Abstract
Introduction
T regulatory (Treg; CD4+FOXP3+) cells constitute a unique subpopulation of CD4+ T cells that inhibit T cell responses and prevent disease development/exacerbation in models of autoimmunity. In the present study, we tested the hypothesis that Treg cells are induced in periapical lesions by dental pulp infection.
Methods
In situ hybridization (ISH) was used to localize FOXP3+ cells on day 21 after pulp exposure of the 1st molar teeth and infection with bacteria from the oral environment. FOXP3/GFP knock-in transgenic mice were used to quantify FOXP3+Treg cells that infiltrate into periapical lesions by flow cytometry on days 7, 14, and 21 after infection. Periodontal ligament from uninfected teeth served as a negative control.
Results
ISH showed strong signals that demonstrated the presence of FOXP3+ cells mainly at the periphery of periapical lesions. In contrast no positive cells were present in the periodontal ligament of uninfected controls. Flow cytometry demonstrated an increase in the number of FOXP3+ Treg beginning between day 7 and day 14 (0.69% of the infiltrate) after infection, and increased to day 21 (0.94%) (p<0.05, p<0.001 respectively vs. uninfected controls). Treg were also increased in number in draining cervical lymph nodes following pulpal infection.
Conclusions
These results demonstrate that Treg cells are induced to infiltrate into periapical lesions by pulpal infection, and suggest that they increase in a time-dependent manner.
Keywords: T regulatory cells, periapical lesions, pulp infection, FOXP3/GFP knock-in, flow cytometry, in situ hybridization
Introduction
The host immune response to bacterial infection of the dental pulp and periapical tissues involves an initial influx of phagocytic leukocytes and the production of inflammatory cytokines as manifestations of innate immunity. As the disease progresses to the chronic stage, an infiltration of most of the canonical cellular and humoral elements of adaptive immunity, including various T cell subsets, is observed.
Sakaguchi et al(1, 2) showed that a minor population of CD4+ T cells, which co-express the interleukin-2 receptor a-chain (CD25), was crucial for the control of auto-reactive T cells in vivo and regulation of the immune response to infection (3). These cells were named ‘T regulatory cells’ (Treg), and were later found to also express the forkhead/winged helix transcription factor (FOXP3), which is uniquely present in this cell type and is essential for Treg differentiation (4, 5). Subsequent studies have shown that CD4+CD25+FOXP3+ T cells are both hypo-responsive and suppressive of a variety of cell functions (6), and prevent disease development/exacerbation in models of autoimmunity (7). Indeed, FOXP3 gene deletion in mice results in generalized autoimmunity and death (8).
Treg (CD4+CD25+FOXP3+) cells have been identified in human systemic infections as well as in periodontal disease tissues (9), although they have not yet been identified in periapical lesions. The purpose of this study was to evaluate the presence of infiltrating Treg cells in induced periapical lesions in mice at various times after dental pulp infection, using in situ hybridization and FOXP3/GFP knock-in mice which express green fluorescent protein under the control of the FoxP3 promoter.
Material and Methods
Animals
C57BL/6 mice, 6–8 weeks old, were obtained from Charles River Laboratories (Wilmington, MA). F0XP3/GFP knock-in (KI) (C57BL/6 background) reporter mice were kindly provided by Dr. M. Oukka, Harvard Medical School, Boston, MA (10). FOXP3/GFP KI mice express green fluorescent protein (GFP) under the control of the FOXP3 promoter and also express full length functional FOXP3. They are immunologically intact and possess normal Treg function (11). All animals were maintained in a specific pathogen free environment at the Forsyth Institute Animal Facility, in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC). All experimental protocols were approved by the Forsyth IACUC.
Periapical lesion induction
To evaluate the presence of Treg cells by in situ hybridization, periapical lesions were induced by pulp exposure of the mandibular 1st molar pulps of C57BL/6 mice (n=5/group). For the quantification of Treg cells by flow cytometry, we used FOXP3/GFP knock-in mice (n=10/group) that were subjected to both 1st and 2nd mandibular molar pulp exposure. Mice were anesthetized via intraperitoneal injection with ketamine HCI (80 mg/kg) and xylazine (10 mg/kg), and were placed on a jaw retraction board. The dental pulps of the molars were exposed using an electric dental hand piece with a no. 1/4 round bur under a surgical microscope (MC-M92; Seiler, St. Louis, MO) as described previously by Yu and Stashenko (12). The exposure site was approximately 1.5 times the diameter of the bur. The pulp chambers were opened until the entrance of the canals could be visualized and probed with a size 6 endodontic file. Exposed dental pulps were left open to the oral environment to permit infection until day 21 for in situ hybridization studies, or until days 7, 14 and 21 for quantification of Treg cells by flow cytometry. Mice with non-exposed, uninfected teeth served as negative controls (day 0).
Tissue sample preparation for in situ hybridization
C57BL/6 mice were sacrificed by CO2 asphyxiation and mandibles were isolated and fixed in fresh 4% paraformaldehyde in PBS for 8 hours, then washed 3 times with PBS. Bone blocks containing periapical tissue were decalcified using 10% formic acid and sodium citrate and embedded in paraffin. Serial sections of 6 micron thickness were cut; every 5th sample was mounted and stained with hematoxylin and eosin (H&E). Sections containing the region of interest (patent root canal with localized periapical lesion) were selected, mounted and processed for in situ hybridization.
In situ hybridization
In situ hybridization was performed as previously described (13, 14) on periapical tissue sections using digoxygenin (DIG)-labeled riboprobes. Briefly, sections were deparaffinized in graded alcohols and rehydrated. Digoxigenin (DIG)-labeled FoxP3 complementary RNAs (cRNA) riboprobes were generated by PCR amplification using the following primers:
FOXP3 fw, gctatttaggtgacactatagactgctggcaaatggagtct;
FOXP3 rev, ttgtaatacgactcactataggg aagtaggcgaacatgcgagt.
Primers included T7 and Sp6 sequences for generation of anti-sense and sense control RNA probes respectively. Sections were treated with 1 µg/mL proteinase K for 20 min. Hybridizations were performed in a humidified chamber for 18 h at 60°C. Signals were detected with BM Purple (Roche, Indianapolis, IN) following the manufacturer’s instructions. Micrographs were taken with a Stemi SV11 microscope (Carl Zeiss Microlmaging, Inc. Thornwood, NY) and a Leica DMLS microscope (Leica Microsystems). The images were captured using Zeiss Axiovision 3.1 software (Stemi SV11) and DC Twain V4.0.2.0 software (Leica DMLS).
Flow cytometry of cells from isolated periapical lesions
For flow cytometric (FACS) analyses of cells present in periapical lesions, FOXP3/GFP KI mice were sacrificed, mandibles were isolated and dissected free of soft tissue. The periapical tissues surrounding the roots (mesial and distal) of the lower 1st and 2nd molars were carefully extracted with the surrounding bone as block specimens under a surgical microscope. The isolated bone blocks then were treated as previously described (15) with collagenase Type IV, 2 mg/ml in PBS (Worthington Biochemical, NJ, USA) for 60 minutes at 37°C to liberate leukocytes according to the manufacturer instructions. Isolated cell suspensions were washed 3 times with PBS, resuspended in PBS, and stained with anti-mouse CD4. Approximately 30,000 cells were analyzed for CD4+FOXP+GFP+ Treg using an EPICS ULTRA flow cytometer (Beckman Coulter, Miami, FL). Single cell suspensions were also prepared from cervical lymph nodes. The nodes were harvested, macerated to obtain single cell suspensions, which were then washed three times with PBS containing 1% bovine serum albumin at 4°C, resuspended in PBS, and stained with anti-mouse CD4. Treg (CD4+FOXP3/GFP+) cells were enumerated as outlined above.
Statistical analysis
Descriptive statistics including the mean and standard error were calculated. Differences in cell numbers were analyzed by Student’s t test (when comparing two groups) or one way ANOVA and Tukey’s multiple comparison tests using Prism software (GraphPad Software, San Diego, CA, USA).
Results
Presence of FOXP3+ cells in periapical lesions
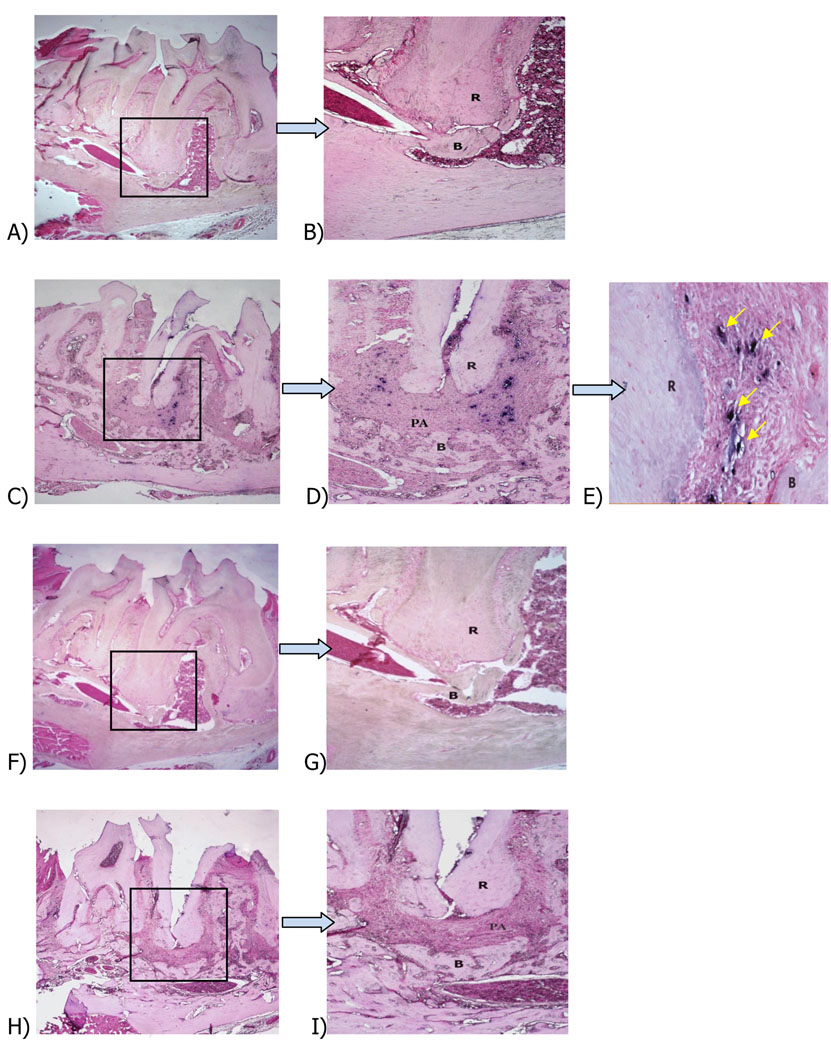
To verify the presence of Treg cells in periapical lesions we chose to target the F0XP3 transcription factor, since this molecule is a unique Treg marker (4, 5). Periapical lesions were induced by pulp exposure of the 1st molar pulp of C57BL/6 mice (n=5/group), and teeth were left open to the oral environment to induce infection by the oral flora. Mice with no pulp exposures were used as controls. Previous studies done in our laboratory have shown that periapical lesions in this model peak in size at approximately 21 days after pulp exposure and infection (12). In situ hybridization for FOXP3+ cells showed strong signals with the anti-sense FOXP3 probe in sections of periapical lesions from mice with exposed pulps (Fig. 1C, D, E). Controls (no pulp exposure) stained with anti-sense probes failed to show cells positive for FOXP3 in the periodontal ligament space (Fig. 1A, B). A few FOXP3+ cells were found in marrow spaces adjacent to teeth with unexposed pulps, likely representing a circulating Treg population (not shown). As a negative control, animals with exposed or with non-exposed pulps stained with the FOXP3 sense probe did not show any signal (Fig. 1 F-I).
Figure 1. In situ hybridization of F0XP3+ cells in periapical lesions.
Representative sections of (A): F0XP3 anti-sense probe, control unexposed pulp, first mandibular molar (×40); (B): higher magnification (×l00 of box in A); (C): FOXP3 anti-sense probe, exposed pulp (×40); (D): higher magnification (×l00) from C; (E): higher magnification (×400) from D; (F): FOXP3 sense probe, control unexposed pulp; (G): higher magnification (×400) from F; (H): FOXP3 sense probe, exposed pulp; (I): higher magnification (×l00) from H; R: root; PA: periapical lesion; B: bone. Eosin counterstain.
It was noted that in tissues from animals with pulp exposures reacted with the anti-sense probe, numerous FOXP3 positive cells were located midway between the periphery of the periapical lesion and the apical foramen, and were also found close to the periphery of the lesion (Fig. 1C, D, E). In addition some lesions showed an interesting pattern of infiltration of FOXP3+ cells, which was distributed around the distal root of the infected first molar but extended toward the mesial root of the second molar (with no exposed pulp).
Influx of Treg in periapical lesions
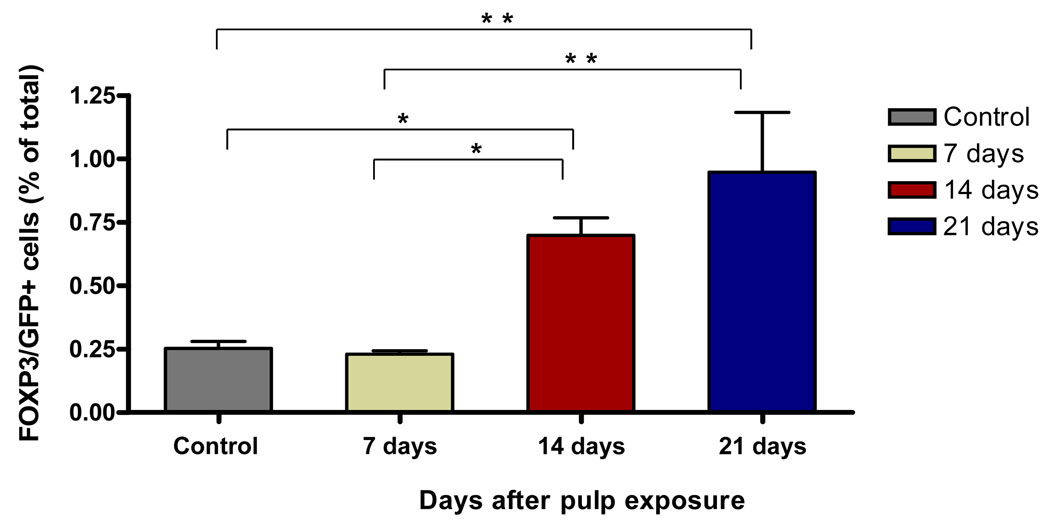
To verify the infiltration of Treg cells into periapical lesions seen by in situ hybridization, we used FOXP3/GFP knock in mice (n=10/group) that were subjected to 1st and 2nd mandibular molar pulp exposure and infection. Unexposed/uninfected animals served as negative controls. Cells were isolated from block sections that included the periapical lesions on day 0 (no pulp exposure) and after 7, 14 and 21 days. As shown in Figure 2, cells isolated from periapical lesions exhibited a time-dependent increase in FOXP3/GFP+ cells in response to pulp exposure and infection. Numbers were increased on day 14 (0.69%, p<0.05 vs. day 0), with a higher numbers on day 21 (0.94%, p<0.001) compared to infected animals on day 7 or uninfected day 0 controls. These results confirm findings by in situ hybridization, and further indicate that Treg infiltration begins between day 7 and day 14 in response to pulpal infection in this model.
Figure 2. Quantitation of Treg in periapical lesions after pulp infection.
Cells were extracted from lesions at the indicated times, and CD4+/FOXP3/GFP+ cells were quantified by flow cytometry. Control: unexposed, uninfected pulp; other groups are exposed and infected. Bars represent mean ± SEM.* p < 0.05; ** p < 0.001 by one way-ANOVA and Tukey’s multiple comparison test.
Effect of pulpal infection on Treg in draining lymph nodes
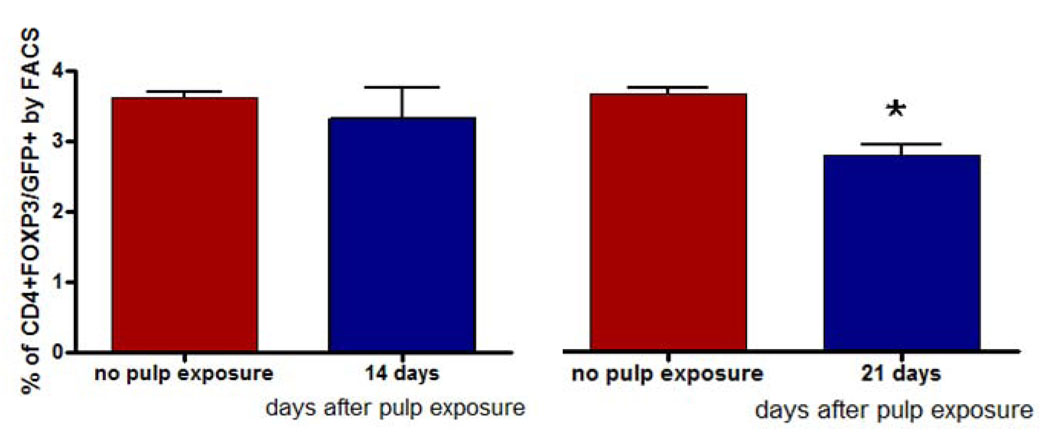
To further evaluate the involvement of Treg cells following dental pulp infection, we enumerated these cells in the draining cervical lymph nodes (CLN). For these experiments animals were sacrificed on days 7 (not shown), 14 and 21 post pulp exposure and infection from the oral cavity. Surprisingly, we found a higher percentage of CD4+FOXP3+/GFP+ cells on both day 14 (p<0.05) and day 21 (p<0.05) in non-infected animals when compared to infected animals (Fig. 3).
Figure 3. Treg numbers in cervical lymph nodes (CLN).
CD4+FOXP3/GFP+ cells were quantified by flow cytometry in CLN 14 and 21 days after pulp exposure and compared to unexposed pulps. Bars represent mean ± SEM. *p < 0.05 by t test.
However, the CLN in animals with pulp exposure were larger and contained more cells than in uninfected animals. When the total number of Treg cells in CLN were calculated, we found that after 21 days Treg (CD4+FOXP3/GFP+) cell numbers were actually significantly higher in the infected animals versus the uninfected controls (Table 1). A similar trend was seen on day 14, although the observed increase was not significant when compared to controls (no pulp exposure). The increase in Treg in CLN on day 21 was significant compared to day 14 (p<0.001). These data indicate that Treg are induced in CLN, which may serve as a source from which they subsequently migrate to the periapical lesion near the site of infection.
Table 1.
Number of Treg in draining cervical lymph nodes.
| Total number of CD4+FOXP3+ cells/CLN (×106) | ||
|---|---|---|
| Days after pulp exposure | ||
| Group | Day 14 | Day 21 |
| No pulp exposure | 0.37 ± 0.08† | 0.58 ± 0.03 |
| Pulp exposure | 0.43 ± 0.04 | 0.92 ± 0.08*‡ |
Mean ± SEM
p<0.05 versus day 21 no pulp exposure (control)
p<0.001 versus day 14 after pulp exposure
Discussion
Treg have been shown to be important in controlling or preventing the development of autoimmunity and other deleterious inflammatory reactions. Accordingly we investigated the presence and time dependence of these cells in the development of periapical lesions using a well characterized mouse model. We took advantage of the recent finding that FOXP3 is a putative ‘master gene’ for Treg (16), and therefore serves as a unique and precise marker of this cell type. We demonstrated that Treg were indeed present in periapical lesions as determined by in situ hybridization, which detected the presence of cells expressing mRNA for FOXP3 in the lesion area (17–20). In contrast, only rare FOXP3+ cells were found around roots of teeth with unexposed pulps, mainly in the adjacent marrow spaces.
To further characterize the presence of Treg in periapical lesions we used flow cytometric analysis of FOXP3+ cells in newly generated FOXP3/GFP knock-in transgenic mice (10). These animals have been genetically engineered to co-express FOXP3 and GFP under the control of the FOXP3 promoter, and are phenotypically and immunologically normal. Using this construct, the presence of FOXP3+ cells in pulpal infection could be precisely quantified. When we analyzed the expression of FOXP3/GFP in periapical lesions at 7, 14 and 21 days after pulpal infection, it was clear that FOXP3+ cells infiltrated into lesions in a time dependent manner beginning between days 7 and 14. At 21 days post-infection the number of cells expressing FOXP3/GFP was three-fold higher than in control animals with no pulp exposure. A few Treg cells were found in the control group, most likely because the bone block specimens taken for analysis included some bone marrow around the lesion. Previous studies have shown that bone marrow contains a recirculating pool of lymphocytes (21) including some Treg cells (22).
In this model of periapical lesion development it takes approximately 1–3 days for innate immune cells (neutrophils and macrophages) to migrate to the periapical region (23–25) and another two or three days for adaptive immune cells to become involved. As noted above, periapical lesion size reaches its peak on about day 21 in this model (12, 26). Although not directly studied in these experiments, our results using FOXP3/GFP expression suggest that Treg infiltration is somewhat delayed (days 7 to 14) compared to the infiltration of innate and other adaptive immune cells, likely as a mechanism to control the development of an overly exuberant inflammatory reaction. In general, Treg numbers parallel periapical bone loss, indicating that their presence could help to control lesion expansion, although this hypothesis remains to be proven. In a chronic infection model caused by Leishmania, natural Treg accumulated at sites of infection to control T effector cells (27). Nakajema et al also showed that FOXP3 expression in periodontitis lesions isolated from human subjects was higher compared with gingivitis lesions (9).
Cervical lymph nodes from animals with infected dental pulps showed elevated numbers of CD4+FOXP3/GFP+ Treg cells compared to non-exposed controls, albeit their proportions were somewhat reduced (Fig. 3 & Table 1). Cervical lymph nodes are the regional sites that drain dentoalveolar infections. There is evidence that adaptive Treg cells can either develop from naive T cells or differentiate from natural Treg under specific conditions of antigen exposure (28). Altered TCR signal transduction or low-affinity antigen may trigger the development of adaptive Treg in the periphery. The recruitment and conversion of peripheral naive CD4+ cells and/or natural Treg cell to adaptive Treg is believed to occur in the lymph nodes draining a tissue undergoing an infection (28). Thus, the Treg in CLN may be those that subsequently migrate to the periapical lesions. The reduced proportions of Treg in the CLN likely reflects that fact that other lymphocyte populations are also expanded in response to the infection, including B cells and a variety of other effector T cells (29). Our results agree with a previous report that Treg numbers increase in a similar time dependent manner both at the site of infection and in draining lymph nodes of mice with genital HSV (30).
In conclusion, our results provide novel information concerning the influx and likely involvement of Treg cells in periapical lesions induced by dental pulp infection. These findings set the stage for further studies of the function of Treg in modulating periapical immune responses and pathogenic bone destruction in vivo.
Acknowledgments
We thank Dr. Xiazohe Han for flow cytometry analysis, Ms. Justine Dobeck for histology and Mr. Subbiah Yoganathan for animal care. This work was supported by grants from the AAE Foundation (E.A), the Krakow Endodontic Research Fund (E.A), ROl DE-09018 (PS.) and K22 DE-16309 (P.P) from the NIDCR/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
"NO FINANCIAL AFFILIATION EXISTS" FOR ALL AUTHORS”
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 2.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGuirk P, Mills KH. Pathogen-specific regulatory T cells provoke a shift in the Thl/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 2002;23:450–455. doi: 10.1016/s1471-4906(02)02288-3. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 5.Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165–168. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 6.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima T, Ueki-Maruyama K, Oda T, Ohsawa Y, Ito H, Seymour GJ, et al. Regulatory T-cells infiltrate periodontal disease tissues. J Dent Res. 2005;84:639–643. doi: 10.1177/154405910508400711. [DOI] [PubMed] [Google Scholar]
- 10.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 12.Yu SM, Stashenko P. Identification of inflammatory cells in developing rat periapical lesions. J Endod. 1987;13:535–540. doi: 10.1016/S0099-2399(87)80033-X. [DOI] [PubMed] [Google Scholar]
- 13.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 14.Pham L, Purcell P, Morse L, Stashenko P, Battaglino RA. Expression analysis of nha-oc/NHA2: a novel gene selectively expressed in osteoclasts. Gene Expr Patterns. 2007;7:846–851. doi: 10.1016/j.modgep.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vernal R, Dezerega A, Dutzan N, Chaparro A, Leon R, Chandia S, et al. RANKL in human periapical granuloma: possible involvement in periapical bone destruction. Oral Dis. 2006;12:283–289. doi: 10.1111/j.1601-0825.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 16.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 17.Pringle JH, Primrose L, Kind CN, Talbot IC, Lauder I. In situ hybridization demonstration of poly-adenylated RNA sequences in formalin-fixed paraffin sections using a biotinylated oligonucleotide poly d(T) probe. J Pathol. 1989;158:279–286. doi: 10.1002/path.1711580403. [DOI] [PubMed] [Google Scholar]
- 18.Mei LX, Jiang Y, Zhao CH, Liu Z, Zhang P. [Relationships between periapical lesion and IL-1, TNF-alpha gene expression in rat] Zhonghua Kou Qiang Yi Xue Za Zhi. 2003;38:345–347. [PubMed] [Google Scholar]
- 19.Lin SK, Kok SH, Kuo MY, Wang TJ, Wang JT, Yeh FT, et al. Sequential expressions of MMP-1, TIMP-1, IL-6, and COX-2 genes in induced periapical lesions in rats. Eur J Oral Sci. 2002;110:246–253. doi: 10.1034/j.1600-0447.2002.11227.x. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K, Lappin DF, MacDonald GD, Kinane DF. Relative distribution of plasma cells expressing immunoglobulin G subclass mRNA in human dental periapical lesions using in situ hybridization. J Endod. 1998;24:164–167. doi: 10.1016/S0099-2399(98)80175-1. [DOI] [PubMed] [Google Scholar]
- 21.Osmond DG. Production and selection of B lymphocytes in bone marrow: lymphostromal interactions and apoptosis in normal, mutant and transgenic mice. Adv Exp Med Biol. 1994;355:15–20. doi: 10.1007/978-1-4615-2492-2_3. [DOI] [PubMed] [Google Scholar]
- 22.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 23.Okiji T, Kawashima N, Kosaka T, Kobayashi C, Suda H. Distribution of la antigen-expressing nonlymphoid cells in various stages of induced periapical lesions in rat molars. J Endod. 1994;20:27–31. doi: 10.1016/s0099-2399(06)80023-3. [DOI] [PubMed] [Google Scholar]
- 24.Akamine A, Hashiguchi I, Toriya Y, Maeda K. Immunohistochemical examination on the localization of macrophages and plasma cells in induced rat periapical lesions. Endod Dent Traumatol. 1994;10:121–128. doi: 10.1111/j.1600-9657.1994.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 25.Kawashima N, Okiji T, Kosaka T, Suda H. Kinetics of macrophages and lymphoid cells during the development of experimentally induced periapical lesions in rat molars: a quantitative immunohistochemical study. J Endod. 1996;22:311–316. doi: 10.1016/S0099-2399(96)80266-4. [DOI] [PubMed] [Google Scholar]
- 26.Huang GT, Do M, Wingard M, Park JS, Chugal N. Effect of interleukin-6 deficiency on the formation of periapical lesions after pulp exposure in mice. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:83–88. doi: 10.1067/moe.2001.115025. [DOI] [PubMed] [Google Scholar]
- 27.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 28.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 29.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 30.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]