Abstract
The clinical use of benzodiazepines (BZs) is hampered by sedation and cognitive deterioration. Although genetic and pharmacological studies suggest that α1- and α5-containing GABAA receptors mediate and/or modulate these effects, their molecular substrate is not fully elucidated. By the use of two selective ligands : the α1-subunit affinity-selective antagonist β-CCt, and the α5-subunit affinity- and efficacy-selective antagonist XLi093, we examined the mechanisms of behavioural effects of diazepam in the tests of spontaneous locomotor activity and water-maze acquisition and recall, the two paradigms indicative of sedative- and cognition-impairing effects of BZs, respectively. The locomotor-activity decreasing propensity of diazepam (significant at 1.5 and 5 mg/kg) was antagonized by β-CCt (5 and 15 mg/kg), while it tended to be potentiated by XLi093 in doses of 10 mg/kg, and especially 20 mg/kg. Diazepam decreased acquisition and recall in the water maze, with a minimum effective dose of 1.5 mg/kg. Both antagonists reversed the thigmotaxis induced by 2 mg/kg diazepam throughout the test, suggesting that both GABAA receptor subtypes participate in BZ effects on the procedural component of the task. Diazepam-induced impairment in the declarative component of the task, as assessed by path efficiency, the latency and distance before finding the platform across acquisition trials, and also by the spatial parameters in the probe trial, was partially prevented by both, 15 mg/kg β-CCt and 10 mg/kg XLi093. Combining a BZ with β-CCt results in the near to control level of performance of a cognitive task, without sedation, and may be worth testing on human subjects.
Keywords: Benzodiazepine, GABAA subtype, learning, memory, sedation
Introduction
All benzodiazepines (BZs) currently in clinical practice act as positive modulators of fast inhibitory neurotransmission mediated through those populations of GABAA receptors which contain α1, α2, α3 or α5 subunits in addition to the γ2 subunit (~80% of all GABAA receptors). The diverse pharmacological effects of BZs: anxiolytic, sedative, hypnotic, muscle relaxant, anticonvulsive and amnesic, stem from the substantial involvement of GABAA receptors in the regulation of vigilance, anxiety, muscle tension, epileptogenic activity, and memory functions (Rudolph & Möhler, 2004; Sieghart & Ernst, 2005).
Although very effective in short-term treatment of different psychiatric and neurological ailments (mostly anxiety disorders, insomnia, muscle spasms and epilepsy), BZs are not free of psychomotor and cognitive impairing effects, those prominent being sedation and anterograde amnesia (Lader, 1999). Sedation is basically related to suppression of the unconditioned psychomotor performance. Sometimes, the effect seen after use of certain doses of BZs is an increase, not a decrease of the tracked activity, the most parsimonious explanation of this phenomenon being related to the disinhibitory properties of BZs (Crawley, 1985). On the other hand, the specific cognitive effect of BZs appears to be more an impairment of learning (acquisition) than an effect on memory (retention) itself, and the term ‘acquisition impairing’ would be more appropriate than ‘amnesic’ (Clement & Chapouthier, 1998).
Based on pharmacological studies with ligands with some degree of GABAA receptor subtype selectivity, such as CL218,872 and zolpidem (e.g. Depoortere et al. 1986; Lippa et al. 1979), it has been hypothesized that the four populations of BZ-binding site-containing GABAA receptors, with their distinct patterns of anatomical distribution in the mammalian brain, may represent differentiable molecular substrates for the various effects of BZs. The recent genetic studies with mice carrying a point mutation (‘knock-in’) of histidine to arginine in α1, α2, α3 or α5 subunits, rendering the respective GABAA receptors selectively insensitive to effects of BZs, substantiated the possibility of a specific contribution of individual receptor subtypes to the spectrum of behavioural actions of the reference BZ, diazepam (reviewed in Rudolph & Möhler, 2004, 2006). The plausibility of selective switching off of, for clinical use, mainly unwanted sedative- and acquisition- impairing effects of BZs is highly desirable, and demands additional knowledge of the molecular and cellular substrates of these effects. Experimental evidence to date, including the screening of newer affinity- and/or efficacy-selective BZ site ligands, suggests that GABAA receptors containing α1 and α5 subunits may be of importance in exerting these two effects in mutated and wild-type animals (McKernan et al. 2000; Rudolph et al. 1999; Savić et al. 2008a; van Rijnsoever et al. 2004). Without questioning the main contribution of the α1 subunit (McKernan et al. 2000, Rudolph et al. 1999), experiments with ligands functionally selective for α2-, α3- and α5-, or essentially selective for α5-containing subtypes of GABAA receptors, suggest that sedation may be partly dependent on activity mediated by α5-containing GABAA receptors (Savić et al. 2008a). Moreover, based on inhibitory (Savić et al. 2008a, b) or excitatory (Hauser et al. 2005; van Rijnsoever et al. 2004) influences of modulation of activity exerted by neurons expressing the α5-subunitcontaining GABAA receptors on locomotor output, existence of certain discontinuous ‘effective windows’ of this modulation, which could enable the ‘on/off switch’ role of these receptors in control of vigilance, was proposed (Savić et al. 2008b).
On the other hand, behavioural studies with subtype selective ligands (Savić et al. 2005a, b, 2008b) and genetically modified animals (Collinson et al. 2002; Crestani et al. 2002; Rudolph et al. 1999) have indicated that both, the α1- and α5-subunit-containing GABAA receptors, comprise the ‘memory-modulating’ population of these receptors. It appears that the impairing effects of BZs on the acquisition of procedural memory, as assessed in the active avoidance paradigm, may predominantly depend on the α1-containing GABAA receptors (Savić et al. 2005b), while the influence on the acquisition of declarative memory, assessed in the passive avoidance paradigm, probably involves the α5 subunit, in addition to the α1 subunit (Rudolph et al. 1999; Savić et al. 2005a).
The aim of the present study was to elucidate, by the use of two selective ligands, the preferential α1- subunit affinity-selective antagonist β-CCt, and the α5- subunit affinity- and efficacy-selective antagonist XLi093, to what extent GABAA receptors containing α1 and α5 subunits contribute to the well-established behavioural effects of diazepam in the tests of spontaneous locomotor activity and water-maze acquisition and recall, the two paradigms mainly, but not exclusively, indicative of sedative- and spatial-cognition- impairing effects of BZs, respectively. The selectivity of β-CCt and XLi093 has been confirmed in in-vitro experiments of affinity and efficacy at recombinant GABAA receptors (Huang et al. 2000; June et al. 2003; Li et al. 2003), as well as in in-vivo studies of inhibition of [3H]flumazenil binding in distinct brain regions, which differ in the GABAA receptor subtype expression (Griebel et al. 1999; Shinday et al. 2008).
Materials and methods
Drugs
XLi093 (4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid, 8-ethynyl-5,6-dihydro-5-methyl-6-oxo-, 1,3-propanediyl ester), the α5-subunit affinity- and efficacy-selective antagonist, and β-CCt (t-butyl-βcarboline- 3-carboxylate), the preferential α1-subunit affinity-selective antagonist were synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin–Milwaukee, as described in detail previously (Cox et al. 1995; Li et al. 2003). Diazepam was obtained from Galenika (Serbia).
Behavioural experiments
Experiments were carried out on male Wistar rats (Military Farm, Serbia), weighing 220–250 g. All procedures in the study conformed to EEC Directive 86/ 609 and were approved by the Ethical Committee on Animal Experimentation of the Faculty of Pharmacy in Belgrade. The rats were housed in transparent plastic cages, six animals per cage, and had free access to pelleted food and tap water. The temperature of the animal room was 22±1 °C, relative humidity 40–70%, illumination 120 lx, with a 12-h light/dark cycle (lights on 06:00 hours). All handling and testing took place during the light phase of the diurnal cycle. Separate groups of animals were used for two behavioural paradigms. The behaviour was recorded by a ceiling-mounted camera and analysed by ANY-maze Video Tracking System software (Stoelting Co., USA). The drugs were dissolved/suspended with the aid of sonication in a solvent containing 85% distilled water, 14% propylene glycol, and 1% Tween-80, and were administered in a total volume of 2 ml/kg, 20 min before behavioural testing. The first treatment indicated in combination was administered into the lower right quadrant of the peritoneum, and the second treatment immediately afterwards into the lower left quadrant of the peritoneum.
Measurement of locomotor activity
Twenty minutes after receiving the appropriate treatment, single rats were placed in a clear Plexiglas chamber (40×25×35 cm). Activity under dim red light (20 lx) was recorded for a total of 30 min, without any habituation period, using ANY-maze software. Besides the total distance travelled, behaviour was analysed by dividing the locomotor activity data into 5-min bins.
Two experiments were performed. In the first, the dose–response curve for diazepam (0, 0.5, 1.5, 5.0 mg/ kg) was determined. In the second experiment, the design included the factors agonist (the same doses of diazepam as those used in the dose–response study) and antagonists (β-CCt at 0, 5, 15 mg/kg, and XLi093 at 0, 10, 20 mg/kg), thus generating 20 experimental groups in total.
Behaviour in the Morris water maze
The water maze consisted of a black cylindrical pool (diameter 200 cm, height 60 cm), with a uniform inner surface. The pool was filled to a height of 30 cm with water at 23 °C (±1 °C). The escape platform of black plastic (15×10 cm) was submerged 2 cm below the water surface. The platform was invisible to rats by being the same colour as the pool wall (Terry, 2000). There were many distal cues in the testing room (doors, pipes on the walls and the ceiling, cupboards, a camera suspended above the centre of the maze). An indirect illumination in the experimental room was provided by white neon tubes fixed on the walls.
The rats received the appropriate treatment 20 min before a swimming block, each day for 5 consecutive days of spatial acquisition. Each block consisted of four trials, lasting a maximum time of 120 s, the inter-trial interval being 60 s. For each trial the rat was placed in the water facing the pool at one of four pseudo-randomly determined starting positions. As during spatial learning the platform was hidden in the middle of the NE quadrant, the four distal start locations chosen were S, W, NW and SE (Fig. 1). Once the rat found and mounted the escape platform it was permitted to remain on the platform for 15 s. The rat was guided to the platform by the experimenter if it failed to locate it within 120 s. To assess the long-term spatial memory at the end of learning, a probe trial for 60 s, with the platform omitted, was given 24 h after the last acquisition day. The probe trial, starting from the novel, most distant SW location (in order to ensure that any spatial bias is a consequence of the spatial memory of escape location, rather than of a specific swim strategy ; Vorhees & Williams, 2006), was performed without any pre-treatment. A drug-free probe trial (cf. McNamara & Skelton, 1993) was chosen because diazepam impairs acquisition, but not retrieval of place preference in the water maze (Anand et al. 2007; McNamara & Skelton, 1991), and confounding effects of possible sensorimotor, i.e. non-cognitive actions of treatment on recall performance were avoided by such a protocol. The tracking software virtually divided the pool into four quadrants, three concentric annuli and a target region consisting of the intersection of the platform quadrant and the platform annulus (Fig. 1). Similar to the approach used by Cain (1997), the central annulus was set up to 10% of the whole area; the platform annulus equalled 40%, whereas the area of the peripheral annulus was 50% of the whole.
Fig. 1.
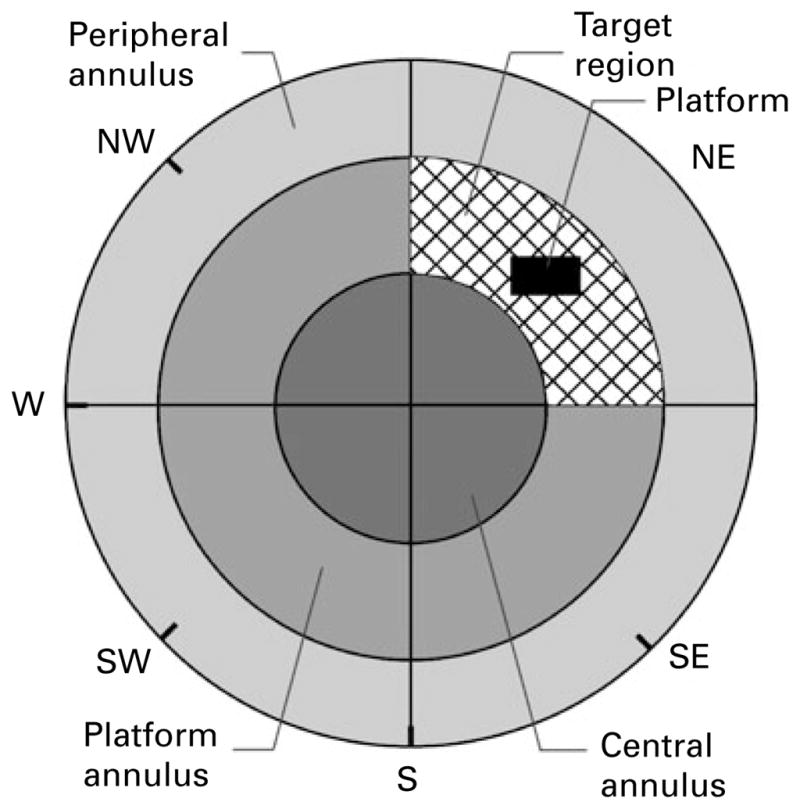
The scheme representing the virtual division of the water maze used in the analysis of rats’ performance.
Dependent variables chosen for tracking during the acquisition trials were: latency to platform (time from start to goal), total distance swam (path length), average swim speed and path efficiency (the ratio of the shortest possible path length to actual path length). All these indices are, to a lesser or greater degree, related to goal-directed behaviour, i.e. spatial learning (Vorhees & Williams, 2006). As thigmotaxis (the tendency to swim or float near the pool wall) represents a factor which accounts for much of the variance in the water-maze performance, and normally weakens during consecutive trials (Vorhees & Williams, 2006), we quantified the persistence of thigmotaxis in the target (NE) quadrant. The loss of thigmotaxis is related to the procedural component of acquisition, and the percent of the distance swum in the target region (away from the wall) of the target quadrant may be seen as a measure of procedural learning.
The indices of memory, assessed during the probe trial, included the distance and time in the platform (target) quadrant, platform ring and target region, as well as the number of entries and distance swum in the area where the platform used to be during training (Fig. 1). In addition, the distance swum during 60 s in the probe trial was taken as a measure of overall activity, while peripheral ring parameters (distance and time) were connected to thigmotaxic behaviour.
Three experiments in the water maze were performed. In the first, the dose–response curve for diazepam (0, 1, 1.5, 2, 5 mg/kg) was determined. In the second experiment, the influences of β-CCt (5, 15 mg/kg) and XLi093 (10, 20 mg/kg) on the effects of 1.5 mg/kg diazepam (the minimal effective dose from the dose–response study) were assessed. The inclusion of the groups treated by the antagonists without diazepam would have made the experiment overly long, on each of five training days. In preliminary experiments with the current protocol, we noticed the lack of behavioural activity of higher doses of β-CCt and XLi093 used here (15, 20 mg/kg, respectively). In the third water-maze experiment, we assessed the capability of β-CCt (15 mg/kg) and XLi093 (10 mg/kg) to antagonize the behavioural effects of a higher dose of diazepam (2 mg/kg).
Statistical analysis
All numerical data presented in the figures are given as the mean± S.E.M. Data from the activity assay were assessed by a one-way or two-way ANOVA, whereas the results from the water-maze test were analysed using a two-way ANOVA with repeated measures. Post-hoc comparisons, where applicable, were performed using Student–Newman–Keuls or Dunnett’s test. Statistical analyses were performed with ANY-maze Video Tracking System software (Stoelting Co., USA).
Results
Motor activity assay
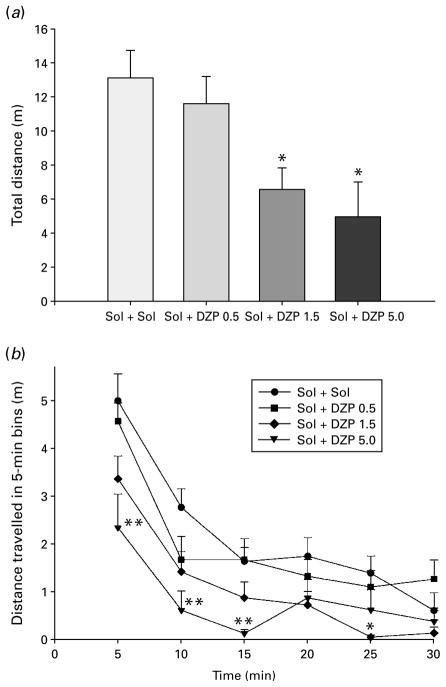
An ANOVA showed a significant effect of diazepam treatment on total distance travelled during 30 min of monitoring [F(3, 28)=5.63, p=0.004] (Fig. 2a). According to Dunnett’s test, the activity-depressing effect of two higher doses of diazepam was significant compared with solvent control. When the analysis of travelled distance was developed into 5-min intervals (Fig. 2b), it was seen that a dose of 5 mg/kg diazepam highly significantly decreased locomotion in the 0– 15 min period, whereas a dose of 1.5 mg/kg was effective in the 20–25 min period.
Fig. 2.
The effects of diazepam (Sol+DZP 0.5, 1.5 and 5.0 mg/ kg) on total distance (a) and distance travelled in 5-min intervals (b). * p<0.05 compared to solvent (Sol+Sol) group; ** p<0.01 compared to solvent. Animals per treatment (n=8).
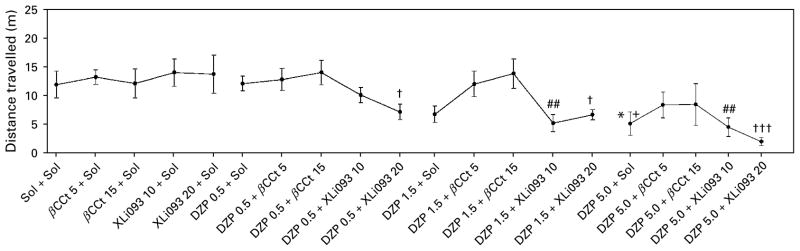
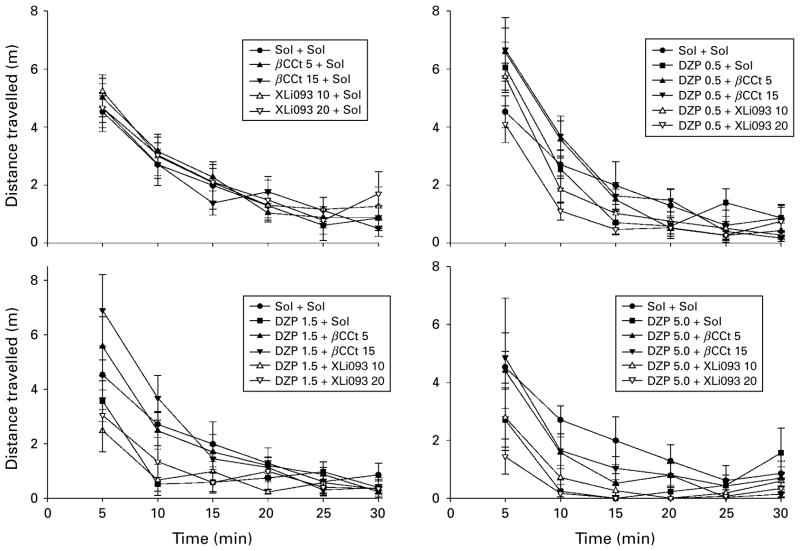
On the other hand, while devoid of discernible activity of their own (Figs 3, 4), β-CCt and XLi093 exerted differential effects on the hypolocomotor effect of diazepam. Their influences were evaluated by separate statistical analyses. A two-way ANOVA for the analysis of the influence of β-CCt has shown a significant effect of dose of diazepam [F(3, 71)=3.95, p= 0.012], whereas the dose of antagonist as a factor, as well as the agonist×antagonist interaction did not reach significance [F(2, 60)=2.30, p=0.109; F(6, 71)= 0.49, p=0.811, respectively]. Post-hoc Student– Newman–Keuls method revealed that the existing significant differences between the levels of diazepam itself (5 vs. 0 mg/kg and 5 vs. 0.5 mg/kg) disappeared when multiple comparisons were made within the 5 mg/kg β-CCt dose (respective p values 0.419 and 0.339), as well as within the 15 mg/kg β-CCt level (respective p values 0.251 and 0.302). When analysing the overall influence of XLi093 as antagonist, there was a significant effect of dose of diazepam [F(3, 71)= 15.323, p<0.001], whereas dose of XLi093 as a factor, as well as the agonist×antagonist interaction were insignificant [F(2, 60)=0.806, p=0.451; F(6, 71)=0.846, p=0.540, respectively]. Contrary to the antagonism exerted by β-CCt, post-hoc analysis revealed that the existing effects of diazepam (5 vs. 0 mg/kg, p=0.027; 5 vs. 0.5 mg/kg, p=0.041) were potentiated by XLi093 (Fig. 3). Namely, comparisons within the 10 mg/kg XLi093 level have shown highly significant differences in the effects of 1.5 and 5 mg/kg doses of diazepam vs. the effect of the antagonist itself (p=0.003 in both cases), whereas within the dose of 20 mg/kg XLi093, all three levels of diazepam (0.5, 1.5, 5 mg/kg) were statistically different from the antagonist (respective p values: 0.013, 0.002,<0.001). Similar conclusions can be reached while statistically analysing (not shown) the data obtained by dividing the locomotor activity into 5-min bins (Fig. 4). As a rule, locomotor activity of animals treated with combination of diazepam+ β-CCt, irrespective of the dose employed, was near to, or slightly above, the control value, whereas XLi093, especially at the higher dose, tended to deepen, or unveil, the sedation induced by diazepam.
Fig. 3.
The effects of combinations of diazepam (DZP), at doses of 0, 0.5, 1.5 and 5.0 mg/kg, and the antagonists β-CCt (0, 5, 15 mg/kg) and XLi093 (0, 10, 20 mg/kg), on total distance travelled in the spontaneous locomotor activity test. * p<0.05, compared to solvent (Sol+Sol) group; + p<0.05 compared to DZP 0.5+Sol group; ## p<0.01 compared to XLi093 10+Sol group; # p<0.05, ### p<0.001 compared to XLi093 20+Sol group. Animals per treatment (n=6).
Fig. 4.
Mean distance travelled in successive 5-min blocks for groups designated as in Fig. 3.
Morris water maze
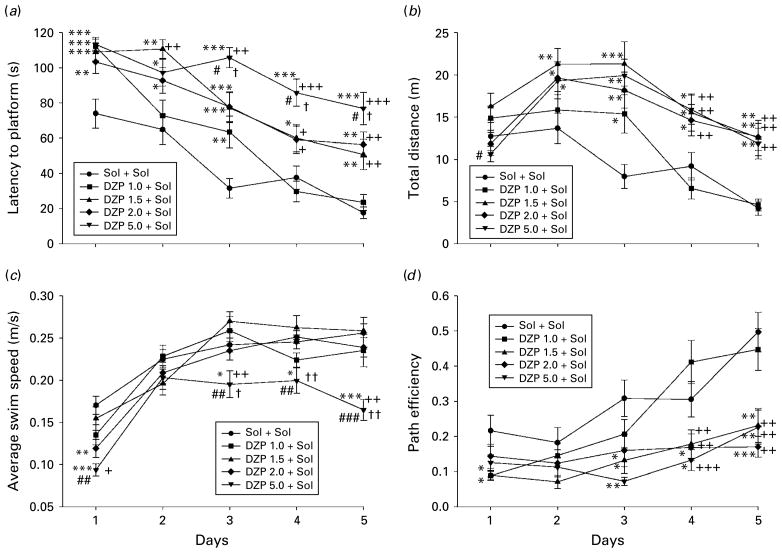
For the dose–response study of diazepam, the factors treatment and days, as well as the interaction treatment×days, were statistically highly significant for the latency to find platform, the distance swum before finding the platform, swim speed and path efficiency; significant differences among treatments during training days are presented in Fig. 5. The results of the post-hoc analysis for the factor treatment are summarized in Table 1. The analysis showed that the lowest effective dose of diazepam was 1.5 mg/kg.
Fig. 5.
The effects of diazepam 1.0, 1.5, 2.0 and 5.0 mg/kg (DZP 1.0+Sol to DZP 5.0+Sol) on (a) latency to platform, (b) total distance, (c) average swim speed and (d) path efficiency of rats during 5 d acquisition trials in the water maze. * p<0.05, ** p<0.01, p<0.001 compared to solvent (Sol+Sol) group; +p<0.05, ++ p<0.01, +++ p<0.001 compared to DZP 1.0+Sol group; # p<0.05, ## p<0.01, ### p<0.001 compared to DZP 1.5+Sol group; # p<0.05, ## p<0.01 compared to DZP 2.0+Sol group. Animals per treatment (n=7).
Table 1.
Significant differences among overall influences (averaged for 5 d acquisition) on the water-maze learning parameters: latency to find the platform (L), distance swam before finding the platform (D), mean swim speed (S) and path efficiency (E) in the dose–response study of diazepam (DZP, mg/kg)
| DZP 1.0+Sol | DZP 1.5+Sol | DZP 2.0+Sol | DZP 5.0+Sol | |
|---|---|---|---|---|
| Sol+Sol | L: p=0.004 | L: p<0.001 | L: p<0.001 | L: p<0.001 |
| D: p<0.001 | D: p=0.001 | D: p=0.002 | ||
| E: p<0.001 | E: p<0.001 | S: p<0.001 | ||
| E: p<0.001 | ||||
| DZP 1.0+Sol | L: p<0.001 | L: p=0.001 | L: p<0.001 | |
| D: p=0.002 | D: p=0.014 | D: p=0.030 | ||
| E: p=0.001 | E: p=0.001 | S: p<0.001 | ||
| E: p=0.001 | ||||
| DZP 1.5+Sol | L: p=0.007 | |||
| S: p<0.001 | ||||
| DZP 2.0+Sol | L: p=0.002 | |||
| S: p<0.001 |
Sol, Solvent.
The incapacitating influences of previous treatment with diazepam were also discernible during the probe trial (Table 2), when a number of indices of memory (time in platform quadrant, time and distance in platform ring, time and distance in target region) were dose-dependently adversely affected. Concomitantly, a significant increase of peripheral ring parameters, i.e. pronounced thigmotaxis (Table 2), has confirmed that learning the required water-maze skills and strategies was impaired under diazepam.
Table 2.
The representative parameters of water-maze performance in the probe trial of the diazepam (DZP, mg/kg) dose-response experiment. The key to regions used in the analysis is given in Fig. 1.
| Sol+Sol | DZP 1.0+Sol | DZP 1.5+Sol | DZP 2.0+Sol | DZP 5.0+Sol | ANOVA F(4, 30) | p | |
|---|---|---|---|---|---|---|---|
| Whole water maze parameters | |||||||
| Distance (m) | 13.97±1.19 | 11.54±0.48 | 15.01±1.26 | 14.51±0.78 | 13.70±0.69 | 2.051 | 0.112 |
| Platform quadrant (NE) parameters | |||||||
| Distance (m) | 3.87±0.45 | 2.33±0.39 | 2.92±0.34 | 2.95±0.37 | 2.34±0.36 | 2.661 | 0.052 |
| Time (s) | 16.26±1.42 | 11.44±1.98 | 11.29±1.23 | 11.94±1.50 | 8.63±1.38* | 3.255 | 0.025 |
| Peripheral ring parameters | |||||||
| Distance (m) | 6.67±0.95 | 5.34±1.13 | 11.79±1.67*++ | 10.67±0.77*++ | 10.62±0.46*++ | 6.914 | <0.001 |
| Time (s) | 30.61±2.37 | 29.41±5.33 | 48.44±3.30**++ | 47.33±2.75**++ | 50.19±1.95**++ | 9.361 | <0.001 |
| Platform ring parameters | |||||||
| Distance (m) | 6.09±0.75 | 5.00±0.61 | 2.59±0.82** | 3.26±0.61* | 2.69±0.51** | 5.371 | 0.002 |
| Time (s) | 24.79±2.37 | 24.90±3.67 | 9.43±2.72**++ | 10.73±2.37**++ | 8.63±1.65**++ | 10.100 | <0.001 |
| Target region parameters | |||||||
| Distance (m) | 2.00±0.26 | 1.01±0.20** | 0.40±0.12*** | 0.67±0.20*** | 0.55±0.20*** | 10.108 | <0.001 |
| Time (s) | 7.81±1.24 | 4.59±0.92* | 1.36±0.41***+ | 2.14±0.74***+ | 1.64±0.60***+ | 10.704 | <0.001 |
| Platform parameters | |||||||
| No. of entries | 1.00±0.38 | 0.43±0.20 | 0.00±0.00 | 0.43±0.30 | 0.29±0.18 | 2.167 | 0.097 |
| Distance (m) | 0.102±0.042 | 0.043±0.022 | 0.000±0.000 | 0.047±0.034 | 0.012±0.008 | 2.261 | 0.086 |
Values are mean ± S.E.M.
p<0.05,
p<0.01,
p<0.001, compared to solvent (Sol+Sol) group.
p<0.05,
p<0.01, compared to DZP 1.0+Sol group.
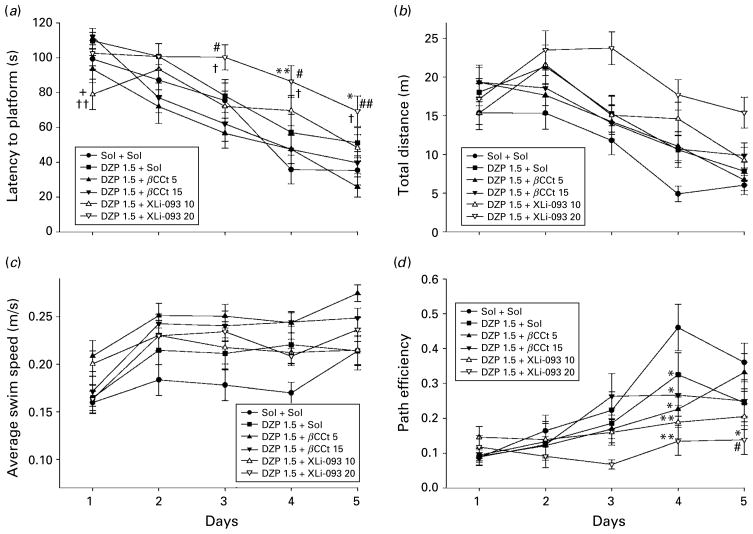
Based on the presented dose–response study, we performed a further experiment in which two doses of each of the antagonists, tested in the locomotor activity assay, were combined with 1.5 mg/kg diazepam. However, in this experiment, the effect of diazepam did not reach significance compared with control, for any of the learning measures calculated. A two-way ANOVA with repeated measures for this antagonism study revealed significant variability in regard to latencies to find the platform [treatment effect: F(5, 138)=6.59, p<0.001 ; day effect: F(4, 552)=50.39, p<0.001 ; and treatment×day interaction : F(20, 552)= 1.80, p=0.018] and path efficiencies across the 5 d [treatment effect: F(5, 138)=4.67, p=0.001; day effect: F(4, 552)=17.61, p<0.001 ; treatment×day interaction : F(20, 552)=1.88, p=0.012]. The respective significant differences among treatments during days are presented in Fig. 6(a, d). The factors, but not the interaction, also reached significance when swim distances (Fig. 6b) and average swim speed (Fig. 6c) were analysed [treatment effect: F(5, 138)=5.42, p<0.001; day effect: F(4, 552)=34.27, p<0.001 ; treatment× day interaction : F(20, 552)=1.50, p=0.075; and treatment effect: F(5, 138)=5.02, p<0.001; day effect: F(4, 552)=21.08, p<0.001 ; treatment×day interaction : F(20, 552)=1.22, p=0.233, respectively]. Bearing in mind especially the latency to platform (Fig. 6a), it appears that antagonism of the effects of diazepam at GABAA receptors containing α5 subunits (1.5 mg/kg diazepam+10 mg/kg XLi093) may enhance acquisition in the earliest stages of spatial learning, while addition of a higher dose of the antagonist (1.5 mg/kg diazepam+20 mg/kg XLi093) may even impair the later phases of learning. Throughout the acquisition trials, there were no discernible effects of adding βCCt, at either dose, to diazepam. In the probe trial, the significant differences in dependent measures of performance were generally absent, probably due to the lack of clear behavioural activity of the used dose of diazepam, and these data are not presented.
Fig. 6.
The effects of diazepam (DZP 1.5+Sol), diazepam and β-CCt (DZP 1.5+β-CCt 5 and DZP 1.5+β-CCt 15) and diazepam and XLi093 (DZP 1.5+XLi093 10 and DZP 1.5+XLi093 20) (all doses in mg/kg) on (a) latency to platform, (b) total distance, (c) average swim speed and (d) path efficiency of rats during 5 d acquisition trials in the water maze. * p<0.05, ** p<0.01 compared to solvent (Sol+Sol) group; + p<0.05 compared to DZP 1.0+Sol group; # p<0.05, ## p<0.01 compared to DZP 1.5+β-CCt 5 group; # p<0.05, ## p<0.01 compared to DZP 1.5+β-CCt 15 group. Animals per treatment (n=6).
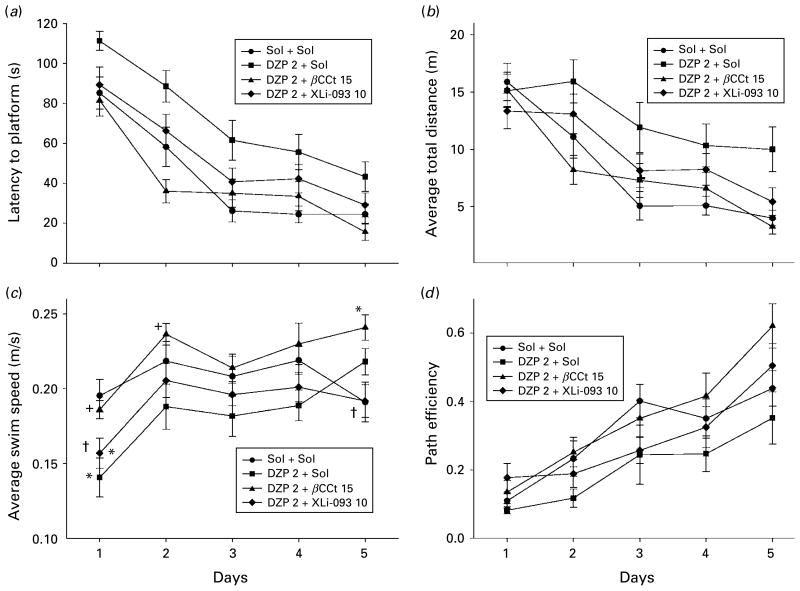
Finally, the results of the experiment with a higher effective dose of diazepam (2 mg/kg), on its own and in combination with 10 mg/kg XLi093 and 15 mg/kg βCCt are shown in Figs 7 and 8, and Tables 3 and 4. A two-way ANOVA with repeated measures of latencies to find the platform across the 5 d (Fig. 7a) revealed the following results [treatment effect: F(3, 100)=11.65, p<0.001; day effect: F(4, 400)=56.74, p<0.001 ; treatment×day interaction : F(12, 400)=0.96, p=0.484]. Similar tendencies were evident when swim distances (Fig. 7b) and path efficiencies (Fig. 7d) were analysed [treatment effect: F(3, 100)=6.34, p=0.001; day effect: F(4, 400)=28.17, p<0.001; treatment×day interaction : F(12, 400)=1.46, p=0.135; and treatment effect: F(3, 100)=5.98, p=0.001 ; day effect: F(4, 400)=27.68, p<0.001 ; treatment×day interaction : F(12, 400)=1.03, p=0.422, respectively]. The interaction only reached significance when swim speed was analysed [treatment effect: F(3, 100)=6.29, p=0.001; day effect: F(4, 400)=14.03, p<0.001; treatment×day interaction : F(12, 400)=1.92, p=0.031], and significant differences among treatments during days are given in Fig. 7c. As treatment as a factor was statistically significant for all four learning parameters illustrated, the respective significances for single treatments are shown in the Table 2. βCCt (15 mg/kg) completely prevented acquisition-impairing actions of diazepam administered at the dose of 2 mg/kg, whereas addition of XLi093 (10 mg/kg) was effective in this sense for all parameters considered, with the exception of the mean swim speed (Table 3). It should be noted that statistical analysis revealed no overall significant difference in maximum speed in treatments; moreover, on the first day, the rats treated with diazepam were even faster, in maximum, than control rats (1.16± 0.44 m/s vs. 0.78±0.14 m/s), which is a hint of transient behavioural disinhibition.
Fig. 7.
The effects of diazepam (DZP 2+Sol), diazepam and β-CCt (DZP 2+β-CCt 15) and diazepam and XLi093 (DZP 2+XLi093 10) (all doses in mg/kg) on (a) latency to platform, (b) average total distance, (c) average swim speed and (d) path efficiency of rats during 5 d acquisition trials in the water maze. * p<0.05 compared to solvent (Sol+Sol) group; + p<0.05 compared to DZP 2+Sol group; † p<0.05 compared to DZP 2+β-CCt 15 group. Animals per treatment, for Sol+Sol to DZP 2+XLi093 10 (n=6, 6, 7, 7, respectively).
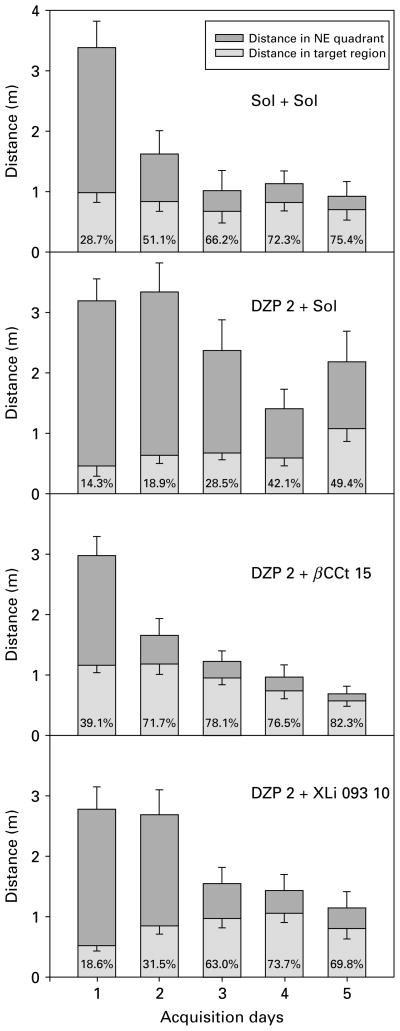
Fig. 8.
The effects of (a) solvent (Sol+Sol) ; (b) diazepam (DZP 2+Sol), (c) diazepam and β-CCt (DZP 2+β-CCt 15) and (d) diazepam and XLi093 (DZP 2+XLi093 10) (all doses in mg/kg) on the distance rats travelled in the SE quadrant and target region during 5 d acquisition trials in the water maze. The numbers inside the columns are the percent of the distance swam inside the target (NE) quadrant which was travelled in the target region.
Table 3.
Significant differences among overall influences (averaged for 5 d of acquisition) of the tested treatments (mg/kg) on the water-maze learning parameters: latency to find the platform (L), distance swam before finding the platform (D), mean swim speed (S) and path efficiency (E)
| Sol+Sol | DZP 2+βCCt 15 | DZP 2+XLi093 10 | |
|---|---|---|---|
| DZP 2+Sol | L: p<0.001 | L: p<0.001 | L: p=0.002 |
| D: p=0.001 | D: p=0.001 | D: p=0.011 | |
| E: p=0.024 | S: p=0.001 | E: p=0.025 | |
| E: p<0.001 | |||
| DZP 2+XLi093 10 | S: p=0.005 |
DZP, Diazepam; Sol, solvent.
Table 4.
The representative parameters of water-maze performance in the probe trial. The key to regions used in the analysis is given in Fig. 1
| Sol+Sol | DZP 2+Sol | DZP 2+βCCt 15 | DZP 2+XLi093 20 | ANOVA, F | p | |
|---|---|---|---|---|---|---|
| Whole water maze parameters | ||||||
| Distance (m) | 10.74±1.62 | 11.74±0.40 | 12.45±0.58 | 11.43±0.96 | 0.531 | 0.67 |
| Platform quadrant (NE) parameters | ||||||
| Distance (m) | 2.11±0.48 | 2.39±0.28 | 1.72±0.47 | 1.65±0.29 | 0.522 | 0.52 |
| Time (s) | 10.50±2.05 | 11.98±1.59 | 7.33±2.13 | 9.31±2.06 | 0.971 | 0.42 |
| Peripheral ring parameters | ||||||
| Distance (m) | 3.80±0.99 | 7.86±0.84** | 5.16±0.60+ | 5.11±0.61+ | 4.77 | 0.010 |
| Time (s) | 29.45±4.18 | 44.82±2.39** | 29.19±2.81++ | 32.20±2.42++ | 5.81 | 0.004 |
| Platform ring parameters | ||||||
| Distance (m) | 6.26±0.91 | 2.98±0.55* | 6.03±0.50+ | 5.08±0.71+ | 4.62 | 0.012 |
| Time (s) | 27.05±3.19 | 11.75±2.32** | 25.87±1.98++ | 22.31±2.29++ | 7.66 | 0.001 |
| Target region parameters | ||||||
| Distance (m) | 1.48±0.44 | 0.78±0.26 | 1.06±0.22 | 1.12±0.27 | 0.86 | 0.48 |
| Time (s) | 5.63±1.82 | 3.20±1.09 | 3.94±1.05 | 4.79±1.24 | 0.61 | 0.62 |
| Platform parameters | ||||||
| No. of entries | 1.33±0.49 | 0.17±0.17 | 0.57±0.20 | 0.86±0.26 | 2.52 | 0.084 |
| Distance (m) | 0.146±0.051 | 0.007±0.007* | 0.051±0.019 | 0.076±0.034 | 3.11 | 0.047 |
DZP, Diazepam; Sol, solvent.
Values are mean ± S.E.M.
DZP, βCCt, XLi093 treatments in mg/kg.
p<0.05 compared to solvent (Sol+Sol) group;
p<0.01 compared to solvent;
p<0.05 compared to DZP 2 mg/kg group (DZP 2+Sol);
p<0.01 compared to DZP 2 mg/kg group.
In Fig. 8, the distances the rats swam in the platform quadrant (NE) during acquisition trials are presented alongside the respective distance in the portion of NE quadrant lying in the platform annulus of the maze (‘the target region’). The rats treated with 2 mg/kg diazepam strikingly lacked the preferential activity in that part of the NE quadrant in which platform finding was possible; even on day 5, only 49.4% of the distance they travelled in NE quadrant was in the target region; the respective values for control, 2 mg/kg diazepam+15 mg/kg βCCt and 2 mg/kg diazepam+ 10 mg/kg XLi093 groups were 75.4%, 82.9% and 69.8%.
In Table 4, a number of parameters calculated from the probe trial performance in the antagonism study with 2 mg/kg diazepam are presented. The total distance swum was not different, and there were also no significant differences among groups regarding distance and time spent in the platform quadrant. On the other hand, animals treated for 5 d with diazepam exerted a strong bias towards the peripheral annulus, which was reversed by both antagonists. Concomitantly, previous treatment with diazepam resulted in significant avoidance of the platform annulus, which was also antagonized by both, βCCt (15 mg/kg) and XLi093 (10 mg/kg). The changes of these two parameters are indicative of influences on the previous days’ behavioural strategies learning, i.e. the procedural component of water-maze spatial memory. There were no significant differences in target region activity, whereas diazepam treatment tended to decrease platform site entries and significantly decreased the distance in platform position. The latter effect, indicative of influence on the declarative spatial component of memory, was attenuated, but not reversed, by both antagonists.
Discussion
The α1- and α5-containing GABAA receptors have been repeatedly implicated, to a different degree, in mediation or modulation of widely known sedative and amnesic effects of agonists at BZ-sensitive GABAA receptors (McKernan et al. 2000; Rudolph et al. 1999; Savić et al. 2008a; van Rijnsoever et al. 2004). The present experiments, using the selective antagonists at BZ site of α1- and α5-containing GABAA receptors, demonstrated that the activity-decreasing propensity of diazepam, as a measure of sedation, is the consequence of its binding at α1-containing GABAA receptors, whereas spatial learning and memory deficits induced by diazepam are related to action at both of these receptor populations.
The findings in the motor activity assay on the predominant role of α1 GABAA receptors are in accord with genetic studies (McKernan et al. 2000; Rudolph et al. 1999). What appears to be the most surprising result of this part of the study, combining diazepam with XLi093, especially with the higher (20 mg/kg) of the two tested doses of the antagonist, potentiated sedation induced by diazepam. The 20 mg/kg dose of XLi093 presumably caused a complete antagonism of effects of diazepam at α5-containing GABAA receptors (cf. occupancy of about 65% of α5 GABAA receptors in mice at 10 mg/kg XLi093 in Shinday et al. 2008). We have recently put forward the hypothesis that locomotor-activity changes induced by ligands possessing a substantial α5 efficacy may be, at least partly, contributed by modulation at GABAA receptors containing this subunit (Savić et al. 2008a). It appears that the role of positive modulation at α5 GABAA receptors depends on the concomitant activity at α1-containing GABAA receptors. Moreover, α5 GABAA receptors may exert a dual control on the state of vigilance : to limit sedative effects elicited by supraphysiological stimulation of α1-containing receptors, and, conversely, to enhance basal/endogenous activation of α1 GABAA receptors, thereby inducing mild sedation. Three sets of data may indirectly support the notion of the modulatory role of this population of receptors. First, it is notable that α5-containing GABAA receptors are at least moderately present in both regions believed to be involved in the sedative properties of GABAA receptor activators (Hentschke et al. 2005; Kiehn, 2006), i.e. ventral horn of the spinal cord (Bohlhalter et al. 1996), and pyramidal neurons of the neocortex, especially layer V (Pirker et al. 2000; Yamada et al. 2007). Second, there is a conspicuous association between α1 and α5 subunits: the co-localization within individual neurons (Bohlhalter et al. 1996), and even within the single GABAA receptor (Araujo et al. 1999). Third, the knock-in mice harbouring the α5 subunit insensitive to diazepam are refractory to development of tolerance to the α1-mediated sedative effect of diazepam at subchronic doses (van Rijnsoever et al. 2004).
The effects of diazepam on the acquisition and retention of place learning in the water maze have been previously assessed in two settings, similar but not identical to the present procedure (Arolfo & Brioni, 1991; Cain, 1997). The lowest dose effective in our experiment (1.5 mg/kg) lies between those found by Arolfo & Brioni (1.0 mg/kg) and Cain (3.0 mg/kg diazepam). However, the antagonism study showed that the 1.5 mg/kg dosage level was a borderline dose of diazepam, unreliable in affecting rats’ behaviour under the conditions used in the current water-maze protocol. Nevertheless, in such settings, an impairment of the later phases of water-maze learning produced by the combination of diazepam (1.5 mg/kg) and the higher dose of XLi093 (20 mg/kg) was revealed. We hypothesize that this finding may be connected with the profound sedation observed with the same combination in the procedure measuring locomotor activity. Namely, it is possible (cf. van Rijnsoever et al. 2004) that the supposed complete antagonism at α5-containing GABAA receptors forestalls development of tolerance to sedation and/or decreased vigilance, and hence impairs learning; this question could be partly resolved with further studies of repeated dosing of diazepam and XLi093 in the locomotor activity test.
In the antagonism study with 2.0 mg/kg diazepam, both antagonists tended to reverse its effects, which may be seen as corroborating previous conclusions that the water-maze acquisition impairment is not due to the sedative effect of diazepam (McNamara & Skelton, 1991). The fact that rats treated with the combination 2 mg/kg diazepam+15 mg/kg β-CCt were even faster swimmers, overall, than the group treated with diazepam and the group treated with the combination 2 mg/kg diazepam+10 mg/kg XLi093, replicates our previous finding with the combination of 2 mg/kg midazolam+30 mg/kg β-CCt, which potentiated inter-trial crossings during the acquisition session of active avoidance paradigm (Savić et al. 2005b). In regard to the cognitive function-related parameters, β-CCt antagonized the inhibitory effect of midazolam on procedural memory tested through active avoidance retention (Savić et al. 2005b), and attenuated the deteriorating effect of the BZ on declarative memory in passive avoidance paradigm (Savić et al. 2005a). In the anxiety-related paradigms (elevated plus maze and acquisition session of active avoidance), potentiation of the anti-anxiety action of midazolam was observed (Savić et al. 2004, 2005b). As the emotionally arousing experiences tend to be well remembered (McGaugh, 2004), it is widely accepted that suppression of arousal and anxiety by BZs may impair some aspects of cognitive functioning (Curran, 1991). However, the present water maze results, together with the previous findings, dismiss the suggestion (Zanotti et al. 1994) that the diazepam-induced place learning impairment may be mainly related to its anxiolytic properties. Despite the fact that the anti-anxiety effect of diazepam may have only been preserved or potentiated, not abolished, by β-CCt, the overall performance during five acquisition days was at least equal to that of the control group. The present and previous results suggest that combining a BZ with β-CCt may result in a near to control level of performance of a cognitive task, without sedation, but with highly desirable preserved anti-anxiety activity.
On the other hand, Cain (1997) suggested that the acquisition deficits may result from the sensorimotor disturbances that diazepam causes. Namely, the author found that non-spatial pretraining without pretreatment eliminated swimming in the periphery of the pool, platform deflections and swimovers, and resulted in the normal, rapid acquisition of the water-maze task under diazepam (Cain, 1997). Nevertheless, McNamara & Skelton (1991) found that treatment with diazepam after the rats acquired the location of the platform did not affect further water-maze activity, while it did impair finding of the newly located platform. Bearing in mind the interaction between arousal, cognitive function and anxiety (Curran, 1991), it is difficult to say that the present results support the view of either pure learning-impairing (McNamara & Skelton, 1991) or non-selective incapacitating (Cain, 1997) effects of diazepam as the explanation for its effects on spatial learning. Both types of influences may be partly operating in the learning impairment induced by diazepam in the Morris water maze.
The results from the probe trial show that the platform quadrant parameters are not a reliable measure of spatial memory influences at the used doses of diazepam (cf. Gerlai, 2001). As an example, rats treated during previous days with 2.0 mg/kg diazepam spent three quarters of the probe trial time in balanced circling throughout the peripheral annulus, and it was not possible to detect any lack of preference for the target quadrant during the 15 s of rest. Suppression of an instinct to swim thigmotaxically appears to be necessary to effectively accomplish the maze task (Cain, 1998). β-CCt as well as XLi093 reversed both the increase of peripheral annulus and the decrease of platform annulus parameters, induced by 2 mg/kg diazepam. The results from the recall trial as well as from acquisition trials suggest that it is sufficient to antagonize the activity of diazepam at either α1- or α5-containing GABAA receptors in order to forestall its influence on learning the required water-maze skills and strategies, i.e. procedural components of this memory task (Cain, 1998; Rossato et al. 2006).
Despite the expected relatively low control group activity in the platform zone on its own (cf. Vorhees & Williams, 2006), the anterograde amnesic influence of previous treatment with 2 mg/kg diazepam still reached statistical significance, and was only partially prevented by both antagonists used, i.e. they attenuated, but did not antagonize, the spatial memory deficit. In fact, the parameters related to the previous platform location in the probe trial are the only ones in the antagonism study with 2 mg/kg diazepam which did not tend to be at least a little more preserved in combination with β-CCt than with XLi093. The water maze is usually seen as a hippocampal-dependent memory model (Gerlai, 2001), and abundant staining in the rat hippocampus was shown for the α1 as well as α5 subunit (Pirker et al. 2000). There are several experimental findings related to the role of the α5 subunit in spatial memory. Thus, the α5 knockout mice, compared to the wild-type animals, performed significantly better in a working-memory protocol of the water maze (Collinson et al. 2002), while an inverse agonist selective for GABAA receptors containing α5 subunits facilitated the acquisition and recall of rats in a similar protocol of working memory (Collinson et al. 2006). The present protocol enabled the long-term consolidation of spatial memory to happen, therefore it can be hypothesized that potentiation of inhibitory transmission at both, the α5- and α1-containing GABAA receptors contributes in an interactive way to impairment in the declarative spatial component of the task (Cain, 1998; Rossato et al. 2006). It is conceivable that besides the hippocampus, with its crucial role in long-term spatial memory (Bird & Burgess, 2008), the α1- and α5-containing GABAA receptors in neocortex (Pirker et al. 2000; Yamada et al. 2007) may be of significance for spatial memory deficits induced by diazepam.
Curran (1991) concluded that sedative effects of BZs in humans are much more easily reversed than amnesic effects; a similar conclusion may have been applied to rats’ behaviour in the active avoidance task (Savić et al. 2005b) and to a certain degree to the present results. It appears that the procedural component (strategy learning) of the water-maze learning deficit induced by diazepam is more prone to reversion by α1- and α5-subtype selective antagonists, the role of α1- containing GABAA receptors being more salient, while the declarative spatial memory component of learning deficit is less prone to attenuation by antagonists, and may be more related to α5-containing GABAA receptors. Considering the previous results with the combination of a non-selective BZ site agonist and the α1 selective antagonist β-CCt (Savić et al. 2004, 2005a, b), it appears that behavioural effects of such a polypharmacy approach may be highly attractive. In the quest for anxioselective anxiolytics, such a combination may be worth testing on human subjects, and it could be especially useful in treating those forms of emotional disorders which are accompanied by psychomotor effects.
Acknowledgments
This work was supported in part by NIMH 46851 (J.M.C.) and by The Ministry of Science, R. Serbia – Grant no. 145022B (M.M.S.). We acknowledge the support of this work by the Research Growth Initiative of the University of Wisconsin – Milwaukee and the Lynde and Harry Bradley Foundation.
Footnotes
Statement of Interest
None.
References
- Anand A, Saraf MK, Prabhakar S. Sustained inhibition of brotizolam induced anterograde amnesia by norharmane and retrograde amnesia by L-glutamic acid in mice. Behavioural Brain Research. 2007;182:12–20. doi: 10.1016/j.bbr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Araujo F, Ruano D, Vitorica J. Native γ-aminobutyric acid type A receptors from rat hippocampus, containing both α1 and α5 subunits, exhibit a single benzodiazepine binding site with α5 pharmacological properties. Journal of Pharmacology and Experimental Therapeutics. 1999;290:989–997. [PubMed] [Google Scholar]
- Arolfo MP, Brioni JD. Diazepam impairs place learning in the Morris water maze. Behavioral and Neural Biology. 1991;55:131–136. doi: 10.1016/0163-1047(91)80133-y. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. Journal of Neuroscience. 1996;16:283–297. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nature Reviews Neuroscience. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Cain DP. Prior non-spatial pretraining eliminates sensorimotor disturbances and impairments in water maze learning caused by diazepam. Psychopharmacology. 1997;130:313–319. doi: 10.1007/s002130050245. [DOI] [PubMed] [Google Scholar]
- Cain DP. Testing the NMDA, long-term potentiation, and cholinergic hypotheses of spatial learning. Neuroscience & Biobehavioral Reviews. 1998;22:181–193. doi: 10.1016/s0149-7634(97)00005-5. [DOI] [PubMed] [Google Scholar]
- Clement Y, Chapouthier G. Biological bases of anxiety. Neuroscience and Biobehavioural Reviews. 1998;22:623–633. doi: 10.1016/s0149-7634(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Collinson N, Atack JR, Laughton P, Dawson GR, Stephens DN. An inverse agonist selective for alpha5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology. 2006;188:619–628. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. Journal of Neuroscience. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E, Hagen T, McKernan R, Cook JM. Bz1 receptor subtype specific ligands. synthesis and biological properties of βCCt, a Bz1 receptor subtype specific antagonist. Medicinal Chemistry Research. 1995;5:710–718. [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neuroscience & Biobehavioral Reviews. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proceedings of the National Academy of Sciences USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV. Benzodiazepines, memory and mood: a review. Psychopharmacology. 1991;105:1–8. doi: 10.1007/BF02316856. [DOI] [PubMed] [Google Scholar]
- Depoortere H, Zivkovic B, Lloyd KG, Sanger DJ, Perrault G, Langer SZ, Bartholini G. Zolpidem, a novel nonbenzodiazepine hypnotic. I. Neuropharmacological and behavioral effects. Journal of Pharmacology and Experimental Therapeutics. 1986;237:649–658. [PubMed] [Google Scholar]
- Griebel G, Perrault G, Letang V, Granger P, Avenet P, Schoemaker H, Sanger DJ. New evidence that the pharmacological effects of benzodiazepine receptor ligands can be associated with activities at different BZ (omega) receptor subtypes. Psychopharmacology. 1999;146:205–213. doi: 10.1007/s002130051108. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Behavioral tests of hippocampal function: simple paradigms complex problems. Behavioural Brain Research. 2001;125:269–277. doi: 10.1016/s0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- Hauser J, Rudolph U, Keist R, Möhler H, Feldon J, Yee BK. Hippocampal alpha5 subunit-containing GABAA receptors modulate the expression of prepulse inhibition. Molecular Psychiatry. 2005;10:201–207. doi: 10.1038/sj.mp.4001554. [DOI] [PubMed] [Google Scholar]
- Hentschke H, Schwarz C, Antkowiak B. Neocortex is the major target of sedative concentrations of volatile anaesthetics : strong depression of firing rates and increase of GABAA receptor-mediated inhibition. European Journal of Neuroscience. 2005;21:93–102. doi: 10.1111/j.1460-9568.2004.03843.x. [DOI] [PubMed] [Google Scholar]
- Huang Q, He X, Ma C, Liu R, Yu S, Dayer CA, Wenger GR, McKernan R, Cook JM. Pharmacophore/receptor models for GABA(A)/BzR subtypes (alpha1beta3gamma2, alpha5beta3gamma2, and alpha6beta3gamma2) via a comprehensive ligand-mapping approach. Journal of Medicinal Chemistry. 2000;43:71–95. doi: 10.1021/jm990341r. [DOI] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, Eiler WJ, Grey C, Carroll MR, McCane S, et al. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–2137. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annual Review of Neuroscience. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Lader MH. Limitations on the use of benzodiazepines in anxiety and insomnia: are they justified? European Neuropsychopharmacology. 1999;9 (Suppl 6):S399–405. doi: 10.1016/s0924-977x(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Li X, Cao H, Zhang C, Furtmüller R, Fuchs K, Huck S, Sieghart W, Deschamps J, Cook JM. Synthesis, in vitro affinity, and efficacy of a bis 8-ethynyl-4H-imidazo[1,5a]-[1,4]benzodiazepine analogue, the first bivalent alpha5 subtype selective BzR/GABA(A) antagonist. Journal of Medicinal Chemistry. 2003;46:5567–5570. doi: 10.1021/jm034164c. [DOI] [PubMed] [Google Scholar]
- Lippa AS, Coupet J, Greenblatt EN, Klepner CA, Beer B. A synthetic non-benzodiazepine ligand for benzodiazepine receptors : a probe for investigating neuronal substrates of anxiety. Pharmacology Biochemistry and Behavior. 1979;11:99–106. doi: 10.1016/0091-3057(79)90304-6. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nature Neuroscience. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. Diazepam impairs acquisition but not performance in the Morris water maze. Pharmacology Biochemistry & Behavior. 1991;38:651–658. doi: 10.1016/0091-3057(91)90028-z. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. Benzodiazepine receptor antagonists fluamzenil and CGS 8216 and inverse-agonist β-CCM enhance spatial learning in the rat : Dissociation from anxiogenic actions. Psychobiology. 1993;21:101–108. [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors : immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Zinn CG, Furini C, Bevilaqua LR, Medina JH, Cammarota M, Izquierdo I. A link between the hippocampal and the striatal memory systems of the brain. Anais de Academia Brasileira de Ciencias. 2006;78:515–523. doi: 10.1590/s0001-37652006000300011. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annual Review of Pharmacology & Toxicology. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Current Opinion in Pharmacology. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Savić MM, Clayton T, Furtmüller R, Gavrilović I, Samardžić J, Savić S, Huck S, Sieghart W, Cook JM. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA(A) receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Research. 2008b;1208:150–159. doi: 10.1016/j.brainres.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savić MM, Huang S, Furtmüller R, Clayton T, Huck S, Obradović DI, Ugresić ND, Sieghart W, Bokonjić DR, Cook JM. Are GABAA receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology. 2008a;33:332–339. doi: 10.1038/sj.npp.1301403. [DOI] [PubMed] [Google Scholar]
- Savić MM, Obradović DI, Ugrešić ND, Cook JM, Yin W, Bokonjić DR. Bidirectional effects of benzodiazepine binding site ligands in the elevated plus-maze: differential antagonism by flumazenil and beta-CCt. Pharmacology Biochemistry and Behavior. 2004;79:279–290. doi: 10.1016/j.pbb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Savić MM, Obradović DI, Ugrešić ND, Cook JM, Yin W, Bokonjić DR. Bidirectional effects of benzodiazepine binding site ligands in the passive avoidance task : differential antagonism by flumazenil and β-CCt. Behavioral Brain Reseasrch. 2005a;158:293–300. doi: 10.1016/j.bbr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Savić MM, Obradović DI, Ugrešić ND, Cook JM, Sarma PVVS, Bokonjić DR. Bidirectional effects of benzodiazepine binding site ligands on active avoidance acquisition and retention : differential antagonism by flumazenil and β-CCt. Psychopharmacology. 2005b;180:455–465. doi: 10.1007/s00213-005-2170-1. [DOI] [PubMed] [Google Scholar]
- Shinday NM, Rallapalli S, Cook JM, Meyer JS, Rowlett JK. 2008 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2008. [Accessed 14 January 2009]. The selective α5 GABAA receptor antagonist Xli-093 reverses diazepam-induced deficits in a holeboard memory task. Program No. 589.18. 2008 ( http://www.abstractsonline.com/plan/ViewAbstract.aspx?sKey=544d3829-25d9-4d17-a2a4-bd2227896cf6&cKey=09468dce-5728-43a9-a3aca536cf357b5a) [Google Scholar]
- Sieghart W, Ernst M. Heterogeneity of GABAA receptors : revived interest in the development of subtype-selective drugs. Current Medicinal Chemistry – Central Nervous System Agents. 2005;5:217–242. [Google Scholar]
- Terry AV., Jr . Spatial navigation (water maze) tasks. In: Buccafusco JJ, editor. Behavioral Methods in Neuroscience. Boca Raton: CRC Press; 2000. pp. 153–166. [PubMed] [Google Scholar]
- van Rijnsoever C, Tauber M, Choulli MK, Keist R, Rudolph U, Möhler H, Fritschy JM, Crestani F. Requirement of alpha5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. Journal of Neuroscience. 2004;24:6785–6790. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Furukawa T, Ueno S, Yamamoto S, Fukuda A. Molecular basis for the GABAA receptor-mediated tonic inhibition in rat somatosensory cortex. Cerebral Cortex. 2007;17:1782–1787. doi: 10.1093/cercor/bhl087. [DOI] [PubMed] [Google Scholar]
- Zanotti A, Arban R, Perazzolo M, Giusti P. Diazepam impairs place learning in native but not in maze-experienced rats in the Morris water maze. Psychopharmacology. 1994;115:73–78. doi: 10.1007/BF02244754. [DOI] [PubMed] [Google Scholar]