Abstract
Objective
As an elevated erythropoietin has been observed in diabetic retinopathy, we sought to investigate the role of hemoglobin (HGB) in predicting proliferative diabetic retinopathy (PDR).
Research Design and Methods
We assessed 426 individuals without PDR at baseline (213 men; 213 women) from the Pittsburgh Epidemiology of Diabetes Complications Study, an 18-year prospective cohort study of childhood onset type 1 diabetes. PDR was determined by stereo fundus photography. Cox proportional hazards modeling with stepwise regression was used to determine the independent association of HGB with PDR. Analyses were conducted gender-specifically.
Results
There were 206 events. Although the incidence of PDR did not vary by gender (48 % in both men and women), in men, HGB exhibited a positive linear relationship with the 18-year incidence of PDR (HR=1.33, 1.10–1.60, p=0.003) while in women HGB exhibited a quadratic relationship with PDR (p=0.0008). After multivariable adjustment for univariately significant covariates, HGB remained significantly predictive of PDR in both men (p=0.004) and women (p=0.04).
Conclusion
Higher HGB predicts the incidence of PDR in Type 1 diabetes, though the association varies by gender, being linear and positive in men and quadratic in women.
Introduction
Proliferative diabetic retinopathy (PDR) is a leading cause of blindness in the United States (NIH), and has been linked to retinal hypoxia and characterized by small blood vessel proliferation, retinal microanerurysms, and vitreous hemorrhaging (1). Lower hematocrit (HCT) has been associated with the progression of diabetic kidney disease (2,3), and with the incidence of PDR (4), suggesting that anemia may increase complication risk. In contrast, however, a stabilization of retinopathy has also been reported in those with end stage renal disease (5,6), a condition associated with anemia. The association between hemoglobin (HGB) and retinopathy is likely therefore to be complex, particularly given the recent observation of unusually high HGB levels, reaching 18.8 g/dl, in nephropathic type 1 diabetes study participants (7).
Hemorheological factors are altered in diabetes and there is some evidence that these disturbances may also be associated with diabetic retinopathy (8). Increased HCT (8) and blood viscosity (9,10) have been observed cross-sectionally in those with diabetic retinopathy. An elevated erythropoietin has also been observed in the vitreous fluid of those with diabetic retinopathy and found to be an angiogenic promoter of PDR (11).
Given these observations, we decided to investigate the association of HGB with the incidence of PDR in type 1 diabetes.
Methods
The EDC study is an 18-year prospective study based on a well-defined cohort with childhood-onset (<17 years) type 1 diabetes living within 100 miles of the University of Pittsburgh at study baseline. The 658 participants (325 females, 333 males) (67% of all those eligible) were diagnosed between January 1, 1950 and May 30, 1980 at Children's Hospital of Pittsburgh, and first seen in EDC between 1986 and 1988, when men age and diabetes duration were 28 and 19 years, respectively. They were then seen biennially for ten years, after which they were followed by survey with exams limited to certain subgroups. Between 2004 and 2007, however, an eighteen year follow-up was conducted for all participants. The design and methods of the study have been previously described (12)
Before attending each cycle of examinations, information was collected by questionnaire concerning demographic characteristics, medical history, and health care behaviors as previously described (13). At each cycle, both a standardized medical history and clinical examination were performed by a trained internist to document complications of diabetes.
Fasting blood samples were assayed for lipids, lipoproteins, glycosylated hemoglobin (HbA1), and HGB. HGB was measured using the Coulter Counter Model S-Plus IV automated blood cell counter. High-density lipoprotein (HDL) cholesterol was determined by a heparin and manganese procedure, a modification of the Lipid Research Clinics method (14). Cholesterol was measured enzymatically. Glomerular filtration rate (GFR) was estimated using the four-variable Modification of Diet in Renal Disease formula (15).
Stable glycosylated HbA1 was originally measured in saline-incubated samples by microcolumn cation exchange chromatography (Isolab, Akron, Ohio, USA). On October 26, 1987, the method was changed to high-performance liquid chromatography (HPLC) (Diamat, Bio-Rad Laboratories, Hercules, CA, USA). The two methods were highly correlated (r = 0.95; Diamat HbA1 = −0.18+1.00 Isolab HbA1). After the 10-year follow-up, HbA1c was measured with the DCA 2000 analyzer (Bayer, Tarrytown, NY, USA). The Diamat and DCA were also highly correlated (r=0.95; DCA [HbA1c]=−0.18+1.00 Diamat HbA1] HbA1 and HbA1c were converted to Diabetes Control and Complications Trial (DCCT) aligned HbA1c values using regression formulas derived from duplicate analyses (DCCT HbA1c = [0.83 * EDC HbA1] + 0.14; DCCT HbA1c = [EDC HbA1c − 1.13]/0.81).
Blood pressure was measured by a random-zero sphygmomanometer according to a standardized protocol [Hypertension Detection and Follow-Up (HDFP)] (16) after a 5-minute rest period. Blood pressure levels were analyzed, using the mean of the second and third readings.
Retinopathy was assessed in three standardized visual fields. Stereoscopic fundus photographs were taken in fields 1, 2, and 4 with a Zeiss camera (Carl Zeiss, Germany), and were read by the Fundus Photography Reading Center, University of Wisconsin, Madison, Wisconsin. Gradings were classified by the modified Arlie House System, with PDR defined as grade 60 or higher in at least one eye. Individuals without gradable fundus photographs were graded according to their medical history data with confirmation by their ophthalmologist if required. Those with panretinal photocoagulation scars and a grade <60 were recorded as having PDR if the medical history indicated that the laser therapy was for PDR. Two step progression, an increase of at least two steps on the modified Arlie House System (17), and clinically significant macula edema were also identified (18).
Nephropathy status was determined based on consistent results from at least two of three timed urine collections (24-hour, overnight, timed samples during exam) and urine albumin excretion rate (AER). The adequacy of urine collection was determined by whether the total amount of creatinine excreted was appropriate for an individual's age, based on body size and gender. Urinary albumin was determined immunonephelometrically (19). All procedures were approved by the Institutional Review Board of the University of Pittsburgh.
Statistical Analyses
Continuous data were compared between groups by the Student's t test with serum creatinine, triglycerides, HDL cholesterol, and non-HDL cholesterol being log transformed prior to testing. Categorical data were compared between groups by chi-square tests. Cox proportional hazards modeling with forward selection was used to determine the association of baseline HGB with the 18-year incidence of PDR. The functional form of HGB was assessed by plotting a restricted cubic spline transformation for HGB against the log hazard ratio separately for men and women using Harrell's (20) SAS macro, %PSPLINET. A Wald test was used to test the assumption of a linear relationship between HGB and the log hazard ratio. Due to the gender difference in HGB distribution, analyses were conducted sex-specifically. Censoring occurred at the time of event or at last follow-up.
Results
HGB data was available for 652 study participants. HGB ranged from 9.2–20.0 g/dl, with a median and interquartile range of 16.2 (15.4–16.9) in men and 14.1 (13.5–15.0) in women. Two hundred and two participants had PDR at baseline and were excluded from further analyses. Of the remaining 450, 24 provided no further PDR information and complete follow-up to 18 years was available for 78% of those still alive, follow-up being censored at last observation for the remainder. Baseline characteristics of the 426 study participants free of PDR at baseline are described in Tables 1a and 1b for men and women, respectively.
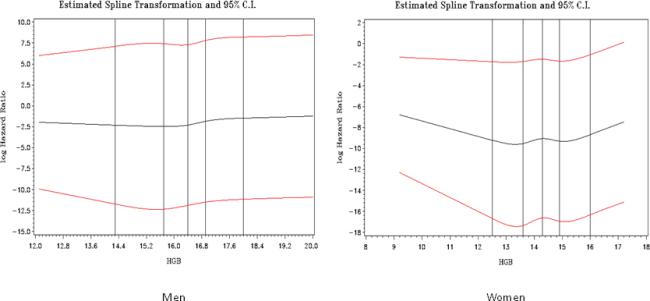
Figure 1.
Parts A–B. Restricted Cubic Spline of Hemoglobin (g/dl) by the Log Hazard Ratio of Proliferative Diabetic Retinopathy by Gender.
Men: Wald Test of Linearity p=0.15
Women: Wald Test of Linearity p=0.02, p-value for quadratic term in Cox model=0.008
Table 1b.
Baseline Characteristics of Participants by Subsequent Proliferative Diabetic Retinopathy (PDR) Status in Women, mean (SD) or N (%).
| PDR (n=103) | No PDR (n=110) | p-value | |
|---|---|---|---|
| Age (years) | 26.0 (7.1)) | 25.7 (8.5) | 0.76 |
| Diabetes duration (years) | 17.0 (6.8) | 17.1 (7.5) | 0.97 |
| Hemoglobin (g/dl) | 14.4 (1.2) | 14.2 (1.0) | 0.24 |
| White blood cells (– 103/mm2) | 6.8 (1.9) | 6.2 (1.8) | 0.03 |
| Body mass index (kg/m^2) | 23.3 (3.1) | 23.2 (3.5) | 0.70 |
| Overt Nephropathy (%) | 33 (35.5) | 15 (14.0) | 0.0004 |
| Estimated GFR (MDRD)* (ml/min/1.73 m2), median (IQR) | 105.2 (86.0–134.3) | 104.2 (87.6–129.4) | 0.92 |
| Albumin excretion rate (μg/min)*, median (IQR) | 12.9 (7.7–71.6) | 9.0 (5.6–19.4) | 0.002 |
| Total cholesterol (mg/dl)* | 195.6 (41.1) | 178.6 (32.0) | 0.0009 |
| Non-HDL cholesterol (mg/dl) | 136.1 (41.3) | 117.8 (30.5) | 0.0003 |
| HDL cholesterol (mg/dl) | 59.5 (13.7) | 60.8 (11.8) | 0.48 |
| Fibrinogen (mg/dl) | 310.7 (90.5) | 270.6 (60.8) | 0.0002 |
| Systolic blood pressure (mm Hg) | 109.9 (13.2) | 105.3 (10.1) | 0.004 |
| Diastolic blood pressure (mm Hg) | 70.3 (10.7) | 67.7 (7.9) | 0.05 |
| HbA1c (%) | 9.2 (1.7) | 8.4 (1.3) | <0.0001 |
| Total insulin injections /day | 2 (1–2) | 2 (1–2) | 0.17 |
| Current smoker (%) | 18 (18.4) | 19 (17.4) | 0.86 |
Natural logarithmically transformed before analysis GFR (MDRD)=estimated glomerular filtration rate, Modification of Diet in Renal Disease formula IQR=interquartile range HDL=high density lipoprotein HbA1c=glycosylated hemoglobin A1c
The cumulative incidence of PDR was 48% after 18 years. Median follow-up were 14.6 years. In men, there was no suggestion of any deviation from a linear relationship between HGB and the log hazard ratio (Wald test of linearity p=0.15), therefore, HGB was entered into Cox models as a continuous linear covariate. In women, however, the Wald test showed a significant deviation from linearity (p=0.02) and graph of the restricted cubic spline suggested a quadratic relationship between HGB and the log hazard ratio (Figure 1) and therefore HGB was entered into Cox models as a quadratic term. Univariately, HGB showed a significant linear association with the risk of developing PDR in men (p=0.003) and a significant non-linear association in women (p=0.008, quadratic term).
In multivariable analyses allowing for univariately significant covariates, HGB remained significantly predictive of PDR in men (HR=1.29, 1.08–1.54, p=0.004) (Table 2). In women, HGB as a quadratic term also remained significantly predictive of PDR (p=0.04). Other significant multivariable predictors included HbA1c and diastolic blood pressure in both genders, albumin excretion rate in men, and systolic blood pressure and fibrinogen in women. Finally, the association of HGB with other components (markers) of retinopathy was also explored. A significant prediction of both macula edema (p<0.0001, 0.03, men and women, respectively) and two-step progression (p=0.03, 0.0009 men and women, respectively) was observed.
Table 2.
Multivariable Prediction of 18-year Incidence of Proliferative Diabetic Retinopathy in Type 1 Diabetes (n=426).
| Men (n=213, 103 events) | Women (n=213, 103 events) | |||
|---|---|---|---|---|
| Variable | HR | 95% CI | HR | 95% CI |
| HGB (g/dl) | 1.29 | 1.08–1.54 | NA | |
| HGB quadratic (g/dl) | N/A | 1.10 | 1.00–1.20 | |
| HbA1c (%) | 1.30 | 1.14–1.49 | 1.32 | 1.16–1.50 |
| Albumin Excretion | 1.34 | 1.20–1.50 | NS | |
| Rate (μ/min)* | ||||
| DBP (mm Hg) | 1.03 | 1.00–1.05 | 2.28 | 1.02–5.07 |
| SBP (mm Hg) | NS | 1.03 | 1.01–1.05 | |
| Fibrinogen (mg/dL) | NS | 1.00 | 1.00–1.01 |
Natural logarithmically transformed before analysis N/A=not applicable N/S=not selected HGB=hemoglobin HbA1c=glycosylated hemoglobin A1c DBP=diastolic blood pressure SBP=systolic blood pressure
The following variables were also made available for multivariable analyses in the stepwise selection model: diabetes duration, body mass index, non-HDL cholesterol, white blood cell count, fibrinogen, and hypertension medication.
Discussion
The cumulative incidence of PDR in this population was 48% after 18 years. EDC incidence rates are similar to those observed in the DCCT/EDIC conventional treatment group of similar diabetes duration (49.7% at 30 years of diabetes duration (21)) and the WESDR population (37% at ~26.6 years of diabetes duration (18)). Although much attention has been given in recent years to anemia and low levels of HGB in diabetes, particularly as it relates to kidney disease (22–28), this is the first study to our knowledge to report high HGB levels to be predictive of the long term incidence of PDR. We have also shown that the relationship of HGB with PDR varies by gender, with HGB demonstrating a linear relationship in men, but a nonlinear, quadratic (p=0.008), relationship in women. These results, if confirmed, may have important clinical relevance as they may both identify new pathogenetic pathways to PDR and influence clinical treatment. There are several potential explanations for these findings.
First, the HGB levels in this population were relatively high, ranging from 9.2–20.0 g/dl. We have observed that HGB levels in our population, including the two hundred ten with PDR and two hundred twenty-eight with overt nephropathy at baseline, were approximately 1 g/dl higher in both men and women compared to the NHANES III Caucasian population limited to the same age range (29). Higher HGB levels in type 1 diabetes were also noted by Pichler et al (30), who observed a higher (0.8 g/dl) difference in HGB levels between children and adolescents with type 1diabetes compared to controls. Factors related to increased HGB, such as testosterone, hypoxia, growth factors, and viscosity may responsible for the PDR associated with increased HGB in this population.
Androgens, in particular testosterone, are a known stimulant of erythropoiesis (31), and testosterone has been fairly consistently shown to be elevated in type 1 diabetes. Haffner et al found higher testosterone levels (32) and lower sex hormone-binding globulin (33) in type 1 diabetes males with PDR compared to those with out PDR. Chaurasia et al (34) also found higher testosterone levels in those with PDR. Factors associated with the anabolic affects of androgens, such as erythropoietin stimulation, may thus accelerate progression to PDR.
Erythropoietin is a glycoprotein produced by the peritubular fibroblasts of the kidney and its production is primarily determined by tissue hypoxia in those with normal renal function (35). Erythropoietin stimulates angiogenesis (11) and has been found to be associated with the retinopathy of prematurity (36,37). In diabetes, Watanabe et al (11) found both erythropoietin and vascular endothelial growth factor to be independently associated with PDR cross-sectionally, while blocking of erythropoietin was found to inhibit retinal neovascularization (38). In preterm infants, Romagnoli et al (36) observed that infants treated with recombinant human erythropoietin, to reduce the need for blood transfusions, were at an increased risk of retinopathy of prematurity with no change in the need for transfusion. Brown et al (37) also noted an increased risk of prematurity in preterm infants given recombinant erythropoietin. In diabetic patients on hemodialysis, Diskin et al (39) observed that recombinant erythropoietin dose/week and hematocrit were positively associated with deterioration of retinopathy. Finally, recent trials have also observed an increase in adverse events, i.e. a deterioration of kidney disease and more cardiovascular events and mortality, associated with the correction of anemia by erythropoietin therapy in those with kidney disease (40,41). Since these target treatment HGB levels were relatively low, this seems to suggest that growth factors stimulated by erythropoietin are more likely to be factors associated with adverse outcomes rather than high HGB per se.
Another potential and related mechanism is that the HGB association with PDR reflects increased erythropoietin production stimulated by general hypoxia (11) which may contribute to the microvascular disease seen in both the retina and the kidneys in type 1 diabetes. Some recent studies have shown increased levels of anemia in type 1 diabetes to be associated with complications of diabetes, including retinopathy, renal impairment, and macrovacular disease. However, these patients were patients from tertiary care clinics and were older (23,28) than our participants. In contrast, other literature suggests increased blood viscosity in diabetes (10, 42, 43, 44), which may lead to tissue ischemia (8). Vekasi et al (8) found higher levels of HCT, plasma fibrinogen, higher plasma and whole blood viscosity in those with diabetic retinopathy compared to non-diabetic controls. Blood viscosity is directly related to HCT (10). As higher HCT and blood viscosity have also been observed in those with retinal vein occlusion (45), and the HGB levels associated with PDR in our population were quite high, it is quite possible that our PDR association may be partly due to increased viscosity and associated sludging. Unfortunately, direct viscosity measures are not available
The gender differences, e.g. HGB demonstrated a nonlinear relationship with PDR in women and a linear relationship in men, is of interest. However, this is likely to just reflect the small sample sizes at the lower ends of the distribution in men (n=5) at levels (≤13.4 g/dl) where an increased risk was seen in women (n=46). A `U' shaped relationship is thus likely to be true and many `U' shaped relationships exist between risk states and outcomes (e.g. weight and mortality) with different pathological pathways operating at each end of the spectrum. Interestingly, a very recent study in type 1 diabetes found a genetic variant promoter of the EPO gene to be associated with both PDR and end stage renal disease (47). Another limitation of our study was that we could not adequately stratify by renal disease status, a complication associated with both HGB levels and diabetic retinopathy, as only 14% (n=56) of those free of PDR at baseline had overt nephropathy. However, we did try to account for kidney disease by controlling for albumin excretion rate, which, while predictive of PDR, did not account for HGB's association with PDR incidence.
In conclusion, these data suggest that high HGB levels may be associated with an increased risk of PDR. Furthermore, these PDR findings are complimented by observations that HGB also predicts two step progression and macula edema. Increased testosterone in type 1 diabetes, growth factors, viscosity, and/or compensation for generalized ischemia/hypoxia may account for the association of high HGB levels with PDR. Taken as a whole, these results suggest that further evaluation of the potential adverse role of high HGB in those with type 1 diabetes is warranted.
Table 1a.
Baseline Characteristics of Participants by Subsequent Proliferative Diabetic Retinopathy (PDR) Status in Men, mean (SD) or N (%).
| PDR (n=103) | No PDR (n=110) | p-value | |
|---|---|---|---|
| Age (years) | 25.1 (6.4) | 24.2 (7.8) | 0.35 |
| Diabetes duration (years) | 16.8 (5.7) | 16.1 (6.7) | 0.43 |
| Hemoglobin (g/dl) | 16.5 (1.2) | 16.1 (1.2) | 0.009 |
| White blood cells (× 103/mm2) | 6.2 (1.6) | 6.2 (1.8) | 0.92 |
| Body mass index (kg/m^2) | 23.7 (2.9) | 22.9 (3.4) | 0.05 |
| Overt Nephropathy (%) | 36 (39.1) | 14 (12.8) | <0.0001 |
| Estimated GFR (MDRD) (ml/min/1.73 m2) *, median (IQR) | 107.1 (92.2–146.2) | 107.1 (88.7–139.1) | 0.70 |
| Albumin excretion rate (μg/min)*, median (IQR) | 14.8 (9.0–65.2) | 8.6 (5.7–21.6) | 0.0003 |
| Total cholesterol (mg/dl)* | 186.1 (37.7) | 174.4 (33.0) | 0.02 |
| Non-HDL cholesterol (mg/dl) | 136.9 (37.0) | 124.1 (33.6) | 0.009 |
| HDL cholesterol (mg/dl) | 49.2 (9.0) | 50.2 (10.0) | 0.42 |
| Fibrinogen (mg/dl) | 262.8 (80.2) | 266.2 (81.6) | 0.76 |
| Systolic blood pressure (mm Hg) | 114.8 (13.6) | 111.3 (12.5) | 0.05 |
| Diastolic blood pressure (mm Hg) | 74.4 (9.5) | 71.0 (9.9) | 0.01 |
| HbA1c (%) | 9.0 (1.5) | 8.5 (1.5) | 0.009 |
| Total insulin injections /day | 2 (1–2) | 2 (1–2) | 0.15 |
| Current smoker (%) | 22 (21.8) | 19 (19.0) | 0.62 |
Natural logarithmically transformed before analysis GFR (MDRD)=estimated glomerular filtration rate, Modification of Diet in Renal Disease formula IQR=interquartile range HDL=high density lipoprotein HbA1c=glycosylated hemoglobin A1c
Acknowledgements
This work was funded by NIH grant DK34818. We are also indebted to the participants of the Pittsburgh Epidemiology of Diabetes Complications Study for their dedication and cooperation to the advancement of knowledge in the scientific community relating to the complications of type 1 diabetes. BC, RM, and TO had full access to the data and take full responsibility of the data and the accuracy of the data analyses. Findings of this study were presented in part at the 67th American Diabetes Association Scientific
References
- 1.Dintenfass L. Blood viscosity factors in severe nondiabetic and diabetic retinopathy. Biorheology. 1977;14:151–157. doi: 10.3233/bir-1977-14401. [DOI] [PubMed] [Google Scholar]
- 2.Mohanram A, Zhang Z, Shahinfar S, Keane W, Brenner, Toto R. Anaemia and End-stage Renal Disease in Patients with Type 2 Diabetes and Nephropathy. Kidney International. 2004;66:1131–1138. doi: 10.1111/j.1523-1755.2004.00863.x. [DOI] [PubMed] [Google Scholar]
- 3.Conway B, Fried L, Costacou T, Orchard T. Hematocrit Predicts End Stage Renal Disase (ESRD) and non-ESRD Mortality in Type 1 Diabetes with Overt Nephropathy. Diabetes. 2005;54(S1):A551. [Google Scholar]
- 4.Davis M, Fisher M, Gangnon R, Barton F, Aiello L, Chew E, Ferris F, Knatterud G. Risk Factors for High-Risk Proliferative Diabetic Retinopathy and Severe Visual Loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest Opthalmol Vis Sci. 1998;39:233–252. [PubMed] [Google Scholar]
- 5.Yoshimoto M, Matsumoto S. Changes in diabetic retinopathy and visual acuity in patients with end-stage diabetic nephropathy after the introduction of hemodialysis. Nippon Ganka Gakkai Zasshi. 2006;110(4):271–275. [PubMed] [Google Scholar]
- 6.Watanabe Y, Yuzawa Y, Mizumoto D, Tamai H, Itoh Y, Kumon S, Yamazaki C. Long-term follow-up study of 268 diabetic patients undergoing haemodialysis, with special attention to visual acuity and heterogeneity. Nephrol Dial Transplant. 1993;8(8):725–734. doi: 10.1093/ndt/8.8.725. [DOI] [PubMed] [Google Scholar]
- 7.Conway B, Fried L, Orchard T. Hemoglobin and Overt Nephropathy Complications in Type 1 Diabetes. Annals of Epidemiology. doi: 10.1016/j.annepidem.2007.07.110. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vekasi J, Marton Zs, Kesmarky G, Cser A, Russai A, Horvath B. Hemorheological alterations in patients with diabetic retinopathy. Clinical Hemorheology and Microcirculation. 2001;24:59–94. [PubMed] [Google Scholar]
- 9.Trope G, Lowe G, McArdle B, et al. Abnormal blood viscosity and haemostasis in long standing retinal vein occlusion. Br J Opthalmol. 1983;67:137–142. doi: 10.1136/bjo.67.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piermarocchi S, Segato T, Bertoja H, Midena E, Zucchetto M, Girolami A, Procidano M, Mares M. Branch Retinal Vein Occlusion: the pathogenic role of blood viscosity. Ann Opthalmol. 1990;22:303–311. [PubMed] [Google Scholar]
- 11.Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, Suxuma I, Hashi H, Ojima T, Murakami T, Kobayashi T, Masuda S, Nagao M, Yoshimura N, Takagi H. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353(8):782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 12.Orchard T, Dorman J, Masser R, Becker D, Drash A, Ellis D, LaPorte R, Kuller L. The prevalence of complications in insulin-dependent diabetes mellitus by sex and duration: The Pittsburgh Epidemiology of Diabetes Complications Study-II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 13.Orchard T, Dorman J, Maser R, Becker D, Ellis D, LaPorte R, Kuller L, Drash A. Factors associated with avoidance of severe complications after 25 years of insulin dependent diabetes mellitus: Pittsburgh Epidemiology of Diabetes Complications Study. I. Diabetes Care. 1990;13:741–747. doi: 10.2337/diacare.13.7.741. [DOI] [PubMed] [Google Scholar]
- 14.National Institute of Health, Department of Health, Education and Welfare U.S. Govt. Printing Office 1975; Washington, D.C.: Lipid Research Clinics Program. 1978 (NIH pub no. 75-628)
- 15.Levey A, Bosch J, Lewis B, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Annals of Internal Medicine. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Borhani N, Kass E, Langford H, Payne G, Remington R, Stamler J. The Hypertension Detection and Follow-up Program. Prev Med. 1976;5:207–215. [Google Scholar]
- 17.Lloyd C, Klein R, Maser R, Kuller L, Becker D, Orchard T. The Progression and Incidence of Retinopathy over Two Years: The Pittsburgh Epidemiology of Diabetes Complications Study. J Diabetes Complications. 1995;9:140–148. doi: 10.1016/1056-8727(94)00039-q. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Klein B, Moss S, Cruickshanks K. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year Incidence and Progression of Diabetic Retinopathy and Associated Risk Factors in Type 1 Diabetes. Opthalmology. 1998;105:1801–1815. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 19.Ellis D, Buffone G. A new approach to the evaluation of proteinuric states. Clin Chem. 1977;23:660–670. [PubMed] [Google Scholar]
- 20.Harrell F. Survrisk.pak with %SPLINET MACRO. 1994 http://biostat.mc.vanderbilt.edu/twiki/pub/Main/SasMacros/survrisk.txt.
- 21.Nathan DM, Zinman B, Cleary PA, Backlund J-YC, Miller R, Orchard TJ. Modern-Day Clinical Course of Type 1 Diabetes Mellitus. The DCCT/EDIC Experience 1983-2008. Diabetologia. 2008;51(Suppl 1):S50A. [Google Scholar]
- 22.Craig K, Williams J, Riley S, Smith H, Owens D, Worthing D, Cavil I, Philips A. Anaemia and Diabetes in the Absence of Nephropathy. Diabetes Care. 2005;28:1118–1123. doi: 10.2337/diacare.28.5.1118. [DOI] [PubMed] [Google Scholar]
- 23.Thomas M, MacIsaac R, Tsalamandris C, Molyneaux L, Goubina I, Fulcher G, Yue D, Jerums G. Anemia in patients with type 1 diabetes. J Clin Endocrinol Metab. 2004;89(9):4359–63. doi: 10.1210/jc.2004-0678. [DOI] [PubMed] [Google Scholar]
- 24.Thomas M, MacIsaac R, Tsalamandris C, Power D, Jerums G. Unrecognized Anaemia in Patients with Diabetes. Diabetes Care. 2003;26:1164–1169. doi: 10.2337/diacare.26.4.1164. [DOI] [PubMed] [Google Scholar]
- 25.Thomas S, Rampersad M. Anaemia in Diabetes. Acta Diabetol. 2004;41:S13–S17. doi: 10.1007/s00592-004-0132-4. [DOI] [PubMed] [Google Scholar]
- 26.Cusick M, Chew E, Hoogwerf B, Agron E, Wu L, Lindley A, Ferris F, Early Treatment Diabetic Retinopathy Study Research Group Risk factors for renal replacement therapy in the Early Treatment Diabetic Retinopathy Study (ETDRS), Early Treatment Diabetic Retinopathy Study Report No. 26. Kidney Int. 2004;66(3):1173–1179. doi: 10.1111/j.1523-1755.2004.00869.x. [DOI] [PubMed] [Google Scholar]
- 27.Rampersad M, Fry J, Gnudi L, Watkins P, Thomas S. Erythropoietin deficiency anaemia is common in early diabetic disease. Diab Med. 2002;19(2S):1–27. A9. [Google Scholar]
- 28.Fischer H, Schroeter W, Nauck M. Anemia in Patients with Type 1 and Type 2 Diabetes. Diabetes. 2005;54(Suppl 1):A832. [Google Scholar]
- 29.Orchard T, Conway B. Are Hemoglobin Levels Elevated in Type 1 Diabetes?. Oral presentation at the 43rd Annual Meeting of the European Diabetes Epidemiology Group of the EASD; April 2008. [Google Scholar]
- 30.Pichler G, Urlesberger B, Jirak P, Zotter H, Reiterer, Muller W, Borkenstein M. Reduced forearm blood low in children and adolescents with type 1 diabetes. Diabetes Care. 2004;27:1942–1946. doi: 10.2337/diacare.27.8.1942. (measured by near-infrared spectroscopy) [DOI] [PubMed] [Google Scholar]
- 31.Krantz S, Jacobson L. Erythropoietin and the Regulation of Erythropoiesis. Chicago; The University of Chicago Press: 1970. [Google Scholar]
- 32.Haffner S, Klein R, Dunn J, Moss S, Klein B. Increased testosterone in type 1 diabetic subjects with severe retinopathy. Opthalmology. 1990;97(10):1270–4. doi: 10.1016/s0161-6420(90)32428-4. [DOI] [PubMed] [Google Scholar]
- 33.Haffner S, Klein R, Dunn J, Klein B. Sex Hormones and the incidence of severe retinopathy in male subjects with type 1 diabetes. Opthalmology. 1993;100(1):1782–6. doi: 10.1016/s0161-6420(93)31398-9. [DOI] [PubMed] [Google Scholar]
- 34.Chaurasia R, Singh R, Agrawal J, Maurya O. Sex hormones and diabetic retinopathy. Ann Opthalmol. 1993;25(6):227–30. [PubMed] [Google Scholar]
- 35.Al-Khoury S, Afzali B, Thomas S, Gusbeth-Tatomir, Goldsmith D, Covic A. Diabetes, kidney disease, and anaemia: time to tackle a troublesome triad? Int J Clin Pract. 2007;61:281–289. doi: 10.1111/j.1742-1241.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 36.Romagnoli C, Zecca E, Gallini F, Girlando P, Zuppa A. Do recombinant human erythropoietin and iron supplementation increase the risk of retinopathy of prematurity? Eur J Pediatr. 2000;159:627–634. doi: 10.1007/pl00008390. [DOI] [PubMed] [Google Scholar]
- 37.Brown M, Baron A, France E, Hamman R. Association between higher cumulative doses of recombinant erythropoietin and risk for retinopathy of prematurity. Journal of AAPOS. 2006;10(2):143–149. doi: 10.1016/j.jaapos.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Takagi H, Watanabe D, Suzuma K, Kurimoto M, Suzuma I, Ohashi H, Ojima T, Murakami T. Novel role of erythropoietin in proliferative retinopathy. Diabetes Res Clin Pract. 2007;77(S1):S62–4. doi: 10.1016/j.diabres.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 39.Diskin C, Stokes T, Dansby L, Radcliff L, Carter T. A hypothesis: Can Erythropoietin Administration Affect the Severity of Retinopathy in Diabetic Patients with Renal Failure? Am J of Med Sci. 2007;334(4):260–264. doi: 10.1097/MAJ.0b013e3180a5e8ed. [DOI] [PubMed] [Google Scholar]
- 40.Drueke T, Locatelli F, Clyne N, Eckardt K, MacDougall I, Tsakiris D, Burger H, Scherhag A, the CREATE Investigators Normalization of Hemoglobin in Patients with Chronic Kidney Disease and Anemia. NEJM. 2006;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 41.Singh A, Szczech L, Tang K, Barnhart H, Sapp S, Wolfson M, Reddan D, the CHOIR Investigators Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 42.Turcyzynski B, Michalska-Malecka K, Slowinska L, Szczesny S, Romaniuk W. Correlations between the severity of retinopathy in patients with diabetic retinopathy and whole blood and plasma viscosity. Clinical Hemorheology and Mircrocirculation. 2003;29:129–137. [PubMed] [Google Scholar]
- 43.Little H, Sacks A. Role of Abnormal Blood Rheology in the Pathogenesis of Diabeteic Retinopathy. Transactions of the American Academy of Opthalmology and Otolaryngology. 1977;83:522–534. [PubMed] [Google Scholar]
- 44.McMillan D. Plasma protein changes, blood viscosity, and diabetic microangiopathy. Diabetes. 1976;25(S2):858–864. [PubMed] [Google Scholar]
- 45.Lip L, Blann A, Jones A, Lip G. Abnormalities in haemorheological factors and lipoprotein (a) in retinal vein occlusion: impications for increased vascular risk. Eye. 1998;12:245–251. doi: 10.1038/eye.1998.58. [DOI] [PubMed] [Google Scholar]
- 47.Tong Z, Yang Z, Patel S, et al. Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proc Natl Acad Sci. 2008;105(19):6998–7003. doi: 10.1073/pnas.0800454105. [DOI] [PMC free article] [PubMed] [Google Scholar]