ligand VCAM-1 also play a critical role in PP organogenesis. α4β1 is expressed by the fetal LTi cells and interactions with VCAM-1 expressed by the stromal organizer cells are believed to be important for maintaining a self sustaining cellular cluster and formation of the PP anlagen(2, 10–12). The similarities between PP and ILF suggest that α4 integrins might also have important and diverse roles in ILF development, however a potential role for α4 integrins in ILF development has not been previously addressed.
We observed that a significant proportion of ILF lymphocytes and LTi like cells express α4β1 and α4β7. In accord with prior observations we found that VCAM-1 expression was primarily restricted to non-hematopoietic stromal cells within the CP and ILFs(39). MAdCAM-1 expression was primarily restricted to non-lymphatic vessels within the ILFs. In comparison to PP HEVs, these vessels universally had low or no PNAd expression, and could represent immature HEVs(40).
Our findings provide convincing evidence of a role for α4β7 and MAdCAM-1 in recruiting B-lymphocytes into the developing ILFs. Animals deficient in β7 and animals receiving blocking antibodies to α4β7 or MAdCAM-1 had similar phenotypes demonstrating a blockage of ILF development at a stage corresponding to the influx of B-lymphocytes. Likewise the bone marrow chimeric mice demonstrated a deficiency in the localization of β7−/− B-lymphocytes to the developing ILFs despite the presence of wildtype CD90+ cells and the ability to recruit dendritic cells to the growing cluster. Furthermore adoptive co-transfer of wildtype and β7−/− mature B-lymphocytes demonstrated that β7−/− B-lymphocytes were less efficient at localizing to the small intestine and the transitioning CP. The recruitment of B-lymphocytes into the developing ILF is of particular importance, as LT dependent signals delivered by this cell type are required for the maturation of ILFs into a functional immune inductive site containing a follicle associated epithelium(29, 30). These observations are consistent with the role of α4β7 for localizing B-lymphocytes to PP and add ILFs as another mucosal site highly dependent upon α4β7 and MAdCAM-1 for lymphocyte entry.
The role of α4β7 expression by the lin− c-kit+ CP cells in ILF development is less critical. Mice deficient in β7 have a normal number of PP that are hypoplastic, suggesting the early events determining PP number are intact(4, 13–15). These early events are dependent upon signals delivered by the fetal LTi cells, therefore implicating that the function of the fetal LTi cells inducing PP anlagen development is intact in β7 deficiency. The lin− c-kit+ CP cells share phenotypic and developmental features with the fetal LTi cells and are felt to function in a similar manner delivering the early signals that subsequently result in ILF development(33). We observed no deficiency in the localization of the lin− c-kit+ cells to the small intestine or the clustering of these cells to form CP in the absence of β7, suggesting that in accord with PP development in the β7−/− mice, these early events are preserved. In contrast to this, previous investigations revealed that MAdCAM-1 blockade during embryogenesis reduced fetal LTi cell recruitment to developing lymphoid tissues, thus implying a role for α4β7/MAdCAM-1 interactions in fetal LTi cell localization (47). Consistent with this our bone marrow chimeric studies demonstrated that β7−/− CP cells were less efficient at localizing to the small intestine and CP when they were required to compete with wildtype CP cells for this niche. This contrasts with our observations that CP development in the neonatal period is intact in animals lacking α4β7/MAdCAM-1 function. In total these observations are consistent with a role for α4β7 in efficiently localizing the lin− c-kit+ cells to the small intestine and a non-essential role for α4β7 in CP development.
Despite identifying α4β1 and VCAM-1 expression by ILF cell types, our observations did not identify a role for VCAM-1 in CP and ILF development in adult and neonatal mice. This is in contrast to studies demonstrating a role for VCAM-1 and β1 in embryonic events required for PP development (2, 47). Our observations could result from a functional difference between PP and CP development, or like α4β7, α4β1 may play a less essential role in CP development that was not revealed by these studies.
Blockade of the α4β7/MAdCAM-1pathway is a potential therapy for inflammatory bowel disease, with the appeal that the effects of blocking this pathway will largely be limited to mucosal sites, and therefore in comparison to more global immunomodulators, systemic toxicity will be reduced. The findings presented here indicate that blocking this pathway will adversely effect the development, and hence the function of ILFs. While the function of these structures is still an ongoing area of investigation, ILFs have been shown to play an important role generating immune responses to luminal antigens, and by extension protection from potential pathogens(29). These observations highlight the duality of the α4β7/MAdCAM-1 pathway contributing to intestinal inflammation and simultaneously playing an essential role in the development of lymphoid tissues that promote immune homeostasis.
Footnotes
This work was supported in part by NIH grant DK-64798 (R.D.N.), the Crohn’s and Colitis Foundation of America (C.W.), the Washington University School of Medicine Digestive Diseases Research Core Center grant (P30-DK52574), and The Siteman Cancer Center High Speed Cell Sorting Core supported in part by an NCI Cancer Center Support Grant (P30 CA91842).
Abbreviations used in this manuscript: LTi, lymphoid tissue inducer; CP, cryptopatch; ILF, isolated lymphoid follicle; lymphotoxin, LT; lymphotoxin beta receptor, LTβR; Peyer’s patch, PP; lin−, lineage marker negative.
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 2.Finke D, Acha-Orbea H, Mattis A, Lipp M, Kraehenbuhl J. CD4+CD3− cells induce Peyer's patch development: role of alpha4beta1 integrin activation by CXCR5. Immunity. 2002;17:363–373. doi: 10.1016/s1074-7613(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 4.Wagner N, Lohler J, Kunkel EJ, Ley K, Leung E, Krissansen G, Rajewsky K, Muller W. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 5.Binion DG, West GA, Ina K, Ziats NP, Emancipator SN, Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology. 1997;112:1895–1907. doi: 10.1053/gast.1997.v112.pm9178682. [DOI] [PubMed] [Google Scholar]
- 6.Binion DG, West GA, Volk EE, Drazba JA, Ziats NP, Petras RE, Fiocchi C. Acquired increase in leucocyte binding by intestinal microvascular endothelium in inflammatory bowel disease. Lancet. 1998;352:1742–1746. doi: 10.1016/S0140-6736(98)05050-8. [DOI] [PubMed] [Google Scholar]
- 7.Keszthelyi E, Karlik S, Hyduk S, Rice GP, Gordon G, Yednock T, Horner H. Evidence for a prolonged role of alpha 4 integrin throughout active experimental allergic encephalomyelitis. Neurology. 1996;47:1053–1059. doi: 10.1212/wnl.47.4.1053. [DOI] [PubMed] [Google Scholar]
- 8.Cupedo T, Kraal G, Mebius RE. The role of CD45+CD4+CD3− cells in lymphoid organ development. Immunol Rev. 2002;189:41–50. doi: 10.1034/j.1600-065x.2002.18905.x. [DOI] [PubMed] [Google Scholar]
- 9.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. Epub 2003 Dec 2021. [DOI] [PubMed] [Google Scholar]
- 10.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 11.Finke D. Fate and function of lymphoid tissue inducer cells. Curr Opin Immunol. 2005;17:144–150. doi: 10.1016/j.coi.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3− cells to colonize lymph nodes. Proc Natl Acad Sci U S A. 1996;93:11019–11024. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schweighoffer T, Tanaka Y, Tidswell M, Erle DJ, Horgan KJ, Luce GE, Lazarovits AI, Buck D, Shaw S. Selective expression of integrin alpha 4 beta 7 on a subset of human CD4+ memory T cells with Hallmarks of gut-trophism. J Immunol. 1993;151:717–729. [PubMed] [Google Scholar]
- 14.Wagner N, Muller W. Functions of alpha 4- and beta 7-integrins in hematopoiesis, lymphocyte trafficking and organ development. Curr Top Microbiol Immunol. 1998;231:23–32. doi: 10.1007/978-3-642-71987-5_2. [DOI] [PubMed] [Google Scholar]
- 15.Williams MB, Butcher EC. Homing of naive and memory T lymphocyte subsets to Peyer's patches, lymph nodes, and spleen. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]
- 16.Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E, Ringler DJ. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158:2099–2106. [PubMed] [Google Scholar]
- 17.Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR, Newman W, Ringler DJ. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuzaki K, Tsuzuki Y, Matsunaga H, Inoue T, Miyazaki J, Hokari R, Okada Y, Kawaguchi A, Nagao S, Itoh K, Matsumoto S, Miura S. In vivo demonstration of T lymphocyte migration and amelioration of ileitis in intestinal mucosa of SAMP1/Yit mice by the inhibition of MAdCAM-1. Clin Exp Immunol. 2005;140:22–31. doi: 10.1111/j.1365-2249.2005.02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol. 1999;65:349–355. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe C, Miura S, Hokari R, Teramoto K, Ogino T, Komoto S, Hara Y, Koseki S, Tsuzuki Y, Nagata H, Granger DN, Ishii H. Spatial heterogeneity of TNF-alpha-induced T cell migration to colonic mucosa is mediated by MAdCAM-1 and VCAM-1. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1379–G1387. doi: 10.1152/ajpgi.00026.2002. [DOI] [PubMed] [Google Scholar]
- 21.Feagan BG, Greenberg GR, Wild G, Fedorak RN, Pare P, McDonald JW, Dube R, Cohen A, Steinhart AH, Landau S, Aguzzi RA, Fox IH, Vandervoort MK. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–2507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- 22.Hesterberg PE, Winsor-Hines D, Briskin MJ, Soler-Ferran D, Merrill C, Mackay CR, Newman W, Ringler DJ. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7. Gastroenterology. 1996;111:1373–1380. doi: 10.1053/gast.1996.v111.pm8898653. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa T, Miura S, Tsuzuki Y, Ogino T, Teramoto K, Inamura T, Watanabe C, Hokari R, Nagata H, Ishii H. Chronic allergy to dietary ovalbumin induces lymphocyte migration to rat small intestinal mucosa that is inhibited by MAdCAM-1. Am J Physiol Gastrointest Liver Physiol. 2004;286:G702–G710. doi: 10.1152/ajpgi.00183.2003. [DOI] [PubMed] [Google Scholar]
- 24.Stopfer P, Obermeier F, Dunger N, Falk W, Farkas S, Janotta M, Moller A, Mannel DN, Hehlgans T. Blocking lymphotoxin-beta receptor activation diminishes inflammation via reduced mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expression and leucocyte margination in chronic DSS-induced colitis. Clin Exp Immunol. 2004;136:21–29. doi: 10.1111/j.1365-2249.2004.02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teramoto K, Miura S, Tsuzuki Y, Hokari R, Watanabe C, Inamura T, Ogawa T, Hosoe N, Nagata H, Ishii H, Hibi T. Increased lymphocyte trafficking to colonic microvessels is dependent on MAdCAM-1 and C-C chemokine mLARC/CCL20 in DSS-induced mice colitis. Clin Exp Immunol. 2005;139:421–428. doi: 10.1111/j.1365-2249.2004.02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Assche G, Rutgeerts P. Antiadhesion molecule therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2002;8:291–300. doi: 10.1097/00054725-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, Yamamoto H, Ishikawa H. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 28.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated Lymphoid Follicle Formation Is Inducible and Dependent Upon Lymphotoxin-Sufficient B Lymphocytes, Lymphotoxin beta Receptor, and TNF Receptor I Function. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 29.Lorenz RG, Newberry RD. Isolated Lymphoid Follicles Can Function as Sites for Induction of Mucosal Immune Responses. Ann N Y Acad Sci. 2004;1029:44–57. doi: 10.1196/annals.1309.006. [DOI] [PubMed] [Google Scholar]
- 30.McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174:5720–5728. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
- 31.Newberry RD, Lorenz RG. Organizing a mucosal defense. Immunological Reviews. 2005;206:6–21. doi: 10.1111/j.0105-2896.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, McDonald KG, McDonough JS, Newberry RD. Murine isolated lymphoid follicles contain follicular B-lymphocytes with a mucosal phenotype. Am J Physiol Gastrointest Liver Physiol. 2006 doi: 10.1152/ajpgi.00525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol. 2005;5:413–420. doi: 10.1038/nri1600. [DOI] [PubMed] [Google Scholar]
- 34.Kweon MN, Yamamoto M, Rennert PD, Park EJ, Lee AY, Chang SY, Hiroi T, Nanno M, Kiyono H. Prenatal blockage of lymphotoxin beta receptor and TNF receptor p55 signaling cascade resulted in the acceleration of tissue genesis for isolated lymphoid follicles in the large intestine. J Immunol. 2005;174:4365–4372. doi: 10.4049/jimmunol.174.7.4365. [DOI] [PubMed] [Google Scholar]
- 35.Pabst O, Herbrand H, Friedrichsen M, Velaga S, Dorsch M, Berhardt G, Worbs T, Macpherson AJ, Forster R. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol. 2006;177:6824–6832. doi: 10.4049/jimmunol.177.10.6824. [DOI] [PubMed] [Google Scholar]
- 36.Fujimura Y, Kamoi R, Iida M. Pathogenesis of aphthoid ulcers in Crohn's disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut. 1996;38:724–732. doi: 10.1136/gut.38.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiserling E. Newly-formed lymph nodes in the submucosa in chronic inflammatory bowel disease. Lymphology. 2001;34:22–29. [PubMed] [Google Scholar]
- 38.Lockhart-Mummery HE, Morson BC. Crohn's disease (regional enteritis) of the large intestine and its distinction from ulcerative colitis. Gut. 1960;1:87–105. doi: 10.1136/gut.1.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor RT, Lugering A, Newell KA, Williams IR. Intestinal cryptopatch formation in mice requires lymphotoxin alpha and the lymphotoxin beta receptor. J Immunol. 2004;173:7183–7189. doi: 10.4049/jimmunol.173.12.7183. [DOI] [PubMed] [Google Scholar]
- 40.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 41.Pabst O, Herbrand H, Worbs T, Friedrichsen M, Yan S, Hoffmann MW, Korner H, Bernhardt G, Pabst R, Forster R. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35:98–107. doi: 10.1002/eji.200425432. [DOI] [PubMed] [Google Scholar]
- 42.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci U S A. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. Epub 2004 Feb 1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrew DP, Rott LS, Kilshaw PJ, Butcher EC. Distribution of alpha 4 beta 7 and alpha E beta 7 integrins on thymocytes, intestinal epithelial lymphocytes and peripheral lymphocytes. Eur J Immunol. 1996;26:897–905. doi: 10.1002/eji.1830260427. [DOI] [PubMed] [Google Scholar]
- 46.Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mebius RE, Schadee-Eestermans IL, Weissman IL. MAdCAM-1 dependent colonization of developing lymph nodes involves a unique subset of CD4+CD3− hematolymphoid cells. Cell Adhes Commun. 1998;6:97–103. doi: 10.3109/15419069809004464. [DOI] [PubMed] [Google Scholar]