Abstract
Antineutrophil cytoplasmic autoantibody (ANCA)-associated small-vessel vasculitis frequently affects the kidney. Here we describe the rates of infection, disease relapse, and death in patients with ANCA small-vessel vasculitis before and after end-stage renal disease (ESRD) in an inception cohort study and compare them to those of patients with preserved renal function. All patients had biopsy-proven ANCA small-vessel vasculitis. Fisher's exact tests and Wilcoxon rank sum tests were used to compare the characteristics by ESRD status. ESRD follow-up included time on dialysis with transplants censored. Over a median follow-up time of 40 months, 136 of 523 patients reached ESRD. ESRD was associated with new-onset ANCA small-vessel vasculitis in 51% of patients, progressive chronic kidney disease without active vasculitis in 43%, and renal relapse in 6% of patients. Relapse rates of ANCA small-vessel vasculitis, reported as episodes/person-year, were significantly lower on chronic dialysis (0.08 episodes) compared with the rate of the same patients before ESRD (0.20 episodes) or with patients with preserved renal function (0.16 episodes). Infections were almost twice as frequent among patients with ESRD on maintenance immunosuppressants and were an important cause of death. Given the lower risk of relapse and higher risk of infection and death, we suggest that immunosuppression be geared to patients with ESRD who present with active vasculitis.
Keywords: ANCA, chronic dialysis, glomerulonephritis
Antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis (ANCA-SVV) frequently affects the kidneys, causing a destructive pauci-immune necrotizing and crescentic glomerulonephritis. Despite improved therapies and outcomes,1–4 ANCA-SVV still results in end-stage renal disease (ESRD) in a quarter of patients over 3–4 years.3,5,6 Information on the course of ANCA-SVV while on chronic dialysis is relatively scant. Risk factors for relapse have been described for patients with ANCA-SVV,3 but it is not clear whether they apply to the prediction of relapse after ESRD is reached. Knowledge about risk and severity of relapse is crucial in determining the need for maintenance immunosuppression. This is of particular importance as the rate of infections is significantly higher among patients on dialysis.7
Chronic dialysis may result in a relative quiescence of the autoimmune process.8–11 It has thus been reported that the relapse rate of ANCA-SVV among patients on chronic dialysis is substantially lower compared with that among patients with preserved renal function.7,12 It is not clear whether this reduced rate of relapse is attributable to continued immunosuppression or simply reflects the natural progression of this disease. Fear of disease relapse has led to the use of preventive maintenance immunosuppressive therapy irrespective of the need for dialysis.7,13 However, morbidity associated with dialysis demands that any benefit from immunosuppression outweighs the risks. If ANCA-SVV disease activity and relapses decrease on chronic dialysis, prolongation of immunosuppression after ESRD may be associated with more risk than benefit.
On the basis of a large inception observational cohort of patients with ANCA-SVV, we describe the clinical course of dialysis-dependent patients compared to patients with preserved renal function with respect to the cause of ESRD, the disease activity, frequency of relapse, morbidity, and mortality.
RESULTS
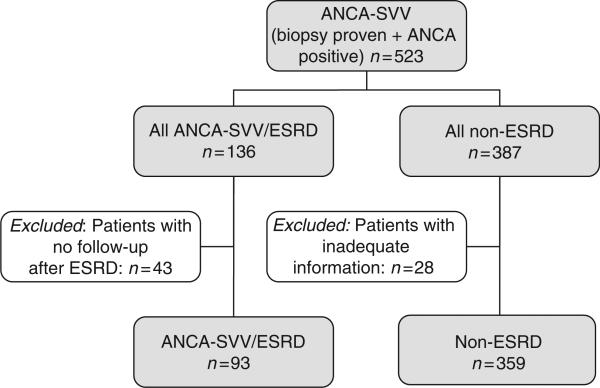
Description of the ANCA-SVV ESRD and non-ESRD groups
Of the 523 ANCA-positive patients with biopsy-proven ANCA-SVV in our registry, 95 needed dialysis at initial presentation. Of these, 40 remained dialysis dependent or died during the first 3 months, and 55 recovered enough renal function to discontinue dialysis. As of 1 October 2007, we identified 136 patients who had reached ESRD (ESRD group) and 387 patients with preserved renal function (non-ESRD group). No clinical information after the start of dialysis was available for 43 patients in the ESRD group; therefore, they were excluded from the analyses. Likewise, 28 patients (7%) with preserved renal function were excluded because of limited follow-up (recently diagnosed cases or lost to follow-up) (Figure 1). The median observation time for the ESRD group, including time after ESRD, was 63 months (interquartile range (IQR): 13–75 months) and for the non-ESRD group was 40 months (IQR: 15–55 months).
Figure 1.
Inception cohort with ANCA vasculitis.
Demographics
As shown in Table 1, demographics, and baseline and disease-related characteristics of the total ESRD group (n = 136) were similar to those of the ESRD group with available follow-up (n = 93). As a consequence, we considered the latter group representative of the total ESRD group and based estimates and comparisons on this subgroup.
Table 1.
Characteristics of patients with ANCA-SVV who reached ESRD
| Characteristic | All ANCA-SVV/ESRD | ANCA-SVV/ESRD with available long-term information |
|---|---|---|
| n (%) or mean ± s.d. | n=136 | n=93 |
| Age at diagnosis (years) (± s.d.) | 57 ± 21 | 56 ± 22 |
| Gender, female (%) | 67 (49%) | 47 (51%) |
| Race | ||
| Caucasian | 113 (83%) | 78 (84%) |
| Other | 23 (17%) | 15 (16%) |
| ANCA typea | ||
| PR3 or C-ANCA | 45 (34%) | 35 (39%) |
| MPO- or P-ANCA | 88 (66%) | 55 (61%) |
| Disease category | ||
| WG | 19 (14%) | 14 (15%) |
| MPA | 67 (49%) | 48 (52%) |
| RL | 49 (36%) | 30 (32%) |
| CSS | 1 (1%) | 1 (1%) |
| Organ involvement, ever | ||
| Lung | 63 (46%) | 47 (51%) |
| ENT | 36 (27%) | 26 (28%) |
| Initial treatmentb | ||
| No treatment | 12 (9%) | 6 (7%) |
| Corticosteroids alone | 23 (17%) | 13 (14%) |
| Prednisone + CYC | 96 (72%) | 70 (77%) |
| Other | 2 (2%) | 2 (2%) |
| Age at initiation of ESRD (years) | 58 ± 21 | 57 ± 22 |
| Duration of disease,# months (± s.d.) | 16.6 ± 27.01 | 16.9 ± 28.8 |
| Median | 3.45 | 2.4 |
| IQR | 0.03, 24.01 | 0.0, 24.01 |
| BVAS at diagnosis of ESRD (± s.d.) | 7.75 ± 8.05 | 8.21 ± 8.25 |
ANCA-SVV, antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis; BVAS, Birmingham Vasculitis Activity Score; CSS, Churg–Strauss syndrome; CYC, cyclophosphamide; ENT, ear, nose, and throat; ESRD, end-stage renal disease; MPA, microscopic polyangiitis; RL, renal-limited disease; WG, Wegener's granulomatosis.
Duration of disease before ESRD among patients who reached ESRD and for the full duration of follow-up for the non-ESRD group.
Three patients were reported as 'ANCA positive,' but had missing information on ANCA specificity. These three patients were among those with long-term follow-up information.
Three patients had missing information on initial treatment, two of whom were among those with long-term follow-up information.
All patients in the ESRD group and 87% in the non-ESRD group had biopsy-proven glomerulonephritis. No significant differences were observed in the distribution of gender and race, with a predominance of Caucasians in both groups (84 and 87%, respectively). Mean age at diagnosis of ANCA-SVV was 56 years in both groups.
In the ESRD group, mean age at initiation of chronic dialysis was 58 years. Over the follow-up time with renal failure, the majority of patients received hemodialysis (n = 83 and 89%), whereas only a minority were treated at any time with peritoneal dialysis (n = 31 and 33%) or had a kidney transplant (n = 19 and 20%).
Characteristics related to ANCA-SVV
The mean Birmingham Vasculitis Activity Score14 (BVAS) at initiation of chronic dialysis was 7.75 ± 8.05, whereas 51.5% of the patients had active disease in at least one organ (BVAS > 1). All patients in the ESRD group (n = 136) were further categorized with respect to the phase of ANCA-SVV, which led to ESRD (Table 2). Sixty-nine patients (51%) reached ESRD due to new-onset ANCA glomerulonephritis (BVAS = 13.76 ± 5.98), with 51 dialysis dependent at presentation and 12 who progressed to renal failure without attaining a remission. Of the 51 patients who required dialysis at presentation, 7 were not treated, whereas the remaining 44 received immunosuppressive therapy. Relapsing disease involving the kidney led to ESRD in eight cases (6%) (BVAS = 7.50 ± 5.92). Fifty-eight patients (43%) reached ESRD due to progressive chronic kidney disease without evidence of active ANCA glomerulonephritis (BVAS = 0) (Tables 1 and 2). Mean time from diagnosis to ESRD in these patients was 24 months (IQR: 12–45 months).
Table 2.
Summary of cause and disease activity among patients reaching ESRD
| ESRD subgroup | n (%) | % With active disease | BVAS (mean ± s.d.) |
|---|---|---|---|
| ESRD groupa | 136 | 51.5 | 7.75 ± 8.05 |
| New-onset GN | 69 (51%) | 100 | 13.76 ± 5.98 |
| Relapsing GN | 8 (6%) | 100 | 7.50 ± 5.92 |
| Progressive CKD without active vasculitis | 58 (43%) | 0 | 0 |
BVAS, Birmingham Vasculitis Activity Score; CKD, chronic kidney disease; ESRD, end-stage renal disease; GN, glomerulonephritis.
One unknown ESRD cause.
Myeloperoxidase ANCA (MPO-ANCA) was more frequent in the ESRD group (61%) than in the non-ESRD group (53%), but this difference was not statistically significant (P = 0.25). The ESRD group comprised a higher proportion of patients with renal-limited disease (33%) and a lower proportion of patients with Wegener's granulomatosis (15%) compared with the non-ESRD group (20 and 27%, respectively; P = 0.0104) (Table 3). A total of 66 ESRD patients (71%) had at least one risk factor previously associated with relapse in a subset of the overall cohort3 (PR3-ANCA positive, pulmonary or upper respiratory involvement), which was similar to the overall group where 274 (76%) had at least one of these risk factors.
Table 3.
Comparison of the ANCA-SVV ESRD and non-ESRD groups
| Characteristica | ANCA-SVV ESRD | ANCA-SVV non-ESRD | |
|---|---|---|---|
| n (%), mean ± s.d., rate with 95% CI, or median and IQR, as noted | n=93 | n=359 | P-value |
| Age at diagnosis (years) | 56 ± 22 | 56 ± 18 | 0.32 |
| Gender, female (%) | 47 (51%) | 164 (46%) | 0.53 |
| Race | |||
| Caucasian | 78 (84%) | 312 (87%) | 0.80 |
| Non-Caucasian | 15 (16%) | 47 (13%) | |
| ANCA type | |||
| PR3 or C-ANCA | 35 (39%) | 164 (47%) | 0.25 |
| MPO- or P-ANCA | 55 (61%) | 188 (53%) | |
| Disease category | |||
| WG | 14 (15%) | 97 (27%) | 0.0104 |
| MPA | 48 (52%) | 187 (53%) | |
| RL | 30 (33%) | 72 (20%) | |
| Organ involvement | |||
| Kidney | 93 (100%) | 339 (94%) | 0.0193 |
| Lung | 47 (51%) | 187 (52%) | 0.82 |
| ENT | 26 (28%) | 143 (40%) | 0.04 |
| Peak serum creatinine at onset (± s.d.) (mg per 100 ml) | 6.9 ± 4.5 | 3.7 ± 2.8 | <0.0001 |
| Initial treatment | |||
| No treatment | 6 (6%) | 8 (2%) | 0.007 |
| Corticosteroids alone | 13 (14%) | 29 (8%) | |
| Prednisone + CYC | 70 (77%) | 300 (84%) | |
| Other | 2 (2%) | 22 (6%) | |
| Treatments with CYC, months | 6.6 ± 7.5 | 9.1 ± 9.4 | 0.0002 |
| Treatment resistant | 52 (56%) | 29 (8%) | <.0001 |
| ANCA-SVV pre-ESRD | 0.20 (0.13–0.26) | 0.16 (0.13–0.17) | 0.20 |
| Relapse rate, episodes per person-year (95% post-ESRD CI) | 0.08 (0.04–0.11) | NA | |
| P-value versus pre-ESRD=0.0012 | |||
| Malignancy rate, episodes per person-year (95% CI) | 0.04 (0.01–0.07) | 0.01 (0.007–0.02) | 0.046 |
| Patients with malignancy | 5 (5%) | 15 (4%) | |
| Total number of malignancies | 8 | 15 | |
| Mortality rate (deaths/person-year) | 0.31 (0.26–0.36) | 0.07 (0.05–0.08) | <0.0001 |
| Deaths, total number (%) | 59 (63%) | 76 (21%) | |
| Median follow-up time in months (IQR) | 63 (13–75) | 40 (15–55) |
ANCA-SVV, antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis; BVAS, Birmingham Vasculitis Activity Score; CI, confidence interval; CSS, Churg–Strauss syndrome; CYC, cyclophosphamide; ENT, ear, nose, and throat; ESRD, end-stage renal disease; IQR, interquartile range (25th and 75th percentile); MPA, microscopic polyangiitis; NA, not applicable; RL, renal limited disease; WG, Wegener's granulomatosis.
One to three values were missing for each variable and were excluded from estimates and comparison tests.
Regarding organ involvement, all patients within the ESRD group and 94% of patients in the non-ESRD group exhibited kidney involvement at some point, but entry serum creatinine was significantly higher in the ESRD group (P < 0.0001). Lung involvement was present with similar frequency, whereas upper respiratory tract involvement was more frequent among the non-ESRD patients.
Compared with the non-ESRD group, more patients in the ESRD group received no immunosuppression (6 versus 2%) and corticosteroids alone (14 versus 8%), whereas fewer in the ESRD group received treatment with combined therapy with corticosteroids and cyclophosphamide (CYC) (75 versus 84%) either orally or intravenously at the time of diagnosis (P = 0.007) (Table 3). Patients in the ESRD group received CYC for a shorter duration (6.6 ± 7.5 months) than did patients in the non-ESRD group (9.1 ± 9.4 months, P = 0.0002). Treatment resistance was more common in the ESRD group (56%) than in the non-ESRD group (8%; P < 0.0001).
Among the ESRD patients, we compared those who required dialysis within 1 month of diagnosis of ANCA-SVV with the patients who reached ESRD later, as a result of relapse or a progressive decline in glomerular filtration rate without active vasculitis or glomerulonephritis (Table 4). Patients with ESRD from new-onset ANCA glomerulonephritis had higher levels of serum creatinine (8.6 ± 4.2 versus 5.3 ± 3.5 mg per 100 ml, P < 0.0001), received treatment with CYC for a shorter period (2.9 ± 3.0 versus 8.4 ± 7.9 months, P < 0.0001), and were more frequently resistant to therapy (86 versus 25%, P < 0.0001) (Table 4).
Table 4.
Characteristics of the patients with ANCA-SVV and ESRD in association with phase of ANCA glomerulonephritis that led to chronic renal failure
| ANCA-SVV/ESRD, n=136 | New-onset disease, n=69a | Relapse or CKD, n=66a | P-value |
|---|---|---|---|
| Peak entry creatinine (± s.d.) | 8.6 ± 4.2 | 5.3 ± 3.5 | <0.0001 |
| Treatment with CYC, months (± s.d.) | 2.9 ± 3.0 | 8.4 ± 7.9 | <0.0001 |
| Treatment resistant (%) | 59 (86%) | 16 (25%)b | <0.0001 |
| Median time from ANCA-SVV diagnosis to ESRD in months, median (IQR) | 0.03 (0–0.9) | 24 (10–39) | <0.0001 |
ANCA-SVV, antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis; CYC, cyclophosphamide; ESRD, end-stage renal disease; IQR, interquartile range.
One patient with unknown disease status when reached ESRD.
Two missing values for treatment resistance.
Relapse rates of ANCA-SVV before and after ESRD
Relapse rates of ANCA-SVV were estimated before and after initiation of chronic dialysis, and for the non-ESRD group. In the ESRD group, relapses occurred at a rate of 0.20 episodes per person-year (95% confidence interval (CI): 0.13–0.26) before dialysis, which was similar to the rate of 0.16 episodes per person-year (95% CI: 0.13–0.17) for the non-ESRD group (P = 0.20). When the non-ESRD group was limited to only patients with renal involvement (n = 339), the relapse rate was similar (0.15 episodes per person-year; 95% CI: 0.14–0.17). Post ESRD, the relapse rate was 0.08 per person-year (95% CI: 0.04–0.11), which was significantly lower than the pre-ESRD rate (P = 0.0012) (Table 3). Similarly, the exclusion of patients with renal-limited ANCA-SVV did not significantly impact the relapse rate in the non-ESRD group (0.17 episodes per person-year; 95% CI: 0.15–0.19) or the relapse rates before and after ESRD (0.20 and 0.08 episodes per person-year, respectively) in the ESRD group.
Among patients who reached ESRD, the relapse rate pre and post ESRD was calculated separately for the subgroups of patients with PR3- and MPO-ANCA (Table 5). For patients with PR3-ANCA, the rate of relapse decreased significantly from 0.34 (95% CI: 0.25, 0.42) episodes per person-year pre ESRD to 0.11 (95% CI: 0.04, 0.18) post ESRD (P = 0.0015). For patients with MPO-ANCA, the rates of relapse were low and did not change significantly pre and post ESRD (0.06 (95% CI: 0.004, 0.12) and 0.04 (95% CI: 0.002, 0.076), respectively (P = 0.47)).
Table 5.
Summary of pre and post relapses in patients who reached ESRD
| ESRD | Group | n (%) | n (relapse) | Follow-up time (years) | Incidence (per patient-year) | 95% CI |
|---|---|---|---|---|---|---|
| Pre | ||||||
| ESRD | 93 | 26 | 130.4 | 0.20 | (0.13, 0.26) | |
| PR3-ANCAa | 35 (39%) | 22 | 65.3 | 0.34 | (0.25, 0.42) | |
| MPO-ANCAa | 55 (61%) | 4 | 62.4 | 0.06 | (0.004, 0.12) | |
| Post | ||||||
| ESRD | 93 | 14 | 184.6 | 0.08 | (0.04, 0.11) | |
| PR3-ANCAa | 35 (39%) | 7 | 65.6 | 0.11 | (0.04, 0.18) | |
| MPO-ANCAa | 55 (61%) | 4 | 101.1 | 0.04 | (0.002, 0.076) |
CI, confidence interval; ESRD, end-stage renal disease.
Data on ANCA antigen specificity missing for 3 of the 93 patients who reached ESRD.
Ten (11%) patients experienced 14 vasculitis relapses after the start of dialysis corresponding to an incidence rate of 0.25 relapses per patient-year (95% CI: 0.17, 0.33). These 10 patients had had a total of eight relapses pre ESRD (0.40 relapses per patient-year (95% CI: 0.26, 0.53)). Three of them had ESRD due to new-onset vasculitis, one due to relapse, and six due to chronic kidney damage. Five of the 14 post-ESRD relapses occurred while the patients were on maintenance immunosuppressive therapy. Organ systems affected by relapses included the lungs (n = 8), upper respiratory tract (n = 3), skin (n = 2), musculoskeletal system (n = 3), and the eye (n = 1). The mean BVAS at relapse was 8.9, excluding three cases for which insufficient information was available for scoring. Immunosuppressive treatment was instituted in 11 of 14 occurrences. Of the three untreated patients, one had unrecognized active vasculitis that was detected on autopsy and one had upper respiratory tract disease managed with a local procedure. The third was an elderly patient with only mild skin manifestations. Relapse outcomes included complete or partial remission in eight patients, death in five (including the relapse detected at autopsy), and an unknown outcome in one patient.
Adverse events
Three hundred and fifty-four episodes of infections occurred in 61 patients on chronic dialysis corresponding to an incidence rate of 1.92 episodes per person-year. Infectious episodes included the respiratory and urinary tracts, the skin, dialysis access sites, bacteremia of unknown origin, and peritonitis. The rate of infection was significantly higher among immunosuppressed patients (1.94 episodes per person-year; 95% CI: 1.61–2.26) than among those not receiving immunosuppressive therapy (1.03 episodes per person-year; 95% CI: 0.87, 1.19; P < 0.0001).
Eight malignancies were diagnosed in five patients in the ESRD group, resulting in an incidence rate of 0.04 episodes per patient-year (95% CI: 0.01–0.07), which was statistically higher than that in the non-ESRD group where 15 malignancies were found in 15 patients, resulting in a rate of 0.01 episodes per person-year (95% CI: 0.007–0.02, P = 0.046) (Table 3).
Mortality rate and patient survival before and after ESRD
In the ESRD group, 59 deaths occurred, corresponding to a mortality rate of 0.31 deaths per person-year (95% CI: 0.26–0.36). Deaths occurred in a median of 43 months from diagnosis of ANCA-SVV and 37 months from the start of chronic dialysis. Causes of death were known in 21 of the ESRD cases, and were attributed to infection (n = 9), active vasculitis (n = 5), cardiovascular disease (n = 3), malignancy (n = 3), and withdrawal from dialysis (n = 1). Within a month of diagnosis, the predominant cause of death was active vasculitis (4 of 5 deaths), whereas subsequent deaths were primarily due to infections (8 of 16 deaths). Death rate among patients who reached ESRD with active vasculitis (new-onset disease or relapse) (37 of 56, 66%) was similar compared with those who reached ESRD with no active vasculitis (22 of 37, 60%, P = 0.66). However, the incidence of death among patients with ESRD from new-onset disease (0.86 deaths per patient-year; 95% CI: 0.70, 1.01) was significantly higher than that for those who reached ESRD later (0.16 deaths per patient-year; 95% CI: 0.12, 0.21) corresponding to an incidence ratio of 5.22 (95% CI: 3.79, 7.21). One-year and 5-year survival rates were 77 and 28%, respectively, for the ESRD group (from disease onset). The proportion of patients with ESRD who died was similar for the three disease categories (63% of patients with either renal-limited disease or microscopic polyangiitis, 65% of patients with Wegener's granulomatosis).
In the non-ESRD group, 76 deaths occurred corresponding to a significantly lower mortality rate (0.07 deaths per person-year; 95% CI: 0.05–0.08; P < 0.0001) than in the ESRD group (0.27, 95% CI: 0.21–0.32 per person-year). Cause of death was unknown in 55 patients. In the 21 with known causes, death was attributed to vasculitis (n = 7), cardiovascular disease (n = 6), malignancy (n = 5), and infections (n = 3). One-year and 5-year survival rates were 92 and 71%, respectively.
DISCUSSION
Despite the improved therapy of ANCA-SVV over the past 2 decades, the frequency of ESRD remains elevated. In our patient population, 26% of patients reached ESRD over a median of 34 months (IQR: 12–75 months). It is noteworthy that 94% of patients who reached ESRD did so in one of two settings: presentation with advanced renal failure requiring dialysis at or near the time of diagnosis, or progression to ESRD without evidence of active glomerulonephritis or vasculitis over a median of 24 months. Eighty percent of patients who required dialysis near diagnosis presented with severe renal insufficiency (mean entry serum creatinine of 8.6 mg per 100 ml) and remained dialysis dependent despite the institution of appropriate immunotherapy with corticosteroids and CYC. This underscores the importance of early diagnosis and prompt treatment to attain recovery of renal function before renal scarring has occurred. It is worth noting that the majority of patients presented before plasmapheresis was used routinely for patients presenting with advanced renal disease,15 which may decrease the frequency of irreversible dialysis dependence in the future.
In our cohort, there was a higher representation of patients with renal-limited disease in the dialysis-dependent group compared with that in the non-ESRD group. In the absence of extrarenal manifestation of disease, patients may not seek medical attention until they develop uremic symptoms associated with advanced renal scarring and a limited chance of recovery. This concept is supported by our finding that patients who require dialysis at or near diagnosis presented with significantly higher serum creatinine levels. It is also supported by previous correlations of poor renal prognosis with histological markers of chronic scarring.3,16,17
Comparison of initial therapy between the ESRD and non-ESRD groups reveals a higher frequency of patients who did not receive immunosuppressive therapy, and a shorter duration of treatment with CYC in the dialysis-dependent group. The higher frequency of non-treated patients reflects the perception that, in patients with advanced renal failure and no extrarenal vasculitis, the risk of immunosuppressive therapy outweighs the potential benefit. However, a recent cohort analysis showed that there was no glomerular filtration rate below which treatment was futile.3 Similarly, a subgroup analysis of patients presenting with advanced renal failure participating in the MEPEX study revealed that therapeutic futility is seen only when 2% or fewer of glomeruli are normal.4
The finding of a shorter initial course of CYC among the ESRD group is a result rather than a cause of dialysis dependence. In the absence of extrarenal manifestations, cytotoxic therapy is usually discontinued when dialysis dependence continues beyond 3 months. Renal function is not likely to recover beyond that point,6,18–20 whereas continuing cytotoxic therapy is associated with serious infections and death.20
An important issue to address is whether dialysis-dependent patients should receive maintenance immunosuppression for relapse prevention. This decision should be based on an assessment of the risk of continued immunosuppression relative to that of relapse. In our cohort, patients with ESRD had a significantly lower relapse rate after the start of dialysis (0.08 episodes per person-year) than that before (0.20 episodes per person-year). Differences in relapse rates between those on dialysis and those pre dialysis have also been reported by Weidanz et al.7, with a rate after dialysis of 0.13 per person-year (95% CI: 0.07, 0.19) and before dialysis of 0.40 per person-year (95% CI: 0.15, 0.98). This lower rate on dialysis reflects only extrarenal relapses, as recurrent or persistent glomerulonephritis is essentially undetectable once ESRD is reached. It may also reflect changes of the autoimmune response in ESRD, as has been suggested for systemic lupus erythematosus.8–11 Our relapse rate among patients with ESRD is similar to that reported in other cohorts (0.0912 and 0.137 per person-year), but substantially lower than that reported by Haubitz et al.13 (0.24 per person-year) among 35 patients with Wegener's granulomatosis who reached ESRD.
The lower rate of relapse after ESRD occurs in the context of a high rate of infections, especially among patients on maintenance immunosuppressants, whose rate was almost twice that of patients not receiving such treatments. Although the total number of vasculitic flares in our ESRD group was too small (14 events in 10 patients) to formally assess the effect of maintenance immunosuppression on relapse, our findings suggest that the risks of maintenance immunosuppression outweigh the potential benefit among patients with ESRD. We contend that immunosuppressive therapy should be restricted to patients with clinical evidence of active extrarenal manifestation of vasculitis.
The 1-year survival rate of 77% for patients in the ESRD group is comparable with rates of 64–83% reported in other cohorts.5–7,7,12,13 In our cohort, the survival rates were over four times worse in the ESRD group compared with those in the non-ESRD group, which is similar to previous reports.6 The 1-year and 5-year survival rates for patients with ANCA-SVV and ESRD (77 and 28%, respectively) are also considerably worse compared with survival rates for adult patients with ESRD from all causes (90 and 48%, respectively).21 The excess mortality among ANCA-SVV ESRD patients is likely attributable to a combination of sequelae of systemic vasculitis, long-term effects of therapy with corticosteroids, CYC and other immunosuppressants, and older age. The significantly higher incidence of death among the patients with ESRD from new-onset disease compared with that of patients reaching ESRD late in their course of disease likely reflects early deaths from severe active vasculitis.
One may argue that a limitation of this study pertains to the composition of our cohort of patients with ANCA-SVV with a large predominance of patients with glomerulonephritis, which may reflect a lower rate of relapse. However, it is unlikely that the small number of disease flares after ESRD were due to an over-representation of patients at low risk (for example, patients with renal-limited disease) as 71% had at least one risk factor of relapse (50% with lung involvement, 28% with upper respiratory tract disease, and 39% PR3-ANCA positive). In addition, the significant decrease in the rate of relapse post ESRD we observed is not due to an over-representation of patients with MPO-ANCA among patients with ESRD, as this decrease in relapse rate was primarily seen among patients with PR3-ANCA. Furthermore, we report a sharp decrease in the rate of relapses after ESRD (compared with that before) based on the same population, which excludes any effect of the cohort composition on the relapse rate post ESRD. Unfortunately, the small number of disease flares does not allow assessment of the influence of these three risk factors on relapse in the population of patients with ESRD.
Other limitations to our study include retrospective BVAS scoring, incomplete data on the causes of death, and possibly incomplete reporting of serious infections. The latter, however, would result in an underestimation of the importance of infections in the outcome of patients with ANCA vasculitis and ESRD. For lack of suitable local or regional data, we were also unfortunately unable to compare the all-cause and cardiovascular mortality of patients with ANCA vasculitis and ESRD with that of a control population of patients with ESRD, controlling for age and race.
In summary, despite advances in treatment, a quarter of patients with ANCA-SVV still suffer from ESRD. ESRD is largely contributed to by new-onset disease with severe renal failure, rather than by persistent or relapsing vasculitis, underscoring the need for prompt diagnosis and therapy. Faster recognition of ANCA-SVV and implementation of a more effective therapy may help reduce the burden of ESRD. Patients with ANCA-SVV and ESRD experience a low rate of relapses, but are at high risk of infectious complications associated with significant morbidity and mortality. These observations support the judicious use of immunosuppression in patients with ANCA-SVV ESRD, with restriction to use only in the setting of active vasculitis.
MATERIALS AND METHODS
Description of patient population and definitions
Patients enrolled in the ANCA-SVV registry as of 1 October 2007 were eligible to be included in this inception cohort study, which was started in 1986. Registry patients were identified at or near initial diagnosis and fulfilled three criteria: native kidney biopsy showing pauci-immune ANCA glomerulonephritis with or without granulomatous inflammation and/or biopsy on any other tissue showing pauci-immune SVV (n = 27; predominantly the lung, sinus, and skin), positive ANCA determination by immunofluorescence microscopy or antigen-specific enzyme-linked immunosorbent assay,22 and signed informed consent for review of medical records. ANCA positivity was further classified as cytoplasmic ANCA and/or anti-proteinase-3 (anti-PR3) ANCA, or perinuclear ANCA and/or anti-myeloperoxidase ANCA (anti-MPO) or both. A perinuclear ANCA alone required a concurrent negative antinuclear antibody test.23,24 Renal biopsy specimens were evaluated by the University of North Carolina Nephropathology Laboratory and patients were followed by physicians of the Glomerular Disease Collaborative Network. The Glomerular Disease Collaborative Network and subsets of the ANCA-SVV registry have previously been described elsewhere.3,18,19
Diagnostic ANCA-SVV categories were defined according to the Chapel Hill Consensus Conference.23 A diagnosis of Wegener's granulomatosis was defined by the presence of necrotizing granulomatous inflammation in any tissue by histology, and/or imaging showing pulmonary nodules or cavities (non-infectious) and/or bony erosions, and/or subglottic stenosis in the upper respiratory tract. Churg–Strauss syndrome was defined by the presence of asthma, eosinophilia, and necrotizing granulomatous inflammation. Microscopic polyangiitis was defined by systemic necrotizing SVV without evidence of granulomatous inflammation or asthma.23,25
Organ involvement was defined by biopsy or by previously described criteria.18,19 BVASs were determined from a review of medical records. Treatment categories were determined by the regimen used at diagnosis and included no therapy, corticosteroids alone, or corticosteroids in combination with CYC or other immunosuppressive therapies.3,18 Duration of immunosuppressive therapy was recorded but patients were considered as treated with a given regimen independent of duration.
Outcomes of interest included treatment resistance, remission on or off therapy, relapse, ESRD, and death, as previously described.3,18 In brief, outcomes after the initial diagnosis of ANCA-SVV were determined as treatment resistance, remission on therapy, remission off therapy, relapse, and ESRD. Treatment resistance was defined as a progressive decline in kidney function with persistence of active urine sediment or new or persistent extrarenal manifestations of vasculitis despite immunosuppressive treatment. Resistance to therapy was determined after at least 1 month of therapy. Remission was defined as the stabilization or improvement of kidney function as measured by serum creatinine levels and resolution of hematuria or other manifestations of systemic vasculitis for more than 1 month. The persistence of proteinuria was not considered indicative of active glomerulonephritis. Remission off therapy was defined as remission with no therapy or with less than 7.5 mg prednisone per day. Relapse could only be recorded among responders (the patients who had achieved remission either on or off therapy) and included recurrent or new signs and symptoms of active vasculitis in any organ.
The cohort of patients with ANCA-SVV was divided into two groups (Figure 1): (1) patients who had reached ESRD, at or any time after the diagnosis of ANCA-SVV, and then maintained on chronic renal replacement therapy (ESRD group) and (2) those with a preserved renal function (non-ESRD group). Three patients who underwent kidney transplantation promptly after the diagnosis of ESRD were excluded. Patients who required acute dialysis at or after the diagnosis of ANCA-SVV, but subsequently recovered renal function and were dialysis free as of 1 October 2007, were included in the non-ESRD group. Conversely, patients who were dialysis dependent at presentation and never came off were included in the ESRD group. Dialysis dependency at presentation was considered in cases with severe renal insufficiency requiring dialysis at, or within, 30 days of diagnosis. The start of chronic dialysis was determined as the date when dialysis was initiated with no subsequent interruption. As our ANCA-SVV database includes information until death or ESRD, and for the purposes of this study, a detailed supplemental clinical information was sought from the treating nephrologists for all cases in the ESRD group from the onset of dialysis to kidney transplantation or death.
All patients in the ESRD group were categorized with respect to the phase of ANCA-SVV at the point they reached ESRD. Hence, each patient could be classified as having reached ESRD due to one of three reasons: (1) new-onset ANCA glomerulonephritis, which primarily includes those who presented at or near dialysis and those patients who were treatment resistant, (2) relapse of ANCA-SVV involving the kidney, which led to ESRD, or (3) progressive chronic kidney disease without evidence of active ANCA glomerulonephritis for greater than 6 months.
In cases with ANCA-SVV and ESRD, any symptoms or signs were considered vasculitic in origin if they had been observed for a month or more after the initiation of dialysis, to differentiate them from uremic symptoms. We recorded all relapses of ANCA-SVV on chronic dialysis, the organs involved, the type and duration of, and response to, prescribed immunosuppressive therapy. Vasculitic activity was assessed using the BVAS system.14
Infections in the ESRD group were determined as those requiring hospitalization. For comparison of infection rates by use of immunosuppressive therapy, a minimum of 1 month of immunosuppressive therapy after the onset of dialysis was required. Malignancies were assessed by review of medical records. Death was documented from available medical records or from the Social Security Death index (http://ssdi.rootsweb.ancestry.com/cgi-bin/ssdi.cgi).
Statistical methods
Descriptive statistics include n, percent, mean, s.d., and IQR. Demographics and clinical characteristics were compared between patients who reached ESRD and those who did not reach ESRD using Fisher's exact tests for categorical measures and Wilcoxon rank sum tests for continuous measures. The same tests were used to compare clinical characteristics between subgroups of ESRD patients. In instances where continuous measures were compared across more than two patient groups, Kruskal–Wallis tests were used. Incidence rates were calculated, taking into account the varying follow-up times, and are reported as per patient-year of follow-up using all events for relapse, infection, malignancies, and mortality. The binomial principles were used to calculate 95% CIs and s.d. for incidence rates. P-values for comparing two incidence rates were calculated by testing the hypothesis that their difference was equal to zero. Kaplan–Meier estimators were used to estimate the probability of ESRD-free survival. Analyses were conducted using SAS 9.1 (SAS Institute, Cary, NC, USA). Exact P-values are reported with a two-sided P-value of 0.05 or less considered statistically significant.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.de Groot K, Adu D, Savage CO. The value of pulse cyclophosphamide in ANCA-associated vasculitis: meta-analysis and critical review. Nephrol Dial Transplant. 2001;16:2018–2027. doi: 10.1093/ndt/16.10.2018. [DOI] [PubMed] [Google Scholar]
- 2.Fauci AS, Haynes BF, Katz P, et al. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98:76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- 3.Hogan SL, Falk RJ, Chin H, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med. 2005;143:621–631. doi: 10.7326/0003-4819-143-9-200511010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Jayne DR, Gaskin G, Rasmussen N, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–2188. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 5.Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41:776–784. doi: 10.1016/s0272-6386(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 6.Weidner S, Geuss S, Hafezi-Rachti S, et al. ANCA-associated vasculitis with renal involvement: an outcome analysis. Nephrol Dial Transplant. 2004;19:1403–1411. doi: 10.1093/ndt/gfh161. [DOI] [PubMed] [Google Scholar]
- 7.Weidanz F, Day CJ, Hewins P, et al. Recurrences and infections during continuous immunosuppressive therapy after beginning dialysis in ANCA-associated vasculitis. Am J Kidney Dis. 2007;50:36–46. doi: 10.1053/j.ajkd.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Correia P, Cameron JS, Ogg CS, et al. End-stage renal failure in systemic lupus erythematosus with nephritis. Clin Nephrol. 1984;22:293–302. [PubMed] [Google Scholar]
- 9.Mojcik CF, Klippel JH. End-stage renal disease and systemic lupus erythematosus. Am J Med. 1996;101:100–107. doi: 10.1016/s0002-9343(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 10.Fries JF, Weyl S, Holman HR. Estimating prognosis in systemic lupus erythematosus. Am J Med. 1974;57:561–565. doi: 10.1016/0002-9343(74)90007-2. [DOI] [PubMed] [Google Scholar]
- 11.Coplon NS, Diskin CJ, Petersen J, et al. The long-term clinical course of systemic lupus erythematosus in end-stage renal disease. N Engl J Med. 1983;308:186–190. doi: 10.1056/NEJM198301273080403. [DOI] [PubMed] [Google Scholar]
- 12.Allen A, Pusey C, Gaskin G. Outcome of renal replacement therapy in antineutrophil cytoplasmic antibody-associated systemic vasculitis. J Am Soc Nephrol. 1998;9:1258–1263. doi: 10.1681/ASN.V971258. [DOI] [PubMed] [Google Scholar]
- 13.Haubitz M, Koch KM, Brunkhorst R. Survival and vasculitis activity in patients with end-stage renal disease due to Wegener's granulomatosis. Nephrol Dial Transplant. 1998;13:1713–1718. doi: 10.1093/ndt/13.7.1713. [DOI] [PubMed] [Google Scholar]
- 14.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87:671–678. [PubMed] [Google Scholar]
- 15.Jayne D. How to induce remission in primary systemic vasculitis. Best Pract Res Clin Rheumatol. 2005;19:293–305. doi: 10.1016/j.berh.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Bajema IM, Hagen EC. Evolving concepts about the role of antineutrophil cytoplasm autoantibodies in systemic vasculitides. Curr Opin Rheumatol. 1999;11:34–40. doi: 10.1097/00002281-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 17.de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, et al. Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: a prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol. 2006;17:2264–2274. doi: 10.1681/ASN.2005080870. [DOI] [PubMed] [Google Scholar]
- 18.Nachman PH, Hogan SL, Jennette JC, et al. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:33–39. doi: 10.1681/ASN.V7133. [DOI] [PubMed] [Google Scholar]
- 19.Hogan SL, Nachman PH, Wilkman AS, et al. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:23–32. doi: 10.1681/ASN.V7123. [DOI] [PubMed] [Google Scholar]
- 20.de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, et al. Chances of renal recovery for dialysis-dependent ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2007;18:2189–2197. doi: 10.1681/ASN.2007010066. [DOI] [PubMed] [Google Scholar]
- 21.National Institute of Diabetes Digestive Kidney Diseases . US Renal Data System, USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health; Bethesda: 2007. [Google Scholar]
- 22.Hagen EC, Andrassy K, Chernok E, et al. The value of indirect immunofluorescence and solid phase techniques for ANCA detection. A report on the first phase of an international cooperative study on the standardization of ANCA assays. EEC/BCR Group for ANCA Assay Standardization. J Immunol Methods. 1993;159:1–16. doi: 10.1016/0022-1759(93)90136-u. [DOI] [PubMed] [Google Scholar]
- 23.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 24.Jennette JC, Wilkman AS, Falk RJ. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol. 1989;135:921–930. [PMC free article] [PubMed] [Google Scholar]
- 25.Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med. 1997;337:1512–1523. doi: 10.1056/NEJM199711203372106. [see comments] [DOI] [PubMed] [Google Scholar]