Abstract
Through the influence of Goldman-Rakic, much research has been focused on the role of the dorsolateral prefrontal cortex in spatial working memory, decision making, and saccade generation, whereas functions of other parts of the frontal lobe including the ventrolateral prefrontal cortex (VLPFC) are less clear. Previous studies in non-human primates have shown that some VLPFC cells are selectively responsive to faces. Recent findings indicate that adjacent to the region where face- and object-selective cells have been recorded are neurons which respond to complex sounds including human and monkey vocalizations. Furthermore, when neurons in this same region are tested with combined face and voice communication stimuli, it is apparent that some cells in VLPFC are multisensory and respond to audiovisual stimuli. The determination that ventral prefrontal neurons are multisensory and responsive to auditory and visual communication stimuli may help to establish an animal model to assist in the investigation of the circuit and cellular basis of human communication. This will also aid in the understanding of general frontal lobe function and the processes that go awry in disorders including autism and schizophrenia, where disturbances in prefrontal function have been noted.
Introduction
The dissociation of the primate prefrontal cortex (PFC) into discrete functional domains has been described by a number of researchers including Fulton (1950), Mishkin (1964), Fuster (1989), Petrides et al. (2002), and Goldman-Rakic (1987, 1996a, 1996b). Goldman-Rakic (1996a) brought evidence from multiple subfields of neuroscience together and emphasized the differential contribution of distinct parallel networks in the service of working memory and argued that multiple working memory domains existed, specialized for the processing of different types of information (Romanski 2004). Her “domain specificity” hypothesis leaned heavily on the previously published theory that visual information could be segregated into dorsal and ventral visual streams (Ungerleider and Mishkin 1982). Anatomical, physiological, lesion, and neuroimaging studies have provided some support for a segregation of dorsal and ventral streams that extend into the dorsal and ventral prefrontal cortices, respectively (Chavis and Pandya 1976; Mishkin and Manning 1978; Barbas 1998; Wilson et al. 1993; Courtney et al. 1996; Ungerleider et al. 1998; Fig. 1; for review see Romanski 2004).
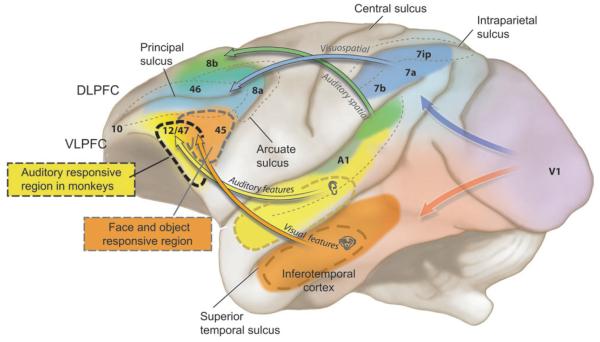
Figure 1.
A schematic of the macaque brain is shown depicting the flow of auditory and visual information through the brain to its ultimate destination in PFC. Regions of the prefrontal cortex are color coded to match those of areas, that project to it. The DLPFC (shown in blue and green) suggested to be essential in spatial working memory receives visual afferents from parietal cortex areas 7a, 7b, and 7ip (shown in blue), which carry visuospatial information, whereas caudal auditory association cortex (green) projects to caudal and dorsal PFC areas 46, 8a, and 8b. VLPFC (orange and yellow areas 12/47 and 45) receive afferents from inferotemporal cortex (orange) carrying information about object features and auditory information from anterior auditory association cortex (yellow). The overlap of auditory and visual domains in VLPFC is indicative of later findings of multisensory processes.
The issue of 2 distinct processing streams in dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC) is somewhat controversial. Some have argued that dorsal and ventral prefrontal cortex receive mixed inputs from both parietal dorsal stream and temporal ventral stream visual cortical regions (Webster et al. 1994; Luppino et al. 2001) and note physiological similarities between neurons in dorsal and ventral prefrontal cortex (Rao et al. 1997). Still others note differences in function when dorsolateral and ventral or orbital frontal regions are recorded from using the same task (Wilson et al. 1993; Wallis et al. 2001; Wallis and Miller 2003). An important issue is the precise delineation of which 2 prefrontal areas are compared (Romanski 2004). For example, when orbital prefrontal regions are compared with DLPFC, differences are much clearer than when the comparison is made with other cytoarchitectonic VLPFC regions. Historically, orbitofrontal cortex has been linked with object processing through lesion studies and it is possible that some VLPFC recordings include some of the regions previously referred to as orbitofrontal cortex. Goldman-Rakic et al. typically recorded from lateral and orbital area 12 in their appreciation of ventral prefrontal object responses (Wilson et al. 1993; O’Scalaidhe et al. 1997; Romanski and Goldman-Rakic 2002). Thus, the main source of controversy may be the area that is referred to as VLPFC when comparisons are drawn. Nonetheless, many agree that areas of the frontal lobe most likely differ with respect to aspects of stimulus or task-related activity.
Although a large number of experiments in nonhuman primates have focused on perceptual visuospatial, saccade, and decision-making processes of the DLPFC (Goldman-Rakic 1987; Funahashi 1990; Chafee and Goldman-Rakic 1998; Williams et al. 2002) fewer studies have probed the functions and responses of neurons in VLPFC, also known as the inferior convexity, which includes areas 12/47 and 45 (Petrides and Pandya 1998). It has been shown that, in the nonhuman primate, VLPFC neurons show little spatial tuning for visual stimuli but do show selectivity for color, shape, or type of stimulus (Pigarev et al. 1979; Rosenkilde et al. 1981; Wilson et al. 1993; O’Scalaidhe et al. 1997, 1999; Nakamura et al. 1998; Hoshi et al. 2000). In some studies, VLPFC neurons with receptive fields that included the fovea were shown to have robust and highly specific responses to pictures of objects and faces (Wilson et al. 1993; O’Scalaidhe et al. 1997, 1999) without evidence of spatial tuning. Additional studies that have compared the processing of spatial and object features across prefrontal regions have supported a bias for object processing in ventral prefrontal cortex including the lateral orbital cortex (Wallis et al. 2001; Wallis and Miller 2003).
The focus of neurophysiological analysis of VLPFC has been on visual working memory or other visual processes, despite the fact that the ventral frontal lobe is the site of speech and language functions in the human brain. Prior to her death in 2003, Goldman-Rakic had begun to address the issue of prefrontal auditory function. In a series of collaborative anatomical and physiological studies, acoustic projections to the prefrontal cortex were mapped and responses to complex auditory stimuli were found in a small VLPFC region. In the current paper, we will review these anatomical and physiological studies in VLPFC. Furthermore, we will discuss new findings indicating that some cells in VLPFC are multisensory and respond to both complex auditory and visual stimuli. The determination that VLPFC neurons are multisensory and responsive to communication stimuli may help us to understand the cellular mechanisms involved in human communication and the processes that occur when it breaks down as can occur in disorders including autism and schizophrenia, where disturbances in prefrontal function have been noted.
Auditory Circuits and Responses in Prefrontal Cortex
In searching for a prefrontal auditory domain in the nonhuman primate brain, knowledge of the auditory afferents to the frontal lobe is essential. The candidate prefrontal areas for acoustic processing in nonhuman primates would be those that have been shown to receive afferents from auditory responsive cortical regions. Although a number of studies have demonstrated connections between anatomically defined auditory cortical regions (Chavis and Pandya 1976; Petrides and Pandya 1988; Barbas 1992), few studies have examined the projections of temporal lobe regions which have been physiologically defined as auditory association cortex. Reexamination and characterization of the organization of the auditory cortex both anatomically and physiologically (Morel et al. 1993; Rauschecker et al. 1995; Hackett et al.1998; Rauschecker 1998; Kaas and Hackett 2000) have reshaped our understanding of the organization of the primate auditory cortex and prompted a reassessment of auditory and prefrontal connectivity. Using this new organizational scheme, recent experiments have examined connections of select regions of the auditory association cortex with the frontal lobe. These anatomical studies confirmed robust projections from the anterior temporal lobe and from the dorsal bank of the superior temporal sulcus (STS) to lateral prefrontal cortex (Hackett et al. 1999; Romanski, Bates, Goldman-Rakic 1999). Additional, albeit lighter, projections originate in earlier portions of auditory association including the parabelt and belt auditory cortex (Romanski, Tian, et al. 1999; Hackett et al. 1999).
Thus a series of cascading auditory afferents originate from auditory association and high-level temporal association cortex and project to key regions of the prefrontal cortex. The earliest projections, from the belt cortex, are light, whereas those from higher association areas such as the anterior temporal lobe and the temporal parietal occipital area (TPO) are dense. Determination of the acoustic nature of these projections is necessary if we are to understand which regions are passing auditory information to particular areas of prefrontal cortex. Some of these projections may not be acoustic in nature. In fact, it is likely that the projections from area TPO are multisensory based on previous physiological recordings (Baylis et al. 1987; Barraclough et al. 2005). Thus characterization of prefrontal afferents as acoustic was undertaken as a collaborative effort. In a combined neurophysiology--anatomical study, the auditory belt and parabelt cortices in macaques were mapped to delineate the anterior (AL), middle (ML) and caudal (CL) auditory belt association fields by Rauschecker and colleagues in a manner similar to their previously published studies (Raushecker et al., 1995) in a manner similar to their previous studies. Similar frequency domains in AL, ML, and CL were injected with distinct anatomical tracers and the resulting projections to and from the prefrontal cortex were charted. These combined anatomical and physiological experiments indicated that several regions of the prefrontal cortex receive afferents from anterior and CL auditory belt/parabelt association cortex including the frontal pole, the rostral principal sulcus, the VLPFC, the lateral orbital cortex, and the frontal eye fields (Romanski, Tian, et al. 1999). Furthermore, these projections are topographically arranged so that rostral and ventral prefrontal cortex receives projections from the anterior auditory association cortex (AL and anterior parabelt) while caudal prefrontal regions are innervated by posterior auditory cortex (CL and caudal parabelt; Fig. 2). Together with recent auditory physiological recordings, these studies suggest that separate auditory streams originate in the anterior and posterior auditory cortex and target rostral, ventrolateral object, and dorsolateral spatial domains in the frontal lobe, respectively (Romanski, Tian, et al. 1999; Rauschecker and Tian 2000; Tian et al. 2001), similar to those of the visual system. Ultimately this also implies that auditory and visual afferents target similar regions of DLPFC and VLPFC (Fig. 1).
Figure 2.
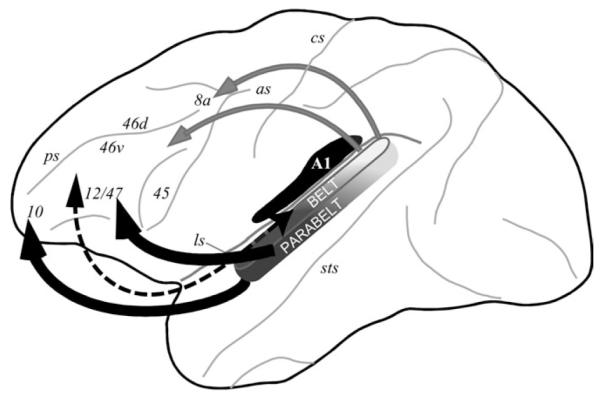
Summary of auditory afferents to the prefrontal cortex. A cascade of increasingly larger projections targets prefrontal cortex (Romanski, Bates, Goldman-Rakic 1999) with light projections (dotted arrows) originating in early auditory belt cortex and heavier projections from parabelt and anterior temporal lobe regions (heavier arrows). The projections are topographic with caudal auditory cortex projecting to DLPFC (gray auditory areas and arrows) and rostral auditory cortex projecting more strongly to anterior and ventral PFC (black auditory areas and arrows) (Romanski, Tian, et al. 1999).
One of the areas which received dense projections from anterior belt and parabelt auditory cortex was the VLPFC, area 12, also referred to as area 12/47 (Petrides and Pandya 1998) or the lateral surface of the inferior convexity, area 12vl (Preuss and Goldman-Rakic 1991). It receives not only dense projections from anterior auditory cortex but also some lighter projections from more caudal auditory regions. In contrast, area 12 orbital receives auditory projections from only anterior auditory areas. The localization of auditory afferents to area 12/47 makes VLPFC an attractive place in which to search for auditory responsive prefrontal neurons in nonhuman primates. We recorded single-unit responses to auditory stimuli in the lateral prefrontal cortex of awake monkeys under controlled conditions. These recordings revealed an acoustically responsive region within area 12/47, which had been shown to receive acoustic projections from auditory association cortex (Romanski, Tian, et al. 1999; Rauschecker and Tian 2000; Romanski and Goldman-Rakic 2002). Although prior studies reported auditory responses in the frontal lobes of nonhuman primates, previously recorded neurons showed only weak responses to auditory stimuli, were seen sporadically, and were not adequately tested with appropriate acoustic stimuli. In our recordings of the awake behaving monkey, we found neurons within a discrete region of the prefrontal cortex, which are robustly responsive to complex auditory stimuli, including species-specific vocalizations (Romanski and Goldman-Rakic 2002; Fig. 3). During these mapping experiments, most of the auditory cells we recorded responded to one or more types of complex stimuli including monkey and human vocalizations but rarely responded to pure tones and band passed noise. The majority of the auditory responsive cells was localized to the anterior VLPFC, in area 12/ 47, and were adjacent to a visually responsive region where neurons have been previously shown to respond to complex stimuli (O’Scalaidhe et al. 1997, 1999) including familiar and unfamiliar objects, patterns, and faces. The juxtaposed auditory and visual responsive regions are shown in Figure 1 superimposed upon the color coding, which indicates the anatomical connections of prefrontal cortex (Fig. 1).
Figure 3.
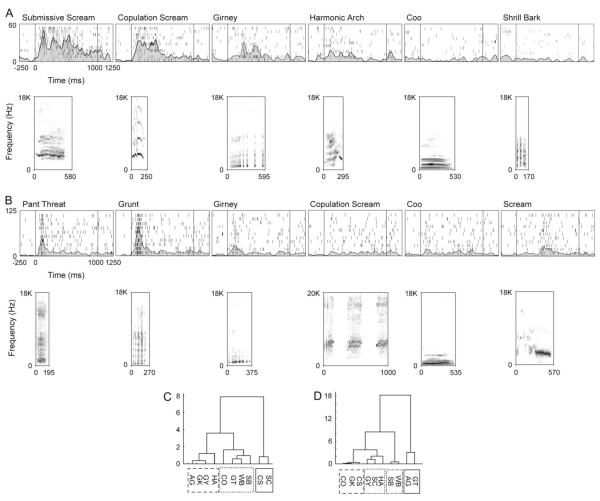
Vocalization responsive neurons in VLPFC. The responses to 5 species-specific vocalizations are shown for 2 example neurons in (A) and (B). The neural response is depicted and the spectrogram for the corresponding vocalization stimulus, which evoked the given response is shown below the raster/spike density plot. The vocalization call type is indicated at the top of each panel. The vocalization onset time was at time 0. The cell in (A) had a response to submissive screams (SC) and to copulation screams (CS). This is also indicated in the dendrogram of the mean response to all 10 call types shown in (C). The cell in (B) responded best to pant threats and grunts. The dendrogram for this cell is shown in (D) and indicates a similar response to the acoustically similar, but functionally different, pants (AG) and grunts (GT). Our data indicated that prefrontal neurons respond with a similar firing rate to calls that have similar acoustic features.
Although VLPFC neurons were initially shown to respond robustly to vocalizations and human speech sounds (Romanski and Goldman-Rakic 2002), the salient features of these complex sounds, which account for prefrontal auditory responses are largely unknown. Species-typical communication stimuli can encode many types of information including vocalization category, caller identity, body size, and reproductive status (Hauser and Marler 1993; Hauser 1996; Bradbury and Vehrencamp 1998; Owings and Morton 1998). Understanding which, if any, of these features is encoded by prefrontal neurons is a pertinent question in characterizing the function of VLPFC. Because the VLPFC receives direct projections from AL and the rostral parabelt auditory association cortices (Hackett et al. 1999; Romanski, Bates, Goldman-Rakic 1999, Romanski, Tian, et al. 1999), it is possible that prefrontal neurons, like those in the lateral belt, also respond to vocalizations with particular acoustic features. However, there remains the possibility that prefrontal neurons respond to species-specific communication sounds based upon their semantic or functional referents. This is supported by human imaging studies of the human inferior frontal gyrus where mnemonic, semantic, and syntactic auditory processes have been localized in positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies (Paulesu et al. 1993; Buckner et al. 1995; Demb et al. 1995; Fiez et al. 1996; Stromswold 1996; Cohen et al. 1997; Gabrieli et al. 1998). Furthermore, in playback experiments using rhesus macaque vocalizations, monkeys responded behaviorally in a similar manner to vocalizations with similar functional referents regardless of acoustic morphology (Hauser 1998; Gifford et al. 2003). The frontal lobe with its widespread connections to sensory and motor systems is a likely candidate to encode some of the behavioral features or functional referents of communication sounds. For example, clusters of nearby cells might all encode food-related vocalizations or agonistic calls. However, it is also possible that cells receiving projections from auditory cortex would more likely encode acoustically similar sounds and vocalizations. This was actually suggested by the fact that neurons responsive to a particular complex sound could often be driven by simpler sounds containing one or more features from the complex sound (Romanski and Goldman-Rakic 2002). An additional question is whether VLFPC cells would respond to species-specific calls that are unfamiliar even though they may be a part of the general macaque repertoire. We tested VLPFC auditory cells in awake, behaving macaque monkeys with a library of rhesus macaque calls recorded from a group of unfamiliar callers. The behavioral and social context under which these calls were produced, as well as their acoustic features, has been well characterized (Hauser and Marler 1993; Gouzoules et al. 1998; Hauser 1998; Ghazanfar and Hauser 1999; Owren and Rendall 2001). We asked whether VLPFC cells would respond to these communication sounds from unfamiliar callers and what type of selectivity prefrontal auditory neurons had with regard to communication-relevant sounds typical of the rhesus macaque repertoire. Further, we asked if prefrontal auditory cells would respond similarly to calls with similar functional referents or if other features of the vocalizations could account for neuronal response specificity.
We determined the selectivity of VLPFC cells for 10 types of rhesus vocalizations and also asked what types of vocalizations cluster together in the neuronal response. There appeared to be a gradient of stimulus selectivity with a small proportion of neurons (8%) responding selectively to only one call type, whereas the majority of the population responded to 2 or more call types (Romanski et al. 2005; Fig. 3). Use of information theoretic approaches to examine vocalization tuning indicates that on average, VLPFC neurons encode information about 2 vocalization types (Romanski et al. 2005). This selectivity of VLPFC neurons is similar to that of neurons found earlier in the auditory hierarchy such as AL (Tian et al. 2001). Further examination of VLPFC auditory responses using a hierarchical cluster analysis of mean firing rate suggests that prefrontal responses to multiple vocalizations is not based strictly on the call function or meaning but may be due to other features including acoustic morphology. When the population of vocalization responsive VLPFC neurons were pooled together it was found that acoustically similar vocalizations, such as “warbles” and “coos” were found to evoke similar responses more frequently in neurons (Fig. 3) than acoustically dissimilar but semantically similar calls such as the high value food calls “warbles” and “harmonic arches” (Romanski et al. 2005). These data are consistent with a role for the primate VLPFC in assessing distinctive acoustic features of complex communication sounds. This would not detract from the assumption that the PFC may categorize complex sounds according to meaning because, in the rhesus macaque vocalization repertoire, specific spectrotemporal features distinguish different call types. Nonetheless some studies have suggested that behavioral context is strictly encoded in prefrontal neurons as evidenced by the similar responses of neurons to semantically similar calls (Gifford et al. 2005). Additional experiments on auditory encoding in the prefrontal cortex may resolve the disparate findings.
The question as to what salient feature is encoded by prefrontal neurons remains unanswered for a number of reasons. One issue is that the stimuli, which are often used to define auditory receptive fields, including pure tones and long white noise sequences, often do not produce reliable responses in prefrontal cortex (Romanski and Goldman-Rakic 2002) or are impractical to use during the awake animal task setting. Second, the selectivity of neurons in higher order sensory areas for complex stimuli implies that the responses are strongly nonlinear functions of the sensory inputs (Tanaka 1993; Rauschecker et al. 1995; Salinas 2000; Bar-Yosef 2002; Lau 2002; Mechler 2002; Sahani and Linden 2003) and, therefore, reverse correlation techniques (Marmarmelis P and Marmarmelis V 1978) may not be effective in approximating the real nonlinearities. A method for reducing the dimensionality of a complex sound and then systematically subtracting the reduced features was described by Averbeck and Romanski (2004) in which the principal components (PC) or independent components (IC) were extracted from complex sounds including species-specific vocalizations. Each PC or IC corresponds to a feature of the vocalization, and due to the way the components are defined, the complete set of PCs or ICs corresponds to all of the features present in the stimuli. The PCs correspond closely to the main Fourier features of the sounds, which are related to the formants of the vocalizations. Conversely, the ICs correspond to features that preserve the relative phase across a set of frequencies (Bell and Sejnowski 1996). Because the features extracted by the 2 techniques can be characterized well, one can directly relate neural responses to PC- and IC-filtered stimuli to specific behavioral (Nearey 1989) and theoretical (Linsker 1988; Lewicki 2002) hypotheses.
Another complementary approach we have employed recently, assumes that prefrontal cortex is involved in discrimination of the vocalizations. Using a set of macaque calls that have been classified into categories based upon their acoustic features and, more importantly, the behavioral context in which they were emitted, we have examined a model which assumes that prefrontal neural responses are a function of how well individual calls conform to or represent individual categories. Using probabilities to characterize category membership, we have shown that prefrontal cortex neural responses can be described as linear functions of the probabilities that individual calls belong to each of the categories (Averbeck and Romanski 2006). This Hidden Markov model was motivated by recent theoretical studies which have examined how to encode probabilities in neural responses (Zemel et al. 1998; Barber et al. 2003; Sahani and Dayan 2003), as well as a large behavioral literature which has examined perceptual processes from a Bayesian probability perspective (Jacobs 2002; Pouget et al. 2003; Knill and Pouget 2004).
Multisensory Responses in VLPFC
The anatomical and physiological evidence for overlapping auditory and visual responsive regions shown in Figure 1 (Wilson et al. 1993; Romanski and Goldman-Rakic 2002; Fig. 1) led us to the hypothesis that some cells in this region might receive convergent inputs and respond to both auditory and visual stimuli, especially face and vocalization communication stimuli. This hypothesis was also strongly motivated by the knowledge that the dorsal bank of the STS sends a direct and robust projection to the lateral PFC (Romanski, Bates, Goldman-Rakic 1999). Multisensory responses to audiovisual stimuli have been previously noted in the STS (Bruce et al. 1981; Baylis et al. 1987; Barraclough et al. 2005). Therefore, some of the STS--PFC projection neurons might carry multisensory information making it possible to elicit responses to audiovisual stimuli in the prefrontal cortex. Moreover, some neurophysiological studies have noted multisensory responses in other portions of the prefrontal cortex (Benevento et al. 1977; Bodner et al. 1996; Fuster et al. 2000), although these responses were not related to face and vocalization stimuli.
Facial gestures, mouth movement, and corresponding vocal stimuli are routinely integrated during communication in animals and humans (Ghazanfar and Logothetis 2003; Izumi and Kojima 2004; Evans et al. 2005). Their combined transmission can affect the information contained in the communication stream, thereby clarifying (Stein and Meredith 1993; Calvert et al. 2001) or altering the message transmitted, as seen in the McGurk effect (McGurk and MacDonald 1976). The widespread connectivity of the frontal lobes makes them a likely candidate for integrating sensory signals related to communication. Furthermore, studies have shown that auditory (Romanski and Goldman-Rakic 2002), visual (Pigarev et al. 1979; Rosenkilde et al. 1981; Wilson et al. 1993; O’Scalaidhe et al. 1997, 1999; Nakamura et al. 1998; Hoshi et al. 2000), and somatosensory (Romo et al. 1999) responsive neurons are located within the VLPFC, suggesting further that VLPFC is multisensory. We chose, therefore, to examine the possibility that single cells in the primate VLPFC were multisensory and responsive to both facial gestures and corresponding vocalizations. We recorded from the VLPFC of awake, behaving rhesus macaques as they were presented with naturalistic audiovisual stimuli. The stimuli consisted of short video clips of familiar monkeys vocalizing. These movies were separated into audio and video streams, and we compared the neural response to the separated unimodal stimuli with that of the combined audiovisual stimuli. A similar naturalistic movie presentation has been used recently in examination of sensory integration in the temporal lobe in both animal electrophysiology (Barraclough et al. 2005; Ghazanfar et al. 2005) and human neuroimaging (Beauchamp et al. 2004).
We found that approximately half the recorded population was bimodal responding to both unimodal auditory and visual stimuli or responding differently to bimodal stimuli than to either unimodal stimuli (Sugihara et al. 2006). VLPFC multisensory neurons exhibited enhancement or suppression (Fig. 4), and it was found that multisensory suppression (73% of cells; Fig. 4B) was more commonly observed than enhancement (27 % of cells; Fig. 4A). Nonetheless, responses varied according to the stimulus exemplar used so that a given cell might show multisensory suppression with one pair of congruent faces and vocalizations and an enhancement with a different pair of stimuli. It was also interesting that face/vocalization stimuli evoked multisensory responses more frequently than nonface/nonvocalization combinations when both were tested. This adds support to the notion that VLPFC may be specialized for integrating face and vocalization information during communication and sets it apart from other brain regions that integrate sensory stimuli of a more general nature. These results, that some VLPFC multisensory neurons are selective for face and voice stimuli, are in agreement with human fMRI studies indicating that a homologous region of the human brain, area 47 (pars orbitalis), is specifically activated by human vocal sounds compared with animal and nonvocal sounds (Fecteau et al. 2005). In contrast, other cortical regions that have been shown to be responsive to face and vocalization stimuli, may not show face/voice selectivity. For example, the STS appears to be specialized for integrating general biological motion in nonhuman primates (Oram and Perrett 1994; Barraclough et al. 2005) rather than solely communication stimuli, whereas the multisensory responses in the auditory cortex, which receives afferents from a number of cortical areas (Petrides and Pandya 1988; Hackett et al. 1999; Romanski, Tian, et al. 1999) may be a product of top-down cortical inputs (Ghazanfar et al. 2005). Thus each cortical node in a sensory integration network may contribute uniquely to the processing of multisensory communication stimuli.
Figure 4.
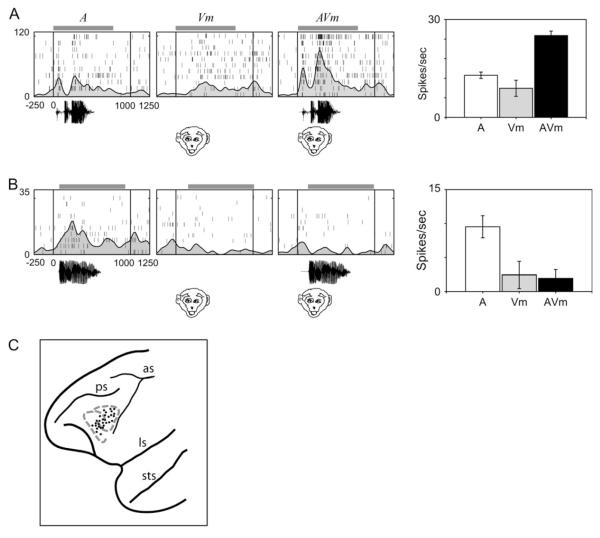
Multisensory responses in VLPFC. The neural responses of 2 neurons are shown in (A) and (B) as raster/spike density functions and a graph of mean firing rate (at right). A multisensory enhanced response is shown in (A) with a strong response to the vocalization (A, white bar in graph), a smaller response to the corresponding face movie (Vm, gray bar in graph), and an increase in responding to the combined AV presentation (AVm, black bar in graph). The cell in (B) had a strong response to the vocalization (A), no response to the face movie (Vm), and exhibited multisensory suppression when the stimuli were combined (AVm, black bar). Multisensory cells were found across previously identified auditory and visual responsive areas (Sugihara et al. 2006) and are shown in the schematic of the prefrontal cortex in (C), as black circles scattered across lower VLPFC. The auditory and visual domains are outlined in gray with dotted lines (similar to Fig. 1).
Prefrontal multisensory and unimodal neurons were both found in VLPFC area 12/47 and were coextensive with previously identified vocalization and face responsive neurons (Sugihara et al. 2006; Fig. 4C). Some cells, which appeared unimodal when tested with auditory or visual stimuli separately, had interesting nonlinear responses to simultaneously presented audiovisual stimuli, suggesting that cells may be incorrectly categorized as unimodal if they are not tested with additional stimuli in an appropriate paradigm. Thus, in future studies, many more VLPFC cells may prove to be multisensory if task and stimulus parameters are appropriately constrained.
Our findings of multisensory cells in the frontal lobe are also in agreement with neuroimaging studies in the human brain, which have shown activation of frontal lobe regions during audiovisual stimulation (Calvert 2001; Homae et al. 2002; Jones and Callan 2003; Miller and D’Esposito 2005). The demonstration that communication-relevant auditory and visual stimulus information reaches single cells of the VLPFC of the rhesus monkey suggests that a communication module exists in the nonhuman primate VLPFC just as it does in the human ventral frontal lobe. Thus, it may provide the animal model necessary to decipher the cellular mechanisms involved during the encoding and integration of communication signals in the human brain during speech and language processes. This is an important first step toward understanding the cellular mechanisms of human communication and in offering clues to the neural changes, which occur when speech and language processes are compromised in autism, schizophrenia, and language disabilities. A number of studies have revealed changes in activation or structural abnormalities in the inferior frontal lobe in schizophrenics. Some studies have revealed specific changes compared with controls when verbal or audiovisual communication stimuli are employed (Surguladze et al. 2001; de Gelder et al. 2003). Audiovisual integration has also been investigated in autistic subjects, and differences have been found in activation compared with control subjects in several brain regions including the frontal lobes (Baranek 1999; Muller et al. 1999; Williams et al. 2004). Notably, there is evidence supporting a frontal lobe deficit in autism with regard to executive function impairments as well as face and language-processing impairments (Ferrari 1982; Hughes et al. 1994; Bennetto et al. 1996; Schultz et al. 2000). Thus, the importance of delineating a “communication module” in the prefrontal cortex of animals may provide clues to the neurophysiological mechanisms which break down when audiovisual communication and comprehension is disrupted by neurological disorders.
Our findings of auditory and multisensory responsive neurons in VLPFC enrich our understanding of the primate frontal lobes. Goldman-Rakic (1996b) firmly believed that structure and function were inextricably linked and that advances on one front would parallel successes in the other. It was her special talent to synthesize information across techniques, disciplines, and the senses in order to understand the role of the frontal lobes in higher cognitive function.
Acknowledgments
Funding National Institutes of Health (DC004845).
The author would like to gratefully acknowledge the inspiration of Patrician Goldman-Rakic and the generous support of Cure Autism Now.
Notes
Conflict of Interest: none declared.
References
- Averbeck BB, Romanski LM. Principal and independent components of macaque vocalizations: constructing stimuli to probe high-level sensory processing. J Neurophysiol. 2004;91:2897–2909. doi: 10.1152/jn.01103.2003. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Romanski LM. Probabilistic encoding of vocalizations in macaque ventral lateral prefrontal cortex. J Neurosci. 2006;26:11023–11033. doi: 10.1523/JNEUROSCI.3466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek GT. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9-12 months of age. J Autism Dev Disord. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J. Comp. Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- Barbas H. Architecture and cortical connections of the prefrontal cortex in the rhesus monkey [review] Adv Neurol. 1992;57:91–115. [PubMed] [Google Scholar]
- Barber MJ, Clark JW, Anderson CH. Generating neural circuits that implement probabilistic reasoning. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68:041912. doi: 10.1103/PhysRevE.68.041912. [DOI] [PubMed] [Google Scholar]
- Barraclough NE, Xiao D, Baker CI, Oram MW, Perrett DI. Integration of visual and auditory information by superior temporal sulcus neurons responsive to the sight of actions. J Cogn Neurosci. 2005;17:377–391. doi: 10.1162/0898929053279586. [DOI] [PubMed] [Google Scholar]
- Bar-Yosef O, Rotman Y, Nelken I. Responses of neurons in cat primary auditory cortex to bird chirps: effects of temporal and spectral context. J Neurosci. 2002;22:8619–8632. doi: 10.1523/JNEUROSCI.22-19-08619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis GC, Rolls ET, Leonard CM. Functional subdivisions of the temporal lobe neocortex. J Neurosci. 1987;7:330–342. doi: 10.1523/JNEUROSCI.07-02-00330.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Argall BD, Martin A. Integration of auditory and visual information about objects in superior temporal sulcus. Neuron. 2004;41:809–823. doi: 10.1016/s0896-6273(04)00070-4. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. Learning the higher-order structure of a natural sound. Network. 1996;7:261–266. doi: 10.1088/0954-898X/7/2/005. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, Rogers SJ. Intact and impaired memory functions in autism. Child Dev. 1996;67:1816–1835. [PubMed] [Google Scholar]
- Benevento LA, Fallon J, Davis BJ, Rezak M. Auditory–visual interaction in single cells in the cortex of the superior temporal sulcus and the orbital frontal cortex of the macaque monkey. Exp Neurol. 1977;57:849–872. doi: 10.1016/0014-4886(77)90112-1. [DOI] [PubMed] [Google Scholar]
- Bodner M, Kroger J, Fuster JM. Auditory memory cells in dorsolateral prefrontal cortex. Neuroreport. 1996;7:1905–1908. doi: 10.1097/00001756-199608120-00006. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of animal communication. Blackwell; Oxford: 1998. [Google Scholar]
- Bruce C, Desimone R, Gross CG. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol. 1981;46:369–384. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Petersen SE. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. J Neurophysiol. 1995;74:2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Hansen PC, Iversen SD, Brammer MJ. Detection of audio-visual integration sites in humans by application of electrophysiological criteria to the BOLD effect. Neuroimage. 2001;14:427–438. doi: 10.1006/nimg.2001.0812. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79:2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Chavis DA, Pandya DN. Further observations on corticofrontal connections in the rhesus monkey. Brain Res. 1976;117:369–386. doi: 10.1016/0006-8993(76)90089-5. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Vroomen J, Annen L, Masthof E, Hodiamont P. Audiovisual integration in schizophrenia. Schizophr Res. 2003;59:211–218. doi: 10.1016/s0920-9964(01)00344-9. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Howell S, Westergaard GC. Auditory—visual cross-modal perception of communicative stimuli in tufted capuchin monkeys (Cebus apella) J Exp Psychol Anim Behav Process. 2005;31:399–406. doi: 10.1037/0097-7403.31.4.399. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Armony JL, Joanette Y, Belin P. Sensitivity to voice in human prefrontal cortex. J Neurophysiol. 2005;94:2251–2254. doi: 10.1152/jn.00329.2005. [DOI] [PubMed] [Google Scholar]
- Ferrari M. Childhood autism: deficits of communication and symbolic development. I. Distinctions from language disorders. J Commun Disord. 1982;15:191–208. doi: 10.1016/0021-9924(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. J Neurosci. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton JF. Functional lobotomy and affective behavior. Norton; New York: 1950. [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Visuospatial coding in primate prefrontal neurons revealed by oculomotor paradigms. J Neurophysiol. 1990;63:814–831. doi: 10.1152/jn.1990.63.4.814. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. Raven Press; New York: 1989. [Google Scholar]
- Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405:347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfar AA, Hauser MD. The neuroethology of primate vocal communication: substrates for the evolution of speech. Trends Cogn Sci. 1999;3:377–384. doi: 10.1016/s1364-6613(99)01379-0. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Logothetis NK. Neuroperception: facial expressions linked to monkey calls. Nature. 2003;423:937–938. doi: 10.1038/423937a. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Maier JX, Hoffman KL, Logothetis NK. Multisensory integration of dynamic faces and voices in rhesus monkey auditory cortex. J Neurosci. 2005;25:5004–5012. doi: 10.1523/JNEUROSCI.0799-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford GW, 3rd, Hauser MD, Cohen YE. Discrimination of functionally referential calls by laboratory-housed rhesus macaques: implications for neuroethological studies. Brain Behav Evol. 2003;61:213–224. doi: 10.1159/000070704. [DOI] [PubMed] [Google Scholar]
- Gifford GW, 3rd, Maclean KA, Hauser MD, Cohen YE. The neurophysiology of functionally meaningful categories: macaque ventrolateral prefrontal cortex plays a critical role in spontaneous categorization of species-specific vocalizations. J Cogn Neurosci. 2005;17:1471–1482. doi: 10.1162/0898929054985464. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, editor. Handbook of physiology. Section 1: The nervous system. Vol. V. Higher functions of the brain. American Physiological Society; Bethesda (MD): 1987. pp. 373–418. [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA. 1996a;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Phil Trans R Soc Lond B Biol Sci. 1996b;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Gouzoules H, Gouzoules S, Tomaszycki M. Agnostic screams and the classification of dominance relationships: are monkeys fuzzy logicians? Anim Behav. 1998;55:51–60. doi: 10.1006/anbe.1997.0583. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 1998;394:475–495. doi: 10.1002/(sici)1096-9861(19980518)394:4<475::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Prefrontal connections of the parabelt auditory cortex in macaque monkeys. Brain Res. 1999;817:45–58. doi: 10.1016/s0006-8993(98)01182-2. [DOI] [PubMed] [Google Scholar]
- Hauser MD. The Evolution of communication. MIT Press; Cambridge (MA): 1996. pp. 92–101. [Google Scholar]
- Hauser MD. Functional referents and acoustic similarity: field playback experiments with rhesus monkeys. Anim Behav. 1998;55:1647–1658. doi: 10.1006/anbe.1997.0712. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Marler P. Food associated calls in rhesus macaques. Macaca mulatta. I. Socioecological factors. Behav Ecol. 1993;4:194–205. [Google Scholar]
- Homae F, Hashimoto R, Nakajima K, Miyashita Y, Sakai KL. From perception to sentence comprehension: the convergence of auditory and visual information of language in the left inferior frontal cortex. Neuroimage. 2002;16:883–900. doi: 10.1006/nimg.2002.1138. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Shima K, Tanji J. Neuronal activity in the primate prefrontal cortex in the process of motor selection based on two behavioral rules. J Neurophysiol. 2000;83:2355–2373. doi: 10.1152/jn.2000.83.4.2355. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32:477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Izumi A, Kojima S. Matching vocalizations to vocalizing faces in a chimpanzee (Pan troglodytes) Anim Cogn. 2004;7:179–184. doi: 10.1007/s10071-004-0212-4. [DOI] [PubMed] [Google Scholar]
- Jacobs RA. What determines visual cue reliability? Trends Cogn Sci. 2002;6:345–350. doi: 10.1016/s1364-6613(02)01948-4. [DOI] [PubMed] [Google Scholar]
- Jones JA, Callan DE. Brain activity during audiovisual speech perception: an fMRI study of the McGurk effect. Neuroreport. 2003;14:1129–1133. doi: 10.1097/00001756-200306110-00006. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill DC, Pouget A. The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci. 2004;27:712–719. doi: 10.1016/j.tins.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lau B, Stanley GB, Dan Y. Computational subunits of visual cortical neurons revealed by artificial neural networks. Proc Natl Acad Sci USA. 2002;99:8974–8979. doi: 10.1073/pnas.122173799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki MS. Efficient coding of natural sounds. Nat Neurosci. 2002;5:356–363. doi: 10.1038/nn831. [DOI] [PubMed] [Google Scholar]
- Linsker R. Self-organization in a perceptual network. IEEE Computer. 1988;21:117. [Google Scholar]
- Luppino G, Calzavara R, Rozzi S, Matelli M. Projections from the superior temporal sulcus to the agranular frontal cortex in the macaque. Eur J Neurosci. 2001;14:1035–40. doi: 10.1046/j.0953-816x.2001.01734.x. [DOI] [PubMed] [Google Scholar]
- Marmarmelis P, Marmarmelis V. Analysis of physiological systems: the white-noise approach. Plenum Press; New York: 1978. [Google Scholar]
- McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264:746–748. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- Mechler F, Reich DS, Victor JD. Detection and discrimination of relative spatial phase by V1 neurons. J Neurosci. 2002;22:6129–6157. doi: 10.1523/JNEUROSCI.22-14-06129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, D’Esposito M. Perceptual fusion and stimulus coincidence in the cross-modal integration of speech. J Neurosci. 2005;25:5884–5893. doi: 10.1523/JNEUROSCI.0896-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Manning FJ. Non-spatial memory after selective prefrontal lesions in monkeys. Brain Research. 1978;143:313–323. doi: 10.1016/0006-8993(78)90571-1. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Preservation of central sets after frontal lesions in monkeys. In: Warren JK, editor. The frontal granular cortex and behavior. McGraw-Hill; New York: 1964. pp. 219–241. [Google Scholar]
- Morel A, Garraghty PE, Kaas JH. Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. J Comp Neurol. 1993;335:437–459. doi: 10.1002/cne.903350312. [DOI] [PubMed] [Google Scholar]
- Muller RA, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, Chugani HT. Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. J Autism Dev Disord. 1999;29:19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sakai K, Hikosaka O. Neuronal activity in medial frontal cortex during learning of sequential procedures. J Neurophysiol. 1998;80:2671–2687. doi: 10.1152/jn.1998.80.5.2671. [DOI] [PubMed] [Google Scholar]
- Nearey TM. Static, dynamic, and relational properties in vowel perception. J Acoust Soc Am. 1989;85:2088–2113. doi: 10.1121/1.397861. [DOI] [PubMed] [Google Scholar]
- Oram MW, Perrett D. Responses of anterior superior temporal polysensory (STPa) neurons to “biological motion” stimuli. J Cogn Neurosci. 1994;6:99–116. doi: 10.1162/jocn.1994.6.2.99. [DOI] [PubMed] [Google Scholar]
- O’Scalaidhe SP, Wilson FAW, Goldman-Rakic PS. Areal segregation of face-processing neurons in prefrontal cortex. Science. 1997;278:1135–1138. doi: 10.1126/science.278.5340.1135. [DOI] [PubMed] [Google Scholar]
- O’Scalaidhe SP, Wilson FAW, Goldman-Rakic PS. Face-selective neurons during passive viewing and working memory performance of rhesus monkeys: evidence for intrinsic specialization of neuronal coding. Cereb Cortex. 1999;9:459–475. doi: 10.1093/cercor/9.5.459. [DOI] [PubMed] [Google Scholar]
- Owings DH, Morton ES. Animal vocal communication: a new approach. Cambridge University Press; Cambridge (MA): 1998. [Google Scholar]
- Owren MJ, Rendall D. Sound on the rebound: bringing form and function back to the forefront in understanding nonhuman primate vocal signaling. Evol Anthropol. 2001;10:58–71. [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Frey S. Differential activation of the human orbital, mid-ventrolateral, and mid-dorsolateral prefrontal cortex during the processing of visual stimuli. Proc Natl Acad Sci USA. 2002;99:5649–5654. doi: 10.1073/pnas.072092299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Pigarev IN, Rizzolatti G, Schandolara C. Neurons responding to visual stimuli in the frontal lobe of macaque monkeys. Neurosci Lett. 1979;12:207–212. doi: 10.1016/0304-3940(79)96063-4. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J. Comp Neurol. 1991;310:429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- Pouget A, Dayan P, Zemel RS. Inference and computation with population codes. Annu Rev Neurosci. 2003;26:381–410. doi: 10.1146/annurev.neuro.26.041002.131112. [DOI] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Parallel processing in the auditory cortex of primates. Audiol Neurootol. 1998;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 1995;268:111–114. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- Romanski LM. Domain specificity in the primate prefrontal cortex. Cogn Affect Behav Neurosci. 2004;4:421–429. doi: 10.3758/cabn.4.4.421. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J Neurophysiol. 2005;93:734–747. doi: 10.1152/jn.00675.2004. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1999;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Goldman-Rakic PS. An auditory domain in primate prefrontal cortex. Nat Neurosci. 2002;5:15–16. doi: 10.1038/nn781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernandez A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399:470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- Rosenkilde CE, Bauer RH, Fuster JM. Single cell activity in ventral prefrontal cortex of behaving monkeys. Brain Res. 1981;209:375–394. doi: 10.1016/0006-8993(81)90160-8. [DOI] [PubMed] [Google Scholar]
- Sahani M, Dayan P. Doubly distributional population codes: simultaneous representation of uncertainty and multiplicity. Neural Comput. 2003;15:2255–2279. doi: 10.1162/089976603322362356. [DOI] [PubMed] [Google Scholar]
- Sahani M, Linden JF. How linear are auditory cortical responses? In: Becker S, Thrun S, Obermayer K, editors. Advances in neural information processing systems. MIT Press; Cambridge, (MA): 2003. pp. 109–116. NIPS. [Google Scholar]
- Salinas E, Hernandez A, Zainos A, Romo R. Periodicity and firing rate as candidate neural codes for the frequency of vibrotactile stimuli. J Neurosci. 2000;20:5503–5515. doi: 10.1523/JNEUROSCI.20-14-05503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The merging of the senses. MIT Press; Cambridge: 1993. [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rauch S. Localization of syntactic comprehension by positron emission tomography. Brain Lang. 1996;52:452–473. doi: 10.1006/brln.1996.0024. [DOI] [PubMed] [Google Scholar]
- Sugihara T, Diltz MD, Averbeck BB, Romanski LM. Integration of auditory and visual communication information in the primate ventrolateral prefrontal cortex. J Neurosci. 2006;26:11138–11147. doi: 10.1523/JNEUROSCI.3550-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, Calvert GA, Brammer MJ, Campbell R, Bullmore ET, Giampietro V, David AS. Audio-visual speech perception in schizophrenia: an fMRI study. Psychiatry Res. 2001;106:1–14. doi: 10.1016/s0925-4927(00)00081-0. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Neuronal mechanisms of object recognition. Science. 1993;262:685–688. doi: 10.1126/science.8235589. [DOI] [PubMed] [Google Scholar]
- Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Natl Acad Sci USA. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. MIT Press; Cambridge (MA): 1982. pp. 549–586. [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH, Massaro DW, Peel NJ, Bosseler A, Suddendorf T. Visual-auditory integration during speech imitation in autism. Res Dev Disabil. 2004;25:559–575. doi: 10.1016/j.ridd.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Wilson FAW, O’Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- Zemel RS, Dayan P, Pouget A. Probabilistic interpretation of population codes. Neural Comput. 1998;10:403–430. doi: 10.1162/089976698300017818. [DOI] [PubMed] [Google Scholar]