Abstract
Objective
To investigate the possible neuroprotective effects of selective cerebral perfusion (SCP) during deep hypothermic circulatory arrest on brain oxygenation and metabolism in newborn piglets.
Methods
Newborn piglets 2–4 days of age, anesthetized and mechanically ventilated, were used for the study. The animals were placed on cardiopulmonary bypass, cooled to 18 °C and put on SCP (20 ml/(kg min)) for 90 min. After rewarming, the animals were monitored through 2 h of recovery. Oxygen pressure in the microvasculature of the cortex was measured by oxygen-dependent quenching of phosphorescence. The extracellular level of dopamine in striatum was measured by microdialysis and hydroxyl radicals by ortho-tyrosine levels. Levels of phosphorylated cAMP response element binding protein (pCREB) in striatal tissue were measured by Western blots using antibodies specific for phosphorylated CREB. The results are presented as mean ± SD (p < 0.05 was significant).
Results
Pre-bypass cortical oxygen pressure was 48.9 ± 11.3 mmHg and during the first 5 min of SCP, the peak of the histogram, corrected to 18 °C, decreased to 11.2 ± 3.8 mmHg (p < 0.001) and stayed near that value to the end of bypass. The mean value for the peak of the histograms measured at the end of SCP was 8 ± 3 mmHg (p < 0.001). SCP completely prevented the deep hypothermic circulatory arrest-dependent increase in extracellular dopamine and hydroxyl radicals. After SCP, there was a statistically significant increase in pCREB immunoreactivity (534 ± 60%) compared to the sham-operated group (100 ± 63%, p < 0.005). Measurements of total CREB showed that SCP did induce a statistically significant increase in CREB as compared to sham-operated animals (168 ± 31%, p < 0.05).
Conclusion
SCP, as compared to DHCA, improved cortical oxygenation and prevented increases in the extracellular dopamine and hydroxyl radicals. The increase in pCREB in the striatum following SCP may contribute to improved cellular recovery after this procedure.
Keywords: Selective cerebral perfusion, Brain oxygenation, Dopamine, Hydroxyl radicals, cAMP response element binding protein
1. Introduction
Surgical outcomes for congenital heart surgery have significantly improved over the past decade. Factors associated with improvement include revised surgical techniques, patient selection and timing of surgery, cardiopulmonary bypass circuitry, modified ultrafiltration and systematized ICU care. The published reports, however, continue to show intermediate and long-term neuropsychologic dysfunction (NPD) associated with congenital heart surgery in children. Several recent studies have demonstrated that the pre-surgical factors such as genetics or reduced cerebral blood flow may be associated with NPD in neonates, infants and children. However, most investigative attention has been directed toward analysis of intraoperative events such as cooling time, deep hypothermic circulatory arrest and low flow cardiopulmonary bypass.
Deep hypothermic circulatory arrest (DHCA) has been extensively studied as a potential source of neuropsychological disability. Both experimental and clinical studies have suggested a relationship between the duration of circulation arrest and NPD. It is generally accepted that 30 min of DHCA is a safe period and if this is exceeded, brain injury can occur.
Selected cerebral perfusion (SCP) has been suggested as a neuroprotective bypass technique during cardiac surgery in infants and children. This technique affords the benefits of DHCA, which is a bloodless cannula-free cardiac field, and brain perfusion, without the deleterious effects of DHCA on the brain. Several clinical and experimental studies have suggested the potential benefit of SCP.
We therefore studied the effect of SCP on brain metabolism in an experimental piglet model of cardiopulmonary bypass. We hypothesized that (1) SCP with DHCA as opposed to DHCA alone would maintain brain tissue oxygenation, reduce tissue production of hydroxyl radicals, and decrease the level of extracellular dopamine; (2) SCP would increase phosphorylation of cAMP response element binding protein (CREB), therefore conferring a protective effect.
2. Materials and methods
2.1. Animal model
Sixteen newborn piglets, 2–4 days of age (1.4–2.5 kg) were assigned to one of the two groups: a SCP group and a sham-operated group. The sham-operated animals did not undergo cardiopulmonary bypass; they were anesthetized and sham-operated. The obtained results were also compared with results from DHCA, previously published by our group [1,2].
After induction with halothane, a tracheotomy was performed, the piglets were placed on a ventilator, and anesthesia was maintained with fentanyl, isoflurane 0.5%, and pancuronium. Femoral venous and arterial cannulas were placed for the collection of blood samples and for monitoring blood pressure. The head of the animal was placed in a stereotaxic holder, the scalp was removed, and a hole approximately 8 mm in diameter, for measuring cortical oxygenation, was made over the right parietal hemisphere. A small hole was drilled over the left parietal hemisphere for implantation of a microdialysis probe into the left striatum. After a 2-h stabilization period, cardiopulmonary bypass was performed. Following bypass, the animals were recovered for 2 h and then euthanized with 4 M KCl. The striatal tissue was then frozen for analysis of the levels of ortho-tyrosine and phosphorylation of CREB.
All animal procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and have been approved by the local Animal Care Committee.
2.2. Cardiopulmonary bypass technique and experimental protocol
The protocols and techniques used during these studies duplicate those practiced in the clinical setting. Anesthesia was maintained using isoflurane (0.5–1.0%) with repeated boluses of pancuronium 0.1 mg/kg (i.v.) and fentanyl infusion of 10 μg/(kg h) (i.v.).
Prior to placing the piglets on bypass, the aortic arch and arch vessels were exposed. Full cardiopulmonary bypass flow was set at 125 ml/(kg min). Once cardiopulmonary bypass was begun the animals were cooled to a nasopharyngeal temperature of 18 °C over 20–25 min. During the cooling phase the pH of the blood was allowed to naturally rise (Alpha-stat management). Once the piglets reach the appropriate study temperature, flow was stopped for less than 1 min. The arterial cannula was advanced into the right carotid artery and snared. The ascending aorta was cross-clamped and the bypass flow rate was lowered to 20 ml/(kg min) for a total SCP time of 90 min. Then, the cross-clamp was removed, snare released, the cannula moved back into the ascending aorta, and bypass flow was slowly increased to 125 ml/(kg min) and the piglets were rewarmed to a temperature of 36 °C over a 30-min period.
2.3. Measurements of oxygen pressure and oxygen distribution by the oxygen-dependent quenching of phosphorescence
Cortical oxygen pressure was measured using oxygen-dependent quenching of phosphorescence [3–6]. The technical basis for determining the distribution of oxygen in the microcirculation of tissue from the distribution of phosphorescence lifetimes in the serum of blood has been described in detail [7]. Briefly, a near infrared oxygen sensitive phosphor, (Oxyphor G2) was injected i.v. at approximately 1.5 mg/kg. The measurements were made using a multi-frequency phosphorescence lifetime instrument (PMOD 5000) using algorithms and software developed by Vinogradov et al. [4]. The excitation light (635 nm), modulated by the sum of 38 sinusoidal waves with frequencies spaced between 100 Hz and 40 kHz, was carried to the tissue through a 3-mm light guide. The phosphorescence (λmax = 790 nm) emitted from the tissue was collected through a second light guide, placed against the tissue at approximately 8 mm (center to center) from the excitation light guide. This positioning of the light guides allowed effective sampling of brain tissue oxygenation down to about 6 mm under the neocortical surface. The phosphorescence was optically filtered and the signal from the detector amplified, digitized and analyzed to give oxygen distribution in the volume of tissue sampled by the light.
2.4. Measurement of extracellular dopamine by in vivo microdialysis
The extracellular level of dopamine in striatum was measured as described in earlier publications [1,8–10]. The microdialysis samples were collected at 15-min intervals. The detection limit of the assay was 5–10 fmol/sample. Identification and quantitation of dopamine were done by comparison with chromatograms of standard solutions. The efficiency of the microdialysis probe was determined in vitro at 36 °C and 18 °C and the relative recoveries were 16 ± 2% and 5 ± 1.6%, respectively (means ± SD for five experiments). The values for dopamine in the dialysate are presented after correction for relative recovery by the microdialysis probe.
2.5. Determination of striatal ortho-tyrosine
Striatal tissue (approximately 1 mg protein/ml) was hydrolyzed with 6N HCl. The hydrolysates were then dried, resuspended in mobile phase and analyzed for ortho-tyrosine by HPLC.
2.6. Western blot measurement of total and phosphorylated CREB
Samples of frozen striatal tissue were homogenized in a buffer containing 2% SDS, 10 mM Tris–HCl (pH 7.4) freshly supplemented with NaF (10 mM), sodium pyrophosphate (10 mM), Na3VO4 (1 mM), Na2MoO4 (1 mM), phenylarsine oxide (1 μM), leupeptin (1 μg/ml) and aprotinin (1 μg/ml), and boiled for 5 min after addition of SDS–PAGE sample buffer. Protein concentration was determined in homogenate samples with a BCA Protein Assay kit (Pierce, IL, USA). An equal amount of protein from each sample was separated by 12% SDS–PAGE and transferred onto a nitrocellulose membrane (Hybond C, Amersham Pharmacia Biotech). Membranes were then incubated in a blocking solution (Blotto: PBS containing 5% non-fat milk powder) for 1 h at room temperature. Blots were then incubated with anti-phosphorylated CREB antibodies (1:200; a-p-CREB; Upstate Biotechnology, NY, USA) at 4 °C overnight. The membranes were then washed three times in PBS containing 0.05% Tween-20 (PBS-T) and incubated for 1 h with anti-rabbit IgG conjugated to HRP (1:1000; Amersham Pharmacia Biotech). Immunoreactivity was detected using enhanced chemiluminescence (ECL) according to manufacturer's instructions (Western Lightning kit, Perkin–Elmer Life Sciences, Boston, MA, USA), and the membranes were exposed to X-ray film. After that, the membranes were washed twice in PBS-T and incubated in a stripping solution (62.5 mM Tris–HCl (pH 6.8), 2% SDS, 100 mM 2-mercaptoethanol) at 50 °C for 30 min as recommended by ECL manufacturer. The stripped membranes were washed twice in PBS-T and incubated in Blotto for 1 h at room temperature. Blots were then incubated with anti-CREB antibodies (1:400; a-CREB; Upstate Biotechnology, NY, USA) at 4 °C overnight. After washing in PBS-T, the membranes were incubated for 2 h with anti-rabbit IgG conjugated to HRP (1:1000). Immunoreactivity was measured using ECL as described above. Autoradiographic films were digitized and analyzed using Scion Image software (NIH, Frederick, MD, USA). Each blot contained two sets of samples, one for an experimental group and another for the control group. The values for experimental groups were normalized to the values obtained for the untreated control group.
2.7. Statistical analysis
All values are expressed as means for “n” experiments ± SD. Statistical significance was determined using one-way analysis of variance with repeated measures by the Wilcoxon signed-rank test. p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of SCP on physiological parameters in newborn piglets
During SCP the pH of the arterial blood increased from 7.43 ± 0.02 to 7.87 ± 0.07 (p < 0.05), but during rewarming and post-bypass recovery it was not significantly different from control (Table 1). The blood pressure decreased from 73.4 ± 1.9 mmHg (control) to 17.4 ± 2.1 mmHg (p < 0.001) at the end of SCP and returned to control level during rewarming. PaCO2 decreased significantly during cooling and SCP from 43.4 ± 1.8 mmHg to 13.5 ± 1.5 mmHg (p < 0.001). PaO2 significantly increased during cooling from 115 ± 18.9 mmHg to 194.4 ± 19.2 mmHg (p < 0.05).
Table 1.
Physiological parameters of newborn piglets during SCP and recovery
| Experimental conditions | Heart rate (beats/min) | pHa | PaCO2 (mmHg) | PaO2 (mmHg) | MABP (mmHg) |
|---|---|---|---|---|---|
| Pre-bypass | 177 ± 7 | 7.43 ± 0.02 | 43.4 ± 1.8 | 115.1 ± 18.9 | 73.4 ± 1.9 |
| Bypass | |||||
| Cooling | 0 | 7.69 ± 0.01 | 20.6 ± 1.6 a | 194.4 ± 19.2 b | 56.0 ± 7.3 b |
| SCP | 0 | 7.87 ± 0.07 b | 13.5 ± 1.5 a | 160.5 ± 33.5 | 17.4 ± 2.1 a |
| Warming | 165 ± 5 | 7.50 ± 0.03 | 31.3 ± 2.4 | 176.5 ± 36.2 | 70.4 ± 3.3 |
| Recovery | |||||
| 1 h | 203 ± 15 | 7.36 ± 0.05 | 42.3 ± 4.3 | 194.6 ± 58.6 | 78.0 ± 4.3 |
| 2 h | 217 ± 16 | 7.38 ± 0.04 | 44.4 ± 6.4 | 103.6 ± 18.5 | 74.0 ± 3.7 |
Abbreviations: pHa: arterial pH; PaCO2: arterial CO2 pressure; PaO2: arterial O2 pressure; MABP: mean arterial blood pressure. The values are the means ± SD for eight experiments.
p < 0.001 for significant difference from pre-bypass conditions.
p < 0.05 for significant difference from pre-bypass conditions.
3.2. Oxygen distribution in brain tissue during SCP and post-bypass reperfusion
Oxygen histograms were measured every 15 min throughout each of the experimental procedures. Prior to bypass, the cortical oxygen pressure was 48.9 ± 11.3 mmHg. During first 5 min of SCP, the peak of the histogram, corrected to 18 °C, decreased to 11.2 ± 3.8 mmHg and stayed near that value to the end of 90 min of SCP. The mean value for the peak of the histograms measured at the end of SCP was 8 ± 3 mmHg (Table 2).
Table 2.
Cortical oxygen pressure during SCP in newborn piglets
| Experimental conditions | Time (min) | Cortical oxygen pressure (mmHg) |
|---|---|---|
| Pre-bypass | 0 | 48.9 ± 11.3 |
| SCP | ||
| 5 | 11.2 ± 3.9 a | |
| 15 | 9.0 ± 7.1 a | |
| 60 | 8.0 ± 5.6 a | |
| 90 | 8.0 ± 3.0 a | |
| Post-bypass recovery | 120 | 37.1 ± 9.9 |
The values are the means ± SD for eight experiments.
p < 0.01 for significant difference from pre-bypass conditions.
In order to provide a measure of the fraction of brain tissue that was seriously deprived from oxygen during the SCP, we have calculated, from the histograms, the fraction of the microcirculation in the cortex with oxygen pressures less than 5 mmHg (Fig. 1). By the end of SCP, 14 ± 6% of microcirculation was below this value.
Fig. 1.
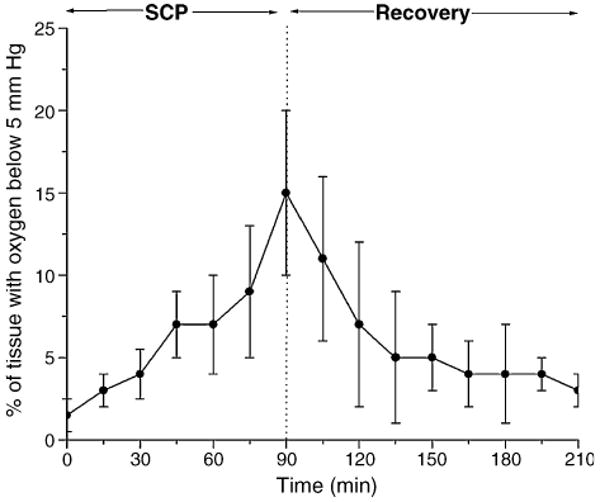
Effect of SCP on fraction of the measured histogram that has oxygen pressures less than 5 mmHg. Oxygen histograms were determined by deconvolution of the distribution of phosphorescence lifetimes for Oxyphor G2 in the microcirculation. The results are means from eight experiments ± SD.
3.3. Changes in extracellular striatal dopamine during SCP
The control value of dopamine was stable prior to bypass with absolute levels of dopamine below 1 pmol/ml. The effect of SCP on the extracellular levels of dopamine is shown in Fig. 2. During SCP, there were no detectable changes in extracellular dopamine level as compared to sham-operated control animals. We had previously shown that during DHCA, the extracellular levels of dopamine increased to 53,000 times the control value.
Fig. 2.
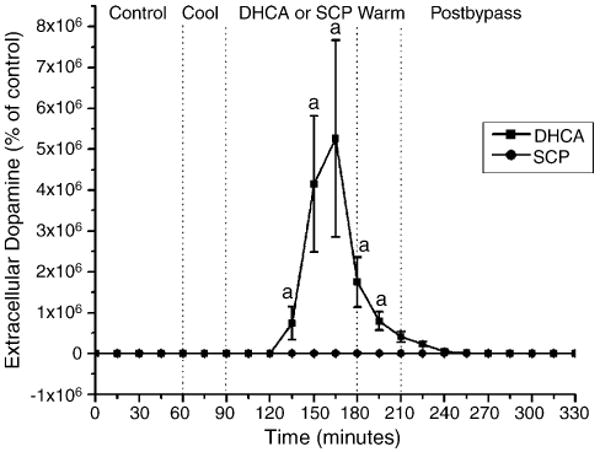
The effect of SCP on the extracellular level of dopamine in striatum of newborn piglets. The three measurements of dopamine during the pre-bypass period were averaged and the value considered as the baseline (100%). The results are means from eight experiments ± SD. ap < 0.001 for significant difference from control (sham-operated) values. The data for DHCA group were taken from Schultz et al. [1].
3.4. Level of striatal ortho-tyrosine following SCP
The level of ortho-tyrosine in the tissue can be used as a reliable measurement of in vivo hydroxyl radical production. This compound is not formed by metabolism, and results only from OH radical attack on the ortho position of free and bound phenylalanine. The level of ortho-tyrosine within the striatum in sham-operated animals was 0.43 ± 0.07 nmol/g striatal tissue. In the SCP group, the level of ortho-tyrosine, measured after 2-h recovery, was 0.46 ± 0.11 nmol/g tissue, a value not significantly different than that for sham-operated animals.
3.4.1. Levels of phosphorylated CREB in striatal tissue measured after 2-h recovery following SCP
Western blots of proteins isolated from striatal tissues of piglets from control group and the group subjected to SCP, probed with anti-pCREB antibodies, are presented in Fig. 3. There was an increase in pCREB immunoreactivity (534 ± 60%) in the striata from SCP animals compared to the sham-operated group (100 ± 63%, p < 0.005) (Fig. 4), and this was statistically significant.
Fig. 3.
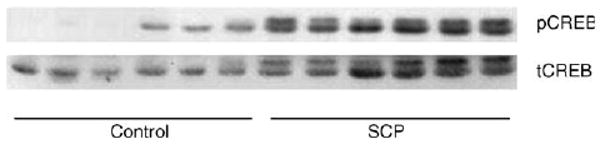
The immunoblots of striatal samples probed with antibodies against phosphorylated and total CREB. The figure presents the Western blots for six control and six SCP piglets, probed with either anti-pCREB antibodies (pCREB) or with anti-CREB antibody (tCREB).
Fig. 4.
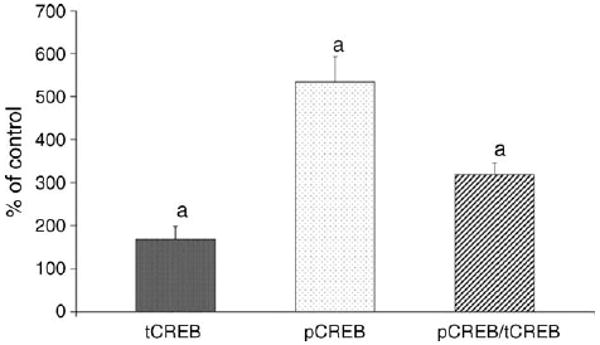
Effect of SCP on levels of total and phosphorylated CREB in striatum of newborn piglets. The results are means from six experiments ± SD. The data are expressed in percentage to the corresponding values (100%) obtained for the control group of piglets (n = 6) and represent measurements from three to five independent experiments. ap < 0.05 for significant difference from control.
To determine whether the total amount of CREB in the striatal tissue was altered, CREB was measured with an antibody that equally recognized both phosphorylated and non-phosphorylated CREB (total CREB (tCREB)). The measurements of tCREB showed that SCP did induce a statistically significant increase in CREB as compared to control (168 ± 31%, p < 0.05) (Fig. 4).
To determine the extent to which the ratio of phosphorylated to dephosphorylated CREB was altered, pCREB levels were normalized to the tCREB. As it can be seen in Fig 4, after normalization the fraction of the tCREB that is phosphorylated was significantly higher following SCP than in the sham-operated group (319 ± 26 vs 100 ± 112; p < 0.01). This means that the elevated levels of pCREB following SCP were due to both increased expression of the CREB protein and increase in the phosphorylated/dephosphorylated CREB ratio. Thus, following SCP, both tCREB protein and the fraction that is phosphorylated were significantly enhanced over those present in sham-operated animals.
4. Discussion
In the present SCP model, the changes in brain oxygenation were correlated with changes in extracellular striatal level of dopamine, striatal tissue level of ortho-tyrosine and with phosphorylation of CREB. The results are directly compared with deep hypothermic circulatory arrest data published in our earlier work [1,2].
Oxygen-dependent quenching of phosphorescence was used to measure oxygen pressure in the microcirculation of the cortex of brain. The measurements are minimally invasive and this is the only available method for repetitively and rapidly measuring oxygen distributions (histograms) in tissue. This ability to measure of the distribution of oxygen within heterogeneous samples, such as tissue, is unique. It is possible because the phosphorescence lifetime is different for each different oxygen pressure, and the phosphorescence emitted from the tissue is the sum of all the different lifetimes (oxygen pressures) in the tissue. The distribution of lifetimes can be mathematically resolved and then the distribution of oxygen pressures (oxygen histogram) calculated. In the present work, we used a multifrequency phosphorometer [4] to collect the data necessary for determining the distributions of lifetimes.
Baseline oxygen histograms have peak values between 45 mmHg and 60 mmHg with essentially no values below 20 mmHg. As the temperature is lowered to 18 °C, the affinity of hemoglobin for oxygen increases about fourfold and cellular metabolic activity decreases three- to fourfold. Thus, although the peak of the histogram at the end of SCP was only 8 ± 3 mmHg, this value indicated that the hemoglobin was largely saturated with oxygen and is an oxygen pressure which is likely to be sufficient to meet the metabolic need. If we assume that an oxygen pressure in the microcirculation of at least 5 mmHg is required to provide oxygen to all of the cells surrounding the vessels at 18 °C, then in SCP only 14 ± 6% of the tissue was oxygen deprived. This is in contrast to DHCA where essentially all of the brain tissue is oxygen deprived. This difference in oxygenation is consistent with SCP suppressing the massive release of dopamine observed in DHCA and providing substantial protection of the brain against cellular injury.
The values of oxygen pressure during SCP presented in this paper are lower than those given in our earlier study [11]. In the present study, the reported values are for the peaks in the oxygen histograms whereas those presented earlier are mean values measured using only two frequency modulations of the excitation light. The mean oxygen pressure was higher than the peak of the histogram due to (1) greater phosphorescence quenching at higher oxygen pressures (decreased signal to noise) resulting in a “tail” extending into high oxygen pressures; (2) blood vessels in the dura, where oxygen from the air can provide oxygen to the blood when there is little or no flow. In the present communication, we have chosen to emphasize the peak of the oxygen histogram because this value is representative of the bulk of the tissue and is not significantly affected by vasculature in the dura or the decrease in signal to noise at higher oxygen pressures.
The observed increase in cortical oxygenation during SCP is consistent with the results of other investigators reporting increase in cerebral oxygen consumption and cerebral oxygen delivery in SCP as compared with DHCA [12–14].
The question arises as to how the changes in brain oxygenation during SCP affected the striatal metabolism. The striatum is the main input site in basal ganglia, which is one of the most important subcortical structures in the motor circuit. Clinical evidence suggests that hypoxic ischemia in the neonatal human infant preferentially damages systems that control tone and movement. Striatal neurons are also very sensitive to hypoxia and ischemia. In a DHCA model, newborn piglets displayed neurologic deficits and revealed histological evidence of brain damage apparent by 6 h of reperfusion [15]. Laptook et al. [16] reported that neurons in striatum and neocortex of the newborn are very vulnerable to normothermic ischemia. In hypothermic global ischemia, striatum continued to be damaged even though the other regions were protected [16,17]. DeLeon et al. [18] showed that, in experiments on dogs, profound hypothermia during CPB caused neuronal loss and degeneration within the cortex and caudate. Similarly, Tseng et al. [19] showed that, in dogs, after circulatory arrest, apoptosis occurred in selected neuronal populations, including the hippocampus, striatum and neocortex. Following cardiac arrest in 1- to 2-week-old piglets, necrosis was the dominant form of cell death, affecting striatum earlier, more uniformly, and to a greater degree than other brain regions [20]. Histological and immunohistochemical analysis showed first signs of cell damage within 6 h after resuscitation, with complete deterioration by 48 h. Damage in the neocortex was barely evident at 24 h and was still increasing as late as 96 h after the insult.
Another reason for choosing the striatum for evaluating the protective effect of SCP was because it is a dopaminergic region of the brain. Our earlier studies have shown that the dopaminergic system within the striatum of a newborn piglet's brain is very sensitive to hypoxia/ischemia [8–10] and therefore provides a very sensitive marker of the adequacy of brain oxygenation. In addition, it has been suggested that release of dopamine during hypoxic/ischemic conditions may play a major role in mediating neuronal damage within the striatum. High levels of dopamine, iron and oxygen are mostly responsible for the generation of free radicals particularly within regions of the brain such as the putamen and caudate nucleus. One of the causes of neuronal cell death after CPB and DHCA appears to be the formation of free radicals. In hypoxia–ischemia in newborn piglets, the increase in hydroxyl radicals observed during recovery was completely abolished by depletion of dopamine in the brain [21].
Our earlier studies showed that DHCA resulted in a massive increase in extracellular dopamine and an about sixfold increase in the level of ortho-tyrosine within the striatum of newborn piglets, the latter indicating increased generation of hydroxyl radicals within the tissue [1]. The results presented in this paper show that SCP at 20 ml/min completely abolished the increase in extracellular dopamine and the increase in hydroxyl radicals.
We reported earlier that although SCP at a flow of 20 ml/min substantially improved cerebral oxygenation, further increase to 40 ml/min lead to even higher oxygen levels [11]. At 40 ml/min, however, a small fraction of the animals developed brain edema, suggesting the pressure in the vessels was high enough to cause significant vascular leakage. The present study shows that a flow of 20 ml/min is sufficient to protect brain metabolism. We conclude that SCP, by improving cortical oxygenation, prevented the increases in the extracellular dopamine and hydroxyl radicals, and by inference, the depletion of cellular energy levels that underlies cellular injury.
Another factor investigated in this study was CREB, a transcription factor involved in the control of neuronal function. Assessment of phosphorylation of CREB is important not only because it is part of one of the regulatory pathways affected by dopamine but also because it is a putative factor in fostering neuronal protection. Several studies involving the over-expression of dominant-negative CREB have suggested that CREB can be a protective factor in various cellular models. A suggested mechanism for the protective effect of phosphorylated CREB could be by increasing a expression of Bcl-2 protein. Several studies have shown that Bcl-2 is positively regulated by CREB via a consensus cyclic AMP response element (CRE), and increased phosphorylation of CREB on Ser-133 induces expression of Bcl-2 protein. Accumulating evidence indicates that over-expression of Bcl-2 provides protection against apoptosis [22] and ischemic neuronal death [23].
The present results show that following 2 h of recovery from SCP the level of CREB phosphorylation is significantly higher than in sham-operated animals. Our early studies showed that LFCPB, which decreased cortical oxygen to the same level as SCP, also caused an increase in CREB phosphorylation [2]. This was in contrast to DHCA data where phosphorylation of CREB was not significantly changed. This is in agreement with studies by other investigators which show that both necrotic and apoptotic neuronal death occurred in vulnerable brain regions after DHCA [15,24,25].
A limitation in our study is that CREB phosphorylation was measured at only one time during the recovery period. Future experiments will characterize the time-dependent changes in CREB phosphorylation during SCP and determine the degree to which the increase in phosphorylated CREB, as measured at 2 h, correlates with cell survival and neuronal injury.
In summary, the increase in CREB phosphorylation following SCP as well as the decreased levels in extracellular dopamine and hydroxyl radicals, are consistent with lesser neuronal injury and better neuronal survival following SCP as compared to DHCA. It can be concluded that in the newborn piglet, SCP provides a significant degree of neuroprotection to the striatum as compared to DHCA and may be of importance for clinical practice.
Acknowledgments
This study was supported by grants HL-58669, HD041484 and NS 31465 from the National Institutes of Health.
References
- 1.Schultz S, Creed J, Schears G, Zaitseva T, Greeley WJ, Wilson DF, Pastuszko A. Comparison of low flow cardiopulmonary bypass and circulatory arrest on brain oxygen and metabolism. Ann Thorac Surg. 2004;77:2138–43. doi: 10.1016/j.athoracsur.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 2.Zaitseva T, Schears G, Schultz S, Creed J, Antoni D, Wilson DF, Pastuszko A. Circulatory arrest and low flow cardiopulmonary bypass alter CREB phosphorylation in piglets brain. Ann Thorac Surg. 2005;80:245–50. doi: 10.1016/j.athoracsur.2005.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson DF, Rumsey W, Green TJ, Vanderkooi JM. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J Biol Chem. 1988;263:2712–8. [PubMed] [Google Scholar]
- 4.Vinogradov SA, Fernandez-Seara MA, Dugan BW, Wilson DF. Frequency domain instrument for measuring phosphorescence lifetime distributions in heterogeneous samples. Rev Sci Instrum. 2001;72:3396–406. [Google Scholar]
- 5.Vinogradov SA, Fernandez-Seara MA, Biruski D, Wilson DF. A method for measuring oxygen distributions in tissue using frequency domain phosphorometry. Comp Biochem Physiol. 2002;32:147–52. doi: 10.1016/s1095-6433(01)00541-4. [DOI] [PubMed] [Google Scholar]
- 6.Schears G, Shen J, Creed J, Zaitseva T, Wilson DF, Pastuszko A. Brain oxygenation during cardiopulmonary bypass and circulatory arrest. Adv Exp Med Biol. 2003;510:325–30. doi: 10.1007/978-1-4615-0205-0_53. [DOI] [PubMed] [Google Scholar]
- 7.Dunphy I, Vinogradov SA, Wilson DF. Oxyphor R2 and G2: phosphors for measuring oxygen by oxygen dependent quenching of phosphorescence. Anal Biochem. 2002;310:191–8. doi: 10.1016/s0003-2697(02)00384-6. [DOI] [PubMed] [Google Scholar]
- 8.Pastuszko A, Lajevardi SN, Chen J, Tammela O, Wilson DF, Delivoria-Papadopoulos M. Effects of graded levels of tissue oxygen pressure on dopamine metabolism in striatum of newborn piglets. J Neurochem. 1993;60:161–6. doi: 10.1111/j.1471-4159.1993.tb05834.x. [DOI] [PubMed] [Google Scholar]
- 9.Pastuszko A. Metabolic responses of the dopaminergic system during hypoxia in newborn brain. Biochem Med Metab Biol. 1994;51:1–15. doi: 10.1006/bmmb.1994.1001. [DOI] [PubMed] [Google Scholar]
- 10.Yonetani M, Huang Ch-Ch, Lajevardi N, Pastuszko A, Delivoria-Papadopoulos M, Wilson DF. Effect of hemorrhagic hypotension on extracellular level of dopamine, cortical oxygen pressure and blood flow in brain of newborn piglets. Neurosci Lett. 1994;180:247–52. doi: 10.1016/0304-3940(94)90531-2. [DOI] [PubMed] [Google Scholar]
- 11.DeCampli WM, Myung R, Schears G, Schultz S, Creed J, Pastuszko A, Wilson DF. Tissue oxygen tension during regional low flow perfusion in neonates. J Thorac Cardiovasc Surg. 2003;125:472–80. doi: 10.1067/mtc.2003.13. [DOI] [PubMed] [Google Scholar]
- 12.Strauch JT, Spielvogel D, Haldenwang PL, Zhang N, Weisz D, Bodian CA, Griepp RB. Impact of hypothermic selective cerebral perfusion compared with hypothermic cardiopulmonary bypass on cerebral hemodynamics and metabolism. Eur J Cardiothorac Surg. 2003;24:807–16. doi: 10.1016/s1010-7940(03)00517-7. [DOI] [PubMed] [Google Scholar]
- 13.Strauch JT, Spielvogel D, Haldenwang PL, Lauten A, Zhang N, Weisz D, Bodian CA, Griepp RB. Cerebral physiology and outcome after hypothermic circulatory arrest followed by selective cerebral perfusion. Ann Thorac Surg. 2003;76:1972–81. doi: 10.1016/j.athoracsur.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Ito T. Effect of deep hypothermia on cerebral hemodynamics during selective cerebral perfusion with systemic circulatory arrest. Jpn J Thorac Cardiovasc Surg. 2002;50:109–15. doi: 10.1007/BF02913471. [DOI] [PubMed] [Google Scholar]
- 15.Kurth CD, Priestly M, Golden J, McCann J, Raghupathi R. Regional patterns of neuronal death after deep hypothermic arrest. J Thorac Cardiovasc Surg. 1999;118:1068–77. doi: 10.1016/S0022-5223(99)70103-0. [DOI] [PubMed] [Google Scholar]
- 16.Laptook AR, Corbett R, Sterett R, Burns DK, Tollefsbol G, Garcia D. Modest hypothermia provides partial neuroprotection for ischemic neonatal brain. Pediatr Res. 1994;35:436–42. [PubMed] [Google Scholar]
- 17.Mujsce DJ, Towfighi J, Heitjan DF, Vanucci RC. Differences in intraischemic temperature influence neurologic outcome after deep hypothermic circulatory arrest in newborn dogs. Stroke. 1994;25:1433–41. doi: 10.1161/01.str.25.7.1433. [DOI] [PubMed] [Google Scholar]
- 18.DeLeon SY, Thomas C, Roughneen PT, King N, Lehne R, DeLeon AM, Walenga J, Pifarre R. Experimental evidence of cerebral injury from profound hypothermia during cardiopulmonary bypass. Pediatr Cardiol. 1998;19:398–403. doi: 10.1007/s002469900335. [DOI] [PubMed] [Google Scholar]
- 19.Tseng EE, Brock MV, Lange MS, Blue ME, Troncoso JC, Kwon CC, Lowenstein CJ, Johnston MV, Baumgartner WA. Neuronal nitric oxide synthase inhibition reduces neuronal apoptosis after hypothermic circulatory arrest. Ann Thorac Surg. 1997;64:1639–47. doi: 10.1016/s0003-4975(97)01110-7. [DOI] [PubMed] [Google Scholar]
- 20.Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997;377:262–85. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Olano M, Song D, Murphy S, Wilson D, Pastuszko A. Relationships of dopamine, cortical oxygen pressure and hydroxyl radicals in brain of newborn piglets during hypoxia and posthypoxic recovery. J Neurochem. 1995;65:1205–12. doi: 10.1046/j.1471-4159.1995.65031205.x. [DOI] [PubMed] [Google Scholar]
- 22.Martinou JC, Frankowski H, Missotten M, Martinou I, Potier L, Dubois-Dauphin M. Bcl-2 and neuronal selection during development of the nervous system. J Physiol. 1994;88:209–11. doi: 10.1016/0928-4257(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 23.Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18:570–9. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Tseng EE, Brock MV, Lange MS, Troncoso JC, Lowenstein CJ, Blue ME, Johnston MV, Baumgartner WA. Nitric oxide mediates neurologic injury after hypothermic circulatory arrest. Ann Thorac Surg. 1999;67:65–71. doi: 10.1016/s0003-4975(98)01363-0. [DOI] [PubMed] [Google Scholar]
- 25.Ditsworth D, Priestley MA, Loepke AW, Ramamoorthy C, McCann J, Staple L, Kurth CD. Apoptotic neuronal death following deep hypothermic circulatory arrest in piglets. Anesthesiology. 2003;98:1119–27. doi: 10.1097/00000542-200305000-00014. [DOI] [PubMed] [Google Scholar]


