Abstract
Purpose
GM-CSF administered locally together with vaccines can augment T cell responses in animal models. Human experience has been limited to small and uncontrolled trials. Thus, a multicenter randomized phase II trial was performed to determine whether local administration of GM-CSF augments immunogenicity of a multipeptide vaccine. It also assessed immunogenicity of administration in one vs. two vaccine sites.
Experimental Design
121 eligible patients with resected stage IIB-IV melanoma were vaccinated with 12 MHC Class I-restricted melanoma peptides (12MP) to stimulate CD8+ T cells, plus an HLA-DR restricted tetanus helper peptide to stimulate CD4+ T cells, emulsified in incomplete Freund’s adjuvant, with or without 110 mcg GM-CSF. Among 119 evaluable patients, T cell responses were assessed by IFN-gamma ELIspot assay and tetramer analysis. Clinical outcomes were recorded.
Results
CD8+ T cell response rates to the 12MP (by day 50), with or without GM-CSF were 34% and 73%, respectively (p<0.001) by direct ELIspot assay. Tetramer analyses corroborated the functional data. CD4+ T cell responses to tetanus helper peptide were higher without GM-CSF (95% vs. 77%, p=0.005). There was no significant difference by number of vaccine sites. Three-year overall and disease-free survival estimates [95% CI] were 76% [67, 83%] and 52% [43, 61%] respectively, with too few events to assess differences by study group.
Conclusions
High immune response rates for this multipeptide vaccine were achieved, but CD8+ and CD4+ T cell responses were lower when administered with GM-CSF. These data challenge the value of local GM-CSF as a vaccine adjuvant in humans.
Keywords: Immunotherapy, Human, Cytokines, T-Lymphocytes, Cytotoxic, Melanoma/im, Antigens, Neoplasm, Tumor vaccines
Introduction
Vaccines of all kinds use adjuvants to increase immune responses; however, the molecular and cellular mechanisms of adjuvants are poorly understood. Mechanisms believed to be important include dendritic cell activation and maturation, and activation of innate immunity through toll-like receptor (TLR) activation. The cytokine GM-CSF has been reported to have potent adjuvant effects, presumed to be mediated through effects on dendritic cells and other antigen-presenting cells. In murine studies, GM-CSF has been an effective adjuvant for cancer and viral vaccines. A syngeneic whole cell vaccine for melanoma was most effective at inducing protective immunity when B16 melanoma cells were transduced with a gene encoding GM-CSF.1 Similarly, immunogenicity of a peptide vaccine for HIV was augmented by inclusion of GM-CSF in the adjuvant emulsion in which the peptides were administered.2
In humans, we found that vaccination with peptides in an emulsion of GM-CSF plus incomplete Freund’s adjuvant (IFA) was more immunogenic than vaccination with peptides pulsed on dendritic cells.3 Other peptide and cell-based vaccines have been administered with local GM-CSF, with encouraging immunologic and clinical outcomes.4-8 On the other hand, a study comparing several adjuvants in sequentially treated cohorts challenged the adjuvant effect of GM-CSF 9. In another study, adding GM-CSF to an adjuvant appeared to increase CXCR3 expression by vaccine-induced T cells.10 The present study was designed to test formally whether immunogenicity of a multipeptide vaccine emulsion was increased by including GM-CSF in the vaccine emulsion. The vaccine includes 12 melanoma peptides restricted by Class I MHC molecules (12MP) plus a tetanus helper peptide restricted by HLA-DR molecules. Thus, this study was designed to test effects of GM-CSF on both CD8+ and CD4+ T cell responses to a synthetic vaccine. The study also was designed to address whether vaccination at one versus two extremity sites affects vaccine immunogenicity.
Materials and Methods
Patients
Patients with resected AJCC stage IIB-IV melanoma arising from cutaneous, mucosal or unknown primary sites were eligible. Inclusion criteria included expression of HLA-A1, A2, or A3 (~80% of patients screened, data not shown), age 12 years and above, ECOG performance status 0-1, adequate liver and renal function, and ability to give informed consent. Exclusion criteria included: ocular melanoma; pregnancy; cytotoxic chemotherapy, interferon, or radiation within the preceding 4 weeks; known or suspected allergies to vaccine components; multiple brain metastases; use of steroids; Class III-IV heart disease; or systemic autoimmune disease with visceral involvement. Patients were studied following informed consent, and with Institutional Review Board (HIC#10524) and FDA approval (BB-IND #9847).
Vaccine composition
All patients received a vaccine comprising 12 melanoma peptides restricted by HLA-A1, -A2, or -A3, as described 11: A1 peptides: DAEKSDICTDEY (Tyrosinase 240-251, which has a substitution of S for C at residue 244), SSDVIPIGTY (Tyrosinase 146-156), EADPTGHSY (MAGE-A1 161-169), EVDPIGHLY (MAGE-A3 168-176); A2 peptides: YMDGTMSQV (Tyrosinase 369-377D), IMDQVPFSV (gp100 209-217, 209-2M), YLEPGPVTA (gp100 280-288), GLYDGMEHL (MAGE-A10 254-262); and A3 peptides: ALLAVGATK (gp100 17-25), LIYRRRLMK (gp100 614-622), SLFRAVITK (MAGE-A1 96-104 ), and ASGPGGGAPR (NY-ESO-1 53-62). The NY-ESO-1 peptide ASGPGGGAPR, originally reported to be immunogenic in association with HLA-A31, is naturally processed and presented also by HLA-A*0301 (A3) on human cells.12 The tetanus helper peptide used was AQYIKANSKFIGITEL.13 This combination of 12 Class I MHC restricted peptides plus 1 Class II restricted peptide is referred to as MELITAC 12.1.
Peptides for vaccines were synthesized and purified (> 95%) under GMP conditions (Multiple Peptide Systems, now NeoMPS, San Diego, CA). After solubilization, each peptide was sterile-filtered, mixed, vialed and lyophilized under GMP conditions by Merck Biosciences AG Clinalfa (Läufelingen, Switzerland) in single-use vials. Vials were submitted to quality-assurance studies including sterility, identity, purity, potency, general safety, pyrogenicity, and stability in accordance with CFR guidelines and BB-IND #9847.
Each vaccine was 2 ml of a stable water-in-oil emulsion consisting of 100 mcg of each of the 12 Class I MHC restricted peptides, 190 mcg of the tetanus helper peptide, and 1 ml Montanide ISA-51 adjuvant (Seppic, Inc., Paris, France/ Fairfield, NJ). For patients in arms B and D, the emulsion also contained 110 mcg GM-CSF (Leukine/Sargramostim; Berlex, Seattle, WA; now Bayer). For arms A and B, the full emulsion was administered to one extremity skin location, whereas for arms C and D, the emulsion was divided in half, with half delivered to each of two extremities. The emulsions were made by use of the two-syringe method. A drop of each emulsion was tested for stability in water. The vaccines were administered on days 1, 8, 15, 29, 36, and 43 (weeks 0, 1, 2, 4, 5, and 6), then at 3, 6, 9, and 12 months. At each injection site, half of the dose was administered subcutaneously (s.c.), and half intradermally (i.d.).
Clinical trial design
Primary objectives of this study were to estimate: (1) whether GM-CSF administered locally changes the immunogenicity of vaccination with multiple synthetic melanoma peptides in an emulsion with incomplete Freund’s adjuvant, and (2) whether vaccination at two extremity sites induces different immunogenicity than vaccination at a single site. Secondary goals were to obtain preliminary data on (3) whether booster vaccination every three months maintains immune responses to a peptide vaccine, and (4) whether cellular immune responses to a 12-peptide vaccine correlate with clinical outcome. This manuscript addresses the first two objectives.
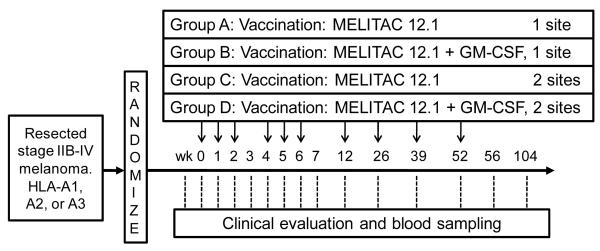
This was a phase II study with randomization to one of four vaccine regimens. A 2×2 factorial design was used to obtain preliminary estimates of possible difference in measures of immune response by factor (a) inclusion/exclusion of GM-CSF and factor (b) number of vaccination sites (1 or 2). It was assumed that there were no clinical or statistical interactions between the two factors. Patients were stratified on stage of disease (IIB/C vs. III vs. IV), and randomization was based on a random assignment within strata with varying block sizes. This is presented schematically in Figure 1.
Figure 1.
Mel43 protocol schema
For this study, we were interested in detecting at least a 2 fold difference in maximum immune response within a patient, between the two primary study groups (GM-CSF vs. not; 1 vs. 2 vaccine sites). Sample size determination was based upon maximum immune response within a patient. For design and analysis purposes, the natural logarithm (In) transformation is used to stabilize the variance. Assuming equal variances, normality and equal standard deviations of In(fold change) of 1.29, approximately 29 eligible patients per arm (116 total) were required to detect at least a 2-fold change in maximum immune response using a t-test at the two-sided 5% level of significance with approximately 80% power. Adjustment of the sample size for a 7.5% ineligibility rate resulted in a target accrual goal of 125 patients. Patients were accrued at the University of Virginia and at 4 other participating institutions.
Toxicity assessment and stopping rules
The trial was monitored continuously for treatment-related adverse events, using NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Dose-modification of GM-CSF was indicated if grade 3 adverse events occurred, other than grade 3 local vaccine site reactions. Protocol treatment was to be discontinued for any grade IV toxicity or for grade 3 toxicities that failed to respond to dose reductions of GM-CSF. Toxicities were recorded by patients using a daily toxicity diary, reviewed by interview with a study clinician weekly. Patients with disease progression requiring other therapy were removed from treatment.
Endpoints for the study
The primary endpoint was the CD8+ T cell response to the 12 melanoma peptides in the vaccine, over the first six vaccines (to day 50). T cell response was assessed by using both a binary indicator of immune response (yes/no) and a continuous measure based upon amount of increase over background (fold-increase) relative to any prevaccine reactivity. Secondary endpoints included CD8+ T cell responses to the 12 melanoma peptides during and after the booster vaccines (> day 50 to 2 years), (2) T cell response to the tetanus helper peptide, (3) disease-free survival and (4) survival.
Collection of peripheral blood mononuclear cells (PBMC)
Peripheral blood (60 ml) was drawn into heparinized tubes for isolation of PBMC, and 10 ml was drawn for serum studies at the time points shown in Figure 1. Lymphocytes were isolated using Ficoll gradient centrifugation, and cryopreserved in 10% DMSO/90% serum by the Tissue Procurement Facility at the University of Virginia. Blood drawn at other participating institutions was shipped in insulated containers near room temperature for overnight delivery and was processed as above.
ELIspot assays
ELIspot assays were performed directly ex vivo, after cryopreservation (direct ELIspot) or after one in vitro sensitization (stimulated ELIspot). Methods for the stimulated ELIspot assay have been reported.11 For direct ELIspot assays, 200,000 PBMC were plated per well, and pulsed with synthetic peptide (10 mcg/ml), in quadruplicate. Controls included irrelevant peptides, a mixture of viral peptides (CEF peptide pool), PMA-ionomycin and PHA. Assessment of immunologic response was based upon a fold-increase over the maximum of two negative controls. Evaluation of T-cell responses was based on the following definitions: Nvax = number T-cells responding to vaccine peptide; Nneg = number T-cells responding to maximum negative control; Rvax = Nvax/Nneg. For evaluations of PBMC, a patient was considered to have a T-cell response to vaccination (binary yes/no), by direct ELIspot assay only if all of the following criteria were met: (1) Nvax exceeded Nneg by at least 20 cells / 100,000 CD4+ or CD8+ cells (0.02%), where CD8 and CD4 counts were based on flow cytometric evaluations of the PBMC samples. (2) Rvax ≥ 2, (3) (Nvax − 1 SD) ≥ (Nneg + 1 SD), and (4) Rvax after vaccination ≥ 2 × Rvax pre-vaccine, as described. The same criteria applied for stimulated ELIspot assays except that the threshold for criterion (1) was higher: such that Nvax had to exceed Nneg by at least 100 cells / 100,000 CD4+ or CD8+ cells (0.1%). Fold-increases less than one (e.g., control counts exceed number of responding T-cells, or fold response compared to baseline is less than one) were set equal to one to indicate no response and to prevent overinflating adjusted fold-increases due to pre-vaccine ratios less than one, or division by zero, while not affecting the determination of response. These methods are consistent with our prior analyses. 14 Continuous measures of immune response denoted as fold-increase must satisfy conditions (1)-(3), and were defined as the amount of Rvax. Cumulative response over all HLA-appropriate peptides, CumRtime, was defined, at each time point, as 1 + the sum of fold-increase exceeding 1 over all patient-specific peptides (eg. at week 3, CumR3 = 1 + (sum over each (Rvax −1) for each peptide for which a response was detected)). When making comparisons across HLA types, this cumulative response is also calculated for the four peptides restricted by each HLA-Class I allele. When making comparisons across patients overall, this is calculated for all HLA-appropriate peptides in the 12MP, which may be 4 or 8 peptides, depending on HLA type.
Interassay CVs were calculated for each of 6 normal donors in their response to the CEF peptide pool. A high responder and a low responder were tested in each assay. For three high responder normal donors (mean number of spots per 100,000 cells plated ranged 123 to 484), CVs were 8%, 24%, 31%. For three low normal donors (means 54-128), CVs were 28%, 30%, and 61%. The weighted mean CVs were 17% for high responders and 34% for low responders. Primary analyses were based upon eligible patients. Maximal response was based upon responses in the blood to the first 6 vaccinations (through day 50). For hypothesis testing, patients who discontinued protocol therapy prior to collection of all blood samples for allergic reactions or adverse events, disease progression, or noncompliance were considered immune response failures if no response was observed in evaluable samples. Immune response was a binary indicator of whether or not the criteria listed above were met, and immune response rates were calculated as the proportion of participants with an immune response. Point estimates and 95% confidence intervals were calculated for all summary parameters. Differences between groups by binary measures of immune response and cumulative response measures were assessed by Chi-square tests and t-tests, respectively.
Analysis for antigen-specific T cells using MHC-peptide tetramers
Enumeration of antigen-specific T cell responses was performed using tetrameric HLA class I/peptide reagents (tetramers, Beckman Coulter, Inc., San Diego, CA). HLA-A2 tetramers used were: MAGE A10254-262 GLYDGMEHL, gp100209-217 ITDQVPFSV, gp100280-288 YLEPGPVTA, and Tyrosinase369-377D YMDGTMSQV; A3 tetramers used were: gp10017-25 ALLAVGATK and gp100614-622 LIYRRRLMK. Tetramers for the other 6 peptides were not studied: two do not recognize functional antigen-reactive T cells (DAEKSDICTDEY, SLFRAVITK); one could not be synthesized (ASGPGGGAPR); two others were not utilized because of the low rates of T cell response observed in ELIspot assay (EADPTGHSY, SSDVIPIGTY), and one worked, but was not evaluated (EVDPIGHLY). Samples from weeks 0 and 6 were selected; if week 6 samples were not available, samples from week 5 or 7 were used. After thawing, PBMC were enriched for CD8+ cells (Miltenyi Biotec, Inc., Auburn CA), washed twice, suspended in PBS/2% FCS (Sigma-Aldrich, St. Louis, MO) and incubated with tetramer for 15 min at room temperature before adding a mixture of CD8 FITC (Clone: SFCI21Thy2D3), CD4 PE-Cy5, CD13 PE-Cy5, and CD19 PE-Cy5 (Beckman Coulter). Samples were transferred to 4°C, incubated 30 min, and washed. Cells were incubated in 7-AAD (Calbiochem, San Diego CA 15) 20 min, washed, fixed in 0.5% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) containing actinomycin D (Calbiochem 16) and acquired on a CyAn™ ADP LX 9 Color flow cytometer (DakoCytomation/Coulter) maintained by the Flow Cytometry Core Facility at the University of Virginia. Gates were set on singlet viable CD8+ T cells. Data were analyzed with FloJo (Treestar, Ashland OR) software. Proportion tetramer-positive cells were calculated as (number tetramer+CD8+ cells)/(total number CD8+ T cells), although it was the square root transformation of this quantity that was used for statistical analysis. Changes were summarized as pre-to-post changes for each peptide and negative control, and cumulatively by summation of changes for each peptide tetramer, across each HLA-A allele. T-tests were performed to determine whether changes in tetramer proportions differed by groups.
Clinical outcome
The product-limit method was used to estimate two and three year survival and disease free survival rates.
Results
Eligibility review
125 patients were enrolled. Four (3.2%) were found ineligible on post-review (low platelet counts (n=2), palpable disease developed before first treatment (n=1), and insufficient time between radiation therapy and registration (n=1)). Analyses of immune response and clinical outcome are limited to 121 eligible patients, which exceeds the target of 116 eligible patients. Patient demographics and clinical presentations were similar across study arms (Supplemental Table 1). Median age was 55, 69% were male and 0.8% African/American; Stage at enrollment was IIB-IIC (14%), IIIA (16%), IIIB/C (55%), or IV (16%). ECOG performance status was 0 in 88% and 1 in 12%.
Summary of clinical toxicities
Treatment-related adverse events are detailed in Supplemental Table 2 for 124 treated patients and were similar to those from our prior experience.11 There were no treatment-related grade 4 toxicities or treatment related deaths. There were no deaths on study, but one patient died approximately 30 days after coming off study because of tumor progression. Nine patients (7%) had unexpected grade 3 treatment-related adverse events requiring IRB reporting: arm A: fever (1); arm B: syncope (1); arm C: rash (2), headache/myalgias/arthralgia (1), injection site reaction with pain (1), fever/cellulitis (1); arm D; fatigue (1), and diarrhea (1). Grade 3 injections site reactions occurred in 6, 9, 13, and 6 patients, respectively, for arms A-D, representing 24% and 31% of those with 1 and 2 vaccine sites, respectively, and 31% and 24% of those without and with GM-CSF, respectively. Arm C patients had the highest estimated rate of treatment-related grade 3 adverse events, but there were no statistical differences in these grade 3 toxicities when arms were evaluated in pairs by the use of GM-CSF or number of vaccine sites.
Seventy-seven patients (64%) completed all protocol treatment. Forty-four came off study before completion of all 10 vaccines (within 1 year of study entry): 38 for disease progression (31%), 4 for adverse events (3%), 1 for refusing further therapy (1%), and 1 for non-compliance (1%). There were no significant differences in rates of completion of treatment or in interrupting treatment for disease progression, between groups (GM-CSF or not; 1 vs 2 vaccine sites). All four patients who stopped treatment early for adverse events were in arm C (no GM-CSF, 2 vaccine sites).
Autoimmune toxicities
Autoimmune toxicities were observed in 27 patients (24%), including increased ANA serum levels (8 patients, 7%), increased RF serum levels (7 patients, 6%), vitiligo (12 patients, 10%), and other autoimmune reactions (15 patients, 14%). These were similar for all study groups: 22% and 26% for those without and with GM-CSF, respectively, and 21% and 27% for 1 or 2 vaccine sites, respectively.
CD8 T cell responses to Class I MHC restricted peptides: direct ELIspot assay
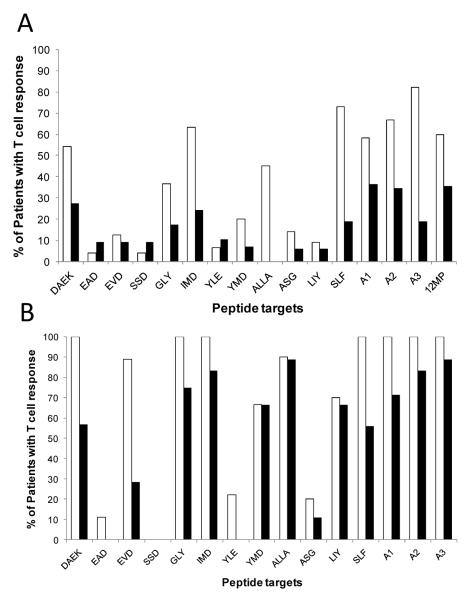
Of 121 eligible patients, 119 (98%) were evaluable for immune responses in PBMC. Immune responses were evaluated by ELIspot assay for production of IFN-gamma in response to each of the 12 peptides restricted by Class I MHC alleles expressed by the patient. ELIspot data are summarized in Supplemental Table 3. These are analyzed based on the fold-increase over background, corrected for any pre-vaccine response. Considering the complete patient population, each of the 12 peptides was immunogenic, with immune response rates ranging from 7 to 50% for each peptide, measured by direct ELIspot assay (Supplemental Table 3). Among the 12 peptides, 8 were immunogenic in more than 10% of patients, and those 8 were more immunogenic for the patients in arms A and C (no GM-CSF) than those in arms B and D (GM-CSF).(Figure 2A) Immune response rates for subsets of 4 peptides relevant for each HLA-A allele (A1, A2, and A3) also were higher for vaccines without GM-CSF, as was the response to the mixture of 12MP in vitro assayed in a subset of 66 patients.(Figure 2A) For patients vaccinated without GM-CSF, immune response rates for the 12 peptides ranged from 4 to 73% (Supplemental Table 3, Figure 2A), whereas they ranged from 0 to 27% for those vaccinated with GM-CSF (Figure 2A). Immune reactivity was greatest to the HLA-A3-restricted peptides, with 82% in arms A and C having reactivity to one or more of the A3 peptides.
Figure 2.
Immune response rates by peptide (3-4 letter abbreviation), by HLA allele (A1, A2, or A3), and overall (12MP) for patients evaluated by direct ELIspot assay (A) or by Stimulated ELIspot assay (B). The proportion of patients meeting criteria for an ELIspot response are plotted for those in Groups A and C (no GMCSF — white bars) and those in Groups B and D (with GM-CSF — black bars). Response rates to each of the 12 HLA-class I peptides are shown, along with data for response rates to one or more peptides per HLA Class I allele (-A1, A2, or A3) or, for the direct ELIspot, to one or more of the 12MP.
Overall, 54% of patients responded to at least one of the 12 melanoma peptides, and the response rate was significantly higher for vaccines without GM-CSF than for those with GM-CSF (73%, 34%, respectively, p < 0.001, chi-square, Table 1). There was a trend to a better response rate with vaccines at two sites rather than one (p = 0.093, chi-square).
Table 1.
Measures of immune response to one or more of the 12 melanoma peptides (Sum 12MP), and to the tetanus helper peptide (119 evaluable patients) - direct ELIspot data through day 50.
| Antigen | Group | N (N pos) | Rate (%) | Median cum. fold increase (median of positives) |
P (Chi-square for rates) |
|---|---|---|---|---|---|
| 12MP | No GM | 60 (44) | 73 % | 5.8 (11.6) | <0.001 |
| 12MP | GM-CSF | 58 (20) | 34 % | 1.0 (7.9) | |
| 12MP | 1 site | 60 (28) | 47 % | 1.7 (10.9) | 0.093 |
| 12MP | 2 sites | 58 (36) | 62 % | 3.4 (7.9) | |
| Tetanus | No GM | 61 (58) | 95 % | 35.4 (38.6) | 0.005 |
| Tetanus | GM-CSF | 57 (44) | 77 % | 10.6 (20.2) | |
| Tetanus | 1 site | 59 (53) | 90 % | 21.3 (23.5) | 0.28 |
| Tetanus | 2 sites | 59 (49) | 83 % | 14.3 (24.8) |
CD8+ T cell responses to Class I MHC restricted peptides: stimulated ELIspot assay
Stimulated ELIspot assays are expected to be more sensitive than direct ELIspot assays and may be compared to our prior experience 3;11;17. They were performed on a subset of 45 patients on this study. Example data for one patient show transient responses by direct ELIspot for some peptides, but a durable response to another peptide (Figure 3A), whereas more of those responses were durable in stimulated ELIspot assays (Figure 3B). Raw data and controls for this patient are provided in Supplemental Figure 1. Results of the stimulated assays also reveal numerically higher immune response rates in the arms without GM-CSF than in those with GM-CSF (Figure 2B, Supplemental Table 3), with immunogenicity rates of 90-100% for 5 of the 12 peptides: Tyrosinase 240-251S (DAEKSDICTDEY), MAGE-A10 254-262 (GLYDGMEHL), gp100 209-2M (IMDQVPFSV), gp10017-25 (ALLAVGATK), and MAGE-A1 96-104 (SLFRAVITK). Immune responses rates across each HLA-A allele were 100% for patients vaccinated without GM-CSF but were only 71%, 83%, and 89%, respectively for those with GM-CSF (Figure 2B).
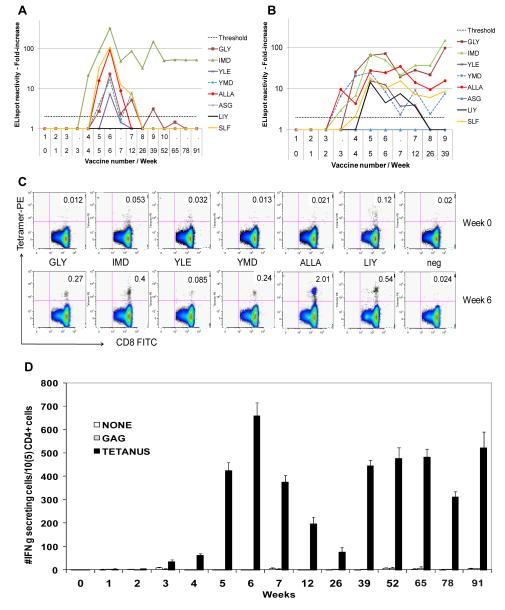
Figure 3. Immunologic data for an immune responder VMM526.
Summary data for the CD8+ T cell reactivity to 8 peptides evaluated for this patient are shown for the calculated fold increase over time, for direct ELIspot data (A) and stimulated ELIspot data (B). The horizontal dotted line represents the 2-fold increase threshold for a positive response. Flow cytometry data on weeks 0 and 6, for tetramer+ cells are shown in (C) for the 6 evaluable tetramers plus negative control tetramer. Numbers in the top right quadrant of each plot represent the percent of CD8+ cells staining with the tetramer. CD4+ T cell responses to the tetanus helper peptide are shown in (D), as measured by direct ELIspot assay.
CD8+ T cell responses to HLA-A-restricted peptides - Tetramer analyses
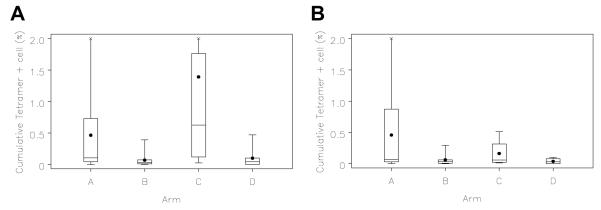
For 6 of the 12 MHC-Class I-restricted peptides, responses were also assessed by quantification of CD8+ T cells staining with MHC Class I tetramers. In some patients, tetramer + T cells approached and exceeded 1% of circulating CD8+ T cells (examples, Figure 3C). Summary data are presented in Figure 4 for each of the 4 study arms. Higher tetramer-detected responses were in patients in arms A and C (without GM-CSF) , for 4 of the 6 tetramers studied, for HLA-A2 peptides overall (p<0.001), and with a strong trend for HLA-A3 peptides overall (p = 0.053), . (Table 2)
Figure 4. Tetramer data Mel43 through day 50, sum of responses to evaluated MHC Class I-restricted peptides.
Peripheral blood mononuclear cell staining for MHC-peptide tetramers reactive to T-cell receptors for (A) 4 HLA-A2 associated peptides (n = 48-53 each) or for (B) 2 HLA-A3 associated peptide (n = 36). Box plots represent the cumulative percent of tetramer+ T cells, among CD8+ cells, for the 4 HLA-A2 & 2 HLA-A3 peptide tetramers by study arm, respectively. Values represent the difference between post vaccine and prevaccine values for each peptide (corrected to 0 if negative), summed among peptides subsetted by . Negative controls were consistently low with median approximately 0.02%. The boxes represent the 25th-75th percentile values, with the mean (symbol) and median (horizontal line) marked, and with the maximum and minimum values at the end of the stems at either end of the boxes. X marks represent high maximal values clipped for panel (A) 2.55% for arm A and 7.02% for arm C, and for panel (B) 2.41% for arm A.
Table 2.
Measures of immune response to 6 melanoma peptides by median change in percentage of tetramer-positive cells from week 0 to week 6.
| No GM-CSF | with GMCSF | P value for differences by group |
||||
|---|---|---|---|---|---|---|
| Antigen | N | Median | N | Median | GM-CSF | # sites |
| GLY | 25 | 0.020 | 26 | 0.002 | 0.011 | 0.53 |
| IMD | 26 | 0.081 | 26 | 0.011 | 0.004 | 0.91 |
| YLE | 24 | 0.002 | 24 | 0 | 0.14 | 0.036 |
| YMD | 26 | 0.024 | 26 | 0.004 | 0.005 | 0.18 |
| ALLA | 21 | 0.046 | 15 | 0.016 | 0.014 | 0.47 |
| LIY | 21 | 0.025 | 15 | 0 | 0.10 | 0.31 |
| Neg | 46 | 0 | 45 | 0 | 0.76 | 0.22 |
| A2 | 26 | 0.23 | 26 | 0.04 | <0.001 | 0.49 |
| A3 | 21 | 0.07 | 15 | 0.03 | 0.053 | 0.64 |
Differences between postvaccine and prevaccine tetramer+ cells are presented as a percentage of CD8+ cells, and are greater than 0 if there was an increase after vaccination. P values for the differences between groups are shown when considering the role of GM-CSF or of the number of vaccine sites. Allowing a limited correction for multiple comparisons, those with p values ≤ 0.025 are underlined.
CD4+ T cell responses to HLA-DR restricted tetanus helper peptide — direct ELIspot assay
The tetanus helper peptide (AQYIKANSKFIGITEL) was used in all patients, and Th1 CD4+ T cell responses were measured by direct IFN-γ ELIspot assay. Responses were detected in 86% of patients, with higher response rates in patients vaccinated without GM-CSF than with GM-CSF (95% vs. 77%, p = 0.005, Table 1). There were no significant differences in CD4+ immune response rates in patients with vaccination based on the number of vaccine sites (p = 0.28, Table 1).
Clinical outcome
For the entire study population, two-year overall and disease-free survival estimates [and 95% CI] were approximately 84% [76, 89%] and 56% [47, 64%] (data not shown). Three-year overall survival and disease free survival estimates were 76% [67, 83%] and 52% [43, 61%] respectively. There have been too few events to assess differences in clinical outcome by study group at this time.
Discussion
In prior work, we found that melanoma vaccines using mixtures of synthetic MHC-associated peptides are immunogenic, and that 10 of the 12 melanoma peptides in MELITAC 12.1 were individually immunogenic.11 The present study enrolled 119 eligible patients who were evaluable for immune responses, and each of the 12 peptides was immunogenic, when assayed directly, without ex vivo stimulation. However, some were weakly immunogenic, and those patterns match our prior studies. Overall, 54% of patients developed immune responses detectable by direct ELIspot assay, which compares favorably to a 37% immune response rate measured in a multicenter clinical trial using three HLA-A2 peptides, using a very similar assay system.18 For the 61 patients vaccinated with peptides in Montanide ISA-51 without GM-CSF, immune responses to the 12 melanoma peptides were detected in 73% of patients (Table 1), which exceeds response rates in prior studies using direct ELIspot.
Also, in prior work, we used an ELIspot assay on lymphocytes stimulated ex vivo, and immune responses detected using this assay did correlate with disease-free survival.11 A direct ELIspot assay is widely considered a more biologically relevant assay; however, that remains to be determined. A stimulated ELIspot assay may be relevant in measuring, in part, proliferative capacity of T cells, which may have some connection with memory responses. In a subset of patients on this trial (n = 45), we performed stimulated ELIspot assays also, which were associated with immune response rates on the order of 90% for the study overall, and 100% for patients vaccinated without GM-CSF (Table 2). In those assays, even for individual peptides, immune response rates were 100% for 4 peptides and close to 90% for two others (Figure 2B, Supplemental Table 3). These findings highlight the immunogenicity of peptide vaccines administered in an incomplete Freund’s adjuvant.
One question addressed by the present study is whether vaccination in two extremity sites leads to better immune responses than vaccination in just one site. There was a trend to greater CD8+ T cell response with 2 vaccine sites, and a weak trend to a greater CD4+ T cell response with one vaccine site, neither of which was statistically significant (Table 1). Thus, we conclude that there is insufficient evidence in the present data to claim that response rates are increased by vaccinating at two sites. However, other data strongly suggest that trafficking of T cells to tumor may depend on where they are primed by a vaccine 19; thus, there is still more work to do to answer the question of where to vaccinate, depending on the location of melanoma metastases.
The main question that was addressed in the present study was whether GM-CSF administered locally in the vaccine emulsion would increase immunogenicity. Prior murine studies found that this strategy increased immune responses to a peptide vaccine 2, and supported the value of GM-CSF delivered in whole-cell vaccines.1 However, recent studies are conflicting on the role of GM-CSF as an immune potentiator or therapeutic agent.20 GM-CSF administered with a heat shock protein vaccine has been implicated in the induction of myeloid-derived suppressor cells in melanoma patients 21, and GM-CSF at high dose, in murine models, may increase myeloid-derived suppressor cells (MDSC).22 However, daily subcutaneous administration of GM-CSF (125 mcg/m2/d) increased circulating mature dendritic cells (DC), but did not increase MDSC in melanoma patients.23
Prior studies of GM-CSF as a vaccine adjuvant were primarily small non-randomized studies, or did not include defined antigens. We designed the present study to address the role of GM-CSF administered locally as a vaccine adjuvant, in a randomized multicenter design, with adequate power to detect a significant immunologic effect. The resulting data show that the circulating T cell response to the multipeptide vaccine is significantly lower in patients whose vaccines included GM-CSF. This was true both for CD8+ T cell responses to 12 melanoma peptides and for CD4+ T cell responses to a tetanus helper peptide. Reasons for this remain to be defined, but the findings cast doubt on the benefit of GM-CSF protein as local adjuvant. This finding is consistent with a prior small study with local administration of GM-CSF using a different delivery strategy 9. It has been suggested that negative immunologic effects of GM-CSF may be associated primarily with high doses of GM-CSF, in the range of 100-500 mcg/day 20. The dose used in this trial was 110 mcg administered only once a week. Since it was given in a slow-release emulsion, the effective daily dose is arguably less than 20 mcg/day. Thus, even low doses of GM-CSF administered with a multipeptide vaccine may have negative immunologic effects. A possible mechanism by which GM-CSF may interfere with immune function is through milk fat globule EGF-8 (MFG-E8). 24;25 GM-CSF induces expression of MFG-E8 by macrophages and DC; MFG-E8 can maintain regulatory T cells and can downregulate Th1 responses.24;25 This mechanism deserves further investigation as a possible explanation for some negative effects of GM-CSF as an adjuvant. Additional studies are also needed to determine if GM-CSF alters the quality of function of vaccine-induced T cells and whether inclusion of GM-CSF with the vaccine may have an impact on clinical outcome.
In addition to questions about whether GM-CSF may have value as an immune adjuvant, it has been suggested that GM-CSF may have therapeutic value when administered subcutaneously as a single-agent therapy for melanoma 26, and that finding is being tested in a randomized phase III trial of systemic subcutaneous GM-CSF for the adjuvant therapy of high-risk melanoma, alone or combined with a multipeptide vaccine (ECOG trial E4697). That study has been closed to accrual, and final analysis is pending. In a recent non-randomized study, clinical outcome of 39 patients with stage III-IV melanoma treated with subcutaneous GM-CSF has been presented as additional support for the therapeutic potential of single agent GM-CSF.23 However, the results of non-randomized clinical studies of small size has been notoriously subject to selection bias, and the clinical outcome in the present study with and without GM-CSF compares favorably to what was reported in that study 23. With longer follow-up, we will be able to address whether clinical outcome mirrors immunologic outcomes.
In summary, we have found that immunization with the MELITAC 12.1 multipeptide melanoma vaccine is immunogenic in a majority of patients with high-risk melanoma, that vaccination in two extremities does not increase T cell response, and that addition of GM-CSF to the vaccine decreases circulating CD4+ and CD8+ T cell responses to the vaccine. Caution should be taken in future use of GM-CSF as a local vaccine adjuvant.
Supplementary Material
Supplemental Figure 1. ELIspot data and controls for immune responder VMM526. The number of IFN-gamma-secreting cells in direct ELIspot assays are shown for peptides restricted by HLA-A2 (A) and HLA-A3 (B), and for CEF peptide controls (C) per 100,000 CD8+ cells, as well as for PHA and PMA/ionomycin (D) and tetanus helper peptide (E) per 100,000 total PBMC. IVS ELIspot assays were also done for this patient, and the number of IFN-gamma-secreting cells per 100,000 CD8+ cells are shown for peptides restricted by HLA-A2 (F) and HLA-A3 (G). Error bars represent standard deviations of replicate wells.
Acknowledgements
We appreciate the work of Drs Margaret von Mehren (Fox Chase Cancer Center and Joseph Cattlett (Washington Hospital Center), who contributed materially to patient care and evaluation on this study. Particular thanks are due to Patrice Neese and Carmel Nail for administering vaccines and for recording and managing toxicities, Donna Deacon for vaccine preparation, Cheryl Murphy, Kelly Smith, Nadejda Galeassi, Sarah Hibbitts, and Naomi Johansen, and Kim Underwood for outstanding ELIspot assay work, and to Elizabeth Coleman for assistance in data management and data entry for the ELIspot assays. We also acknowledge the careful expertise of Chantel McSkimming and JoAnne Lannigan in the tetramer analyses. The regulatory and auditing work was supported and overseen by a great team including Robyn Fink, Elizabeth Woodson, Sarah Lewis, Christine Schulte, Scott Boerner, Erin Farris, Beverely Turner, and Kim Underwood.
Funding support. This study was funded by NIH/NCI grant R21 CA103528 (to C.L.S). (Principal investigator: Craig L. Slingluff, Jr.) Support was also provided by the University of Virginia Cancer Center Support Grant (NIH/NCI P30 CA44579, Clinical Trials Office, Tissue Procurement Facility, Flow Cytometry Core, and Biomolecular Core Facility); the UVA General Clinical Research Center (NIH M01 RR00847). Peptides used in this vaccine were prepared with philanthropic support from the Commonwealth Foundation for Cancer Research and Alice and Bill Goodwin. Additional philanthropic support was provided from the James and Rebecca Craig Foundation, George S. Suddock, Richard and Sherry Sharp, and the Patients and Friends Research Fund of the University of Virginia Cancer Center. GM-CSF (made by Berlex/now Bayer) and Montanide ISA-51 (made by Seppic, Inc.) were used in the vaccines in this trial, but these were paid for by the University of Virginia. No corporate funding support was provided for this study. Berlex has provided support (in the form of GM-CSF at no charge) for a different clinical trial, for which Dr. Slingluff is the study chair; so he has a relationship with Berlex related to that separate support.
Footnotes
This project was presented as an oral abstract #3008 at the 2009 Annual meeting of the American Society of Clinical Oncology (J Clin Oncol 27:15s, 2009)
Reference List
- (1).Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Ahlers JD, Dunlop N, Alling DW, Nara PL, Berzofsky JA. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: granulocyte-macrophage colony-stimulating factor and TNF-alpha synergize with IL-12 to enhance induction of cytotoxic T lymphocytes. JI. 1997;158:3947–3958. [PubMed] [Google Scholar]
- (3).Slingluff CL, Jr., Petroni GR, Yamshchikov GV, et al. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–4026. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- (4).Jager E, Ringhoffer M, Dienes HP, et al. Granulocyte-macrophage-colony-stimulating factor enhances immune responses to melanoma-associated peptides in vivo. Int J Cancer. 1996;67:54–62. doi: 10.1002/(SICI)1097-0215(19960703)67:1<54::AID-IJC11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- (5).Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Laheru D, lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Salgia R, Lynch T, Skarin A, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol. 2003;21:624–630. doi: 10.1200/JCO.2003.03.091. [see comment] [DOI] [PubMed] [Google Scholar]
- (8).Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Scheibenbogen C, Schadendorf D, Bechrakis NE, et al. Effects of granulocyte-macrophage colony-stimulating factor and foreign helper protein as immunologic adjuvants on the T-cell response to vaccination with tyrosinase peptides. Int J Cancer. 2003;104:188–194. doi: 10.1002/ijc.10961. [DOI] [PubMed] [Google Scholar]
- (10).Na IK, Keilholz U, Letsch A, et al. Addition of GM-CSF to a peptide/KLH vaccine results in increased frequencies of CXCR3-expressing KLH-specific T cells. Cancer Immunol Immunother. 2007;56:391–396. doi: 10.1007/s00262-006-0198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Slingluff CLJ, Petroni GR, Chianese-Bullock KA, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- (12).Hogan KT, Sutton JN, Chu KU, et al. Use of selected reaction monitoring mass spectrometry for the detection of specific MHC class I peptide antigens on A3 supertype family members. Cancer Immunol Immunother. 2005;54:359–371. doi: 10.1007/s00262-004-0592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Slingluff CLJ, Yamshchikov G, Neese P, et al. Phase I trial of a melanoma vaccine with gp100(280-288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–3024. [PubMed] [Google Scholar]
- (14).Chianese-Bullock KA, Irvin WP, Jr., Petroni GR, et al. A multipeptide vaccine is safe and elicits T-cell responses in participants with advanced stage ovarian cancer. Journal of Immunotherapy. 2008;31:420–430. doi: 10.1097/CJI.0b013e31816dad10. [DOI] [PubMed] [Google Scholar]
- (15).Schmid I, Uittenbogaart CH, Krall WJ, Braun J, Giorgi JV. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry. 1992;13:204–208. doi: 10.1002/cyto.990130216. [DOI] [PubMed] [Google Scholar]
- (16).Fetterhoff TJ, Holland SP, Wile KJ. Fluorescent detection of non-viable cells in fixed cell preparations. Cytometry. 1993;14(Suppl 6):27. [Google Scholar]
- (17).Slingluff CL, Jr., Petroni GR, Yamshchikov GV, et al. Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol. 2004;22:4474–4485. doi: 10.1200/JCO.2004.10.212. [DOI] [PubMed] [Google Scholar]
- (18).Kirkwood JM, Lee S, Moschos SJ, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/-granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Annals of Oncology. 2007;18:232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- (21).Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- (22).Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response throught he recrutiment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- (23).Daud AI, Mirza N, Lenox B, et al. Phenotypic and functional analysis of dendritic cells and clinical outcome in patients with high-risk melanoma treated with adjuvant granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2008;26:3235–3241. doi: 10.1200/JCO.2007.13.9048. [DOI] [PubMed] [Google Scholar]
- (24).Jinushi M, Nakazaki Y, Dougan M, Carrasco DR, Mihm M, Dranoff G. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest. 2007;117:1902–1913. doi: 10.1172/JCI30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Jinushi M, Nakazaki Y, Carrasco DR, et al. Milk fat globule EGF-8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment. Cancer Res. 2008;68:8889–8898. doi: 10.1158/0008-5472.CAN-08-2147. [DOI] [PubMed] [Google Scholar]
- (26).Spitler LE, Grossbard ML, Ernstoff MS, et al. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2000;18:1614–1621. doi: 10.1200/JCO.2000.18.8.1614. [see comments] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. ELIspot data and controls for immune responder VMM526. The number of IFN-gamma-secreting cells in direct ELIspot assays are shown for peptides restricted by HLA-A2 (A) and HLA-A3 (B), and for CEF peptide controls (C) per 100,000 CD8+ cells, as well as for PHA and PMA/ionomycin (D) and tetanus helper peptide (E) per 100,000 total PBMC. IVS ELIspot assays were also done for this patient, and the number of IFN-gamma-secreting cells per 100,000 CD8+ cells are shown for peptides restricted by HLA-A2 (F) and HLA-A3 (G). Error bars represent standard deviations of replicate wells.