Abstract
Misediting of the serotonin (5HT) 2C receptor (5HT2CR) has been implicated in both depression and anxiety. The adenosine deaminases that act on double stranded RNAs (ADARs) are reported to modify the 5HT2CR by RNA editing. Transgenic mice misexpressing the RNA editing enzyme ADAR2 show an adult onset obese phenotype due to chronic hyperphagia, but little more than this is known about the behavior of these animals. The present experiments examined whether affect-associated behaviors are also altered in ADAR2 transgenic mice. Age- and weight-matched transgenic mice misexpressing ADAR2 were tested for signs of behavioral despair with the forced swim (FST) and tail suspension (TST) tests, and for anxiety by evaluating spontaneous exploration in a novel environment and by elevated plus maze performance. Plasma corticosterone was also determined by radioimmunoassay. Transgenic mice of both sexes displayed indications of increased behavioral despair on first exposures to the TST and the FST. Behavioral despair persisted in ADAR2 mice in that it was also observed in the FST in tests administered 24 hr and 1 week following the initial TST and FST. ADAR2 transgenic mice also displayed behaviors associated with anxiety as indicated by decreased entry into the open arms in an elevated plus maze test. Both sexes of ADAR2 transgenic mice displayed elevated plasma corticosterone. Taken together, the results suggest that ADAR2 transgenic mice represent a novel rodent model of endogenous behavioral despair and anxiety accompanied by elevated hypothalamo-pituitary adrenal axis activity.
Keywords: Elevated plus maze, obesity, animal models, behavioral despair, tail suspension test, forced swim test, anxiety
1. Introduction
The World Health Organization[82] predicts that within a decade depression will be a leading cause of disability, second only to heart disease. At the present time, depression constitutes a monumental health problem, and the direct costs of depression are a significant burden to the economy [43]. Since the 1960s, major depression has been diagnosed based on symptomatic criteria set forth in the Diagnostic and Statistical Manual of Mental Disorders [1]. Most pharmacological treatments for depression modulate brain noradrenergic and serotonergic neurotransmitter systems, and many effective antidepressants act on noradrenergic and serotonergic signal transduction pathways to affect G protein-coupled receptors and associated second messenger systems.
Multiple functional studies have provided evidence of a role for the G protein-coupled serotonin (5HT) 2C receptor (5HT2CR) in the therapeutic actions of antidepressant drugs [37,72]. 5HT2CR are widely distributed throughout the brain, have been implicated in affective disorders such as depression and/or anxiety [21,57,66] and represent an important therapeutic target. 5HT2CR are the only known G protein-coupled receptors to be subjected to a post transcriptional modification referred to as RNA editing. RNA editing changes produce a 5HT2CR with altered signaling properties [9,20,53,59,71,88], and the subsequent change in receptor second messenger function has been suggested to underlie depression and anxiety [18,31,40].
RNA editing of 5HT2CR is catalyzed by a family of enzymes known as adenosine deaminases that act on RNAs (ADARs) [4,27]. This family of enzymes facilitates the conversion of adenosine to inosine residues (A-to-I) in mRNA to effectively alter the nucleotide sequence of mature mRNAs. As a result, the amino acid coding potential of the relevant mRNA is modified, thus leading to altered biological activity of translated proteins [17]. Multiple cDNA isoforms of ADAR2 exist in rats, mice and humans as a result of alternative splicing mechanisms that may affect protein expression and function [24,46,74]. ADAR2 can also autoedit its own transcript to create an alternative splice acceptor site to down-regulate its own expression [19,74].
A recent report using bioinformatics suggests that there may be more than 2600 mRNAs in humans that can potentially undergo A-to-I RNA editing, but as of yet such mRNAs have not had their functional roles identified [48]. At the present time, A-to-I RNA editing has mainly been identified in only a few receptors and ion channels in the central nervous system. These include the 5HT2CR, a glutamate receptor, a GABA receptor, and a potassium channel [6,8,9,61,76,81]. Interestingly, many of these potential editing changes appear in neural systems implicated in the pathophysiology of major mental disorders such as schizophrenia, bipolar disorder, anxiety and major depression [22,31,39,47,60,64,80].
In order to study whether misexpression of ADAR2 results in aberrant editing of ADAR2 target RNAs, a transgenic mouse was generated expressing an epitope FLAG tagged rat ADAR2b cDNA under the control of a human cytomegalovirus promoter [79]. ADAR2 transgenic mice display a mature onset obese phenotype due to chronic hyperphagia [79]. Prior to obesity, ADAR2 transgenic mice have normal plasma glucose, insulin and leptin levels. Little is known about the behavior of ADAR2 transgenic mice [79] other than that they display normal locomotor activity in the non-obese and obese states and that they have hyperphagia in comparison to their control littermates, (see [79] supplementary data Fig. S1). Recently in the course of handling ADAR2 transgenic mice, we observed that the animals were extremely docile and submissive. This led us to speculate that ADAR2 transgenic mice may display elevated behavioral despair and prompted us to conduct further behavioral analyses of this model.
In rats and mice the forced swim test (FST) and the tail suspension test (TST) are two commonly applied behavioral despair paradigms used to screen compounds for pharmacological antidepressant potential [67–69,83]. In these tests decreased activity under experimental conditions where rodents would normally struggle to escape is used as an indication of behavioral despair. To further characterize the behavioral phenotype of ADAR2 transgenic mice and to determine if they display signs of behavioral despair, we used both the FST and the TST in experimentally age- and weight-matched naïve transgenic mice and their control littermates. In addition, since anxiety and depression are often frequently comorbid [89], the present study also tested for anxiety-associated behaviors [49] by examining locomotor activity in a novel environment and performance in the elevated plus maze (EPM). Finally since activity of the hypothalamo-pituitary-adrenal (HPA) axis is altered in states associated with depression and anxiety, basal plasma corticosterone levels were determined in ADAR2 transgenic and control mice.
2. Methods
2.1 Transgenic mice and housing
All studies were conducted on hemizygous transgenic or wild-type mice (e.g., control littermates) that were maintained on C57BL/6J X DBA2 hybrid background by back-crossing to the C57BL/6J X DBA2 (F1) parent strain [79]. Mice were individually housed with a 12:12h light:dark cycle with lights turned on at 0600 h. ADAR2 transgenic mice have mature onset obesity due to chronic hyperphagia [79]. Consequently in the present study, mice were maintained ad libitum on a leaner modified diet (NIH-31 6% mouse/rat diet; 7013, Harlan, Indianapolis) in order to delay the onset of the obese phenotype. This allowed us to assess the behavior of ADAR2 transgenic mice prior to frank obesity. Tap water was available ad libitum.
2.2 Genotyping
For genotype analysis of transgenic mice by polymerase chain reaction (PCR), genomic DNA from whole blood was amplified with the REDExtract-N-Amp™ Blood PCR Kit (Sigma-Aldrich, St. Louis, MO) using sense and antisense primers corresponding to positions 258–275 and 639–659 relative to the start codon of rat ADAR2, respectively. The 402 bp PCR amplicon was subsequently digested with Apa I to generate 313 and 89 bp fragments for the rat ADAR2 transgene, or an uncut 402 bp fragment for mouse ADAR2-derived sequences [79].
2.3 Behavioral assays
At 7 weeks of age, weight-matched control and transgenic mice were tested for indications of behavioral despair in both the FST [68,69] and TST [83]. The duration of each test was 5 min, and these tests were video recorded for later analysis by an observer blind to the genotype of the mice.
Forced swim test
The FST was modified from the original report [68,69]. Mice were placed individually into a Plexiglas cylinder (46 cm height, 21.5 cm diameter) filled with water to a depth of 15 cm. The water temperature was maintained at 23–25°C. After the mouse was placed in the water, it was left undisturbed for the test session.
Tail suspension test
Adhesive tape was used to suspend the mice by their tail from a rod for the TST [83]. The height of the rod was 50 cm.
Elevated plus maze test of anxiety
The EPM was made of black Plexiglas® with arms 30 cm long and 5 cm wide extending from a central platform (5 cm square). Two opposite arms were enclosed by black Plexiglas® walls (15 cm high), and two arms were open with a small ledge (0.25 cm high). The apparatus was situated 40 cm from the floor. The mouse was placed on the central platform (5×5 cm) facing an enclosed arm, and its behavior was video recorded over a 5 min period. Videotaping was conducted under white light illumination with a video camera located 100 cm above the maze. The total amount of time spent in the closed arms and open arms during the 5 min session was scored from videotapes by an observer blind to the genotype of the mice [63,73].
Locomotor activity in a novel environment
In a dark room, free exploration and spontaneous locomotor activity were recorded in a novel environment. This testing was conducted in a chamber that had the following dimensions: 40.5 cm in length × 20 cm in width and was covered with a ventilated lid. Inside the chamber was a center island that measured 24.8 cm length × 4.2 cm width × 20 cm height. Two infra-red beams with associated detectors were each located 5 cm from the two ends of the chambers. As the mice locomotioned around the island, they interrupted the photocell beams. Beam breaks were cumulatively recorded over the course of a 120 min test session.
2.4 Corticosterone assay
After the behavioral assays, the mice were allowed to recover in their home cages. At the termination of the experiments, the mice were decapitated and trunk blood was collected at 0900 h, centrifuged and kept at −80°C for determination of plasma corticosterone [7]. Corticosterone was measured using a Coat-A-Count Rat Corticosterone solid-phase radioimmunoassay (RIA) kit from Diagnostics Products Inc. (Los Angeles, CA). Sensitivity of the corticosterone RIA was 5.7 ng/ml and the intra- and inter-assay coefficients of variation averaged 7% and 9%, respectively.
2.5 Experimental Protocols
Protocol 1 (Group I mice)
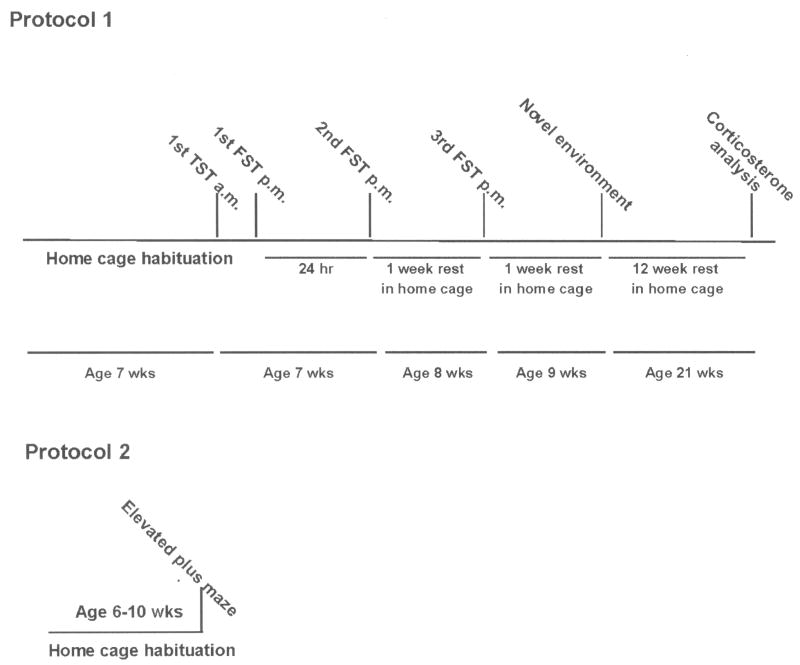
After 4 weeks of habituation in their home cages, post weaned groups of age- and weight-matched control and transgenic mice at 7 weeks of age (i.e., both sexes) were subjected to an initial behavioral despair test (the TST) at 0900 h. After a 2 hr rest period following the TST, a FST was then conducted. 24 hr following the first behavioral despair tests, a second FST was carried out. The animals were then allowed to rest from the behavioral assays in their home cages for 1 week at which time a third FST was conducted. Again, mice were returned to their home cages and allowed to recover from the FST for an additional week. Following this period, they were tested in a novel environment where their locomotor activity was recorded for 120 min. Following the novel environment test the mice were returned to their home cage and allowed to rest for several weeks. At 21 weeks of age with minimum disturbance, the mice were decapitated, and trunk blood was collected for corticosterone analysis at 0900 h (Fig. 1).
Figure 1.
Outline of protocols employed in the analysis of behavioral phenotyping of ADAR2 transgenic mice. In protocol 1, groups of weight-matched (e.g., both sexes) control and transgenic mice were used in the behavioral despair test, novel environment, elevated plus maze and plasma analysis. In protocol 2, a group of naïve control and transgenic mice were used in the elevated plus maze test. TST = tail suspension test; FST = forced swim test.
Protocol 2 (Group II mice)
In a second group of naïve animals the EPM was used to determine if anxiety-associated behaviors were present before any other tests were conducted (Fig. 1) [2].
2.6 Statistical methods
Experimental results are expressed as mean ± SEM. Statistical analyses of differences between groups were performed using two-way ANOVA for body weights at the times animals were tested in the novel environment in the TST and FST, on the EPM, and when blood was collected for the determination of corticosterone. The linear mixed model analysis for repeated measures was used for body weight at the behavioral test, and immobility time on the FST. For the EPM and novel environment activity, negative binomial regression analysis was used to compare the number of beam breaks between the transgenic and control mice for three different time periods. To account for the correlation of responses from the same mice for three time periods, the negative binomial regression model was fitted using the method of generalized estimating equations (GEE). The outcome involving choice of going into the closed or open arm from the center, which represents a binomial outcome, was analyzed using logistic regression. This model was also fitted using the GEE method to account for correlations of responses from the same mice. From the fitted logistic model, the estimate of the probability of entry into the closed arm for transgenic and control mice were obtained, with the genotype effect expressed as the odds ratio of entry into the closed arm of transgenic relative to control mice. For all these models, when there was a significant interaction effect (sex × genotype or time × genotype), genotype difference based on the fitted model was tested by sex or at each time interval. To account for the number of tests performed (i.e., 2 tests for the by sex comparisons), the p-values for these tests have been adjusted using the Bonferroni method. All the statistical analyses were performed using SAS (Version 9.13 SAS Institute, Inc 2003). All tests of significance were two-tailed tests, with P<0.05 considered as statistically significant.
3. Results
3.1 Body weights of mice at the times of behavioral assays and plasma analysis
The mean body weights by genotype and sex at the time of each test are shown in Table 1. Linear mixed model analysis for repeated measures with genotype, sex, and time as the fixed effects was used to compare mean body weights between the genotype groups and between the sexes of the mice at the times of the behavioral despair and anxiety tests and at the time of corticosterone measurement. This model also included all two-factor and three-factor interactions. There was no significant genotype × sex × time interaction [F(1,51)=1.06; P=0.31], genotype × time [F(1,51)=0.07; P=0.80], and sex × time [F(1,51)=1.76; P=0.19] interaction effect. This indicates that genotype and sex differences did not significantly change over the one week interval. However, there was a significant genotype × sex interaction effect [F(1,51)=5.97; P=0.018], where genotype difference in mean body weight was significantly greater in male mice (transgenic smaller by 1.4±0.7 gm; Bonferroni adjusted P=0.08) than female mice (transgenic larger by 1.0±0.7 gm; Bonferroni adjusted P=0.35). Overall, there was no significant genotype effect [F(1,51)=0.24; P=0.63], with mean body weight differences observed only between male and female mice [F(1,51)=31.07 P<0.0001]. Because differences between genotypes in body weight were not present at this age, any alterations in behavior were unlikely to be due to adiposity or body buoyancy.
Table 1.
Mean body weights of control and transgenic mice used in behavioral assays.
| Group/Sex | Behavioral Despair | Novel Environment 9 wks | Elevated Plus Maze 6–10 wks | Corticosterone 21 wks | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | 7 wks* | 8 wks | |||||||
| Mean | Mean | n | Mean | n | Mean | n | Mean | ||
| Control male | 13 | 20.5±0.7 | 21.9±0.6 | 8 | 22.0±0.8 | 22 | 24.6±0.8 | 8 | 34.7±1.7 |
| Transgenic male | 14 | 18.8±0.6 | 20.7±0.7 | 13 | 22.2±0.5 | 15 | 27.5±1.2 | 14 | 41.4±2.5 |
| Control female | 17 | 16.6±0.2 | 17.9±0.4 | 6 | 17.8±0.5 | 12 | 18.9±1.0 | 17 | 24.4±0.8 |
| Transgenic female | 11 | 17.7±0.4 | 18.7±0.5 | 3 | 19.8±0.6 | 12 | 22.8±0.7 | 11 | 32.3±2.6 |
weeks of age
The two-way ANOVA with genotype and sex as the factors was used to compare mean body weight at the times the mice were tested in the novel environment, EPM, and when the corticosterone was measured. This showed no significant genotype × sex interaction effects [F(1,26)=1.10, P=0.30 for novel environment; F(1,57)=0.22, P=0.64 for EPM; and F(1,46)=0.08, P=0.78 for corticosterone]. For the novel environment assay, there was no significant difference in body weight between the genotype groups, with a mean difference in weight of 1.1±0.9 grams between transgenic and control groups [F(1,26)=1.69; P=0.21]. For both the EPM and the corticosterone studies, the mean body weights of transgenic mice were significantly greater compared to control mice. In the EPM, the transgenic mice were 3.4±1.0 grams heavier than control mice [F(1,57)=11.21; P=0.001]. In the corticosterone study, the mean weight difference was 7.3±2.0 grams [F(1,46)=12.95; P=0.0008]. For all of these studies, male mice were significantly heavier than female mice [sex main effect: F(1,26)=14.93, P=0.0007 for novel environment; F(1,57)=27.04, P<0.0001 for EPM; and F(1,46)=22.92, P<0.0001 for corticosterone study].
3.2 Tail suspension test (TST)
A two-way ANOVA, with genotype, sex, and genotype × sex interaction, as the factors in the model, was used to test for differences in mean immobility on TST between the ADAR2 transgenic mice and control (Fig. 2). A test for genotype × sex interaction showed a significant interaction [F(1,56)=4.08; P=0.048] with a greater genotype effect in male mice compared to female mice. For male mice, mean immobility time on TST was 1.5±0.2 min longer for the transgenic mice compared to control (2.9±0.1 min vs. 1.4±0.2 min; Bonferroni adjusted P<0.0001). The difference in mean immobility time on the TST between the transgenic and control female mice was 0.8±0.2 (2.3±0.2 min for transgenic vs. 1.5±0.1 min for control; Bonferroni adjusted P=0.002). Overall, averaging across male and female mice, mean immobility time on the TST was significantly longer for the transgenic mice compared to the control mice by 1.2±0.2 min [F(1,56)=47.55; P<0.0001].
Figure 2.
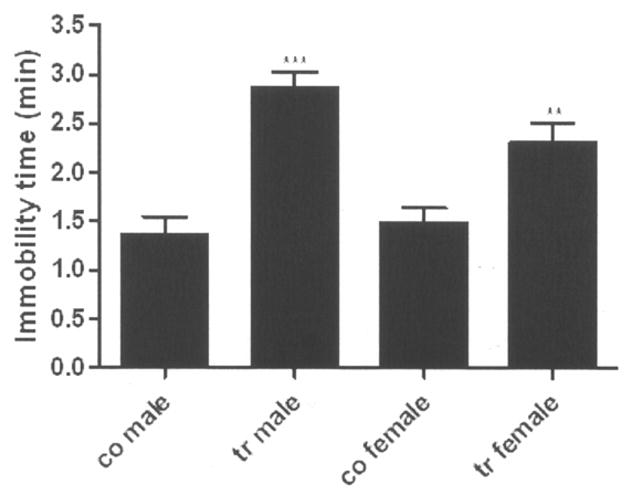
Immobility time on TST in male and female mice with their mean ± SEM. N/group = co male 12; tr male 19; co female 18; and tr female 11. co = control; tr = transgenic. ***p ≤ 0.0001; **p ≤ 0.002.
3.3 Forced swim test (FST)
The linear mixed model analysis for repeated measures was used to compare mean immobility on FST between the ADAR2 transgenic mice and control at three time points (first exposure, 24 hour FST, and 1 week post-exposure; Fig. 3a and 3b). The fixed effects in the model were genotype, sex, and time, with the model that also included all two-factor interactions and the three-factor interaction. There was no significant genotype × sex × time interaction effect [F(2,102)=0.04; P=0.96], and no two factor interactions of time with sex [F(2,102)=1.23; P=0.30] and with genotype [F(2,102)=0.86; P=0.43]. This implies that sex differences or genotype differences did not significantly change with time. There was a significant time effect [F(2,102)=3.15; P=0.047], where there was a significant increase in mean immobility time from first exposure to 24 hours of 0.4±0.2 min (Bonferroni adjusted P=0.047). There was no significant genotype × sex interaction [F(1,51)=1.54; P=0.22], where the test for genotype effect showed a significantly longer immobility time on FST for the transgenic mice compared to the control mice by 1.6±0.1 min [F(1,51)=144.32; P<0.0001].
Figure 3.
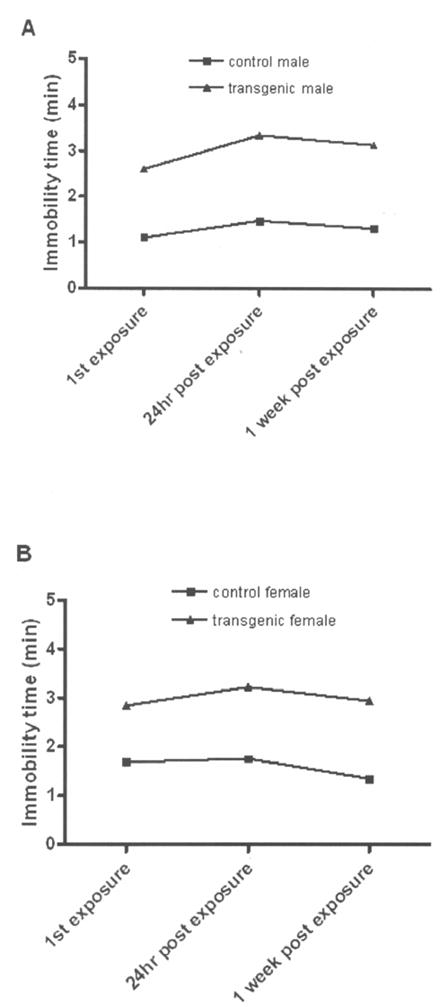
Immobility time on the forced swim test as a function of time in both sexes. a) mean ± SEM male mice b) mean ± SEM female mice. N/group = control male 13; transgenic male 14; control female 17; and transgenic female 11.
3.4 Elevated plus maze
The rate of entry (number of entries over a 5 min period) into either closed or open arms of the EPM was analyzed using negative binomial regression (Table 2). This analysis is applicable for use in cases in which the dependent variable represents a count of the number of events over a known time interval. The independent variables in the model included genotype (control vs. transgenic), sex and genotype × sex interaction. There was no significant sex × genotype interaction [Chi-square (1)=0.17; P=0.68] which indicates that genotype differences were not found to differ significantly between male and female mice. There also was no significant sex main effect [Chi-square (1)=0.67; P=0.41]. However, there was a significant difference in the two genotypes in mean rate of entry into the closed arm, with a lower rate for the transgenic group (4.7±0.5; 95% CI: 1.3, 5.8) compared to the control group (9.9±0.8; 95% CI: 8.5, 11.2) [Chi-square (1)=29.5; P<0.0001]. The rate of entry into the open arm showed no significant sex × genotype interaction [Chi-square (1)=0.15; P=0.703] and no significant difference between sex [Chi-square (1)=0.01; P=0.941]. There was a significant difference in mean rate of entry into the open arms between the control and transgenic mice with the transgenic group also having a lower rate (1.3±0.2; 95% CI: 0.9, 1.8) compared to the control group (4.5±0.5; 95% CI: 3.7, 5.8) [Chi-square (1)=35.8; P<0.0001]. These results suggest that there was less exploration of either arm displayed by the transgenic mice regardless of their sex.
Table 2.
Elevated Plus Maze. Time spent in open arms, number of entries into open and closed arms and the probability of entries into the closed arms are presented for control and transgenic mice.
| Genotype | Percentage of time in open arm median interquartile range (25th–75th percentile) | Number of entries in open arm Mean±SEM | Number of entries in closed arm Mean±SEM | Probability of entries into closed arms (percentage) |
|---|---|---|---|---|
| Control mice | 16.2% (10.8–33.2) | 4.5±0.5 | 9.9±0.8 | 79% |
| Transgenic mice | 11.2% (8.4–15.5) | 1.3±0.2 | 4.7±0.5 | 69% |
The probability of entry into the closed arms (from the center) was compared between control and transgenic mice using logistic regression analysis fitted by the generalized estimating equations method to account for the correlation of responses by the same mice. The independent variables in the model were genotype, sex, and genotype × sex interaction. There was no significant genotype × sex interaction [Chi-square (1)=0.01; P=0.91], and no significant difference in the probability of going into the closed arms between male and female mice [Chi-square (1)=0.37; P=0.54]. There was a significant difference between the control and transgenic mice in the probability of entry into the closed arms with the transgenic mice more likely to go into the closed arms than the control mice (odds ratio of 1.66; 95% CI: 1.11, 2.50; P=0.014). Transgenic mice went into the closed arms 79% (95% CI: 72%, 84%) of the time compared to 69% (95% CI: 65%, 73%) for the control mice. These results suggest that regardless of sex the transgenic mice were more likely to enter the closed arms.
Percentage of time spent in open arms by transgenic mice was significantly lower than control mice (Wilcoxon two sample test, median interquartile range 11.2% vs. 16.2%, P<0.008). Taken together these results suggest that transgenic mice showed elevated anxiety-like behaviors on EPM.
3.5 Activity in the novel environment
Negative binomial regression analysis was used to compare the number of beam breaks over a 120 min test period (i.e., mean beam breaks per min) divided into 3 phases (the first 5 min, 6–60 min, and 61–120 min) between the ADAR2 transgenic and control mice. The model included genotype, sex, phase, all two-factor interactions and the three-factor interaction. There was no significant group × sex × phase interaction [Chi-square (2)=1.23; P=0.54], genotype × phase interaction [Chi-square (2)=3.18; P=0.20], and genotype × sex interaction [Chi-square (1)=0.58; P=0.45]. Having no significant genotype × phase interaction indicates that the statistical test was not able to detect a significant change over the 3 phases in the magnitude of the difference in mean beam breaks between transgenic and control mice (Fig. 4a). For the first 5 min, the mean number of breaks per min was 18.4%±10.0% lower in transgenic mice (4.8±0.5) compared to control mice (5.9±0.4) (Bonferroni adjusted P=0.29) (Fig. 4b). At the 6–60 min and 61–120 min intervals, mean breaks per min was higher in transgenic than control mice by 6.4%±6.8% (2.9±0.1 vs. 2.4±0.1; Bonferroni adjusted P>0.99) and 7.0%±17.6% (1.7±0.2 vs. 1.6±0.2; Bonferroni adjusted P>0.99), respectively. Overall, there was no significant genotype effect with the mean number of breaks per min 2.4%±8.5% lower in transgenic mice relative to controls (P=0.78). However, in the tests involving the sex effect, the data suggested a possible sex × phase interaction [Chi-square (2)=4.64; P=0.095]. Testing for sex effects at each phase showed no significant difference at the first 5 min with the male mean being 13.8%±12.2% lower compared to that of female mice (5.0±0.3 vs. 5.7±0.6; Bonferroni adjusted P=0.65). In contrast, at the 6–60 min and 61–120 min intervals, mean breaks per min was significantly lower in male mice compared to female mice by 29.5%±4.5% (2.4±0.1 vs. 3.4±0.2; Bonferroni adjusted P<0.0001) and 46.8%±8.8% (1.2±0.1 vs. 2.3±0.3; Bonferroni adjusted P=0.0002), respectively. These results suggest that there is no significant genotype difference but there is a sex difference where male mice showed less exploration compared to female mice for most of the test period.
Figure 4.
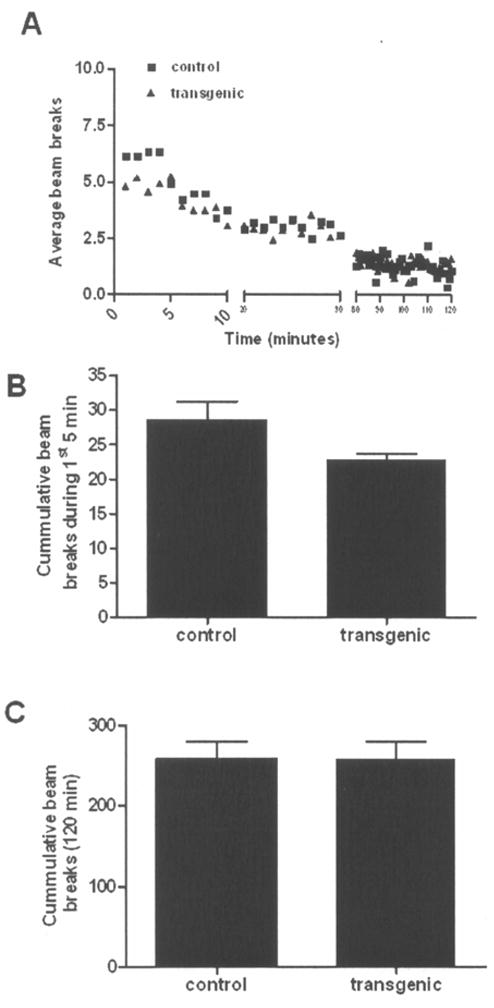
Reduced exploration and locomotor activity in the novel environment of control and ADAR2 transgenic mice with mean ± SEM following 1 week of recovery from behavioral despair tests. a) Graph showing the mean beam breaks of control and transgenic mice depicting their activity shown minute by minute during the entire 120 min of the test. b) Mean ± SEM cumulative beam breaks during first 5 min of exposure to the novel environment. c) Mean ± SEM cumulative beam breaks during the 120 min tests in control and ADAR2 transgenic mice. N/group = control 14 and transgenic 16.
3.6 Corticosterone levels in plasma
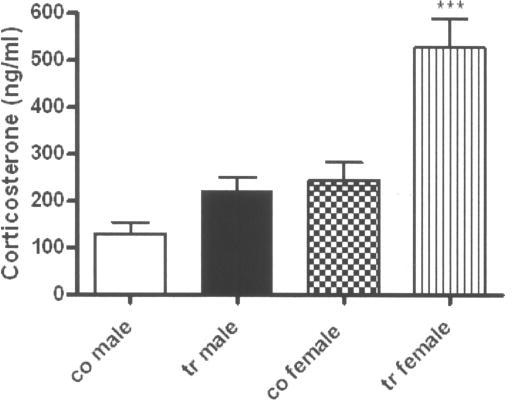
A two-way ANOVA, with genotype, sex, and genotype × sex interaction, as the factors in the model was used to test for differences in mean corticosterone levels between the ADAR2 transgenic mice and controls (Fig. 5). A test for genotype × sex interaction showed a significant interaction effect [F(1,46)=4.89; P=0.032]. For female mice, mean corticosterone was 284±57 ng/ml higher in the transgenic mice compared to control (528±45 ng/ml vs. 244±36 ng/ml; Bonferroni adjusted P<0.0001). There was no significant difference in mean corticosterone between transgenic and control male mice (222±40 ng/ml for transgenic vs. 130±52 ng/ml for control; Bonferroni adjusted P=0.34).
Figure 5.
Vertical plot showing the corticosterone levels with mean ± SEM in each group of mice. N/group = co male 8; tr male 14; co female 17; and tr female 11. co = control; tr = transgenic. ****p ≤ 0.0001
4. Discussion
RNA editing involving the conversion of A-to-I in pre-mRNA results in altered nucleotide sequences in mature mRNA. Such changes can produce altered RNA coding potential and consequently proteins with modified biological activities. This concept led to the generation of the ADAR2 transgenic mouse [79]. The present studies examined behavioral traits and resting corticosterone levels of ADAR2 transgenic mice. The results of this work indicate first, that male and female ADAR2 transgenic mice in comparison to sex-, weight- and age-matched controls showed increased immobility in two experimental paradigms, the FST and the TST. In both of these assays, decreased activity is considered to be an indication of behavioral despair. Second, ADAR2 transgenic mice also demonstrated behavioral changes associated with anxiety as assessed on the EPM. Third, ADAR2 transgenic mice had increased plasma corticosterone. Taken together, the results are consistent with the hypothesis that adult male and female ADAR2 mice manifest both behavioral and endocrine signs of endogenous depression and anxiety.
ADARs catalyze the conversion of A-to-I in mRNAs that have a double-stranded RNA structure. Under normal physiological conditions A-to-I modification has been observed in several components of the mammalian nervous system. In particular, such alterations have been associated with glutamate gated-ion channels, the KV1.1 potassium ion channel, the alpha 3 subunit of the GABA receptor and the 5HT2CR [6,8,61,76,81]. Of all of these neural elements, the 5HT2CR is the one most extensively implicated in the states of depression and anxiety [18,23,31,32,40].
As well as being frequently associated with affective disorders [54], the 5HT system has also been implicated in the alterations of appetite, energy, sleep, libido, and cognitive functions [50]. Serotonergic neurotransmission is mediated by the interaction of 5HT with at least 14 receptor subtypes [37], and of these the 5HT2CR is the only one that undergoes ADAR mediated A-to-I modification. Such an alternation results in reduced G protein-coupling of the phospholipase C signaling pathway [9]. Recent studies in humans and in rodent models have implicated editing of messages for the 5HT2CR in both depression [20,30,38,75,92] and anxiety [32]. Interestingly antidepressant treatment has also been shown to alter 5HT2CR RNA editing [23,75,86].
Many animal models of depression use prior exposure to stressors to induce depression-related behavioral changes [67–69]. For example, it has been demonstrated that chronic mild stress induces a state of anhedonia which is a core sign/symptom of depression in humans [28,90,91]. The FST [68,69], the TST [83] and uncontrollable shock (learned helplessness) [78] all involve subjecting rodents to stressors and are based on observations that animals normally try to escape or avoid aversive stimuli or conditions. As conventionally employed, both the FST and the TST require an initial exposure to the stressor before the actual test session. That is, a 15 min period of preexposure of forced swimming the day before the FST or a 5 min session of tail suspension the day preceding the TST are commonly used. Behavioral despair tests conducted with prior exposure to such stressors have been used to demonstrate the efficacy of many clinically employed antidepressant drugs. The fact that the FST and TST consistently screen and identify antidepressant drugs has been used to establish the high predictive validity of these tests [68]. Recently the FST has been modified to eliminate the prior exposure period and use only a single exposure to the stressor as the actual test period to determine if a depression-related phenotype is endogenous (i.e., occurs without preconditioning) in a given strain or model [11–13]. In the present behavioral despair studies, we used 5 min FST and TSTs without prior exposure to the stressors. Tested in this manner ADAR2 transgenic mice irrespective of sex showed decreased escape behaviors on both of the behavioral despair tests. The “locomotion” per se was not altered in ADAR2 transgenic mice as indicated by activity during the 120 min in a novel environment. In a previous study neither pre-obese nor obese transgenic mice showed altered locomotor activity as compared to control littermates [79]. These results suggest that the motor activity of the transgenic animals is similar to that of control mice and therefore not likely to confound the results of the behavioral despair tests. Our studies also indicate that ADAR2 transgenic mice consistently showed signs of increased behavioral despair on not only the first TST but in the subsequent FST and in additional TSTs. These observations indicate that behavioral despair is persistent in ADAR2 mice. This is consistent with previous findings showing that in spite of repeated exposures to stressors behavioral despair does not subside [10,14,15,84].
In the present studies, the stage of the estrous cycle of female mice during testing was not established. Female mice are reported to be more active during estrus [42,77] and differences in activity could potentially affect the results of behavioral despair assays. Future experiments with staging of the female estrous cycle in female mice will be helpful in determining the nature of the sex effects in ADAR2 transgenic mice and the effects of the estrous cycle in behavioral despair models.
Depression is often comorbid with anxiety [89]. Two commonly employed behavioral tests of anxiety are reduced locomotion in novel environments and reduced exploration in the EPM. The present study used both of these methods to assay anxiety-like behaviors in the ADAR2 transgenic mouse. The EPM test is based on the natural tendency of rodents to explore novel environments while at the same time avoiding brightly lit, elevated, open areas [33,49]. The portion of time spent in the open arm of the EPM and rate of arm entries have been used as measures of anxiety [63,73]. In the EPM, ADAR2 transgenic mice showed a significant decrease in the percentage of time spent in the open arm, lower rate of open and closed arm entries and an increased probability of entering the closed arms. This pattern suggests that anxiety-like behaviors are increased in ADAR2 transgenic mice as compared to control mice. In a novel environment ADAR2 transgenic mice display only a slight decrease in activity during the 1st five minutes which did not reach a statistical significance. After the initial 5 min period, the activity of the ADAR2 transgenic mice was comparable to their control littermates. Some investigators suggest that the number of closed arm entries in the EPM is an indication of activity, rather than “anxiety per se” [25,56]. Taken together, both the performance on the EPM and the failure to find a significant change in locomotor activity in a novel environment suggest that ADAR2 transgenic mice manifest behavioral signs of increased anxiety. Because indications of anxiety-like behavior are evident in naïve ADAR2 transgenic mice when the EPM was conducted as an initial test (Experiment 2), ADAR2 mice can be considered to display signs of endogenous anxiety.
Both anxiety and depression are often associated with altered HPA axis activity as reflected by high levels of glucocorticoids [3,29,52,58,70]. Cortisol and corticosterone are metabolic hormones in humans and rodents, respectively, that play key roles in the physiological responses to stressors [16,26,65,93]. Both sexes of ADAR2 transgenic mice, compared to their control littermates, showed significantly elevated corticosterone levels. Female ADAR2 transgenic mice had higher corticosterone levels than males. Sex differences in cortisol and corticosterone levels in humans and rodents have been reported in both depression and anxiety [44,45,51,62,87]. It should be noted that the mean body weights of ADAR2 mice was significantly greater than those of control animals and that this might affect glucocorticoid levels. However, the increased corticosterone in ADAR2 mice is likely to be independent of the differences in body weight. In a previous unpublished study, ADAR2 mice with body weights matched to controls also showed significantly elevated glucocorticoid levels (Singh, unpublished data).
Mood disorders such as depression and anxiety are often associated with behavioral changes such as altered appetite and sleep, obesity, and reduced cognitive function. Human depression, anxiety, hyperphagia and obesity have been associated with low 5HT signaling and disordered serotonergic signaling through the 5HT2CR [5,18,23,30,32,34–36,38,40,41,50,52,55,57,59,60,75,85,86]. Previous studies of 5HT2CR null mice indicate that they have maturity onset obesity, type II diabetes, spontaneous seizures, anxiety-related phenotype, cognitive defects and hyperactivity but normal corticosterone levels [35,36,85]. The phenotype of ADAR2 transgenic mice is clearly distinct from the 5HT2CR null mouse. For example, the ADAR2 transgenic mouse in comparison to the 5HT2C-R null mouse develops a maximum body weight of 90 to 125 grams [79] rather than the approximately 40 gram body mass observed in the receptor knock-out model [85]. ADAR2 transgenic mice have a form of adult onset obesity but without any apparent metabolic dysregulation prior to the inordinate weight gain [79].
The fact that ADAR2 transgenic mice have hyperphagia [79] and elevated plasma corticosterone along with behavioral despair and anxiety makes them an attractive model for investigating this important set of co-morbidities. The appearance of the phenotypes in a single model raises the question of whether there is a single molecular disruption that accounts for all of these signs. One hypothesis to be considered is that altered RNA editing of the 5HT2CR in the brain is key to all of these phenotypic alterations. ADAR2 transgenic mice misexpress ADAR2 in all areas of the brain that have been examined [79]. In an earlier study [79] RNA was pooled from both whole brain and from the entire hypothalamus to determine if there were changes in 5HT2CR RNA editing in ADAR2 mice. In these studies no differences in 5HT2CR RNA were observed in the samples studied [79]. However, in these studies it is possible that any differences between groups were diluted because of the large size of the tissue samples studied. Only a subpopulation of neurons in any given brain region express 5HT2CR, and misediting of this receptor in specific cells at particular brain sites may be required for the phenotypes of depression, anxiety, hyperglucocorticoidism, hyperphagia and obesity. Alternatively, it is also possible that other neuronal substrates (e.g., other receptors or ion channels) may also have undergone RNA editing changes, and these alterations may also contribute to the physiological and behavioral phenotype of ADAR2 transgenic mice. In future studies a detailed examination of changes in RNA editing in several brain regions will be required to begin to fully understand the phenotypic disorders characterized in the ADAR2 mouse.
Acknowledgments
This research was supported by National Heart, Lung, and Blood Institute HL-14388 (AKJ) and National Institute of Diabetes and Digestive and Kidney Diseases DK-66086 (AKJ). The authors would also like to thank Dr. Mark E. Anderson for financial support from the National Heart, Lung, and Blood Institute HL-079031, HL-62494, and HL-70250.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 2.Andreatini R, Bacellar LF. The relationship between anxiety and depression in animal models: a study using the forced swimming test and elevated plus-maze. Braz J Med Biol Res. 1999;32:1121–1126. doi: 10.1590/s0100-879x1999000900011. [DOI] [PubMed] [Google Scholar]
- 3.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 4.Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg KA, Cropper JD, Niswender CM, Sanders-Bush E, Emeson RB, Clarke WP. RNA-editing of the 5-HT(2C) receptor alters agonist-receptor-effector coupling specificity. Br J Pharmacol. 2001;134:386–392. doi: 10.1038/sj.bjp.0704255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhalla T, Rosenthal JJ, Holmgren M, Reenan R. Control of human potassium channel inactivation by editing of a small mRNA hairpin. Nat Struct Mol Biol. 2004;11:950–956. doi: 10.1038/nsmb825. [DOI] [PubMed] [Google Scholar]
- 7.Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- 9.Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 10.Chourbaji S, Brandwein C, Vogt MA, Dormann C, Gass P. Evaluation of effects of previous exposure to an acute stressor before testing for depression-like behaviours in mice. Stress. 2008;11:170–175. doi: 10.1080/10253890701560119. [DOI] [PubMed] [Google Scholar]
- 11.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 12.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 14.Dal-Zotto S, Marti O, Armario A. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav Brain Res. 2000;114:175–181. doi: 10.1016/s0166-4328(00)00220-5. [DOI] [PubMed] [Google Scholar]
- 15.Deak T, Bellamy C, D’Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160:125–134. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Delbende C, Delarue C, Lefebvre H, Bunel DT, Szafarczyk A, Mocaer E, Kamoun A, Jegou S, Vaudry H. Glucocorticoids, transmitters and stress. Br J Psychiatry Suppl. 1992;15:24–35. [PubMed] [Google Scholar]
- 17.Emeson RB, Singh M. Adenosine to inosine RNA editing: substrates and consequences. In: Bass BL, editor. RNA Editing: Frontiers in Molecular Biology. London: Oxford University Press; 2001. pp. 109–138. [Google Scholar]
- 18.Englander MT, Dulawa SC, Bhansali P, Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25:648–651. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng Y, Sansam CL, Singh M, Emeson RB. Altered RNA editing in mice lacking ADAR2 autoregulation. Mol Cell Biol. 2006;26:480–488. doi: 10.1128/MCB.26.2.480-488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald LW, Iyer G, Conklin DS, Krause CM, Marshall A, Patterson JP, Tran DP, Jonak GJ, Hartig PR. Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacology. 1999;21:82S–90S. doi: 10.1016/S0893-133X(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 21.Fone KC, Shalders K, Fox ZD, Arthur R, Marsden CA. Increased 5-HT2C receptor responsiveness occurs on rearing rats in social isolation. Psychopharmacology (Berl) 1996;123:346–352. doi: 10.1007/BF02246645. [DOI] [PubMed] [Google Scholar]
- 22.Frey BN, Andreazza AC, Nery FG, Martins MR, Quevedo J, Soares JC, Kapczinski F. The role of hippocampus in the pathophysiology of bipolar disorder. Behav Pharmacol. 2007;18:419–430. doi: 10.1097/FBP.0b013e3282df3cde. [DOI] [PubMed] [Google Scholar]
- 23.Gardiner K, Du Y. A-to-I editing of the 5HT2C receptor and behaviour. Brief Funct Genomic Proteomic. 2006;5:37–42. doi: 10.1093/bfgp/ell006. [DOI] [PubMed] [Google Scholar]
- 24.Gerber A, O’Connell MA, Keller W. Two forms of human double-stranded RNA-specific editase 1 (hRED1) generated by the insertion of an Alu cassette. RNA. 1997;3:453–463. [PMC free article] [PubMed] [Google Scholar]
- 25.Gerits N, Van Belle W, Moens U. Transgenic mice expressing constitutive active MAPKAPK5 display gender-dependent differences in exploration and activity. Behav Brain Funct. 2007;3(1–15):58. doi: 10.1186/1744-9081-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez F, Lahmame A, de Kloet ER, Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–337. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- 27.Gott JM, Emeson RB. Functions and mechanisms of RNA editing. Annu Rev Genet. 2000;34:499–531. doi: 10.1146/annurev.genet.34.1.499. [DOI] [PubMed] [Google Scholar]
- 28.Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–316. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Groenink L, Dirks A, Verdouw PM, Schipholt M, Veening JG, van der Gugten J, Olivier B. HPA axis dysregulation in mice overexpressing corticotropin releasing hormone. Biol Psychiatry. 2002;51:875–881. doi: 10.1016/s0006-3223(02)01334-3. [DOI] [PubMed] [Google Scholar]
- 30.Gurevich I, Englander MT, Adlersberg M, Siegal NB, Schmauss C. Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J Neurosci. 2002;22:10529–10532. doi: 10.1523/JNEUROSCI.22-24-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002;34:349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- 32.Hackler EA, Airey DC, Shannon CC, Sodhi MS, Sanders-Bush E. 5-HT(2C) receptor RNA editing in the amygdala of C57BL/6J, DBA/2J, and BALB/cJ mice. Neurosci Res. 2006;55:96–104. doi: 10.1016/j.neures.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa S, Nishi K, Watanabe A, Overstreet DH, Diksic M. Brain 5-HT synthesis in the Flinders Sensitive Line rat model of depression: an autoradiographic study. Neurochem Int. 2006;48:358–366. doi: 10.1016/j.neuint.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Yeo GS, O’Rahilly S, Colmers WF, Elmquist JK, Tecott LH. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci. 2007;27:6956–6964. doi: 10.1523/JNEUROSCI.2584-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 2007;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 37.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 38.Iwamoto K, Bundo M, Kato T. Estimating RNA editing efficiency of five editing sites in the serotonin 2C receptor by pyrosequencing. RNA. 2005;11:1596–1603. doi: 10.1261/rna.2114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwamoto K, Kato T. RNA editing of serotonin 2C receptor in human postmortem brains of major mental disorders. Neurosci Lett. 2003;346:169–172. doi: 10.1016/s0304-3940(03)00608-6. [DOI] [PubMed] [Google Scholar]
- 40.Iwamoto K, Nakatani N, Bundo M, Yoshikawa T, Kato T. Altered RNA editing of serotonin 2C receptor in a rat model of depression. Neurosci Res. 2005;53:69–76. doi: 10.1016/j.neures.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Jenck F, Bos M, Wichmann J, Stadler H, Martin JR, Moreau JL. The role of 5-HT2C receptors in affective disorders. Expert Opin Investig Drugs. 1998;7:1587–1599. doi: 10.1517/13543784.7.10.1587. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins JA, Williams P, Kramer GL, Davis LL, Petty F. The influence of gender and the estrous cycle on learned helplessness in the rat. Biol Psychol. 2001;58:147–158. doi: 10.1016/s0301-0511(01)00111-9. [DOI] [PubMed] [Google Scholar]
- 43.Kind P, Sorensen J. The costs of depression. Int Clin Psychopharmacol. 1993;7:191–195. doi: 10.1097/00004850-199300730-00010. [DOI] [PubMed] [Google Scholar]
- 44.Kirschbaum C, Wust S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. J Clin Endocrinol Metab. 1992;75:1526–1530. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- 45.Kurina LM, Weiss LA, Graves SW, Parry R, Williams GH, Abney M, Ober C. Sex differences in the genetic basis of morning serum cortisol levels: genome-wide screen identifies two novel loci specific to women. J Clin Endocrinol Metab. 2005;90:4747–4752. doi: 10.1210/jc.2005-0384. [DOI] [PubMed] [Google Scholar]
- 46.Lai F, Drakas R, Nishikura K. Mutagenic analysis of double-stranded RNA adenosine deaminase, a candidate enzyme for RNA editing of glutamate-gated ion channel transcripts. J Biol Chem. 1995;270:17098–17105. doi: 10.1074/jbc.270.29.17098. [DOI] [PubMed] [Google Scholar]
- 47.Lan MJ, McLoughlin GA, Griffin JL, Tsang TM, Huang JT, Yuan P, Manji H, Holmes E, Bahn S. Metabonomic analysis identifies molecular changes associated with the pathophysiology and drug treatment of bipolar disorder. Mol Psychiatry. 2009:269–279. doi: 10.1038/sj.mp.4002130. [DOI] [PubMed] [Google Scholar]
- 48.Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, Shemesh R, Fligelman ZY, Shoshan A, Pollock SR, Sztybel D, Olshansky M, Rechavi G, Jantsch MF. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol. 2004;22:1001–1005. doi: 10.1038/nbt996. [DOI] [PubMed] [Google Scholar]
- 49.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 50.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 51.Magiakou MA, Mastorakos G, Webster E, Chrousos GP. The hypothalamic-pituitary-adrenal axis and the female reproductive system. Ann N Y Acad Sci. 1997;816:42–56. doi: 10.1111/j.1749-6632.1997.tb52128.x. [DOI] [PubMed] [Google Scholar]
- 52.McAllister-Williams RH, Ferrier IN, Young AH. Mood and neuropsychological function in depression: the role of corticosteroids and serotonin. Psychol Med. 1998;28:573–584. doi: 10.1017/s0033291798006680. [DOI] [PubMed] [Google Scholar]
- 53.McGrew L, Price RD, Hackler E, Chang MS, Sanders-Bush E. RNA editing of the human serotonin 5-HT2C receptor disrupts transactivation of the small G-protein RhoA. Mol Pharmacol. 2004;65:252–256. doi: 10.1124/mol.65.1.252. [DOI] [PubMed] [Google Scholar]
- 54.Meltzer HY. Role of serotonin in depression. Ann N Y Acad Sci. 1990;600:486–499. doi: 10.1111/j.1749-6632.1990.tb16904.x. [DOI] [PubMed] [Google Scholar]
- 55.Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- 56.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreau JL, Bos M, Jenck F, Martin JR, Mortas P, Wichmann J. 5HT2C receptor agonists exhibit antidepressant-like properties in the anhedonia model of depression in rats. Eur Neuropsychopharmacol. 1996;6:169–175. doi: 10.1016/0924-977x(96)00015-6. [DOI] [PubMed] [Google Scholar]
- 58.Musselman DL, Nemeroff CB. Depression and endocrine disorders: focus on the thyroid and adrenal system. Br J Psychiatry Suppl. 1996;30:123–128. [PubMed] [Google Scholar]
- 59.Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J Biol Chem. 1999;274:9472–9478. doi: 10.1074/jbc.274.14.9472. [DOI] [PubMed] [Google Scholar]
- 60.Niswender CM, Herrick-Davis K, Dilley GE, Meltzer HY, Overholser JC, Stockmeier CA, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-HT2C receptor: alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology. 2001;24:478–491. doi: 10.1016/S0893-133X(00)00223-2. [DOI] [PubMed] [Google Scholar]
- 61.Ohlson J, Pedersen JS, Haussler D, Ohman M. Editing modifies the GABA(A) receptor subunit alpha3. RNA. 2007;13:698–703. doi: 10.1261/rna.349107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 63.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 64.Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 65.Poland RE, Rubin RT, Lesser IM, Lane LA, Hart PJ. Neuroendocrine aspects of primary endogenous depression. II. Serum dexamethasone concentrations and hypothalamic-pituitary-adrenal cortical activity as determinants of the dexamethasone suppression test response. Arch Gen Psychiatry. 1987;44:790–795. doi: 10.1001/archpsyc.1987.01800210034005. [DOI] [PubMed] [Google Scholar]
- 66.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 67.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 68.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 69.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 70.Porter RJ, Gallagher P, Watson S, Young AH. Corticosteroid-serotonin interactions in depression: a review of the human evidence. Psychopharmacology (Berl) 2004;173:1–17. doi: 10.1007/s00213-004-1774-1. [DOI] [PubMed] [Google Scholar]
- 71.Price RD, Weiner DM, Chang MS, Sanders-Bush E. RNA editing of the human serotonin 5-HT2C receptor alters receptor-mediated activation of G13 protein. J Biol Chem. 2001;276:44663–44668. doi: 10.1074/jbc.M106745200. [DOI] [PubMed] [Google Scholar]
- 72.Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 73.Rodgers RJ, Johnson NJ, Champion AJ, Mills S. Modulation of plus-maze behaviour in mice by the preferential D3-receptor agonist 7-OH-DPAT. Pharmacol Biochem Behav. 1996;54:79–84. doi: 10.1016/0091-3057(95)02110-8. [DOI] [PubMed] [Google Scholar]
- 74.Rueter SM, Dawson TR, Emeson RB. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- 75.Schmauss C. Serotonin 2C receptors: suicide, serotonin, and runaway RNA editing. Neuroscientist. 2003;9:237–242. doi: 10.1177/1073858403253669. [DOI] [PubMed] [Google Scholar]
- 76.Schmauss C, Howe JR. RNA editing of neurotransmitter receptors in the mammalian brain. Sci STKE. 2002;2002:PE26. doi: 10.1126/stke.2002.133.pe26. [DOI] [PubMed] [Google Scholar]
- 77.Schneider T, Popik P. Increased depressive-like traits in an animal model of premenstrual irritability. Horm Behav. 2007;51:142–148. doi: 10.1016/j.yhbeh.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 78.Seligman ME, Maier SF. Failure to escape traumatic shock. J Exp Psychol. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- 79.Singh M, Kesterson RA, Jacobs MM, Joers JM, Gore JC, Emeson RB. Hyperphagia-mediated obesity in transgenic mice misexpressing the RNA-editing enzyme ADAR2. J Biol Chem. 2007;282:22448–22459. doi: 10.1074/jbc.M700265200. [DOI] [PubMed] [Google Scholar]
- 80.Sodhi MS, Burnet PW, Makoff AJ, Kerwin RW, Harrison PJ. RNA editing of the 5-HT(2C) receptor is reduced in schizophrenia. Mol Psychiatry. 2001;6:373–379. doi: 10.1038/sj.mp.4000920. [DOI] [PubMed] [Google Scholar]
- 81.Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- 82.Spiessl H, Hubner-Liebermann B, Hajak G. Depression, a widespread disease. Epidemiology, care situation, diagnosis, therapy and prevention. Dtsch Med Wochenschr. 2006;131:35–40. doi: 10.1055/s-2006-924919. [DOI] [PubMed] [Google Scholar]
- 83.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 84.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 85.Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 86.Tohda M, Nomura M, Nomura Y. Molecular pathopharmacology of 5-HT2C receptors and the RNA editing in the brain. J Pharmacol Sci. 2006;100:427–432. doi: 10.1254/jphs.cpj06005x. [DOI] [PubMed] [Google Scholar]
- 87.Torpy DJ, Chrousos GP. The three-way interactions between the hypothalamic-pituitary-adrenal and gonadal axes and the immune system. Baillieres Clin Rheumatol. 1996;10:181–198. doi: 10.1016/s0950-3579(96)80014-8. [DOI] [PubMed] [Google Scholar]
- 88.Wang Q, O’Brien PJ, Chen CX, Cho DS, Murray JM, Nishikura K. Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin2C receptors. J Neurochem. 2000;74:1290–1300. doi: 10.1046/j.1471-4159.2000.741290.x. [DOI] [PubMed] [Google Scholar]
- 89.Wetzler S, Katz MM. Problems with the differentiation of anxiety and depression. J Psychiatr Res. 1989;23:1–12. doi: 10.1016/0022-3956(89)90013-7. [DOI] [PubMed] [Google Scholar]
- 90.Willner P, Muscat R, Papp M. An animal model of anhedonia. Clin Neuropharmacol. 1992;15(Suppl 1 Pt A):550A–551A. doi: 10.1097/00002826-199201001-00286. [DOI] [PubMed] [Google Scholar]
- 91.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 92.Yang W, Wang Q, Kanes SJ, Murray JM, Nishikura K. Altered RNA editing of serotonin 5-HT2C receptor induced by interferon: implications for depression associated with cytokine therapy. Brain Res Mol Brain Res. 2004;124:70–78. doi: 10.1016/j.molbrainres.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 93.Zarkovic M, Stefanova E, Ciric J, Penezic Z, Kostic V, Sumarac-Dumanovic M, Macut D, Ivovic MS, Gligorovic PV. Prolonged psychological stress suppresses cortisol secretion. Clin Endocrinol (Oxf) 2003;59:811–816. doi: 10.1046/j.1365-2265.2003.01925.x. [DOI] [PubMed] [Google Scholar]