Abstract
Organizing centers in the developing brain provide an assortment of instructive patterning cues, including Sonic hedgehog (Shh). Here we characterize the forebrain phenotype caused by loss of Ttc21b, a gene we identified in an ENU mutagenesis screen as a novel ciliary gene required for retrograde intraflagellar transport (Tran et al., 2008). The Ttc21b mutant has defects in limb, eye and, most dramatically, brain development. We show that Shh signaling is elevated in the rostral portion of the mutant embryo, including in a domain in or near the zona limitans intrathalamica. We demonstrate here that ciliary defects seen in the Ttc21b mutant extend to the embryonic brain, adding forebrain development to the spectrum of tissues affected by defects in ciliary physiology. We show that development of the Ttc21b brain phenotype is modified by lowering levels of the Shh ligand, supporting our hypothesis that the abnormal patterning is a consequence of elevated Shh signaling. Finally, we evaluate Wnt signaling but do not find evidence that this plays a role in causing the perturbed neurodevelopmental phenotype we describe.
Keywords: Mouse, telencephalon, sonic hedgehog, Ttc21b, zona limitans intrathalimica, intraflagellar transport
INTRODUCTION
The mammalian telencephalon develops at the rostral end of the neural tube as part of the early forming prosencephalon. After specification and separation into two distinct hemispheres, which will give rise to the future cerebral vesicles, the telencephalon undergoes a process of massive expansion and differentiation. The development of the telencephalon is under the guidance of a series of morphogens in and around the forebrain. These cues include Bone Morphogenetic Proteins (BMPs), Wnts, Fibroblast Growth Factors (Fgfs) and Sonic Hedgehog (Shh). Despite this information, much about the molecular regulation of forebrain development is still not well understood.
Phenotype-driven mutagenesis screens in the mouse present a method to identify genes involved in specific developmental or physiological processes. As mutations which cause the phenotype are ascertained without prior knowledge of the underlying genetic basis, this strategy facilitates the identification of previously undiscovered molecular contributors to developmental pathways. Our laboratory conducted a screen focused on phenotypes occurring in the late stages of mouse embryogenesis (Herron et al., 2002). One of the mutants generated in this screen is the alien (aln) mutant, which features developmental anomalies that include preaxial polydactyly, cleft palate, partially penetrant micro-opthalmia, and, most strikingly, defects in the brain including profound disruption of the neocortex/ telencephalon. We identified alien to be due to a mutation in a novel gene named Ttc21b (tetratricopeptide repeat domain 21B, Tran et al., 2008; formerly called Thm1, tetratricopeptide repeat–containing hedgehog modulator-1). Ttc21b encodes the ortholog of Chlamydomonas reinhardtii flagellar associated protein 60, which has been identified in a recent Genbank entry (ABU95018) as the complex A protein IFT139, important for retrograde IFT.
Here we describe the brain phenotype in the aln mutants. We show that the aln forebrain is reduced in size, while the midbrain is expanded. The telencephalon shows a disorganized cortex with loss of dorsal structures and increased ventral character. Neurodevelopmental defects are visible as early as E9.5 in the aln embryos. Shh and Shh target genes are upregulated in the aln brain, as is Fgf8. We finally show that Shh is required for development of the aln phenotype.
MATERIALS AND METHODS
Mouse husbandry and genotyping
Alien mice were originally generated by ENU mutagenesis of A/J mice and then outcrossed to FVB/J mice for many generations and then maintained by intercross (Herron et al., 2002). Genotyping was done with microsatellite markers near the mutation (see Tran et al., 2008), or using an AvaII restriction site caused by the single nucleotide change present in the aln mutants. Most aln mutants are readily distinguishable by morphology of the forebrain and limbs. Younger embryos were initially genotyped with DNA purified from yolk sacs. The C57BL/6J Ptc1-lacZ mouse and R26R Cre reporter mice were obtained from the Jackson laboratory (Bar Harbor, ME) and intercrossed with aln heterozygous mice; Ptc1-lacZ genotyping was done with standard lacZ primers and R26R mice were genotyped as described (www.jax.org). The introduction of the B6 genetic background with the Ptc1-lacZ decreased the incidence of exencephaly previously noted in the alien mouse (Herron et al., 2002). Therefore, we continued to introduce the B6 genetic background to our colony to reduce the incidence of exencephaly. Our experiments described here focus on non-exencephalic aln mutants. The Shh null allele and the Wnt1-Cre allele were on mixed backgrounds and genotyped as described (www.jax.org). All animals were housed in accordance with the Harvard Medical School ARCM regulations. Timed matings were checked for signs of copulation in the morning; vaginal plugs were noted and noon of that day was established as embryonic day (E)0.5. The Shh null allele was created by crossing Shh conditional allele-carrying mice to the EIIa-Cre germline Cre expressing mouse (Lakso et al., 1996) and genotyping offspring for the lox-P recombined (null) alleles (www.jax.org).
Histology & Immunohistochemistry
Embryos used for histological analysis were fixed with Bouin’s fixative for at least forty-eight hours and processed for paraffin embedding using a Leica TP1020 automated tissue processor. Sections were cut at a thickness of 14µm and stained for hematoxylin and eosin using standard techniques. Annotations of figures were done with the assistance of published materials (Kaufman, 1992) and primary literature. Sections for analysis of cilia were fixed in 4% paraformaldehyde for 15 minutes at room temperature and cryo-embedded. Sections were 10 µm thick and stained with Polaris Ab (1:1000; gift of B. Yoder) for 60 min at RT. Secondary antibody staining was with Goat anti-rabbit Alexa-Flour 488 (Molecular Probes) and slides were mounted with vectashield. Microscopy was done with a Zeiss AxioImage with ApoTome.
In Situ Hybridization and LacZ staining
Whole mount in situ hybridization was done as previously described (Belo et al., 1997) using a BioLane HTI for post-hybridization and with BM Purple (Roche, Indianapolis, IN) for visualization of riboprobes. All probes with the exception of those used for Ttc21b and Mash1 are published:, Dlx2 (Porteus et al., 1991), Fgf8 (Crossley and Martin, 1995), Foxa2 (Sasaki and Hogan, 1993), Foxg1 (constructed as described in (Gray et al., 2004)), Gli1 (Hui et al., 1994), Lhx5 (Sheng et al., 1997), Ngn2 (Sommer et al., 1996), Nkx2.1 (Shimamura et al., 1995), Olig2 (Zhou et al., 2000), Pax6 (Wawersik et al., 1999), Ptc (Goodrich et al., 1996), Shh (Echelard et al., 1993), Wnt1 (McMahon and McMahon, 1989), and Wnt3a (Roelink and Nusse, 1991). Mash1 probes were generated from Open Biosystems (Huntsville, AL) clone #1361975. Two probes were constructed for in situ hybridization analysis of the alien gene product. A 5’ sequence (primers: 5’probe F -acaaaaatgaaggagcaacg; 5’probeR - gccgagctctgtagcaattt) and a sequence in the middle of the gene were PCR amplified (mid-probeF - ccaggaatgaggagaagcag; mid-probeR - gctctcgagcctgctgtaat) and TA-cloned into pCR-II (Invitrogen, Carlsbad, CA). Sense probes were also generated from these plasmids to test the specificity of the aln probes. LacZ staining was performed using standard procedures (Hogan et al., 1994). Younger embryos (E11.5 and younger) were stained with lacZ and then processed for paraffin histology as described above. Older embryos were fixed in 4% paraformaldehyde, cryoembedded in OCT, sectioned at 20 µm and stained on slides.
Forebrain measurements
To measure the size of the forebrain, whole brains were micro-dissected at the appropriate stages and photographed from the dorsal aspect at identical magnification. Outlines of the forebrain were traced using NIH Image J software and the area of each was calculated. Standards were used to convert Image J measures to approximate square centimeters.
Westerns
Heads of E10.5 and E13.5 wt and aln mice were homogenized in passive lysis buffer (Promega). Protein extracts were run on a 7% polyacrylamide gel and Westerns were performed as described (Tran et al. 2008), using a mouse monoclonal anti-β-catenin antibody (1:4000 dilution; BD Transduction Laboratories 610153), and a mouse monoclonal anti-α-tubulin antibody (1:6000 dilution; Abcam ab7291 DM1A) for a loading control.
RESULTS
Loss of alien results in decreased forebrain size
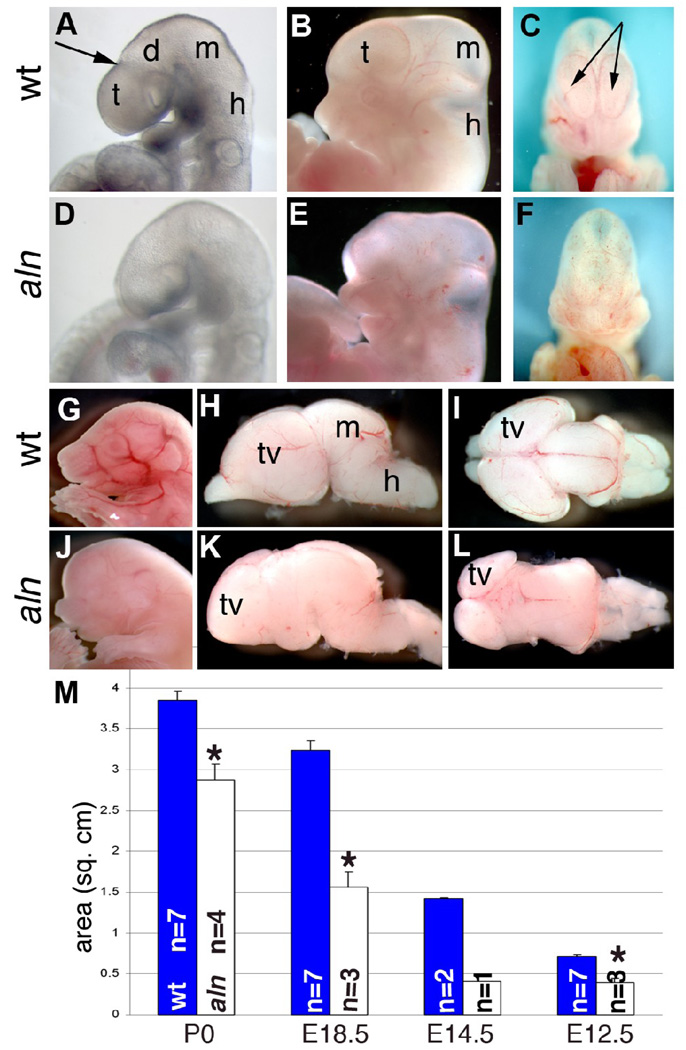
We first observed defects in the brain of aln mutants at E9.5 when a distinct morphology normally indicates the separation of the telencephalon and diencephalon (arrow in Fig. 1A). Aln brains do not have a telencephalon obviously separate from the diencephalon, although we did not see any other gross differences in the midbrain and hindbrain at this stage (Fig. 1D). At E12.5, the forebrain appears slightly enlarged and protrudes from the anterior head of aln mutants when compared to wild-type embryos (Fig. 1B,E). At this stage the midbrain also appears slightly larger in aln mutants while the hindbrain appears normal. A closer observation of wild-type embryos from the coronal aspect revealed distinct telencephalic vesicles (arrows in Fig. 1C), which were not distinguishable in aln mutants (Fig. 1F). At E18.5, the aln head continued to appear enlarged rostrally, protruding over the frontonasal mass, giving the mutant the appearance of having increased forebrain tissue (Fig. 1J,K,L). However, microdissection of the brain at this stage showed the telencephalic vesicles are markedly smaller and the olfactory bulbs are missing in the mutants. We have measured the size of the telencephalic vesicles from E12.5 to P0; this confirmed that the mutant forebrain is significantly smaller than wild-type littermates at all stages examined (Fig. 1M).
Figure 1. Forebrain defects in alien mutants.
Wild-type (A–C, G–I) and alien (D–F, J–L) embryos at E9.5 (A,D) and E12.5 (B,C,E,F). Arrow in A indicates demarcation in wild-type embryos between telencephalon and diencephalon. C,F are coronal views of embryos in B and E, arrows indicate telencephalic ventricles. (G,J) Wild-type (G) and alien (J) embryos at E18.5. Brains were removed from the skull and are shown from the lateral (H,K) and dorsal (I,L) aspects. (M) The telencephalic area of multiple brains was measured at multiple stages (*,p<0.003). All paired pictures are at the same magnification: d:diencephalon, m:midbrain, h:hindbrain, t:telencephalon, tv: telencephalic vesicles.
Expression of the Ttc21b gene in the embryonic brain
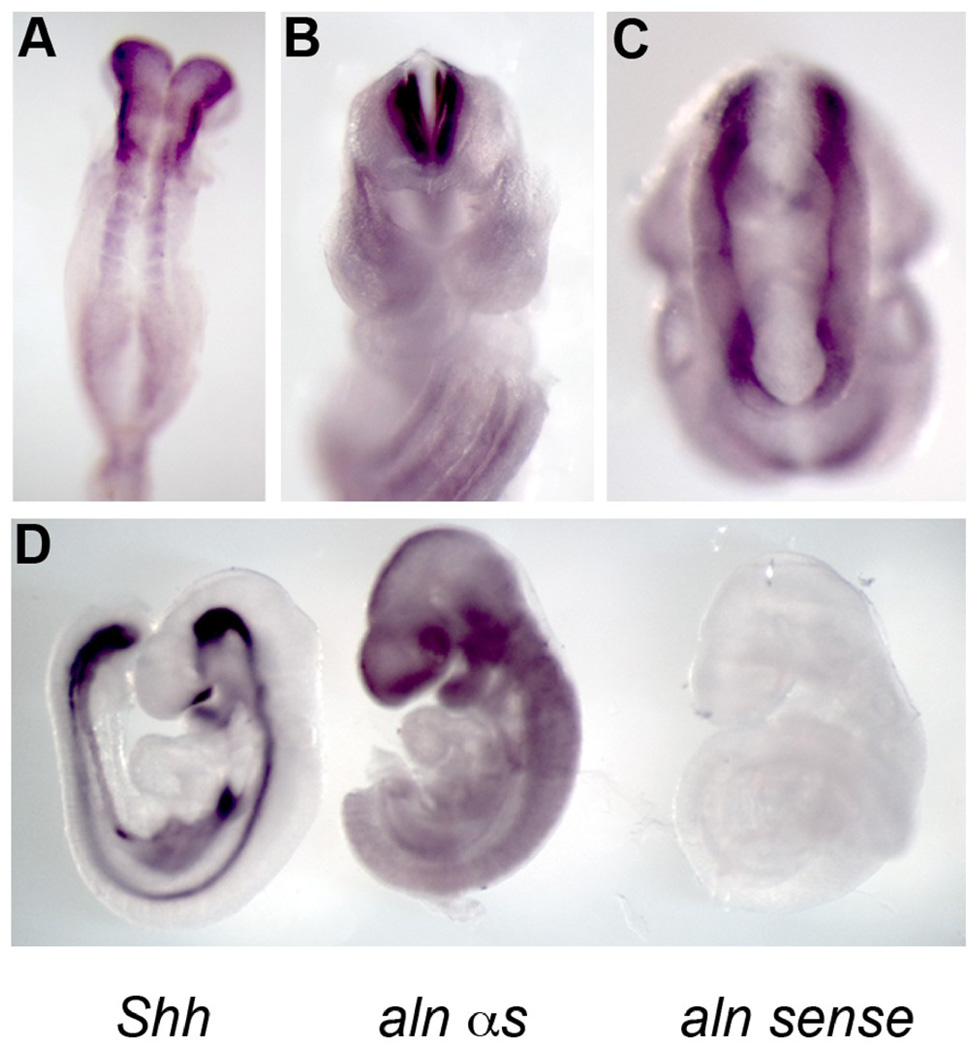
In order to determine the expression pattern of Ttc21b, we have performed in situ hybridization with RNA probes for the Ttc21b product (see also (Tran et al., 2008). At E8.5 we note expression in the anterior neural ectoderm and in the early somites (Fig. 2A). We detect widespread expression at E9.5 in the neural epithelium all along the anterior-posterior axis and expression is also noted in the ectoderm of the pharyngeal arches, the limb bud and diffusely in the axial ectoderm around the neural tube (Fig. 2D, data not shown). To confirm that this low-level, diffuse expression is truly representative of endogenous gene expression, we have microdissected embryos for in situ hybridization to minimize artifacts of staining and still note robust staining throughout the neural ectoderm (Fig. 2B,C). To further test the specificity of these probes, we have used three probes for Shh, Ttc21b, and a sense control for the Ttc21b probe and visualized them simultaneously to test for background staining (Fig. 2D). The Shh probe specifically stained known Shh-expressing tissue and the Ttc21b sense control shows no signal, validating our finding of wide-spread, low-level Ttc21b expression. Taken together, these data indicate Ttc21b is widely expressed in the developing nervous system at low to moderate levels.
Figure 2. Expression of the Ttc21b gene.
Whole mount in situ hybridization for alien at E8.5 (A) and E9.5 (B–D). (B,D) E9.5 embryo microdissected to show expression of Ttc21b in the neural tube (B) and forebrain tissue. (C) Dorsal view of E9.5 to highlight expression throughout the anterior nervous tissue. (D) Embryos individually hybridized with Shh (left), Ttc21b antisense (middle) and Ttc21b sense control (right) probes to test specificity of Ttc21b probe immunoreactivity.
The cortex develops abnormally in alien embryos
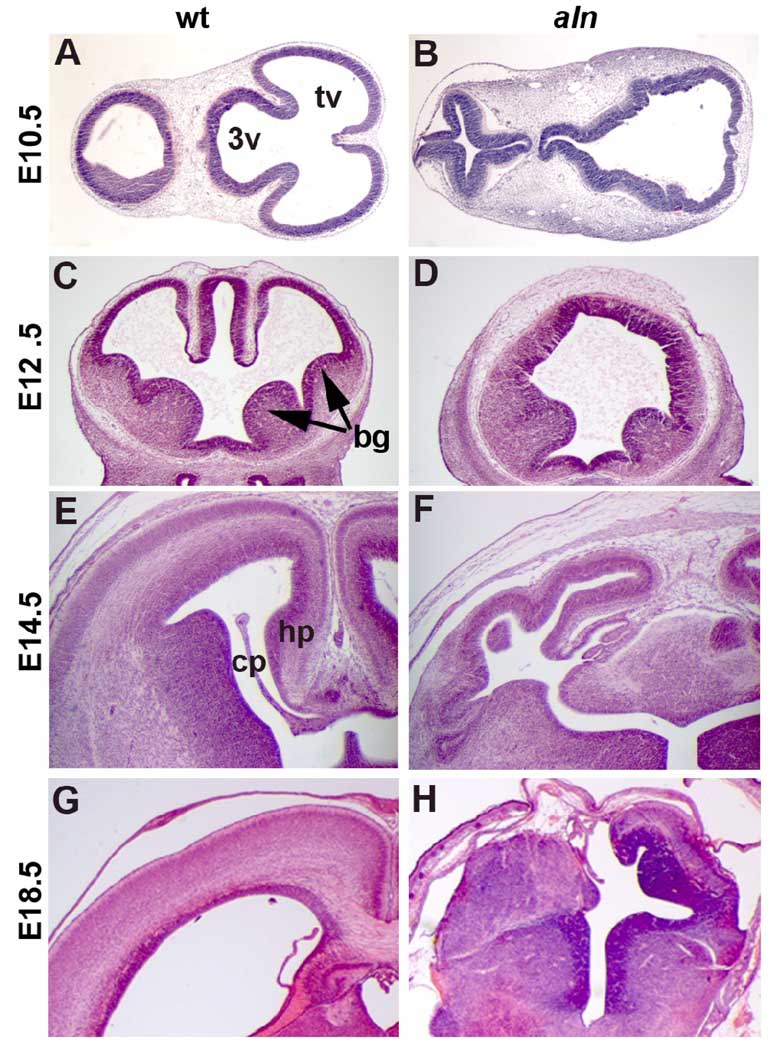
To further analyze the consequences of the aln mutation, we performed a histological analysis of the aln brain during multiple stages of development. At E10.5 (Fig. 3.A,B) the wild-type embryos have clearly distinct lateral ventricles (presumptive telencephalic vesicles) and third ventricles. In contrast, there are not such clear distinctions between the lateral ventricles and the third ventricle in sections from aln mutant embryos (Fig. 3B). At E12.5, the wild-type embryo shows formation of the basal ganglia in the ventral telencephalon and invagination of the dorsal midline tissue that will form the hippocampus and choroid plexus (Fig. 3C). The aln brain, however, shows no such dorsal midline structures and has defects in basal ganglionic structure (Fig. 3D). We do note some variability in the severity of the aln phenotype and some mutants show less significant loss of dorsal midline tissues. The reduction in dorsal structure formation is still evident in E14.5 and E18.5 aln mutants (Fig. 3F,H). Wild-type embryos at these stages (Fig. 3E,G) have robust hippocampal structures and choroid plexus tissue formation while aln brains have no easily recognizable hippocampus, disorganized choroid plexus tissue, and significantly reduced neocortical tissue.
Figure 3. Histological analysis of alien brain development.
Paraffin sections of wild-type (A,C,E,G) and alien (B,D,F,H) brains at E10.5 (A,B), E12.5 (C,D), E14.5 (E,F), and E18.5 (G,H). Arrows in (C) indicate basal ganglia. Sections are in the transverse (A,B) and coronal (C–H) planes. bg: basal ganglia, cp: choroid plexus, hp: hippocampus, tv: telencephalic vesicles, 3v: third ventricle.
The alien forebrain shows defects in dorsal-ventral patterning
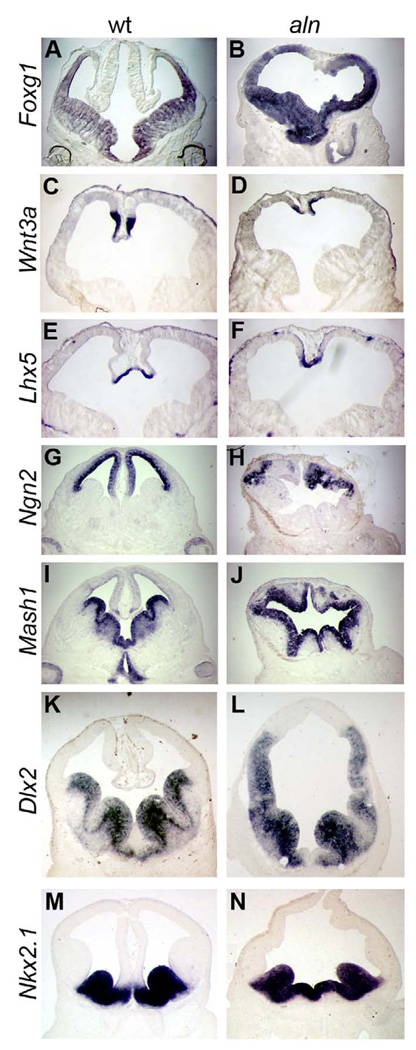
To further characterize the aln phenotype, we performed a molecular marker analysis. First we confirmed that the anterior neural tissue is indeed telencephalic, as both control and aln embryos express Foxg1, a marker of general telecephalic fate (Fig. 4A,B). Next, we examined markers of dorsal-ventral (D-V) patterning as histological analysis showed defects in dorsal midline tissues as well as sub-pallial tissues (Fig. 3). Wnt3a and Lhx5 are markers of extreme dorsal tissue, the cortical hem, and were expressed in the aln mutants at lower levels (Fig. 4C–F). Consistent with our histological observations, the dorsal cortex, highlighted by the expression of Ngn2, is extremely reduced and disorganized in aln mutants (Fig. 4G,H). In contrast, expression of Mash1 as a marker of ventral telencephalon is expanded in aln mutant tissue (Fig. 4I,J). Dlx2 is expressed in the ventral telencephalon in the medial and lateral ganglionic eminences (MGE and LGE, respectively), structures of the subpallium which give rise to tangentially migrating interneurons, forming connections to the neocortex. In aln embryos the expression of Dlx2 is seen throughout a greatly expanded portion of the telencephalon, reaching almost the extreme dorsal portion of the forebrain (Fig. 4K,L), but no distinct LGE is visible. Nkx2.1 is expressed in the MGE of wild-type embryos (Fig. 4M) and expression of Nkx2.1 in aln embryos is slightly expanded in the most ventral portion but does not expand beyond the borders of the MGE (Fig. 4N). Taken together, these results show that while the aln forebrain does maintain a dorsal-ventral pattern, dorsal fates are reduced and ventral fates are expanded. As we saw with our histological analysis, ventral aln tissue does not form the distinct ganglionic eminences which we see in wild-type, and shows a more dramatic change in morphology of the LGE as compared to the MGE.
Figure 4. Dorsal – ventral patterning defects in alien mutants.
Coronal sections of E12.5 wild-type (A,C,E,G,I,K,M) and aln (B,D,F,H,J,L,N) embryos showing expression patterns of indicated markers. The forebrain does retain telencephalic character in aln mutants (A,B). We note the presence of all markers analyzed but a decrease in dorsal gene expression (C–H: Wnt3a, Lhx5, Ngn2) and an increase in ventral markers Mash1 (I,J) and Dlx2 (K,L). The expression of Nkx2.1 (M,N) does not extend significantly beyond the wild-type domain.
Midbrain and diencephalic tissues are expanded in alien embryos
Given the evidence suggesting enlargement of the midbrain at E12.5 (Fig. 1E), we further examined its development in aln embryos. Histological analysis at E12.5 confirmed an expansion of the tegmentum at the floor of the midbrain and the thalamus dorsally (Fig. 5B). At E16.5, the telencephalon is clearly smaller in the mutant (Fig. 5D) and displaced rostrally when compared to wild-type (Fig. 5C). The loss of olfactory bulbs in aln mutant tissue is visible at this stage as well (Fig. 5D). To determine whether this increase in midbrain character was due to an expansion of early-commited midbrain cells or a change in fate of presumptive forebrain cells, we employed a lineage tracing strategy. The Wnt1Cre transgene marks the midbrain tissue (Danielian et al., 1998) from the time of its initial formation. We generated embryos homozygous for aln as well as heterozygous for the Wnt1Cre transgene and the R26R-Cre reporter. In these embryos, the lacZ expression serves as a persistent marker of midbrain tissue throughout the life of the embryo. In Wnt1Cre;R26R wild-type embryos, the lacZ positive cells are present in the midbrain and neural crest cells populating the head, while the dorsal telencephalon is completely devoid of lacZ positive cells (Fig. 5E,G). In the mutants, however, the Wnt1Cre;R26R lacZ positive cells mix into the forebrain to nearly the rostral-most extent of the tissue (Fig. 5F,H).
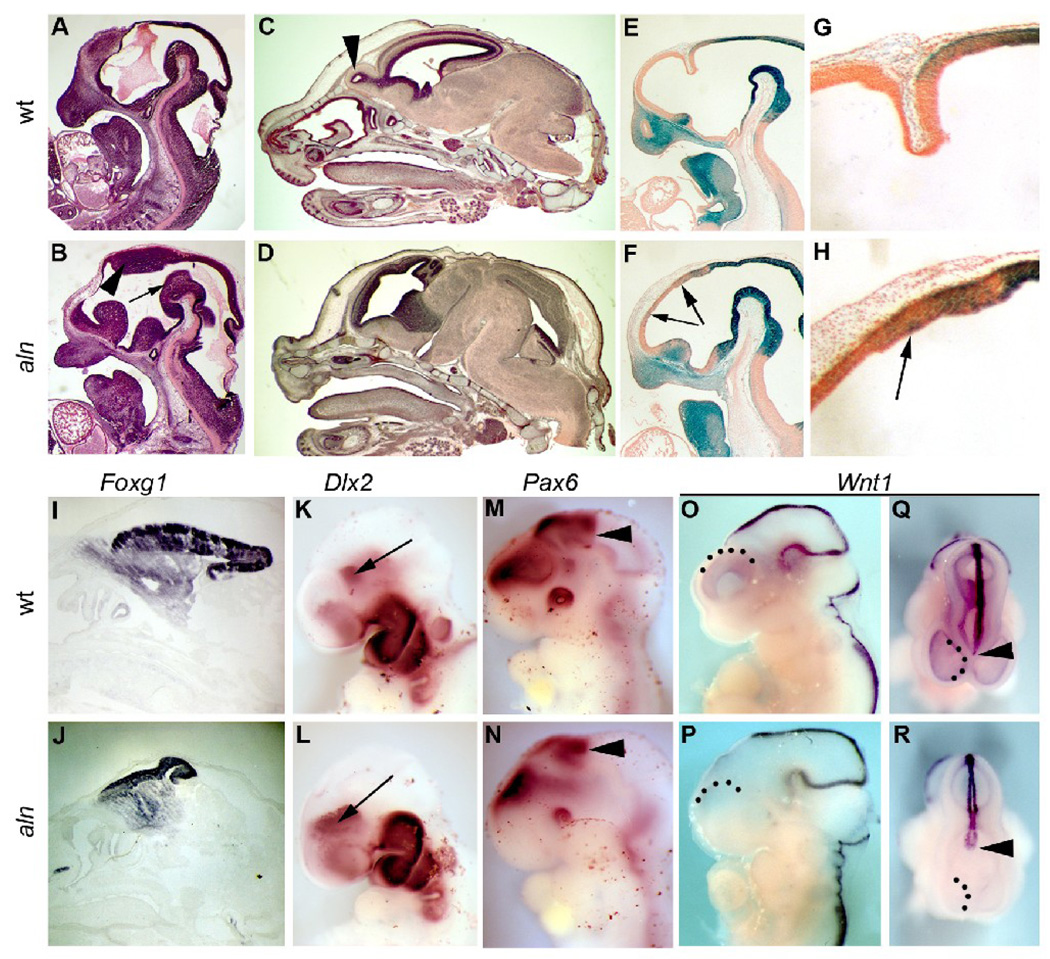
Figure 5. Rostral – caudal patterning defects in alien mutants.
Sagittal sections of wild-type (A,C) and mutant (B,D) embryos at E12.5 (A,B) and E16.5 (C,D). Arrow and arrowhead in B indicate tegmentum/ floor of the midbrain and dorsal thalamus, respectively. Arrowhead in C indicates the olfactory bulb. (E–H) Lineage mapping of the midbrain with the Wnt1-Cre transgene and R26R Cre reporter in wild-type (E,G) and aln (F,H) E12.5 embryos. (G,H) High magnification views of forebrain tissue in embryos shown in E,F. Arrows in mutant embryo (H) indicate β-gal positive cells in the telencephalon. (I–R)Whole mount and section in situ hybridization at E16.5 (I,J) and E10.5 (K–R) for Fog1 (I,J), Dlx2 (K,L: arrows indicate ventral thalamus), Pax6 (M,N; arrowhead indicate caudal extent of Pax6 expression) and Wnt1 (O–R) in wild-type (I,K,M,O,Q) and mutant embryos (J,L,N,P,R). Q,R are dorsal views of embryos shown in O,P and arrowheads indicate the rostral extent of Wnt1 expression while dotted lines indicate the telencephalic boundaries.
Reduction of the telencephalon is confirmed by the expression of Foxg1 at E16.5. The Foxg1 domain in aln mutants represents a smaller portion of the brain tissue and is displaced anteriorly in comparison to wild-type(Fig. 5I,J), a molecular correlate to our gross and histological findings described above. Molecular analysis further confirmed a change in rostral-caudal patterning. Expression of Dlx2 in the subpallium is expanded and expression in the ventral thalamus is shifted rostrally in mutants as compared to wild-types (Fig. 5K,L; also see Fig. 4L). Similarly, the caudal limit of Pax6 expression, marking the boundary between the diencephalon and midbrain, is at a more anterior position in the mutant embryos (Fig. 5M,N). Finally, we examined the expression of Wnt1 which is expressed at the dorsal midline and in wild-type embryos extends through the diencephalon (Fig. 5O–Q). In aln mutants, the Wnt1 expression in the caudal embryo through the midbrain appears unchanged. In the diencephalon however, the expression domain appears severely truncated (Fig. 5P,R). While our data suggest an expansion of the diencephalon, we propose the loss of dorsal Wnt1 in this domain is a consequence of reduced dorsal character in the developing forebrain (see also Fig. 4). All of these findings suggest that the midbrain and diencephalon are inappropriately expanded at the expense of more anterior, rostral telencephalon tissue in aln mutants. Thus, defects in both dorsal-ventral and anterior-posterior patterning appear in the developing aln anterior nervous system.
Sonic hedgehog signaling is elevated in alien forebrain
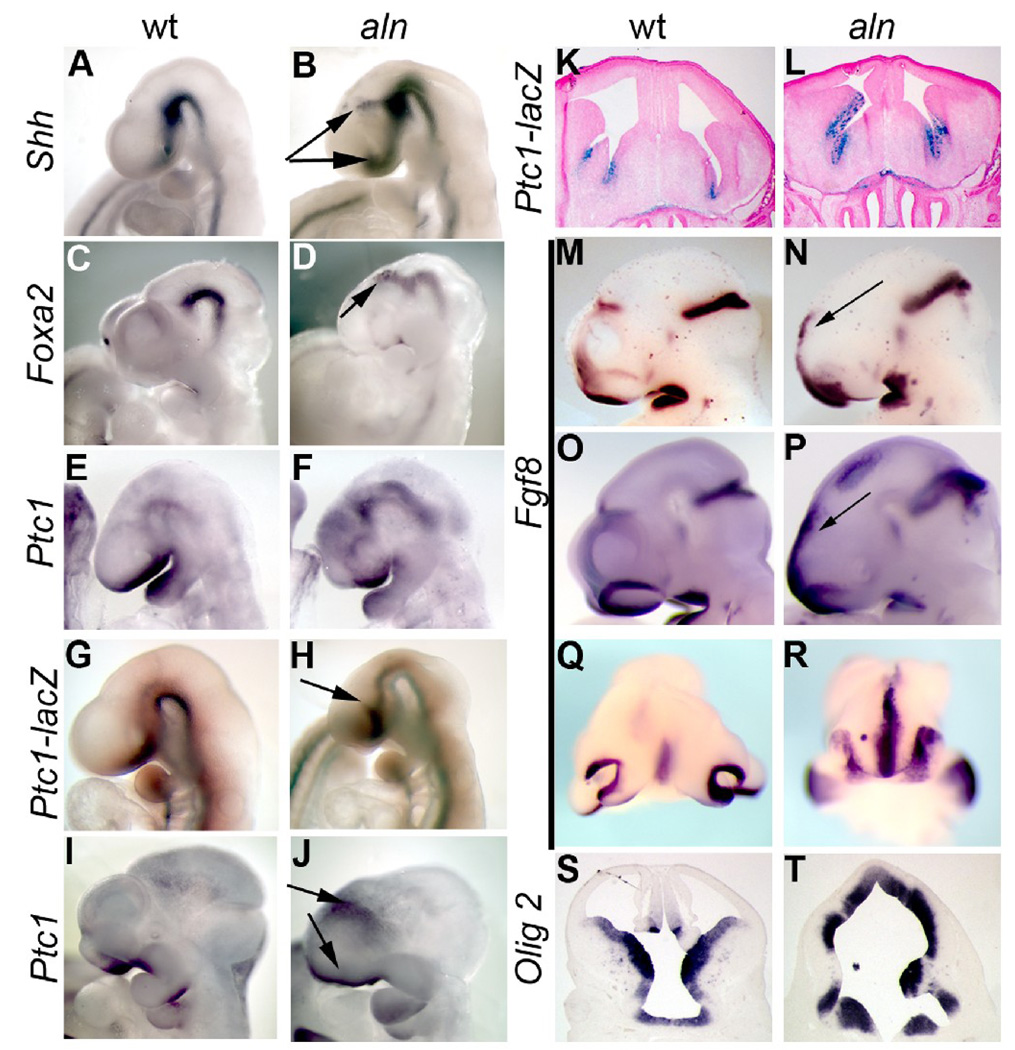
We have previously noted that Shh signaling is upregulated in the aln embryo (Tran et al., 2008) and such an upregulation may contribute to the change in dorsal-ventral patterning we observe. To determine if this is also true in the developing forebrain, we performed in situ hybridization for Shh and Shh target genes in early stages of brain development. Shh is clearly expressed at higher levels in the aln embryo both ventral to the telencephalon and in a more limited region between the telencephalon and diencephalon (arrows in Fig. 6B). This latter expression domain may correspond to an enlarged zona limitans intrathalamica (ZLI), a known organizing center of the developing brain (Kiecker and Lumsden, 2004; Zeltser, 2005). Similarly, the Shh targets, Foxa2 and Patched1 are expressed at higher levels in the developing aln brain region (Fig. 6C–F). The Foxa2 expansion is more discrete and focused around the region of the ZLI (Fig. 6D), while the Ptc1 increase is more widespread and visible in both the telencephalon and along the floor of the neural tube throughout the head (Fig. 6F). To more fully analyze the pattern of increased Ptc1 expression, we created aln mutants which also carried the Ptc1-lacZ reporter allele (Goodrich et al., 1997). The introduction of the Ptc1-lacZ allele did not affect the aln phenotype at any of the stages we examined. The Ptc1-lacZ reporter at E9.5 showed a similar expansion in the aln mutant as Shh expression itself, most prominently around the telencephalon ventrally and between the telencephalon and diencephalon (Fig. 6H). We used the alien;Ptc1-lacZ embryos to determine the earliest stage at which the Shh pathway was activated and did not see any increase in beta-galactosidase activity before E9.5 (data not shown). We next determined whether this activation of the Shh pathway was a prolonged change, or an acute response to the loss of the alien gene product. Whole mount in situ hybridization for Ptc1 at E11.5 showed similar results as at earlier stages: expression was elevated around the telencephalon and craniofacial tissue (Fig. 6J). Furthermore, the Ptc1-lacZ reporter showed increased expression in the forebrain at E14.5, the latest stage we examined (Fig. 7L). These data suggest that the loss of forebrain and expansion of diencephalon and midbrain in aln mutants is the result of an increase in Shh activity in early embryos in tissues known to pattern the developing brain. Furthermore, this increased activation of the Shh pathway continues through later stages of neurodevelopment.
Figure 6. Sonic hedgehog and targets are expressed at increased levels in ventralized alien brains.
Whole mount in situ hybridization for Shh (A,B), Foxa2 (C,D), Ptc1 (E,F,I,J), and Fgf8 (M–R), in wild-type (A,C,E,I,M,O,Q) and alien (B,D,F,J,N,P,R) embryos at E9.5 (A,B,E,F,M,N), E10.5 (C,D,O–R), and E11.5 (I,J). Ptc1-lacZ expression in wild-type (G,K) and alien embryos (H,L) at E9.5 (G,H) and E14.5 (K,L). Arrows in B,D,H,J,N,P indicate regions of elevated Shh pathway and Fgf8 signaling. (Q,R) Coronal view of frontonasal tissue highlighting increase in Fgf8 expression in aln anterior tissue. (S,T) Section in situ hybridization of coronal sections of E12.5 wild-type (S) and aln (T) embryos showing expression of Olig2 extending further dorsally in aln mutants.
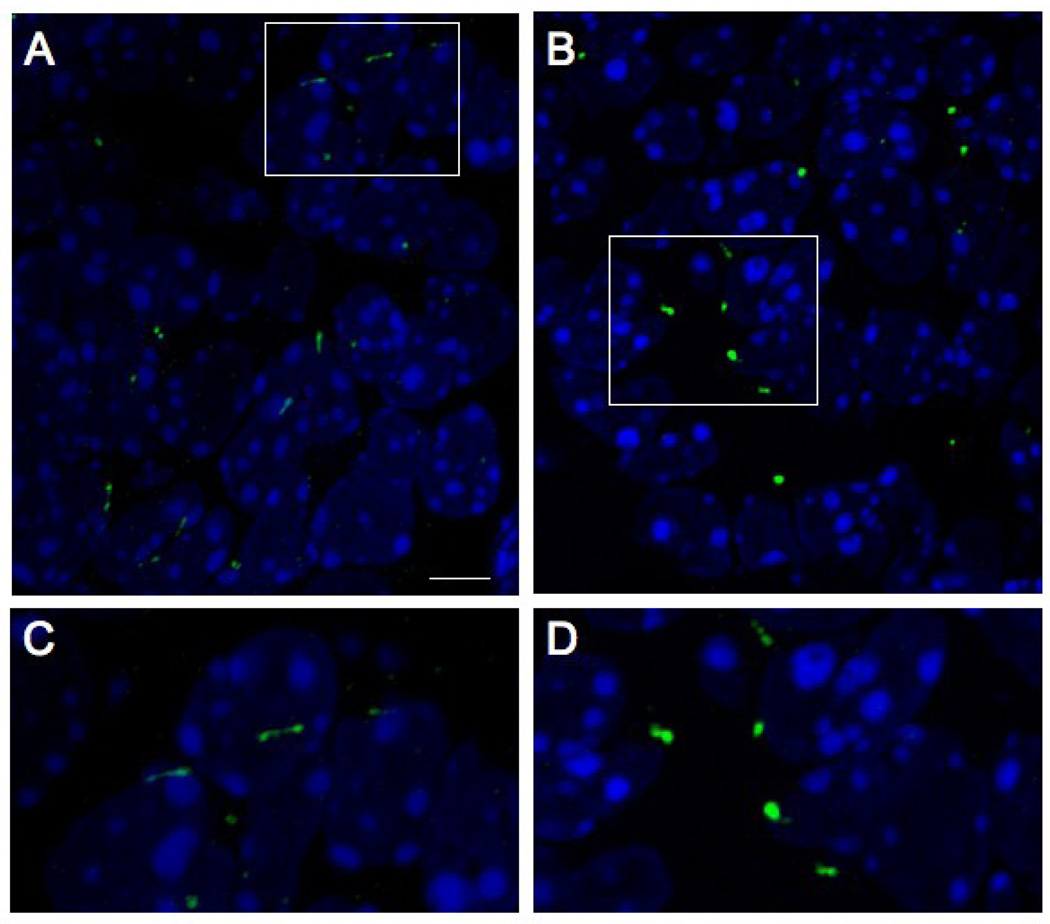
Figure 7. Cilia are abnormal in alien neural tissue.
Immunohistochemistry for Polaris in wild-type (A,C) and aln (B,D) cilia from E9.5 midbrain (nuclei are shown with DAPI). Mutant cilia are more bulbous than wild-type and have larger aggregates of Polaris, rather than a distribution along the entire length of cilia, consistent with a defect in retrograde transport. Approximate areas enlarged in C,D are indicated in A,B respectively by rectangles. Scale bar is 5 µm in A,B and approximately 2.0 µm in C,D.
Fibroblast growth factor 8 (Fgf8) has been shown to be an important molecular regulator of growth and patterning in the rostral forebrain (Garel et al., 2003; Storm et al., 2006; Storm et al., 2003). A mouse mutant with a phenotype similar to that of the aln mouse shows significant increases in Fgf8 signaling (Aoto et al., 2002). To determine if a change in Fgf8 expression may contribute to the aln phenotype, we observed its expression in aln embryos. At E9.5 and E10.5, wild-type embryos have high levels of Fgf8 expression in the commissural plate (the extreme anterior of the closing neural tube), the developing olfactory pits, midbrain/hindbrain junction, and the oral ectoderm (Fig. 6M,O,Q). Aln mutant embryos have elevated Fgf8 expression in all of these domains at E9.5 through E11.5, most strikingly in the commissural plate (Fig. 6N,P,R and data not shown). The olfactory pits are more closely apposed and the rostral expression of Fgf8 is continuous in aln mutant embryos (Fig. 6R). We also note an ectopic domain of Fgf8 expression in the dorsal diencephalon in some embryos at E10.5 (Fig. 6P). Finally, we examined the expression of Olig2, a marker of oligodendrocyte glial fate which is responsive to Shh signaling. In wild-type embryos at E12.5, Olig2 expression is confined to the ventral telencephalon (Fig. 6S), but in aln embryos Olig2 expression extends to the dorsal-most portion of the brain (Fig. 6T), similar to the changes in Dlx2 and Mash1 expression we described earlier (Fig. 4). Thus, we conclude that increased Shh pathway activity, at least in part, leads to the changes in patterning we see in the developing aln brain.
Cilia are abnormal in alien embryonic brain
We previously demonstrated that primary cilia are abnormal in aln embryos; specifically, they show an accumulation of proteins at the distal tip that normally traffic freely along the length of the cilia (Tran et al., 2008). While the consequences of ciliary defects have been widely characterized in the embryonic node, neural tube, kidney and limb bud of the developing embryo, this has been shown much less frequently in the brain (May et al., 2005). We assessed whether the molecular defect in the aln brain is the same as in the rest of the embryo by immunostaining sections for Polaris/IFT88, which typically localizes at the ciliary base and distal ends and punctately throughout the axoneme. In wild-type cilia in the brain, Polaris staining was seen in an elongated fashion consistent with localization along the length of the cilium (Fig. 7A,C). In aln, Polaris accumulated in more intense, bulbous structures (Fig. 7B,D). This is consistent with our previous S.E.M. and immuno-histochemical data showing that aln cilia are shorter with an accumulation of protein at the distal end, including Polaris (Tran et al., 2008). This has previously been observed in the alien limb and results from a defect in retrograde trafficking (Tran et al., 2008). These data suggest that the molecular mechanism underlying the aln phenotype in the brain is the same as in other tissues studied.
Reducing the level of Shh partially rescues the alien phenotype
We hypothesize the increased expression of Shh and Shh target genes are likely to be a cause of the aln brain phenotype. We therefore reasoned we may be able to reduce the severity of the phenotype by genetically reducing the amount of available Shh ligand. We created embryos which were homozygous for aln and heterozygous for a null allele of Shh, hereafter referred to as aln,Shh mutants (aln/aln; Shh/+). At E12.5 we observe a subtle decrease in the severity of the aln phenotype in aln,Shh mutants with a slightly less severe expansion and displacement of the telencephalon by the midbrain (Fig. 8C). This subtle rescue is further evident in sagittal sections (Fig. 8D–F). Over the course of further embryonic development, the rescue in the aln,Shh mutants becomes more pronounced as the cortex is less disrupted and dorsal midline structures are more robust when compared to aln mutants, including a visible choroid plexus and more developed hippocampal tissue at E14.5 (Fig. 8G–I). We recovered two aln,Shh mutants at E18.5 and P0.5; both of these had polydactyly, but the head and brain appear relatively normal, with olfactory bulbs present, and large, distinct telencephalic vesicles (Fig. 8L,O). Sagittal sections at E18.5 show the telencephalon of the aln,Shh mutant is in a position much more similar to that of wild-type telencephalon (Fig. 8R) while the aln cortex is much smaller and displaced anteriorly (Fig. 8Q).
Figure 8. Partial rescue of alien phenotype by heterozygosity for Shh.
Wild-type (A,D,G,J,M,P), aln/aln (B,E,H,K,N,Q) and aln/aln;Shh/+ (C,F,I,L,O,R) embryos at E12.5 (A–F), E14.5 (G–I) and E18.5 (J–R). Sections in D–F and P–R are in the sagittal plane and G–I are coronal. Arrowheads in D–F indicate the caudal extent of the telencephalon, arrows in G,I show choroids plexus tissue, and arrows in M,O,P,R identify olfactory bulbs. (S–U) Whole mount in situ hybridization shows Fgf8 expression in wild-type (S), aln (T), and aln,Shh mutant (U) embryos.
We further used the aln,Shh mutants to determine whether elevated Shh expression is required for the increased rostral expression of Fgf8 in alien mutants. Aln,Shh mutant embryos at E9.5 (Fig. 8U) do not show the increased Fgf8 expression found in alien mutants (Fig. 8T; also see Fig. 6M–R). Thus, our data show that elevated Shh expression is a requirement for the abnormal brain development seen in alien mutants, which is likely mediated by inducing the upregulation of Fgf8.
Taken together, our data show that elevated Shh expression and pathway activity is indeed a requirement for the abnormal brain development seen in aln mutants.
Wnt signaling is reduced in the alien forebrain
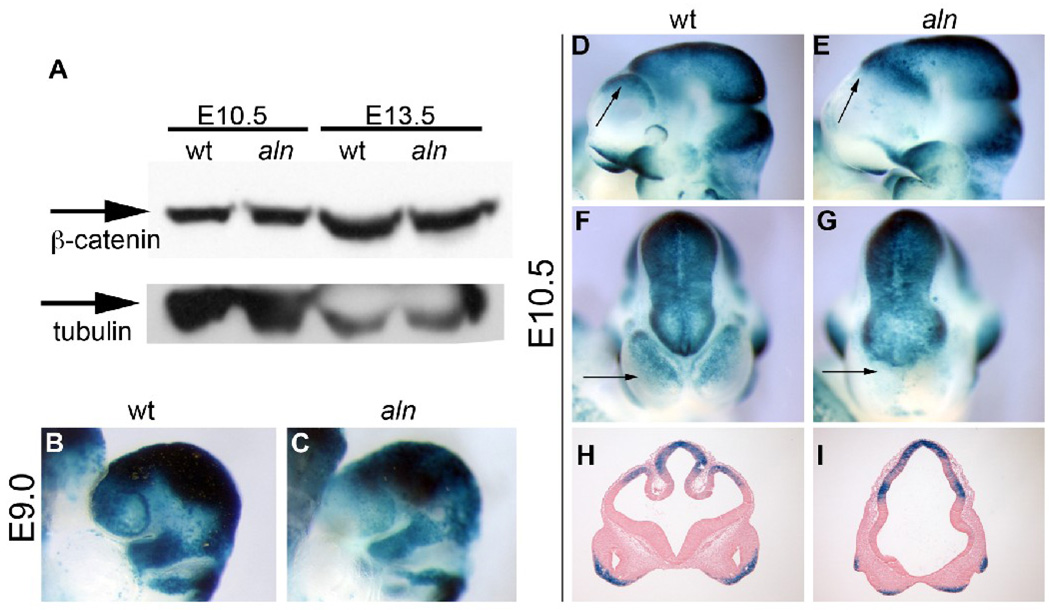
Cilia have recently been implicated in mediating multiple developmental signaling pathways though the role of cilia in mediating Wnt signaling remains unresolved. Loss of cilia due to the absence or reduction of the kinesin subunit, Kif3a, results in increased canonical Wnt signaling in E9.5 Kif3a−/− mutants and in Kif3a-deficient cells (Corbit et al., 2008; Gerdes et al., 2007). However, mice with ciliary defects due to a mutation in the complex B protein IFT172 do not show a change in Wnt reporter activity at E10.5 (Eggenschwiler and Anderson, 2007). To assess whether the change in midbrain character we observe in aln embryos could be due to dysregulated Wnt signaling, we examined the levels of β-catenin in cell lysate from aln head tissue from E10.5 and E13.5. We saw no difference in accumulation of β-catenin at either stage (Fig. 9A). To observe Wnt signaling with more resolution in the developing embryo, we generated mice carrying both the aln mutation and the Bat-gal reporter (Maretto et al., 2003) – a transgene with multiple TCF-LEF binding sites upstream of lacZ which is responsive to Wnt activity. In our experiments, this reporter showed little or no variation in expression among wild type embryos, validating its utility as a reporter of Wnt signaling (data not shown). We generated embryos which were homozygous for the alien mutation and heterozygous for the Bat-gal transgene at multiple stages: E9.0 (Fig. 9B,C) and E10.5 (Fig. 9D–I). In contrast to the increased Wnt signaling seen in mice carrying mutations of Kif3a (Corbit et al., 2008), we saw a decrease in apparent Wnt activity in the dorsal forebrain of aln mutant embryos at all stages examined. We suggest this is not actually reflective of a decrease in Wnt signaling, but, rather, the disrupted dorsal character of the developing anterior nervous system in aln embryos as a result of dysreguled Shh signaling, similar to the decreased expression of dorsal markers described above (Fig. 4,Fig. 5). The conclusion that Wnt signaling levels are not significantly altered in aln mice is supported by our finding of similar levels of β-catenin protein in lysates from wild-type and mutant embryos.
Figure 9. Wnt signaling in aln embryos.
(A) Immunoblot for total β-catenin protein in wild-type (lanes 1,3) and aln (lanes 2,4) at E10.5 (lanes 1,2) and E13.5 (lanes 3,4). Alpha-tubulin is included as a loading control. At both stages, total β-catenin protein levels appear unchanged in aln as compared to wild-type. (B–I) Aln;Bat-Gal embryos to show Wnt signaling levels in vivo. We generated wild-type and aln embryos carrying the Bat-Gal reporter transgene at E9.0 (B,C), and E10.5 (D–I). We note a general decrease in Bat-Gal activity in anterior brain tissue. Arrows in D–G indicate the anterior telencephalon which is significantly reduced in size, dorsal character, and Wnt signaling activity in aln mutants (E,G,I) as compared to wild-type littermates (D,F,H). Coronal sections in H,I are from embryos in D/F and E/G, respectively.
DISCUSSION
We have recently described the early embryonic patterning defects of an ENU-induced mutant mouse, alien, which is notable for having both abnormal primary cilia and overactivation of the SHH pathway (Tran et al., 2008). The aln mutant is due to a defect in the gene Ttc21b and we demonstrated that aln has partially defective retrograde transport resulting in accumulation of IFT proteins. Anterograde transport is functional, which permits entry of SHH components into cilia and results in an increase in SHH pathway activity. This is in marked contrast with previously reported ciliary mutations, in which SHH signaling is generally decreased (Garcia-Garcia et al., 2005; Huangfu et al., 2003; May et al., 2005). In this report we describe the brain phenotype in embryos mutant for Ttc21b. We show that loss of TTC21B results in a smaller forebrain with reduced dorsal structures and expanded ventral character. Furthermore, this telencephalic reduction is concomitant with an expansion of the diencephalon and midbrain. The expression levels of Shh and Shh target genes are elevated in the aln mutant, as is Fgf8, most notably in the commissural plate. Importantly, reduction of Shh expression ameliorates the aln phenotype. Thus, TTC21B is required to restrict Shh pathway expression in the rostral mesendoderm and midbrain, possibly restricting the size of the zona limitans intrathalamica (ZLI), and thereby prevent inappropriate patterning of both the midbrain and forebrain.
Increased Shh and Fgf8 signaling contribute to the alien brain phenotype
We suggest the forebrain phenotype observed in aln embryos is the result of increased activity in both Shh and Fgf8 signaling. The increase in Shh pathway activity in both the anterior neural tissue and ZLI (see below; Fig. 6B) is likely mediated by an alteration in the ratio of activated GLI2 to GLI3 repressor (GLI3-R) in the absence of TTC21B protein (Tran et al., 2008). This loss of relative GLI3-R activity, in turn, decreases repression of Fgf8 (see below; (Gutin et al., 2006) and also leads to further expression of Shh itself. In the ventral forebrain, Shh (McMahon et al., 2003) and Fgf8 (Storm et al., 2006) are both known to impart ventral character, which is dramatically increased in aln tissue. Interestingly, the most ventral cell types (i.e., the Nkx2.1-positive floor plate and MGE; Fig 4N) do not appear to be the most profoundly affected. Rather, there is a more dramatic expansion of other ventral tissue including the LGE (Mash1 and Dlx2 expression, Fig. 4I–L), accompanied by loss of more dorsal tissues (Wnt3a, Lhx5, Ngn2 expression, Fig. 4C–H).
The upregulation of Fgf8 is somewhat puzzling. While consistent with an increase in ventral character (and possibly with increased Foxg1 expression levels in the remaining telencephalon in alien embryos), increased Fgf8 signaling has been demonstrated to expand the area of the forebrain (Storm et al., 2003). Similar defects to those seen in the aln mutants have been noted in other mutations with an increase in Fgf8 (Aoto et al., 2002). The changes in forebrain tissue may be due to any number of other signaling targets affected by either retrograde trafficking defects, as a result of Shh dysregulation, or as an immediate effect of Fgf8 upregulation (e.g. possible compensatory Sprouty induction). FGF signaling is quite complex (Storm et al., 2003) and even different FGF ligands in the forebrain can have quite different effects (e.g. Borello et al., 2008).
The loss of TTC21B results in a defect in ciliary retrograde transport and subsequent activation of the Shh signaling pathway (Tran et al., 2008), which in turn leads to the brain phenotype we describe here. Notably, the aln brain defects are quite different from those found in mice mutant for Dync2h1 (dynein heavy chain) or flexo (IFT88/polaris), in which Shh signaling is decreased. Dync2h1 mutants show a dorsal-ventral patterning defect in the forebrain at E12.5 with a reduction in Nkx2.1 expression (May et al., 2005). The IFT88 mutant shows reduced Ptc1-lacZ expression at E9.5, complementary to our findings (Huangfu et al., 2003). Thus the CNS patterning defects in these mutants, which have reduced Shh signaling, are the opposite of those we see in aln mutants, consistent with our conclusion that the aln phenotype is due to increased Shh activity.
Ttc21b may help control the size of the ZLI
Concurrent with the dorsal-ventral cell fate change, we see evidence for a change in rostral-caudal patterning in the aln brain (Fig. 5). We see an increase in Shh expression in early embryos in or near the ZLI, a known organizing center of the brain (Fig. 6B; Vieira et al., 2005; Vieira and Martinez, 2006). Increased Shh activity in this region can have a profound effect on rostral-caudal patterning. Just as we observe an increase in Dlx2 expression in aln embryos (Fig. 4L, 5L), implantation of Shh-expressing cells induces Dlx2 expression in the chick. Interestingly, in the chick implant experiments, this effect is seen when the implant is in the prethalamus portion of the diencephalon, anterior to the ZLI, but not in the thalamus posterior to the ZLI (Vieira et al., 2005). This correlates with our finding of more profound patterning effects in the more anterior compartments of the aln brain, which are perhaps more responsive to SHH signals (Vieira et al., 2005). The aln mutation is unique with regard to this expansion of Shh in the ZLI, as very few mutants have been found that show an expansion of the ZLI. Shh is also not classically thought to play a significant role in anterior-posterior patterning of the nervous system.
Alien and Shh interact genetically
The increased Shh expression seen in the aln mutants led us to test the hypothesis that genetically reducing the availability of SHH ligand may rescue the aln phenotype. Aln/aln; Shh/+ mutants showed markedly less brain dysmorphology than aln/aln mutants, while retaining the polydactyly, suggesting the elevated SHH signaling we observe does indeed contribute to the ontogeny of the brain phenotype in aln mutants and is not a secondary effect. This rescue is much more pronounced at later stages of development (E18.5 vs. E12.5). We believe this is true because the cell-autonomous aln defect in intraflagellar transport leads to a hyperactivation of the SHH pathway, possibly among other molecular consequences. As embryonic development proceeds and this SHH hyperactivation continues (e.g. Fig. 6L), the phenotype of aln mutants becomes more severe and deviates further from wild-type. In aln;Shh mutants, the reduced availability of SHH ligand during this later period results in the phenotype being less severe than aln mutants at similar late stages.
Alien mutants resemble Gli3 mutants
The phenotype described here is very similar to that of the extra-toes mutation of Gli3. Similar features include polydactyly, a smaller, ventralized forebrain with loss of dorsal midline structures, increased Fgf8 expression, and partially penetrant exencephaly (Grove et al., 1998; Tole et al., 2000). Our observations suggest that Ttc21b and Gli3 are largely coexpressed (Aoto et al., 2002). We have previously shown that loss of the Ttc21b gene product results in a loss of the proper ratio of activated GLI3 to repressor GLI3 (Tran et al., 2008). As the main function of Gli3 is thought to be as a repressor, we presume that aspects of the aln phenotype (e.g. preaxial polydactyly) are due to a significant decrease in Gli3-mediated repression of the Shh pathway.
A further important similarity between alien and Gli3 mutants is the expansion of Fgf8 expression in the anterior, craniofacial region, as well as in the dorsal diencephalon in the Gli3 mutant. The expression pattern of Fg8, and its ability to induce ventral cell fate in cortical explants (even in the presence of cyclopamine) suggests that this may be another morphogen capable of instructing ventral cell fate in the developing telencephalon (e.g. Kuschel et al., 2003). The change in Fgf8 expression in mutant Gli3 brains is thought to affect the dorsal-ventral patterning information mediated by BMPs and Wnts and contribute to the ventralization of the mutant Gli3 brain (Kuschel et al., 2003). We would propose similar effects leading to the ventralization of the aln brain. Interestingly, this expanded Fgf8 expression is maintained in a Shh;Gli3 double mutant (Aoto et al., 2002; Rash and Grove, 2007) which suggests that, although Shh can induce Fgf8 expression in the anterior, it is not required. This implies a direct link between GLI3-R signaling and Fgf8 regulation whereby normal levels of GLI3-R serve to repress Fgf8, as has recently been proposed by Gutin and colleagues in their analysis of FGFR mutations in the forebrain (Gutin et al., 2006). Some aspects of the Gli3 phenotype differ markedly, however, from the alien mutation. Unlike Gli3xtj mutants, aln mutants have overactivated GLI2 and GLI3-A, (activator) presumably accounting for some of the difference. Gli3xtj mutants do not show an increase in activity of the Shh pathway in the brain (Tole et al., 2000).
Another mutation affecting SHH signaling, a non-cholesterol modified SHH (SHH-N), has a phenotype similar to our aln mutation (Huang et al., 2007). ShhN heterozygotes have defects in dorsal midline structures of the forebrain along with an expansion of Fgf8, preaxial polydactyly, and craniofacial abnormalities. In many respects, the aln mutation is almost a phenocopy of this heterozygous animal: reduced forebrain structures, increased Shh expression in the ZLI, increased Shh target gene expression, expanded LGE, the polydactyly and craniofacial phenotypes A portion of our most severely affected aln embryos do show dorsal midline defects as dramatic as those seen in ShhN heterozygous mice (e.g., Fig. 3D). A human patient with a nonsense mutation in SHH thought to produce a protein acting similarly to the ShhN allele has a mild form of holoprosencephaly (semi-lobar HPE; Nanni et al., 1999). A further variant of HPE is middle interhemispheric holoprosencephaly (MIHV) and affected individuals have features reminiscent of both the aln and ShhN mouse phenotypes (e.g. Simon et al., 2002,Lewis et al., 2002, Pulitzer et al., 2004). These similarities support our interpretation of the increased SHH pathway activity as causing the aln phenotype and suggests this may be a candidate allele for some cases of human HPE which do not have mutations in known HPE genes.
Our data suggest that dysregulated Wnt signaling does not play a major role in causing the abnormalities in the developing aln brain, although it is possible that the retrograde trafficking in Ttc21b-mutant cilia affects other signaling molecules. Thus, we conclude that, while Ttc21b and Gli3 ultimately play a similar role in forebrain development restricting the potent patterning information imparted by the SHH morphogen, distinct differences may remain in their mode of action. Further study of the role of the Ttc21b gene in brain development may potentially yield very interesting information about the control of Shh signaling as well as the establishment of a relatively little-studied patterning center in the brain: the ZLI.
ACKNOWLEDGEMENTS
We are grateful for the generous gifts of reagents for in situ probe production and antibodies from D. Anderson, B. Hogan, G. Martin, R. Maas, A. McMahon, J. Rubenstein, C. Tabin, and H. Westphal. This work was supported by the N.I.H. with grants HD36404 and MH081187 to D.B. and HD531982 to R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aoto K, Nishimura T, Eto K, Motoyama J. Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev Biol. 2002;251:320–332. doi: 10.1006/dbio.2002.0811. [DOI] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Borello U, Cobos I, Long JE, Murre C, Rubenstein JL. FGF15 promotes neurogenesis and opposes FGF8 function during neocortical development. Neural Develop. 2008;3:17. doi: 10.1186/1749-8104-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia MJ, Eggenschwiler JT, Caspary T, Alcorn HL, Wyler MR, Huangfu D, Rakeman AS, Lee JD, Feinberg EH, Timmer JR, Anderson KV. Analysis of mouse embryonic patterning and morphogenesis by forward genetics. Proc Natl Acad Sci U S A. 2005;102:5913–5919. doi: 10.1073/pnas.0501071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, Badano JL, Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- Goodrich L, Milenkovic L, Higgins K, Scott M. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes and Development. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Gutin G, Fernandes M, Palazzolo L, Paek H, Yu K, Ornitz DM, McConnell SK, Hebert JM. FGF signalling generates ventral telencephalic cells independently of SHH. Development. 2006;133:2937–2946. doi: 10.1242/dev.02465. [DOI] [PubMed] [Google Scholar]
- Herron BJ, Lu W, Rao C, Liu S, Peters H, Bronson RT, Justice MJ, McDonald JD, Beier DR. Efficient generation and mapping of recessive developmental mutations using ENU mutagenesis. Nat Genet. 2002;30:185–189. doi: 10.1038/ng812. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo. New York: Cold Spring Harbor Press; 1994. [Google Scholar]
- Huang X, Litingtun Y, Chang C. Ectopic sonic hedgehog signaling impairs telencephalic dorsal midline development: implication for human holoprosencephaly. Hum Mol Genetics. 2007;16:1454–1468. doi: 10.1093/hmg/ddm096. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. San Diego: Academic Press, Inc.; 1992. [Google Scholar]
- Kiecker C, Lumsden A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat Neurosci. 2004;7:1242–1249. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]
- Kuschel S, Ruther U, Theil T. A disrupted balance between Bmp/Wnt and Fgf signaling underlies the ventralization of the Gli3 mutant telencephalon. Dev Biol. 2003;260:484–495. doi: 10.1016/s0012-1606(03)00252-5. [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AJ, Simon EM, Barkovich AJ, Clegg NJ, Delgado MR, Levey E, Hahn JS. Middle interhemispheric variant of holoprosencephaly: a distinct cliniconeuroradiologic subtype. Neurology. 2002;59:1860–1865. doi: 10.1212/01.wnl.0000037483.31989.b9. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- McMahon JA, McMahon AP. Nucleotide sequence, chromosomal localization and developmental expression of the mouse int-1-related gene. Development. 1989;107:643–650. doi: 10.1242/dev.107.3.643. [DOI] [PubMed] [Google Scholar]
- Nanni L, Ming JE, Bocian M, Steinhaus K, Bianchi DW, de Die-Smulders C, Giannotti A, Imaizumi K, Jones KL, Del Campo M, Martin RA, Meinecke P, Pierpont MEM, Robin NH, Young ID, Roessler E, Muenke M. The mutational spectrum of the Sonic Hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly. Hum Mol Genetics. 1999;13:2749–2488. doi: 10.1093/hmg/8.13.2479. [DOI] [PubMed] [Google Scholar]
- Porteus MH, Bulfone A, Ciaranello RD, Rubenstein JL. Isolation and characterization of a novel cDNA clone encoding a homeodomain that is developmentally regulated in the ventral forebrain. Neuron. 1991;7:221–229. doi: 10.1016/0896-6273(91)90260-7. [DOI] [PubMed] [Google Scholar]
- Pulitzer SB, Simon EM, Crombleholme TM, Golden JS. Prenatal MR findings of the middle interhemispheric variant of holoprosencephaly. Am J Neuroradiol. 2004;25:1034–1036. [PMC free article] [PubMed] [Google Scholar]
- Rash BG, Grove EA. Patterning the dorsal telencephalon: a role for sonic hedgehog? J Neurosci. 2007;27:11595–11603. doi: 10.1523/JNEUROSCI.3204-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H, Nusse R. Expression of two members of the Wnt family during mouse development--restricted temporal and spatial patterns in the developing neural tube. Genes Dev. 1991;5:381–388. doi: 10.1101/gad.5.3.381. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Bertuzzi S, Chiang C, Shawlot W, Taira M, Dawid I, Westphal H. Expression of murine Lhx5 suggests a role in specifying the forebrain. Dev Dyn. 1997;208:266–277. doi: 10.1002/(SICI)1097-0177(199702)208:2<266::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- Simon EM, Hevner RF, Pinter JD, Clegg NJ, Delgado M, Kinsman SL, Hahn JS, Barkovich AJ. The middle interhemispheric variant of holoprosencephaly. Am J Neuroradiol. 2002;23:151–156. [PMC free article] [PubMed] [Google Scholar]
- Sommer L, Ma Q, Anderson DJ. neurogenins, a novel family of atonal-related bHLH transcription factors, are putative mammalian neuronal determination genes that reveal progenitor cell heterogeneity in the developing CNS and PNS. Mol Cell Neurosci. 1996;8:221–241. doi: 10.1006/mcne.1996.0060. [DOI] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Storm EE, Rubenstein JL, Martin GR. Dosage of Fgf8 determines whether cell survival is positively or negatively regulated in the developing forebrain. Proc Natl Acad Sci U S A. 2003;100:1757–1762. doi: 10.1073/pnas.0337736100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tole S, Ragsdale CW, Grove EA. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes(J) Dev Biol. 2000;217:254–265. doi: 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, Yoder BK, Beier DR. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira C, Garda AL, Shimamura K, Martinez S. Thalamic development induced by Shh in the chick embryo. Dev Biol. 2005;284:351–363. doi: 10.1016/j.ydbio.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Vieira C, Martinez S. Sonic hedgehog from the basal plate and the zona limitans intrathalamica exhibits differential activity on diencephalic molecular regionalization and nuclear structure. Neuroscience. 2006;143:129–140. doi: 10.1016/j.neuroscience.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Zeltser LM. Shh-dependent formation of the ZLI is opposed by signals from the dorsal diencephalon. Development. 2005;132:2023–2033. doi: 10.1242/dev.01783. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]