Abstract
Background
It has been shown that the activation of estrogen receptor-β (ER-β) plays an important cardioprotective role against ischemia-reperfusion (I/R) injury. However, the mechanism for this protection is not clear. We hypothesize that estrogen protects by ER-β activation, which leads to S-nitrosylation (SNO) of key cardioprotective proteins.
Methods and Results
We treated ovariectomized C57BL/6J mice with an ER-β selective agonist, 2,2-bis(4-hydroxyphenyl)-proprionitrile (DPN), 17β-estradiol (E2) or vehicle using Alzet minipumps for two weeks. Isolated hearts were Langendorff perfused and subjected to ischemia and reperfusion. Compared with vehicle-treated hearts, DPN and E2-treated hearts had significantly better post-ischemic functional recovery and decreased infarct size. To test the specificity of DPN, we treated ER-β knockout (βERKO) mice with DPN. However, no cardioprotective effect of DPN was found in βERKO mice, indicating the DPN-induced cardioprotection occurs through the activation of ER-β. Using DyLight-maleimide fluors and a modified biotin switch method, we employed a two dimensional DyLight fluorescence difference gel electrophoresis (2D DyLight DIGE) proteomic method to quantify differences in SNO of proteins. DPN and E2-treated hearts showed an increase in SNO of a number of proteins. Interestingly, many of these proteins had also been shown to have increased SNO in preconditioned hearts. In addition, the DPN-induced cardioprotection and increased SNO were abolished by treatment with a nitric oxide (NO) synthase inhibitor.
Conclusion
The activation of ER-β by DPN treatment leads to increased protein S-nitrosylation and cardioprotection against I/R injury, suggesting that chronic estrogen exposure protects hearts largely via activation of ER-β and NO/SNO signaling.
Keywords: Estrogen receptor, S-nitrosylation, Cardioprotection
Introduction
Premenopausal women have a reduced incidence of cardiovascular disease including ischemic heart disease and heart failure. However, this incidence increases after menopause suggesting a protective role for endogenous estrogen.1,2 Several animal studies have shown estrogen to be cardioprotective during ischemia-reperfusion (I/R). These include studies looking at acute protective effects of estrogen treatment in perfused hearts as well as literature reporting decreased I/R injury in intact females exposed chronically to estrogen.3-6 However, estrogen’s lack of protection in recent clinical trials, such as the Women’s Health Initiative (WHI) contrast with the protection seen in premenopausal women and the described animal studies.7 To understand this difference, we need to better understand the mechanism of estrogen-mediated protection in these animal models.
Traditionally, the effects of estrogen have been attributed to estrogen binding to the classical nuclear estrogen receptors (ER) which act as ligand-gated transcription factors. Binding of estrogen to these receptors (both ER-α and ER-β) leads to altered protein expression. In addition to these genomic effects, estrogen has rapid effects in the heart leading to activation of acute signaling pathways. We have previously reported that females have increased activity of nitric oxide synthase (NOS) in the heart6 while other studies have found that selective ER-β activation upregulates nitric oxide (NO) production in cardiomyocytes.8 Recent literature reports that estrogen binding to either ERs which are localized in the plasma membrane or an estrogen activated G-protein coupled receptor (termed GPR30) can lead to acute activation of signaling cascades such as phosphatidylinositol-3-OH kinase.9 Thus estrogen can alter the level of proteins involved in cardioprotection as well as enhance protection through the activation of acute signaling pathways.
We have previously found that intact female mice exhibit reduced I/R injury compared to males and ovariectomized females.10,11 This protection is lost in hearts from female mice that lack the ER-β receptor, suggesting a role for ER-β in cardioprotection.12 Additionally we have found that this cardioprotection observed in females can be blocked by inhibition of NOS.10,13 Taken together, these data support a role for estrogen acting via ER-β through an NO dependent mechanism to mediate cardioprotection.
NO is an important signaling molecule in cardioprotection.14 In addition to its effects via guanylyl cyclase, a number of recent studies have shown that NO can lead to reversible post-translational protein modifications, such as S-nitrosylation.10,15-17 S-nitrosylation is the covalent attachment of an NO moiety to protein sulfhydryl residues resulting in the formation of an S-nitrosothiol (SNO) group. It is a common post-translational protein modification, analogous to phosphorylation18,19 and has been shown to alter protein activity leading to a cardioprotective state.10,15,16 In this study we aim to test the hypothesis that ER-β, acting through NOS, leads to increased S-nitrosylation of proteins involved in cardioprotection in females. We used DyLight-maleimide fluors in a modified biotin switch method and employed 2D fluorescence difference gel electrophoresis (DIGE) proteomic methods to identify proteins that were S-nitrosylated by estrogen and the ER-β selective agonist 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN). We reasoned that if estrogen protection is mediated by ER-β activation of NOS, then estrogen and DPN should lead to a similar pattern of increased protein S-nitrosylation.
Materials and Methods
Animals
C57BL/6J wild type (WT) and ER-β knockout (βERKO) female mice were obtained from Taconic Laboratories. Animals were bilaterally ovariectomized at 8 weeks of age and delivered to our laboratory at 9 weeks of age. Micro-osmotic ALZET pumps (model 1002) were implanted subcutaneously into the mice. Each pump delivered a constant dose of either vehicle (50% DMSO in 85% Saline), E2 (0.1 mg/kg/day), or DPN (0.8 mg/kg/day) for 2 weeks. These dosages were chosen based on previous work from Nikolic et al.11 All animals were treated in accordance with NIH guidelines.
I/R protocol, post-ischemic functional recovery and infarct size determination
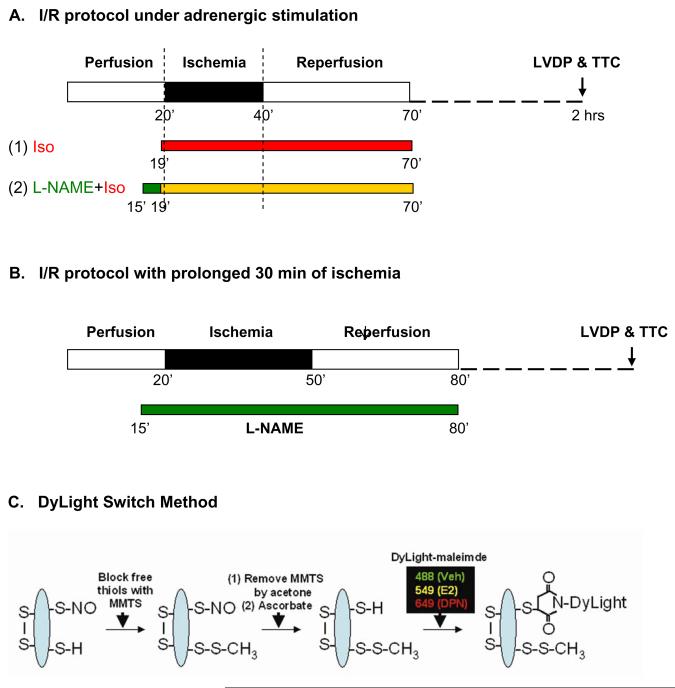
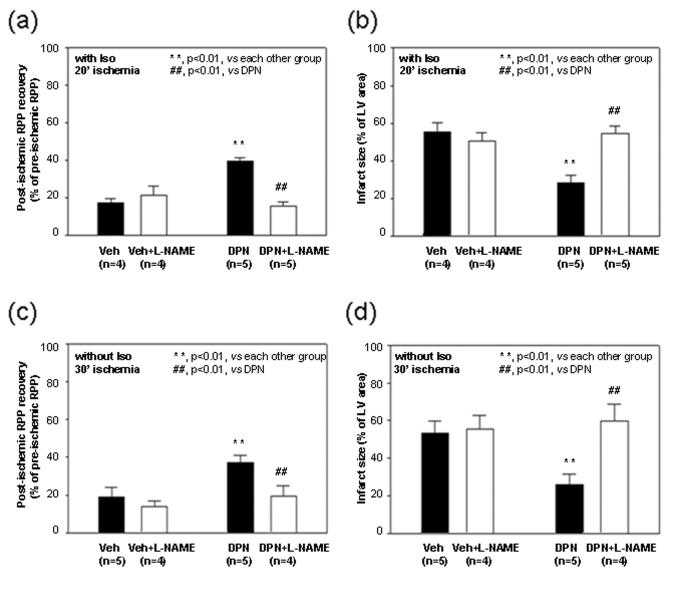
The Langendoff heart perfusion, hemodynamic and infarct size measurements were performed as previously described by Sun et al.16 Treatment protocols are shown in Figure 1. Two groups (with and without 10 μmol/L of N-nitro-L-arginine methyl ester (L-NAME), a NOS inhibitor) underwent a standard I/R protocol with adrenergic stimulation (Figure 1a). Hearts were equilibrated for 20 minutes including treatment with 10 nmol/L isoproterenol 1 minute prior to 20 minutes of no-flow ischemia followed by 30 minutes of reperfusion with drugs and then an additional 90 minutes of reperfusion without drugs. When indicated, L-NAME was added 5 minutes prior to ischemia and perfusion with L-NAME was continued for the first 30 minutes of reperfusion. Another two groups underwent a 30 minutes of no-flow ischemia I/R protocol without adrenergic stimulation (Figure 1b). Hearts were equilibrated for 20 minutes and then subjected to 30 minutes of no-flow global ischemia followed by 2 hours reperfusion. When indicated, hearts were treated for 5 minutes with 10 μmol/L L-NAME prior to 30 minutes of ischemia followed by 30 minutes of reperfusion with L-NAME and then 90 minutes of reperfusion without L-NAME.
Figure 1. Ischemia reperfusion protocol and modified biotin switch method using DyLight-maleimide fluors.
All hearts were subjected to a standard 20 minutes of no-flow global ischemia with adrenergic stimulation (a), or a prolonged 30 minutes of no-flow global ischemia without adrenergic stimulation (b) I/R protocol on the Langendorff apparatus. Heart samples were snap frozen in liquid nitrogen immediately after 1 minute of isoproterenol treatment for our 2D proteomic studies. Additional hearts were collected after 120 minutes of reperfusion. Functional recovery was measured and infarct size was determined using TTC stain. (c) Using a modified biotin switch method, S-nitrosylated proteins were labeled with one of three DyLight-maleimide dyes, each with its own unique excitation/emission wavelength.
Identification of S-nitrosylated proteins by 2D DyLight DIGE method
Total homogenates of each sample were prepared as previously described by Sun et al.16 In some studies, samples were pretreated with 1 mmol/L ascorbic acid prior to the initial thiol blockade as negative control. The modified biotin switch method20 using DyLight-maleimide sulfhydryl-reactive fluors (Pierce Biotechnology, Rockford, IL, Figure 1c) and the 2D DyLight DIGE proteomic method for identification of S-nitrosylated proteins were previously described by Sun et al.16
Protein expression differences using 2D CyDye DIGE method
A 2D DIGE proteomic method with CyDye fluors (GE Healthcare/Amersham Biosciences, Piscataway, NJ) was used to determine protein expression differences between DPN treated group and vehicle control group. An internal standard containing equal amounts of protein from the DPN and vehicle groups was labeled with Cy2 as an internal standard. The labeling reaction was performed on ice for 30 minutes in the dark and then quenched with 10 mmol/L lysine. Equal quantities of labeled protein from each group (Cy2, Cy3, and Cy5) were combined together and separated using a 2D system involving IEF (pH3-10) on an Ettan IPGphor3 (GE Healthcare/Amersham Biosciences) and 5-20% SDS-PAGE (NextGen, Ann Arbor, MI) on an Ettan DaltTwelve System (GE Healthcare/Amersham Biosciences). Each gel was scanned on a Typhoon 9400 variable mode imager (GE Healthcare/Amersham Biosciences) at a resolution of 100 μm. The gel was post-stained overnight with SYPRO Ruby stain and scanned. Images from each gel were aligned and analyzed using Progenesis Discovery software (Nonlinear Dynamics, Newcastle upon Tyne, UK). Protein spots were chosen for identification with mass spectrometry if they exhibited greater than a 1.5 fold difference in fluorescence volume intensity between DPN and vehicle with a p-value<0.05. Automated protein extraction and protein identification are the same as in the 2D DyLight DIGE method described earlier by Sun et al.16
Results
DPN and E2 treatment is cardioprotective after I/R in ovariectomized hearts
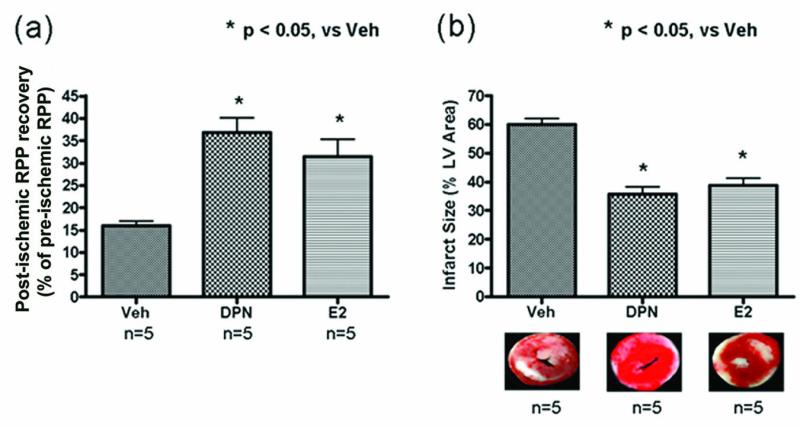
Initial measurements of hemodynamic parameters prior to ischemia demonstrated no baseline differences in HR or LVDP among our treatment groups (see Supplemental Material). Using a Langendorff perfused heart model of I/R, we find that compared to the vehicle control, DPN or E2 treatment of ovariectomized female mice reduces I/R injury (Figure 2). The functional recovery, expressed as % initial rate pressure product (RPP), was significantly higher in DPN and E2 groups compared to vehicle (36.9±3.2 and 31.4±4.0 respectively vs 16.0±1.1, p<0.05). Consistent with higher post-ischemic recovery, infarct size, expressed as % of left ventricular area was decreased in DPN and E2 groups versus vehicle (35.8±2.4 and 38.8±2.5, respectively vs 60.1±2.1, p<0.05). These results show that DPN and E2 treatment are both cardioprotective in a female ovariectomized mouse model with no significant differences in the amount of cardioprotection provided by either treatment.
Figure 2. Post-ischemic functional recovery and infarct size in I/R hearts.
(a) Post-ischemic functional recovery, measured after 120 minutes of reperfusion was significantly higher in DPN and E2 groups compared to vehicle control. (b) Infarct size, also measured after 120 minutes of reperfusion was significantly decreased in DPN and E2 groups compared to vehicle control.
DPN and E2 treatment increases protein S-nitrosylation
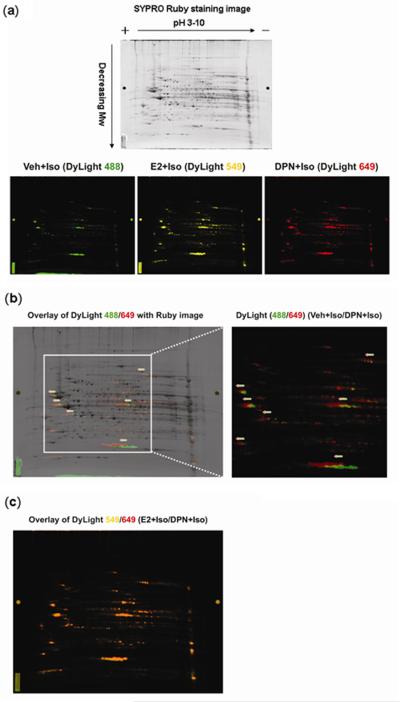
We and others have reported that ER activation leads to increased NOS production and NO availability.6,8 While NO can signal through different mechanisms in the cell potentially leading to cardioprotection, one of its actions is protein S-nitrosylation (SNO), a redox-based reversible post-translational modification.21-23 We therefore examined whether treatment with DPN or E2 led to changes in protein SNO. We employed a recently developed 2D DyLight DIGE method16 to determine SNO differences between various treatments. As seen in Figure 3a, S-nitrosylated proteins in each of three adrenergic stimulated hearts, i.e., DPN, E2, or vehicle, were labeled with a DyLight-maleimide fluor with a distinct excitation/emission wavelength. A representative 2D DyLight DIGE gel is scanned at each of these distinct wavelengths showing the pattern of protein S-nitrosylation for that particular treatment group. S-nitrosylated protein spots in the vehicle group (labeled by DyLight 488) fluoresce a green color while S-nitrosylated protein spots in the DPN (labeled by DyLight 649) and E2 (labeled by DyLight 549) groups fluoresce a red and yellow color respectively. In Figure 3b we show a representative overlay of the DPN/vehicle images (DyLight 649/488) over a Ruby image which stains all protein spots in the 2D gel. Proteins with increased DyLight labeling will exhibit a slight shift to the positive/acidic (left) end of the 2D gel because each molecule of DyLight dye carries ~3-4 negative charges. In this overlay, there are several S-nitrosylated proteins shown as green spots in the vehicle group labeled by DyLight 488, which are slightly shifted compared to the original spot in the Ruby image suggesting small amounts of protein S-nitrosylation. The red spots, indicating increased levels of protein S-nitrosylation in the DPN-treated sample labeled by DyLight 649, are further shifted to the positive/acidic (left) end of the 2D gel compared to vehicle group. Figure 3c shows an overlay of E2-treated samples (labeled by DyLight 549, yellow spots in Figure 3a) with the DPN-treated samples (labeled red with DyLight 649). The orange color (red+yellow=orange) on this overlay (Figure 3c) shows that E2 treatment leads to a similar pattern of protein S-nitrosylation as DPN. These combined data provide evidence that DPN and E2 treatment increase S-nitrosylation of similar proteins in the mouse heart.
Figure 3. S-nitrosylated proteins identified by 2D DyLight DIGE.
(a) A representative 2D DyLight DIGE gel was scanned at each of these distinct wavelengths of DyLight fluors showing a pattern of protein S-nitrosylation for that particular treatment group. The gel was then post-stained overnight with SYPRO Ruby for proteins. (b) Because each DyLight dye has a mass of ~1 KDa and carries ~3-4 negative charges, S-nitrosylated proteins labeled with the DyLight fluor up-shift towards the positive/acidic (left) end of the 2D gel. Several proteins in the vehicle group (green spots, DyLight 488) are shifted from their original protein position seen in the Ruby stain (dark spots). S-nitrosylated proteins in DPN groups (red spots, DyLight 649) exhibit an additional left-ward shift (arrows) indicating increased labeling and increased S-nitrosylation of these proteins compared to vehicle. (c) The orange spots show a complete overlay of DPN (red spots) and E2 (yellow spots) groups, suggesting a similar set of proteins have increased S-nitrosylation in these two samples.
Selected protein spots found to be S-nitrosylated in our 2D DyLight DIGE studies were extracted from the 2D gels and identified by mass spectrometry. SNO levels were quantified and expressed as ratios of the three different treatment groups. As seen in Table 1, DPN or E2 treated hearts showed an increase in SNO of a number of proteins. Interestingly, many of these proteins have also been shown to exhibit increased SNO in preconditioned hearts.16 Additionally, we identified three proteins which exhibit decreased S-nitrosylation in DPN and E2 treated groups compared to vehicle (Table 1).
Table 1.
S-nitrosylation (SNO) levels of identified proteins by 2D DyLight DIGE studies.
| SNO level (arbitrary ratio of DyLight intensity) |
||||||
|---|---|---|---|---|---|---|
| Protein Name | Protein ID | Mass | pI | DPN/Veh | E2/Veh | DPN/E2 |
| (i) S-nitrosylated proteins with increased SNO levels in DPN and E2 treated hearts | ||||||
| Aconitase | Q99KI0 | 86151 | 7.87 | 11.0 | 4.8 | 2.3 |
| Cyt C oxidase subunit 5A | P12787 | 16101 | 6.08 | 8.1 | 4.6 | 1.8 |
| Electron transfer flavoprotein β | Q9DCW4 | 27834 | 8.24 | 7.1 | 1.9 | 3.7 |
| α-enolase | P17182 | 47453 | 6.37 | 6.6 | 2.6 | 2.5 |
| Malate dehydrogenase | P14152 | 36659 | 6.16 | 6.4 | 4.1 | 1.6 |
| Heat shock protein 27 kDa | P14602 | 23014 | 6.12 | 5.2 | 4.4 | 1.2 |
| F1-ATP synthase α1 subunit | Q03265 | 59753 | 9.22 | 5.1 | 3.5 | 1.5 |
| Creatine kinase M-type | P07310 | 43045 | 6.58 | 4.6 | 2.7 | 1.7 |
| Heat shock protein 60 KDa | P63038 | 61088 | 5.91 | 2.4 | 1.4 | 1.8 |
| Heat shock protein 70 KDa | P63017 | 71055 | 5.37 | 1.8 | 1.5 | 1.3 |
| Myoglobin | P04247 | 17116 | 7.07 | 1.8 | 2.2 | 0.9 |
| (ii) S-nitrosylated proteins with decreased SNO levels in DPN and E2 treated hearts | ||||||
| α-actin-2 | P62737 | 42009 | 5.24 | 0.4 | 0.4 | 1.0 |
| Myosin light chain 1 | P09542 | 22521 | 5.03 | 0.6 | 0.2 | 3.0 |
| Pyruvate dehydrogenase E1 β | Q9D051 | 38937 | 6.41 | 0.6 | 1.0 | 0.6 |
All proteins were positively identified (peptide count 2 or more, best ion score > 95%) in three individual 2D DyLight DIGE.
DPN and E2 treatment alter protein levels
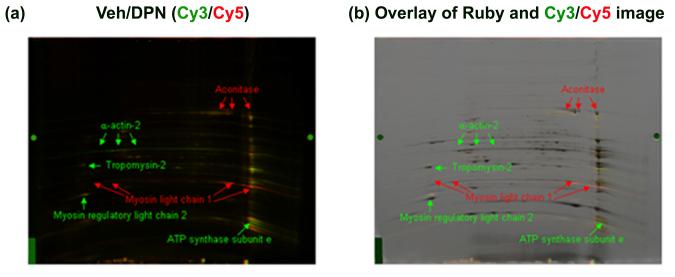
As described earlier, estrogen activation of the classical estrogen receptors can alter the expression of many genes.11 If estrogen and DPN treatment reduce protein expression, the decrease in S-nitrosylation might be due to decreased levels of available protein for S-nitrosylation. As a result, the actual ratio of protein S-nitrosylation per level of total protein might be unchanged. In order to evaluate whether DPN alters protein levels in the ovariectomized female mouse hearts we used a 2D CyDye DIGE method to measure differences in protein expression. Figure 4 shows a representative 2D CyDye DIGE gel comparing vehicle and DPN treated hearts. As listed in Table 2, DPN treatment increased protein expression of aconitase and myosin light chain 1, while it decreased protein expression of α-actin-2, myosin regulatory light chain 2, tropomyosin-2, and ATP synthase subunit e. Interestingly, as seen in Table 1, α-actin-2 exhibits decreased levels of protein S-nitrosylation in DPN and E2 treated groups. This suggests that the decreased protein S-nitrosylation of α-actin-2 may result from decreased protein expression of α-actin-2. On the other hand, the increased protein expression of aconitase might contribute to its significant increase in protein S-nitrosylation. Finally, the increase in protein levels of myosin light chain 1 coupled with decreased S-nitrosylation suggest that this protein has reduced S-nitrosylation under conditions of DPN treatment.
Figure 4. Representative 2D CyDye DIGE comparing protein expression between DPN and vehicle treated groups.
Vehicle control sample was labeled by Cy3 and DPN-treated sample was labeled by Cy5. (a) Overlay images of vehicle control (Cy3, green) with DPN (Cy5, red); (b) Overlay images of Ruby with CyDye images. The significant changes in protein expression are identified and indicated with an arrow. Green and red colors indicate proteins that have decreased and increased protein expression in DPN-treated sample compared to vehicle control.
Table 2.
DPN-induced changes in protein expression identified by 2D CyDye DIGE studies.
| Protein Name | Protein ID | Mass | pI | Arbitrary ratio of Cy3/Cy5 |
|---|---|---|---|---|
| (i) Increased protein expression by DPN treatment | ||||
| Aconitase | Q99KI0 | 86151 | 7.87 | 1.65 ± 0.08 |
| Myosin light chain 1 | P09542 | 22521 | 5.03 | 1.52 ± 0.03 |
| (ii) Decreased protein expression by DPN treatment | ||||
| α-actin-2 | P62737 | 42009 | 5.23 | 0.28 ± 0.06 |
| Myosin regulatory light chain 2 | P51667 | 18852 | 4.86 | 0.40 ± 0.07 |
| Tropomyosin-2 | P58774 | 32930 | 4.66 | 0.58 ± 0.04 |
| ATP synthase subunit e | Q06185 | 8230 | 9.34 | 0.60 ± 0.05 |
All proteins were positively identified (peptide count 2 or more, best ion score > 95%) in three individual 2D CyDye DIGE.
The DPN-induced cardioprotection is dependent on the activation of NO/SNO signaling
As we have shown that females have increased NOS activity6 and others have demonstrated that estrogen increases NOS expression,8 a NOS inhibitor, L-NAME was used to test whether the blockade of NOS would abolish the DPN-induced cardioprotection. As shown in Figure 5, 10 μmol/L of L-NAME abolished the DPN-induced cardioprotection in these I/R hearts under adrenergic stimulation, i.e., the post-ischemic functional recovery (Figure 5a) and infarct size in hearts treated with L-NAME (Figure 5b) were comparable to vehicle treated control. Since the increased intracellular Ca2+ associated with adrenergic stimulation might also lead to the activation of Ca2+-dependent NOS and NO/SNO formation, it is important to test whether DPN can activate SNO and protect the hearts against I/R injury in the absence of isoproterenol. With 20 minutes of no-flow global ischemia the protection observed in females only occurs under conditions of increased contractility.5,6, 10-13 As others have reported protection in females in the absence of increased contractility, but with longer periods of ischemia, we considered whether a more severe ischemic injury might reveal protection in females in the absence of isoproterenol. We therefore increased the time of global ischemia from 20 to 30 minutes. As shown in Figure 5, with 30 minutes of ischemia in the absence of isoproterenol, DPN treatment significantly increased post-ischemic functional recovery (Figure 5c) and decreased infarct size (Figure 5d), which could also be blocked by L-NAME pretreatment.
Figure 5. The DPN-induced cardioprotection is abolished by L-NAME.
Pretreatment with L-NAME (10 μmol/L) 5 minutes prior to ischemia abolished cardioprotective effect of DPN in I/R hearts with (a, b) or without (c, d) adrenergic stimulation: post-ischemic LVDP recovery (a, c) and infarct size (b, d).
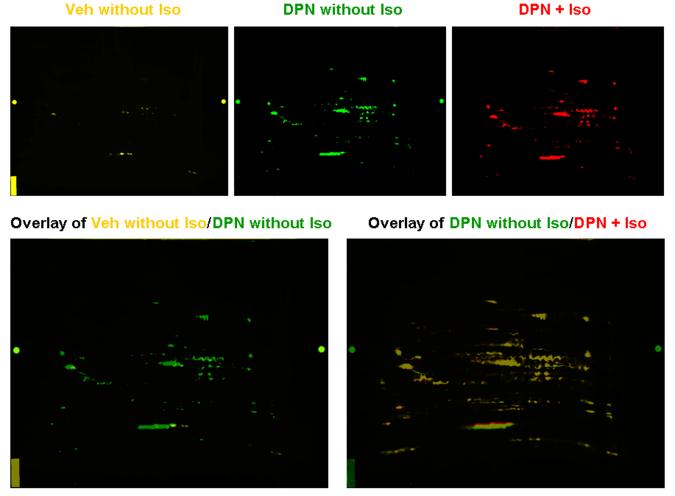
Based on the data in Figure 5, we were interested in determining the levels of SNO that occurred with DPN treatment in the absence of isoproterenol. A representative 2D DyLight DIGE gel as shown in Figure 6 clearly demonstrated that DPN treatment alone without isoproterenol resulted in a significantly increased SNO level, i.e., only a few yellow spots in Veh without Iso (labeled with DyLight 549) vs multiple spots and increased fluorescence intensity in DPN without Iso (labeled with DyLight 488). However, adrenergic stimulation did not further increase SNO level in these DPN-treated hearts, which was indicated by the yellow overlay images of DPN without Iso (green) vs DPN+Iso (labeled with DyLight 649, red). Thus, isoproterenol treatment is apparently not necessary for the DPN-induced increase in SNO.
Figure 6. The DPN-mediated increase in SNO is not dependent on the presence of isoproterenol.
The overlay of Veh without Iso (DyLight 549, yellow) and DPN without Iso (DyLight 488, green) shows that DPN treatment alone significantly increases SNO levels. However, the overlay of DPN without Iso (DyLight 488, green) and DPN+Iso (DyLight 649, red) suggest that adrenergic stimulation by 10 nmol/L isoproterenol did not further increase SNO in DPN-treated hearts,
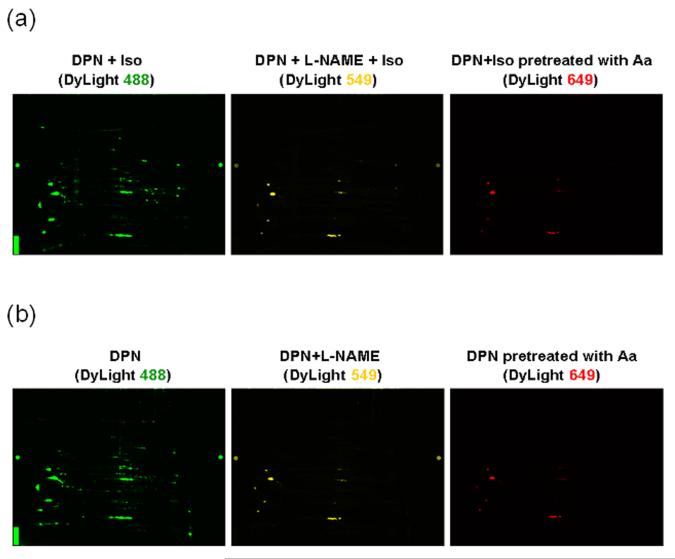
Since L-NAME pretreatment abolished the DPN-induced cardioprotection (Figure 5), we were interested in determining whether L-NAME would also block the DPN mediated increase in SNO. As shown in Figure 7a, the SNO level in L-NAME-pretreated isoproterenol stimulated hearts was significantly decreased to low basal levels. In addition, the pattern of SNO modifications in DPN-treated hearts without isoproterenol stimulation (Figure 7b) was very similar to that found in DPN+Iso samples, and the increase in SNO levels induced by DPN were significantly attenuated by L-NAME pretreatment. To confirm the specificity of the DyLight/Biotin Switch method for labeling SNO, we performed a control in which the extract was pretreated with ascorbic acid before blocking free thiols in the modified DyLight Switch. Ascorbic acid has been used as a specific reducing agent to decompose SNO.20 This sample, which was labeled with DyLight 649 as shown in right panel of Figure 7 shows little or no fluorescence thus demonstrating the specificity of the method for labeling SNO.
Figure 7. The DPN-mediated increase in SNO is blocked by L-NAME pretreatment.
SNO levels in mouse hearts with (a) or without (b) adrenergic stimulation under different treatment by 2D DyLight DIGE: DPN (DyLight 488, green), DPN+L-NAME (DyLight 549, yellow), and DPN sample pretreated with 1 mmol/L ascorbic acid as negative control (DyLight 649, red).
The DPN-induced cardioprotection does not occur in hearts lacking ER-β
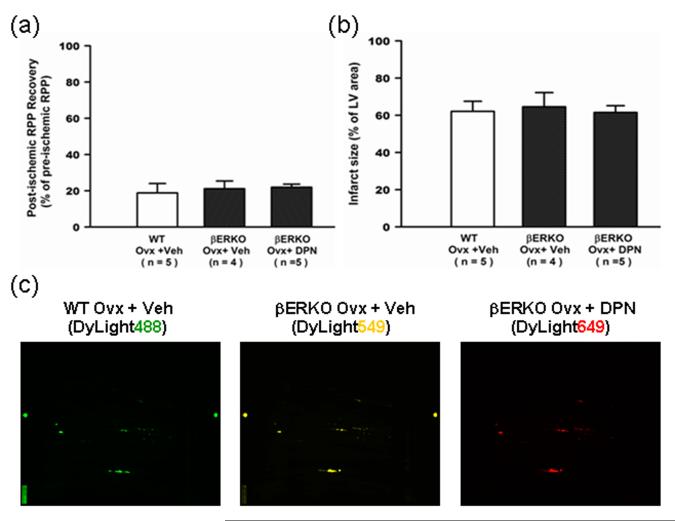
We initially measured uterine weight/body weight ratio in each of our treatment groups (see Supplemental Material) to confirm that DPN, a selective ER-β agonist, was acting only through ER-β and not ER-α.11 To further confirm the DPN-induced cardioprotection was due to the specific activation of ER-β, DPN or vehicle containing minipumps were implanted into ovariectomized βERKO mice, and after two weeks of treatment, the hearts were Langendoff perfused and subjected to 30 minutes of ischemia without adrenergic stimulation. No cardioprotective effect of DPN was found in ovariectomized βERKO mice (Figure 8a,b), indicating that DPN mediates cardioprotection through activation of ER-β. In addition, the similar pattern and fluorescence intensity of DyLight labeling (Figure 8c) in DPN and vehicle treated ovariectomized βERKO mice indicated that DPN did not lead to increase of NO/SNO signaling in βERKO mice, suggesting that the DPN-induced activation of SNO is also dependent on activation of ER-β.
Figure 8. The DPN-induced cardioprotection and increased SNO is lost in ovariectomized βERKO hearts.
The Langendorff perfused heart from βERKO mice were subjected to 20 min of perfusion, 30 min no-flow global ischemia, and 2 hours of reperfusion. There were no significant differences in post-ischemic functional recovery (a), infarct size (b), and SNO level (c) between vehicle-treated and DPN-treated ovariectomized βERKO hearts.
Discussion
Estrogen has been shown to be cardioprotective in a number of different animal studies.3-6 Previous reports suggest that this cardioprotection is mediated by ER-β12 in an NO dependent manner.10 In this study, we find that chronic treatment with DPN is cardioprotective in ovariectomized female mice to a similar extent as E2, and that DPN and E2 treated hearts have similar protein S-nitrosylation patterns. Additionally, the DPN-mediated cardioprotection is abolished in βERKO mice. Taken together, these results provide evidence that ER-β is the primary receptor responsible for long-term cellular changes leading to cardioprotection in females.
We find that ovariectomized female mice treated for 2 weeks with either E2 or DPN have reduced post-ischemic contractile dysfunction and smaller infarcts than vehicle treated mice following I/R. However, this protection is abolished in DPN-treated mice that are pre-treated with the NOS inhibitor, L-NAME. Additionally, our 2D DIGE studies show that E2 and DPN result in increased S-nitrosylation of an overlapping set of cardiac proteins. These observations lend strong support to the hypothesis that estrogen leads to cardioprotection via ER-β specifically and does so through an increase in NO signaling. Many of the proteins found to be S-nitrosylated by DPN and estrogen, such as aconitase, heat shock protein, mitochondrial F1-ATPase, creatine kinase, and malate dehydrogenase, have also been shown to be S-nitrosylated in other models of cardioprotection.16
As shown in Table 2, we also identified three proteins which exhibit decreased S-nitrosylation in the DPN and E2 treated groups compared to vehicle. SNO levels are determined by S-nitrosylation and denitrosylation pathways.24, 25 Estrogen clearly leads to activation of S-nitrosylation pathways, as illustrated by the decrease in SNO in the presence of L-NAME. It is also possible that estrogen might selectively activate or target denitrosylation mechanisms, which might contribute to reduced SNO of these proteins. Another possibility for this decreased S-nitrosylation may be that estrogen alters protein levels leading to decreased availability of protein for S-nitrosylation. To address this question, we performed a 2D CyDye DIGE to determine whether DPN altered protein expression levels compared to vehicle. Of interest, we noted that DPN causes a decrease in protein expression of α-actin-2 which was found to have decreased S-nitrosylation in our E2 and DPN treated groups. This finding would suggest that the decreased levels of α-actin-2 reduce the availability of this protein for S-nitrosylation. As a result, the overall level of protein S-nitrosylation decreases. However, a decrease in protein level does not account for the decrease in S-nitrosylation of myosin light chain 1 and pyruvate dehydrogenase E1 β subunit.
The effects of estrogen have typically been attributed to estrogen-ER mediated changes in gene expression. We were therefore surprised by the small number of proteins that were substantially increased or decreased with DPN treatment; only 2 proteins were significantly increased and 4 proteins were decreased with DPN treatment. This small number of changes at the protein level contrast with the large number of changes (~145) at the mRNA level identified by microarray.11 There are several possible reasons for this discrepancy. Many of the changes at the mRNA level were in transcription factors and relatively low abundance signaling proteins that are not readily observed in the 2D CyDye DIGE method, which is biased towards high abundance proteins. It is also likely that some of the changes in gene expression do not result in significant changes in protein levels. In spite of these differences, it is interesting that most of the protein changes observed with the 2D CyDye DIGE method were contractile proteins. In our previous microarray study, we also observed a large number of changes in contractile proteins.11 This alteration in contractile proteins is intriguing in light of the study by Petre et al reporting sex differences in contractile reserve.26
We have shown that chronic E2 and DPN treatment mediate cardioprotection in female mice and also alter the S-nitrosylation of several cardiac proteins. How does altered protein S-nitrosylation potentially contribute to cardioprotection? S-nitrosylation can have two primary effects on a protein. It can alter the activity of enzymes through changes in protein structure or function and it can protect the modified cysteine from further oxidation. These two principles have been suggested to contribute to S-nitrosylation mediated cardioprotection.16,22,23 One protein of interest is creatine kinase which was found to have increased S-nitrosylation in DPN and E2 treated hearts as well as preconditioned hearts.16 The S-nitrosylation of creatine kinase might prevent the target cysteine residue(s) from further irreversible oxidative damage upon reperfusion.
We also find increased S-nitrosylation of the F1-ATPase α1 subunit in E2 and DPN treated hearts. Sun et al recently reported that preconditioning and GSNO treatment leads to the S-nitrosylation of mitochondrial F1-ATPase, reversibly decreasing its activity.16 As much as 50% of the ATP generated during ischemia by glycolysis is consumed by the F1-ATPase functioning in reverse mode. The decrease in F1-ATPase activity, conferred by increased S-nitrosylation, may therefore reduce the ischemic consumption of ATP.16 Several other important mitochondrial proteins were found to have increased S-nitrosylation in our E2 and DPN treated groups compared to vehicle. Future investigation will focus on whether S-nitrosylation of these mitochondrial proteins alters their activities leading to potential cardioprotection.
There are some interesting clinical implications from these findings. While estrogen is cardioprotective in females, it also has a number of detrimental effects including an increased risk for breast and uterine cancer, both of which are largely mediated by ER-α activation. The finding that DPN, an ER-β selective agonist, is both cardioprotective and increases S-nitrosylation of the same set of cardiac proteins as E2 offers a potentially unique therapy in terms of a selective estrogen receptor modulator that confers cardioprotection without the increased risk for cancer. Additionally, estrogen mediated protein S-nitrosylation may provide a partial explanation for the failure of the WHI to show cardioprotection with hormone replacement therapy. It has been suggested that NO signaling might decrease in aging because of decreased levels of tetrahydrobiopterin27 or an increase in arginase activity.28
Commentary of CIRCULATIONAHA/2009/868729
Premenopausal women have reduced cardiovascular disease and this protection is lost as women enter menopause. A number of studies in animal models have shown that estrogen treatment is cardioprotective. In contrast, the Women’s Health Initiative showed no protection with hormone replacement therapy, although some questions have been raised about the age of the women at treatment. This difference emphasizes the need for a better understanding of the mechanism by which estrogen protects in animal studies. This study shows that estrogen acts via the β-estrogen (β-ER) receptor in the heart to increase the S-nitrosylation of many proteins that have been shown to be cardioprotective. S-nitrosylation is a common post-translational protein modification with covalent attachment of a nitric oxide (NO) moiety to protein sulfhydryl residues. S-nitrosylation not only leads to changes in the structure and function of target proteins, but also prevents the modified cysteine residue(s) from further oxidative modification. These data might prompt future researchers to investigate whether age related alterations in NO signaling contribute to the lack of protection by estrogen treatment in older women. It has been suggested that NO signaling might decrease with age because of a decrease in the levels of tetrahydrobiopterin or an increase in arginase activity. Additionally, the finding that a β-ER specific agonist is protective provides a potential novel treatment therapy that might promote the beneficial effects of estrogen without specific adverse effects on other tissues such as the uterus.
Acknowledgements
We thank the Proteomics Core Facility in NHLBI for help with 2D-DIGE analysis and peptide mass fingerprinting.
Funding Sources: J.L. was supported by the Sarnoff Foundation and Harvard Medical School. C.S. was supported in part by NIH grant HL-39752. E.M. and J.S. were supported by the NIH Intramural Program.
Footnotes
Conflict of Interest Statement: None
References
- 1.Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46:28–49. doi: 10.1016/s0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 2.Konhilas JP, Leinwand LA. The effects of biological sex and diet on the development of heart failure. Circulation. 2007;116:2747–2759. doi: 10.1161/CIRCULATIONAHA.106.672006. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Kloner RA. Is there a gender difference in infarct size and arrhythmias following experimental coronary occlusion and reperfusion? J Thromb Thrombolysis. 1995;2:221–225. doi: 10.1007/BF01062713. [DOI] [PubMed] [Google Scholar]
- 4.Booth EA, Obeid NR, Lucchesi BR. Activation of estrogen receptor-alpha protects the in vivo rabbit heart from ischemia-reperfusion injury. Am J Physiol. 2005;289:H2039–H2047. doi: 10.1152/ajpheart.00479.2005. [DOI] [PubMed] [Google Scholar]
- 5.Cross HR, Lu L, Steenbergen C, Philipson KD, Murphy E. Overexpression of the cardiac Na+/Ca2+ exchanger increases susceptibility to ischemia/reperfusion injury in male, but not female, transgenic mice. Circ Res. 1998;83:1215–1223. doi: 10.1161/01.res.83.12.1215. [DOI] [PubMed] [Google Scholar]
- 6.Cross HR, Murphy E, Steenbergen C. Ca2+ loading and adrenergic stimulation reveal male/female differences in susceptibility to ischemia-reperfusion injury. Am J Physiol. 2002;283:H481–H489. doi: 10.1152/ajpheart.00790.2001. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Nuedling S, Karas RH, Mendelsohn ME, Katzenellenbogen JA, Katzenellenbogen BS, Meyer R, Vetter H, Grohe C. Activation of estrogen receptor beta is a prerequisite for estrogen-dependent upregulation of nitric oxide synthases in neonatal rat cardiac myocytes. FEBS Lett. 2001;502:103–108. doi: 10.1016/s0014-5793(01)02675-8. [DOI] [PubMed] [Google Scholar]
- 9.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel α1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 11.Nikolic I, Liu D, Bell JA, Collins J, Steenbergen C, Murphy E. Treatment with an estrogen receptor-beta-selective agonist is cardioprotective. J Mol Cell Cardiol. 2007;42:769–780. doi: 10.1016/j.yjmcc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005;38:289–297. doi: 10.1016/j.yjmcc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Cross HR, Murphy E, Koch WJ, Steenbergen C. Male and female mice overexpressing the β2-adrenergic receptor exhibit differences in ischemia/reperfusion injury: role of nitric oxide. Cardiovasc Res. 2002;53:662–671. doi: 10.1016/s0008-6363(01)00528-4. [DOI] [PubMed] [Google Scholar]
- 14.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res. 2007;101:1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 17.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane P, Hao G, Gross SS. S-nitrosylation is emerging as a specific and fundamental posttranslational protein modification: head-to-head comparison with O-phosphorylation. Science’s STKE. doi: 10.1126/stke.2001.86.re1. 2001/86/re1. [DOI] [PubMed] [Google Scholar]
- 19.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 20.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;86:PL1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 21.Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:509–517. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, Steenbergen C, Murphy E. S-nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid Redox Signal. 2006;8:1693–1705. doi: 10.1089/ars.2006.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J. Protein S-nitrosylation: a role of nitric oxide signaling in cardiac ischemic preconditioning. Acta Physiol Sinica. 2007;59:544–552. [PubMed] [Google Scholar]
- 24.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005;280:19888–19894. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 25.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petre RE, Quaile MP, Rossman EI, Ratcliffe SJ, Bailey BA, Houser SR, Marqulies KB. Sex-based differences in myocardial contractile reserve. Am J Physiol. 2007;292:R810–R818. doi: 10.1152/ajpregu.00377.2006. [DOI] [PubMed] [Google Scholar]
- 27.Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol. 2004;287:H2448–H2453. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- 28.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]