Abstract
Relapse to drug seeking and drug taking is elicited by exposure to stress, drug-associated cues, or drugs of abuse themselves. According to the clinical literature, relapse also can be elicited in humans by sleep deprivation. Even so, the effect of sleep deprivation on drug–seeking and drug-taking behaviors has received relatively little attention in the laboratory (i.e., currently, no animal model exists) and the underlying circuitry remains unexplored. In the present study, 42 naïve male Sprague-Dawley rats were trained to self-administer cocaine and were then divided, on the basis of their behavior, into low (n=20) and high (n=22) drug-taking groups. Self-administration behavior was extinguished, and the effect of acute sleep deprivation (0, 4, or 8 h) on drug-induced reinstatement and on progressive ratio responding (i.e., on the motivation to work for drug) was investigated. The results showed that, relative to low drug-takers, high drug-takers took more drug in acquisition, made more infusion attempts during drug-induced reinstatement, worked harder for drug, and exhibited greater goal-directed behavior. Acute sleep deprivation had little impact on high drug-takers beyond increasing the rate of infusions self-administered during progressive ratio (PR) testing. Conversely, in low drug-takers, acute sleep deprivation completely abolished cocaine-induced reinstatement during extinction testing. During PR testing, however, sleep deprivation increased the speed with which low drug-taking rats initiated responding for drug, increased the rate of infusions, and increased goal-directed behavior. It did not, however, increase the perceived value of the cocaine reward (i.e., neither sleep-deprived low drug-takers nor high drug-takers exhibited a higher break point for cocaine than their non-deprived counterparts). These data are the first to demonstrate a direct link between sleep deprivation and responding for cocaine, particularly in subjects that would otherwise respond little for drug.
Keywords: sleep deprivation, cocaine, self-administration, drug-induced reinstatement, progressive ratio
Substance abuse and addiction have become major health concerns in the United States. It has been reported that 17% of Americans meet the diagnostic criteria for some form of substance dependence, excluding tobacco dependence (Anthony and Helzer, 1991), which is almost double the incidence of depression (9.5%; National Institute of Mental Health, 2000) and 17 times greater than that of schizophrenia (1%; National Institute of Mental Health, 2006). Substance abuse incurs an estimated $484 billion in annual expenses to our nation (diabetes and cancer impose costs of $132 billion and $172 billion, respectively; National Institute on Drug Abuse, 2005). Addiction is an extremely difficult disease to treat because it is a syndrome comprised of several maladaptive behaviors that exploit the existing reward circuitry of the brain. The severity of the problem (and difficulty of treatment) is further compounded by the fact that addiction is a chronic, relapsing disease that induces long-lasting changes in brain function (O’Brien, 1997). In fact, it has been reported that up to 90% of addicted humans will relapse to drug seeking, even after a prolonged period of abstinence (DeJong, 1994). Three types of relapse have been extensively described in the scientific literature: relapse induced by exposure to environmental stressors, relapse induced by exposure to drug-associated cues, and relapse induced by exposure to drugs themselves.
The clinical literature suggests that sleep deprivation is another factor that can induce relapse. Both subjective (self-administered questionnaire scores) and objective (polysomnographic sleep parameters) measures of poor sleep quality, in general, have been shown to predict relapse (Brower, 2001; Clark et al., 1998; Foster and Peters, 1999; Gillin et al., 1994). This problem is compounded by the overall poor sleep quality experienced by the majority of our society. Data collected in the recent 2008 Sleep in America Poll conducted by the National Sleep Foundation showed that 65% of Americans report experiencing symptoms of a sleep problem several nights per week. The population of individuals reporting poor sleep likely overlaps with the substance-dependent population. Yet, no animal models have been developed and relatively little work has been done to ascertain the mechanisms by which sleep deprivation induces relapse.
In addition, several studies purport insomnia as a reliable predictor of relapse in substance-abusing humans (Brower, 2003; Brower et al., 2001; Ford and Kamerow, 1989; Foster and Marshall, 1998; Gillin, 1998; Hohagen et al., 1993; Longo and Johnson, 1998; Malcolm et al., 2007; Teplin et al., 2006). Admittedly, there is a very clear distinction between frank insomnia and forced sleep deprivation. Insomnia is primarily a disorder of hyperarousal, often linked with high levels of psychological stress and anxiety, among other comorbid conditions. Sleep deprivation refers to the simple suppression or prevention of sleep. However, both represent a profound loss of sleep and poor sleep quality, overall. While the effects of insomnia on relapse cannot simply be generalized to those of sleep deprivation, it seems likely that the two conditions do, in fact, have similar effects on drug-seeking behaviors. Such an inference is not unreasonable given the aforementioned studies showing that both subjective and objective measures of general poor sleep quality also have been shown to predict relapse (Brower, 2001; Clark et al., 1998; Foster and Peters, 1999; Gillin et al., 1994). Given the impact of insomnia and general poor sleep quality on drug seeking and drug taking reported in the clinical literature, we hypothesized that sleep deprivation would augment cocaine-induced reinstatement and cocaine seeking in drug-experienced rats. While chronic sleep deprivation may be more akin to that experienced by the human population (Girardin et al., 2000), we thought it prudent to begin this investigation with the study of acute sleep deprivation. The present study, then, used drug-induced reinstatement and progressive ratio testing to investigate the effects of acute sleep deprivation (0, 4, or 8 h) on drug seeking and drug taking in rats that had been trained to self-administer cocaine.
Methods
Subjects
The subjects were 42 naïve, male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC), approximately three months of age at the beginning of the experiment. Except where otherwise noted, the rats were housed individually in standard wire mesh cages, in a colony room with temperature, humidity, and ventilation controlled automatically. The rats were maintained on a 12-h light-dark cycle, with lights on at 0700 h. They were allowed ad lib access to food (Harlan Teklad, Madison, WI) and water, except where otherwise noted.
Catheter Construction and Implantation
Self-administration catheter
Intra-jugular catheters were custom-made in our laboratory as described by Grigson and Twining (2002).
Catheter implantation
Rats were anesthetized and catheters were implanted into the jugular vein as described by Grigson and Twining (2002) and Liu and Grigson (2005). Following surgery, rats were allowed at least two days to recover. General maintenance of catheter patency involved daily examination and flushing of catheters with heparinized saline (0.2 ml of 30 IU/ml heparin). Catheter patency was verified, as needed, using 0.2 ml of propofol (Diprivan 1%) administered intravenously.
Apparatus
Each rat was trained in one of twelve identical operant chambers (MED Associates, St. Albans, VT) described by Grigson and Twining (2002). Each chamber measured 30.5 cm in length × 24.0 cm in width × 29.0 cm in height, and was individually housed in a light- and sound-attenuated cubicle. The chambers consisted of a clear Plexiglas top, front, and back wall. The side walls were made of aluminum. Grid floors consisted of nineteen 4.8-mm stainless steel rods, spaced 1.6 cm apart (center to center). Each chamber was equipped with two retractable sipper spouts that entered through 1.3-cm diameter holes, spaced 16.4 cm apart (center to center). A stimulus light was located 6.0 cm above each tube. Each chamber was also equipped with a houselight (25 W), a tone generator (Sonalert Time Generator, 2900 Hz, Mallory, Indianapolis, IN), and a speaker for white noise (75 dB). Cocaine reinforcement was controlled by a lickometer circuit that monitored empty spout licking to operate a syringe pump (Model A, Razel Scientific Instruments, Stamford, CT). A coupling assembly attached the syringe pump to the catheter assembly on the back of each rat and entered through a 5.0-cm diameter hole in the top of the chamber. This assembly consisted of a metal spring attached to a metal spacer with Tygon tubing inserted down the center, protecting passage of the tubing from rat interference. The tubing was attached to a counterbalanced swivel assembly (Instech, Plymouth Meeting, PA) that, in turn, was attached to the syringe pump. Events in the chamber and collection of data were controlled on-line with a Pentium computer that used programs written in the Medstate notation language (MED Associates).
Drug Preparation
Individual 20-ml syringes were prepared for each self-administration chamber prior to each daily session by diluting 4.0 ml of cocaine HCl stock solution (1.24 g cocaine HCl + 150 ml saline) with 16.0 ml of heparinized saline (0.1 ml 1000 IU heparin/60.0 ml saline) for a dose of 0.33 mg/infusion. This dose of cocaine supports marked and orderly cocaine self-administration behavior (Grigson & Twining, 2001; Liu & Grigson, 2005; Wheeler et al., 2008).
Data Collection
In an effort to be consistent with other self-administration data collected in our laboratory (e.g., Grigson & Twining, 2002; Liu and Grigson, 2005), habituation, self-administration training, extinction training, and all experimental testing were conducted during the light phase of the light/dark cycle. As such, it could be argued that all animals experienced some degree of sleep deprivation. However, daily training during the light cycle is standard practice for the development of self-administration behaviors. Training sessions were only 60–90 minutes in duration. In addition, sleep deprivation was not forced during the sessions. The animals were free to sleep in the chambers if they so chose (in fact, most did). Thus, these training sessions imposed little, if any, sleep deprivation.
Habituation Procedure
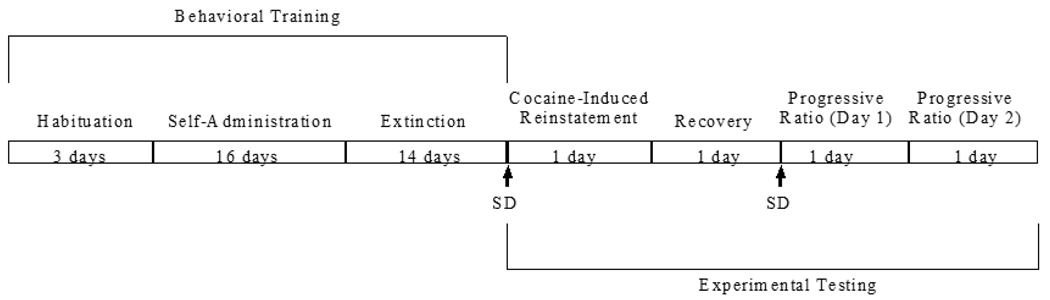
Rats were habituated to the operant chambers for 1 h/day for three days prior to the beginning of self-administration training. During this time, each rat was maintained on a water-deprivation regimen in which they received 1-h daily access to water in the operant chamber from the right spout during the habituation session and 25.0 ml of water in the home cage overnight. Thereafter, rats were returned to ad lib access to water for the duration of the study. See Figure 1 for a summary of behavioral training and experimental testing.
Figure 1.
Timeline of behavioral training and experimental testing.
Self-Administration and Extinction Procedures
Self-administration training
Self-administration training began immediately following the 3-day habituation phase. Each rat was trained during daily 90-min sessions for 16 days. Specifically, rats were placed in the operant chambers in darkness. Immediately upon initiation of the 90-min session, the white noise was turned on, both spouts advanced into the chamber, and the cue light above the active spout was illuminated. The right spout was termed the “active” spout, while the left spout was termed the “inactive” spout. A fixed ratio (FR) 10 schedule of reinforcement was implemented initially (Days 1–12). During this time, completion of 10 licks on the “active” spout was followed by a single intravenous (i.v.) infusion of 0.33 mg cocaine over six seconds. Drug delivery was signaled by offset of the stimulus light, spout retraction, and onset of the tone and houselight. The tone and houselight remained on for a 20-sec timeout period. Responding on the “inactive” spout was without consequence throughout each session. During the final four days of training (Days 13–16), the reinforcement schedule was increased to an FR20 to fully distinguish between active and inactive responding. Following each self-administration training session, the rats were returned to their home cages.
Extinction training
Extinction training began immediately following self-administration training, as previously described (Liu and Grigson, 2005), and continued for 14 days. Conditions matched those employed during self-administration training, except completion of the required number of licks on the FR20 schedule of reinforcement was no longer followed by an i.v. infusion of cocaine. Behavior was considered to be extinguished when the number of responses made on the previously active and inactive spouts did not differ significantly for at least three consecutive extinction trials.
Acute Sleep Deprivation Procedure and Experimental Testing Procedures
Acute sleep deprivation
Acute sleep deprivation was conducted using the novel object method described by Cirelli and colleagues (Cirelli et al., 1995; Cirelli & Tononi, 2000; Vyazovskiy et al., 2007). The standard wire mesh cages that served as home cages were not conducive to this method. Therefore, following the fifth day of extinction training, all rats were housed in Plexiglas home cages (46.0 cm in length × 25.0 cm in width × 21.5 cm in height) with corncob bedding (Harlan Teklad, Madison, WI). They remained in these Plexiglas home cages for the duration of the study, thereby providing ample time for the animals to become habituated to these new home cages prior to the imposition of acute sleep deprivation. During acute sleep deprivation, novel objects were introduced into the Plexiglas home cages of the rats. All rats explored and interacted with these objects, even at the expense of sleep. All animals were under constant investigator supervision for the entire duration of acute sleep deprivation, such that objects could be replaced if their novelty was lost (i.e., if rats stopped attending to those objects). Investigators also introduced folded paper towels into the cages, which prompted interaction by the rats. This approach provides stimulation to the animal, but with less stress than that incurred by other sleep deprivation methods. These methods have been employed reliably for periods of time ranging from 1 – 24 h (Tononi et al., 1994; Cirelli et al., 1999; Cirelli & Tononi, 2000). Rats were divided into three groups: the SD0 group (n=14) did not receive any sleep deprivation; the SD4 group (n=14) received 4 h of total sleep deprivation; and the SD8 group (n=14) received 8 h of total sleep deprivation.
Cocaine-induced reinstatement testing
Twenty-four hours following the final day of extinction training, the rats were acutely sleep deprived, as described, and immediately tested for cocaine-induced reinstatement. Testing occurred during the light phase. During the cocaine-induced reinstatement test, rats were placed in the operant chambers for a single 90-min session, with conditions identical to those employed during extinction training. However, 45 min into the 90-min extinction session, a 0.33 mg infusion of cocaine was passively administered i.v. Responding on the active and inactive spout was measured both before and after the passive infusion.
Progressive ratio testing
An additional method that can be used to assess the impact of acute sleep deprivation on responding for drug is the introduction of a PR schedule of reinforcement to test how hard sleep-deprived rats are willing to work for the drug. Thus, following cocaine-induced reinstatement testing, rats were allowed one day to recover from sleep deprivation. Rats in the same sleep deprivation groups were then subjected to a second bout of acute sleep deprivation. Progressive ratio testing took place immediately thereafter. During PR testing, rats were placed in the operant chambers with conditions identical to those of self-administration training, except the number of active responses required to receive each infusion progressively increased by a multiple of five for up to ten infusions (1, 1+5=6, 6+10=16, 16+15=31, 31+20=51, 51+25=76, 76+30=106, 106+35=141, 141+40=181, 181+45=226). Thereafter, the number of required responses increased by 50 for each successive infusion (226+50=276, 276+50=326, 326+50=376, etc.). During this PR session, rats were allowed to self-administer cocaine (0.33 mg/infusion) until a period of 30 min elapsed without receipt of an infusion. Rate of drug self-administration (inter-infusion interval and load-up latency), as well as goal-directed responding were measured (see Results for a more detailed description).
Data Analysis
All data were analyzed with Statistica (StatSoft, Tulsa, OK) using mixed factorial and one-way analysis of variance (ANOVA) tests, as well as Student’s t-tests. Newman-Keuls post hoc tests were conducted on significant ANOVAs, when appropriate, with α set at 0.05.
Results
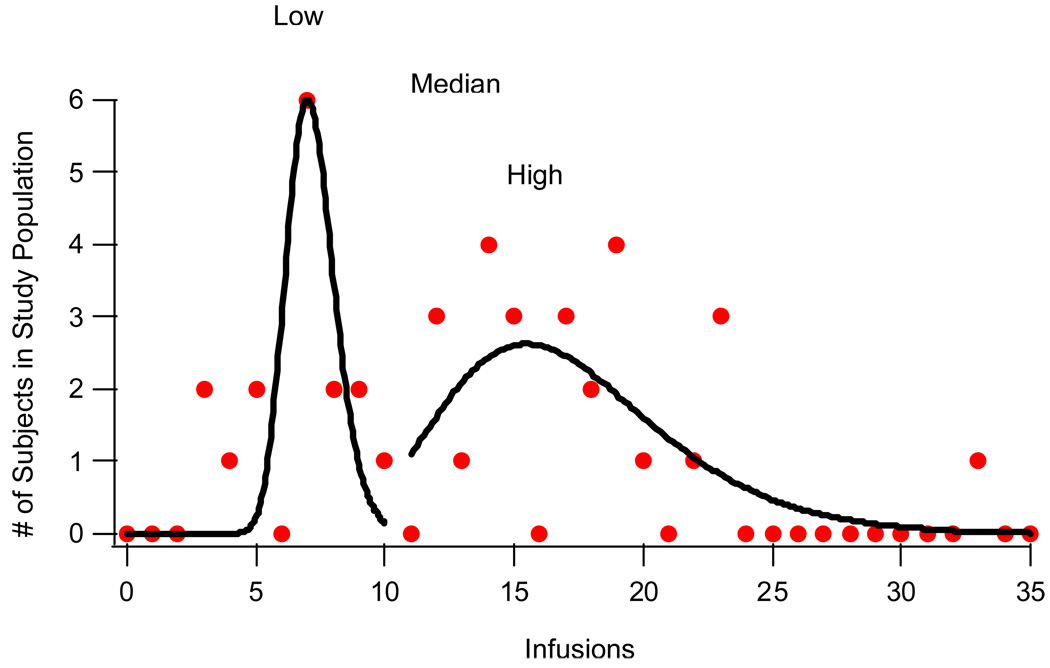
When examining the acquisition data, it became evident that there were likely two separate groups of rats. Thus, prior to data analysis, the number of infusions received during the final two days of self-administration training was averaged, and the rats were separated into two groups using the median split of those means. Consistent with previous findings (Grigson & Twining, 2002), this allowed for the identification of two distinct sub-populations within our sample population: low drug-takers (n=20) and high drug-takers (n=22). These two sub-populations can be seen in Figure 2, which illustrates the bimodal distribution of cocaine self-administration.
Figure 2.
Bimodal distribution of mean number of SA infusions (Low curve: y0 = 0; A = 6; μ = 7; σ = 0.184; χ2 = 21.42; High curve: y0 = −0.0126; A = 2.62; μ = 15.479; σ = 0.3678; χ2 = 27.037; Median: 13).
Thus, during the terminal acquisition period, most rats in the low drug-taking group took between 4 – 9 (median = 5.5, mean = 6) infusions of cocaine/90-min session, while most rats in the high drug-taking group took between 18 – 24 (median = 19, mean = 21) infusions/90-min session.
Acquisition and Extinction
Acquisition: Number of Responses
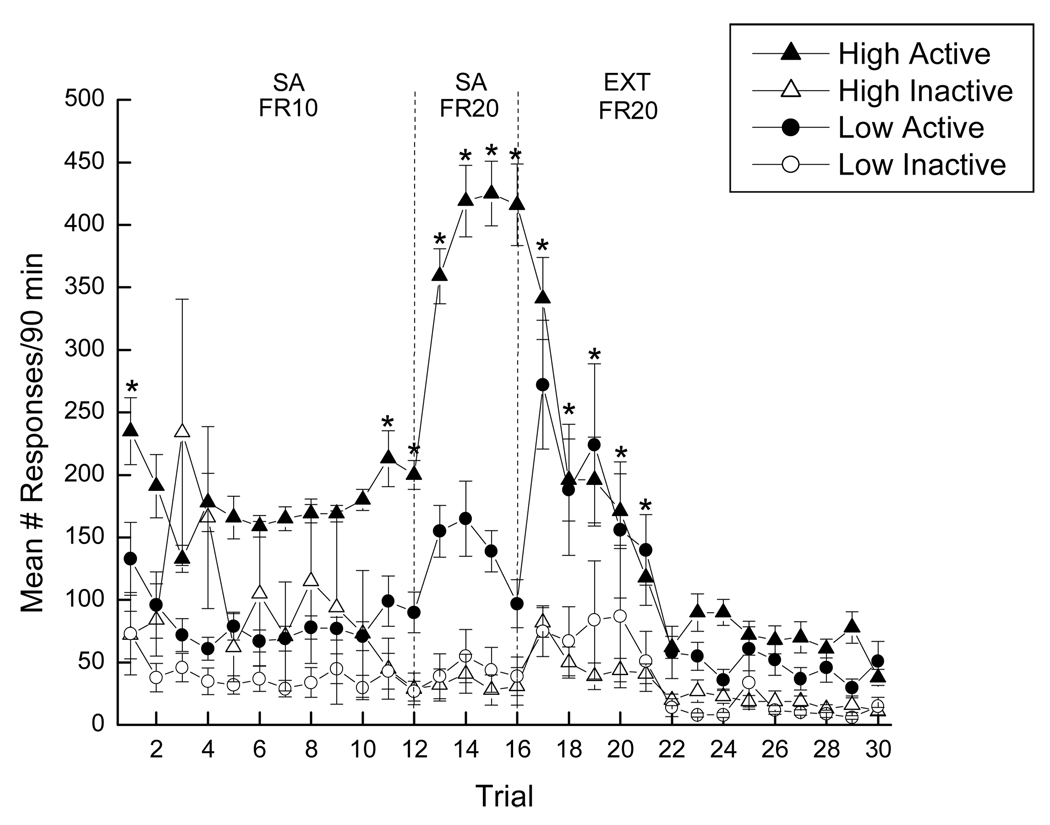
Acquisition of cocaine self-administration was analyzed using a 2 × 2 × 16 mixed factorial ANOVA varying group (low drug-takers or high drug-takers), spout (active or inactive), and trials (1–16). Significant main effects of group, spout, and trial were obtained (see Figure 3).
Figure 3.
Mean active and inactive responding throughout self-administration and extinction training. Closed symbols represent active responses, while open symbols represent inactive responses. High drug-takers (n=22) are represented by triangles, while circles represent low drug-takers (n=20). * denotes statistical significance (p < 0.05).
Overall, high drug-takers responded more than low drug-takers, F(1, 78)=23.53, p < 0.01, more active responses were made than inactive responses, F(1, 78)=36.55, p < 0.01, and responding increased as self-administration trials progressed, F(15, 1170)=5.11, p < 0.01. In addition, significant two-way interactions were seen between group and spout, as well as between spout and trial. Post hoc tests of the significant group × spout interaction, F(1, 78)=7.26, p < 0.01, indicated that high drug-takers exhibited more active responses than low drug-takers, overall, p < 0.05. Post hoc analysis of the significant spout × trials interaction, F(15, 1170)=10.90, p < 0.01, revealed that active responding was greater than inactive responding across the 16 trials of acquisition overall, ps < 0.05. Finally, the group × spout × trial interaction also was significant, F(15, 1170)=5.60, p < 0.01. Post hoc Newman-Keuls tests of this three-way interaction revealed that high drug-takers responded more on the active than the inactive spout on Day 1 and on Days 11–16, ps < 0.01 (see Figure 3). However, low drug-takers never displayed this behavior. That is, the differences between active and inactive responding displayed by low drug-takers were not statistically significant throughout the 16 days of self-administration training.
Acquisition: Response Latency
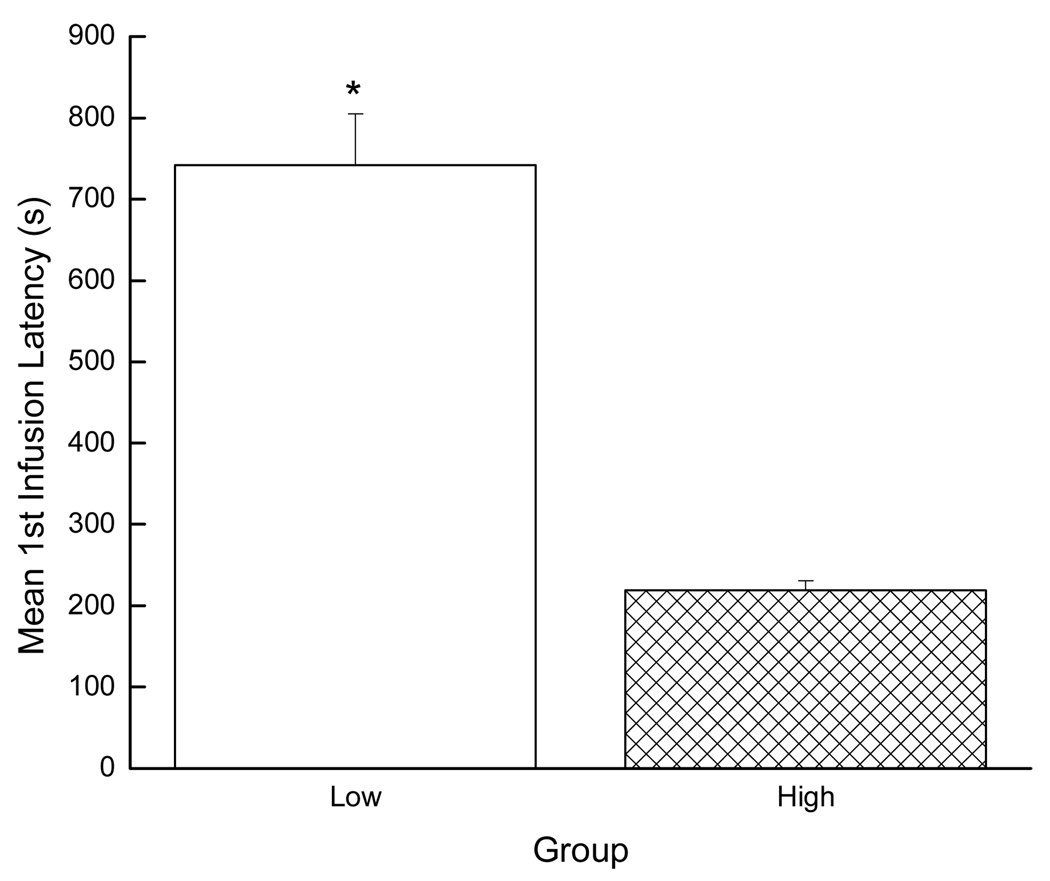
Acquisition data also were evaluated by examining the effect of group on the latency to self-administer the first infusion of cocaine using a Student’s t-test. In accordance with the infusion data, the high drug-takers also were faster to take the first infusion (i.e., exhibited a shorter latency) than the low drug-takers (p < 0.01; see Figure 4).
Figure 4.
Mean latency to first SA infusion. * denotes statistical significance (p < 0.01).
Extinction
Extinction of self-administration training was analyzed similarly using a 2 × 2 × 14 mixed factorial ANOVA varying group (low drug-takers or high drug-takers), spout (active or inactive), and trials (17–30). Significant main effects of spout and trial were seen (see Figure 3). Overall, active responses remained greater than inactive responses, F(1, 80)=28.30, p < 0.01, and responding decreased as extinction trials progressed, F(13, 1040)=35.14, p < 0.01. However, neither the main effect of group, nor the group × spout interaction was significant, Fs < 1. A significant two-way interaction was seen between spout and trial, F(13, 1040)=10.65, p < 0.01, and post hoc tests showed that all rats responded more on the active than the inactive spout during trials 17 – 21, ps < 0.01. In accordance, the group × spout × trials interaction did not attain statistical significance, F < 1. This finding confirmed that the high and the low drug-takers responded similarly on the active and the inactive spouts throughout extinction. Thus, while the low drug-takers failed to make significantly more responses on the active than the inactive spout during acquisition, they exhibited a marked increase in responding on the active spout early in extinction training when the drug was removed. These data suggest that even the low drug-takers were self-administering drug, but simply at a lower level than the high drug-takers.
Cocaine-Induced Reinstatement
Cocaine-induced reinstatement was analyzed using a 2 × 2 × 3 mixed factorial ANOVA varying group (low drug-takers or high drug-takers), test period (pre-drug or post-drug), and SD condition (SD0, SD4, or SD8; see Figure 5).
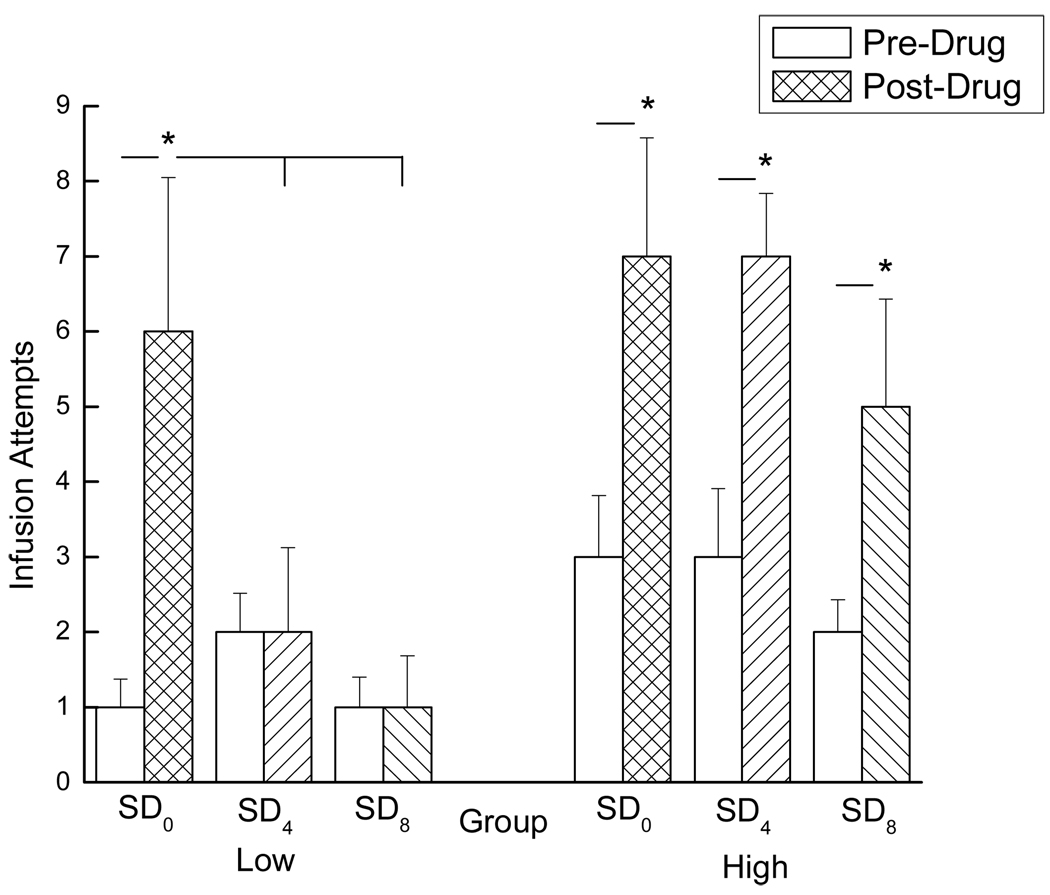
Figure 5.
Mean number of infusion attempts made by rats in all three SD conditions (SD0, SD4, and SD8) during the 45 min extinction period before (open bars) and after (closed bars) the passive infusion of cocaine. Rats with a history of low drug-taking (SD0: n=7, SD4: n=6, and SD8: n=7) can be viewed in the left panel, while rats with a history of high drug-taking (SD0: n=7, SD4: n=8, and SD8: n=7) can be viewed in the right panel. * denotes statistical significance (p < 0.05).
Significant main effects of group and test period were obtained, showing that the high drug-takers responded more than the low drug-takers, overall, F(1, 36)=8.19, p < 0.01, and post-drug responding (i.e., cocaine-induced reinstatement) was greater than pre-drug responding, overall, F(1, 36)=34.21, p < 0.01. The main effect of sleep deprivation condition was not statistically significant, F < 1. Significant two-way interactions, however, were found between test period and sleep deprivation condition, F(2, 36)=3.34, p < 0.05, as well as between test period and group, F(1, 36)=4.51, p < 0.05. Post hoc tests of the test period × group interaction showed that while pre-drug responding did not differ between the high and low drug-takers ( p > 0.05), post-drug responding (i.e., cocaine-induced reinstatement) was greater in the rats with a history of high drug taking compared to those with a history of low drug taking, p < 0.05.
These observations warranted closer inspection of the high and low drug-taker groups, individually. Thus, the low and high drug-takers were analyzed separately using 2 × 3 mixed factorial ANOVAs varying test period (pre-drug or post-drug) and sleep deprivation condition (SD0, SD4, or SD8). When the low drug-taker group was analyzed alone, a significant two-way interaction was obtained between test period and sleep deprivation condition, F(2, 17)=4.06, p < 0.05. Post hoc Newman-Keuls tests revealed that pre-drug responding did not differ across sleep deprivation condition for the low drug-takers, ps > 0.05 (see open bars in left panel of Figure 5). However, post-drug responding was significantly greater in the SD0 group compared to the SD4 and SD8 groups, ps < 0.03, which did not differ from one another, ps > 0.05 (see hatched bars in left panel of Figure 5). In addition, pre- vs. post-drug responding did not differ in either the SD4 or SD8 groups, ps > 0.05, while it did differ for rats in the SD0 group, p < .05. This finding confirms that, unlike the non-sleep-deprived low drug-taking controls, sleep-deprived low drug-takers failed to exhibit cocaine-induced reinstatement (see left panel of Figure 5).
A different pattern of behavior emerged for the high drug-takers. Specifically, a similar analysis of the data from the high drug-taker group revealed that the test period × SD condition interaction was not significant, F < 1 (see right panel of Figure 5). The main effect of test period, however, was statistically significant, F(1, 19)=40.70, p < 0.01, showing higher post-drug responding than pre-drug responding, regardless of SD condition. Student’s t-tests confirmed that post-drug responding was significantly greater than pre-drug responding for rats in all three SD conditions (SD0, SD4, or SD8), ps < 0.05. Acute sleep deprivation, then, prevented cocaine-induced reinstatement in the low drug-takers. High drug-takers, on the other hand, were motivated to seek drug following the cocaine challenge whether they were, or were not, acutely sleep-deprived.
Progressive Ratio testing
Total number of infusions and inter-infusion intervals (i.e., the amount of time between infusions) were analyzed for the PR data using 2 × 3 mixed factorial ANOVAs varying group (low drug-takers or high drug-takers) and SD condition (SD0, SD4, or SD8). Goal-directed behavior, measured by comparing the number of active and inactive responses as a percentage of total PR responding, also was analyzed using a 2 × 2 × 3 mixed factorial ANOVA varying group (low drug-takers or high drug-takers), spout (active or inactive), and sleep deprivation condition (SD0, SD4, or SD8). Student’s t-tests were employed where necessary.
Total Infusion Number
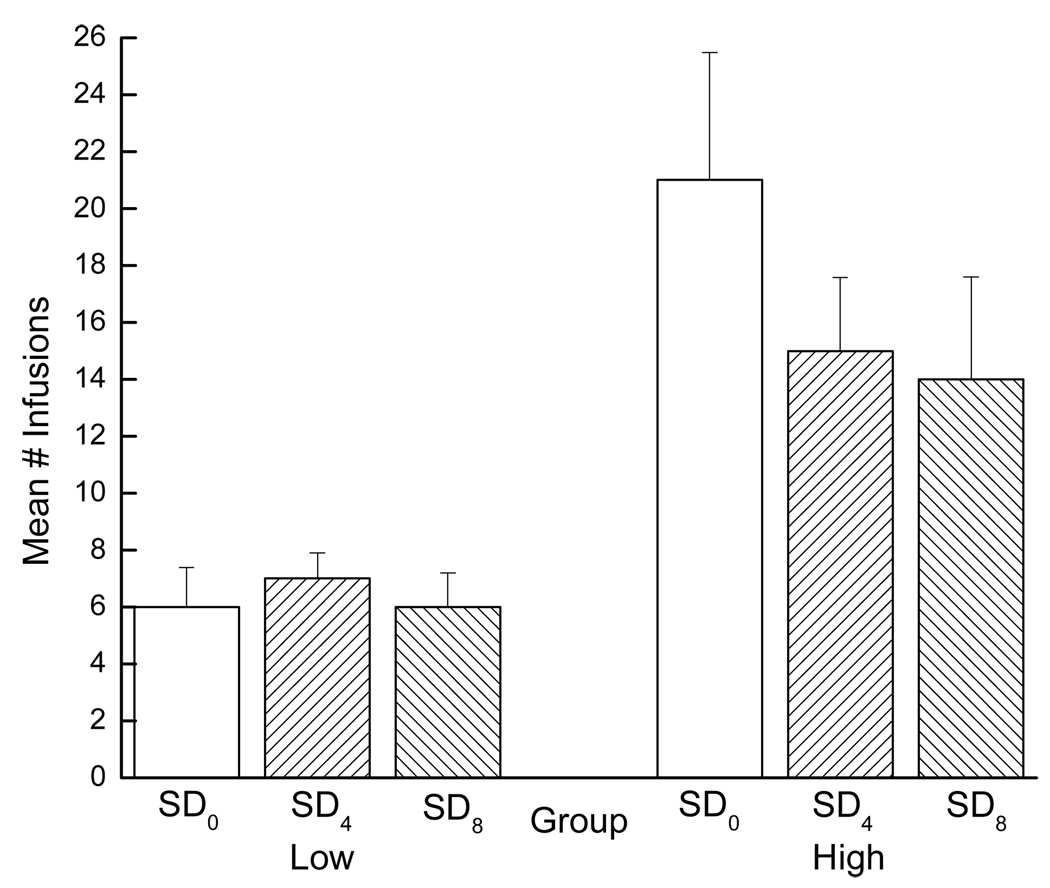
During PR testing, the high drug-takers exhibited higher break points, overall, than did the low drug-takers. Consequently, the high drug-takers took more infusions than did the low drug-takers during the PR session (see Figure 6).
Figure 6.
Mean number of infusions self-administered by low and high drug-taking rats across the three SD conditions during PR testing. Low drug-takers (SD0: n=7, SD4: n=6, and SD8: n=7) can be viewed in the left panel and high drug-takers (SD0: n=7, SD4: n=8, and SD8: n=7) in the right panel.
This observation was confirmed by a significant main effect of group, F(1, 36)=20.50, p < 0.01. Sleep deprivation condition, however, had no significant effect on PR responding as indicated by a non-significant main effect of SD condition, F < 1, and a non-significant group × SD condition interaction, F < 1. Simply put, all high drug-takers worked for drug, while all low drug-takers did not, and acute sleep deprivation was without effect on this measure. Indeed, even Student’s t-tests failed to reveal significant differences in PR responding between the SD0 group and the SD4 group, p > 0.05, or between the SD0 group and the SD8 group, p > 0.05, for the high drug-takers. Acute sleep deprivation, then, did not significantly increase or significantly decrease, a drug-experienced rat’s willingness to work for drug across a protracted PR session.
Inter-Infusion Interval
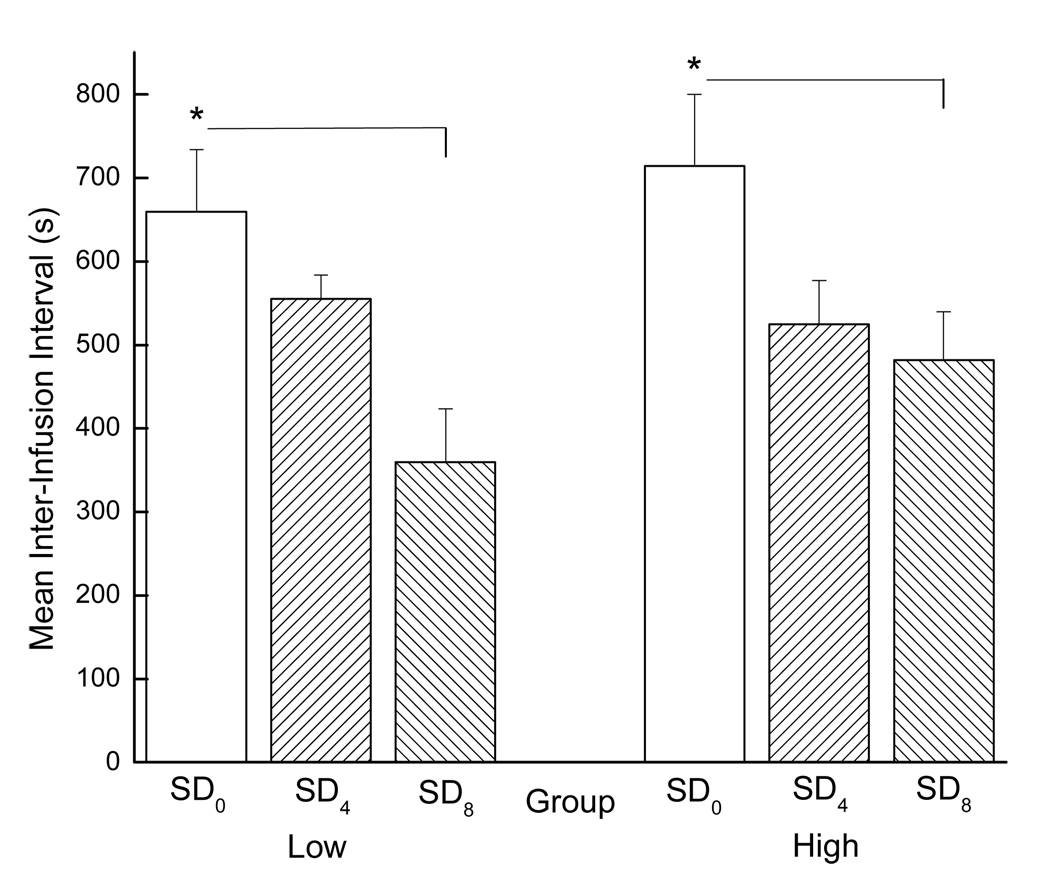
As indicated, we also were interested in the rate at which the rats took drug when given the opportunity. When evaluating inter-infusion intervals across the entire session, acute sleep deprivation had a clear and marked facilitative effect (see Figure 7).
Figure 7.
Mean inter-infusion intervals for low and high drug-taking rats across all SD conditions during PR testing. Low drug-takers (SD0: n=7, SD4: n=6, and SD8: n=7) are shown the left panel and high drug-takers (SD0: n=7, SD4: n=8, and SD8: n=7) are shown in the right panel. * denotes statistical significance (p < 0.05).
The results of a 2 × 3 mixed factorial ANOVA varying group (low drug-takers or high drug-takers) and SD condition (SD0, SD4, or SD8) showed that the main effect of SD condition was significant, F(2, 36)=8.74, p < 0.01, indicating that, overall, inter-infusion intervals were shorter for sleep-deprived versus non-sleep-deprived rats. Post hoc Newman-Keuls tests revealed that inter-infusion intervals for the SD0 group were significantly longer than inter-infusion intervals for the SD4 and SD8 groups, ps < 0.05, overall, which did not differ from one another, p > 0.05. Thus, acute sleep deprivation increased the rate at which the rats self-administered the drug when tested on a PR schedule of reinforcement. The group × SD condition interaction, however, was not significant, F < 1, indicating that the facilitative effect of sleep deprivation on the rate of drug infusion was the same for the low and the high drug-takers.
Goal-Directed Behavior
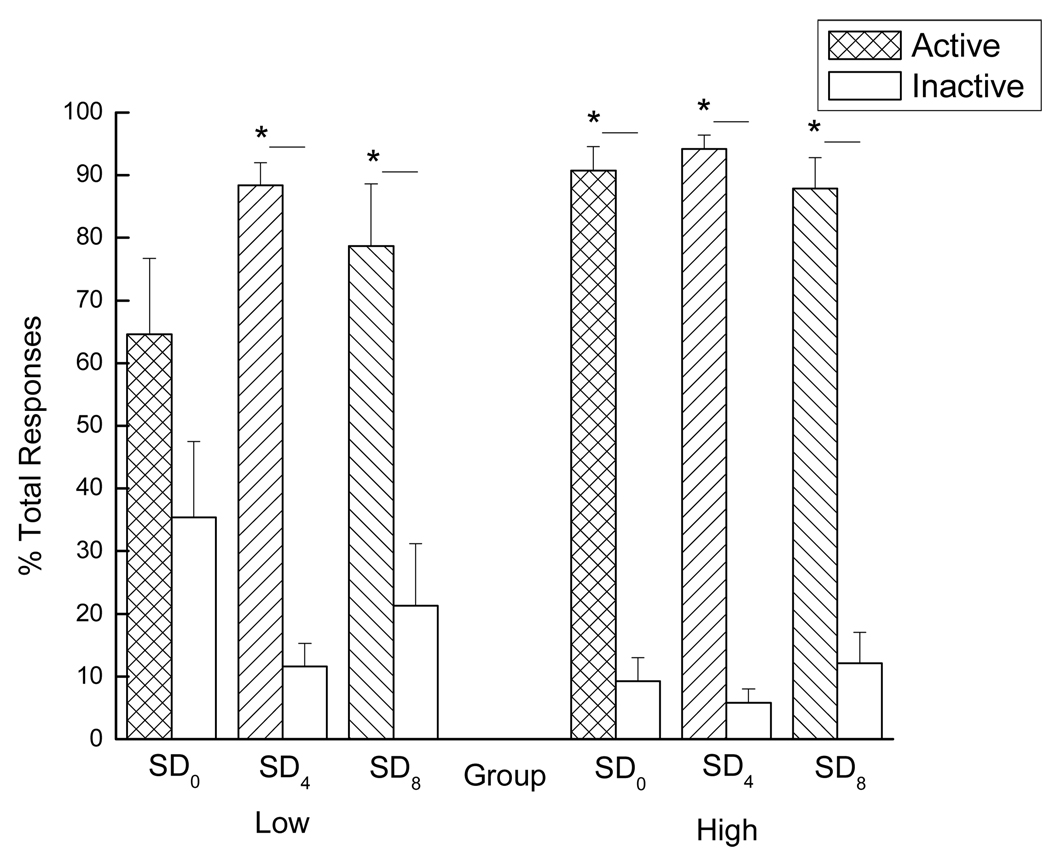
Goal-directed behavior was analyzed by comparing active and inactive responding during PR testing, both represented as percentages of the total number of responses made during the testing period. A significant main effect of spout was seen, F(1, 36)=118.61, p < 0.01, indicating that, overall, more responses were made on the active spout than on the inactive spout (see Figure 8).
Figure 8.
Goal-directed behavior of low (Left panel: SD0: n=7, SD4: n=6, and SD8: n=7) and high (Right panel: SD0: n=7, SD4: n=8, and SD8: n=7) drug-taking rats across all SD conditions presented as the comparison of active and inactive responses (both represented as percentages of the total number of responses made) during PR testing. * denotes statistical significance (p < 0.05).
This high level of active responding was neither affected by group (group × spout interaction, F < 1) nor by SD condition (SD condition × spout interaction, F < 1). Even so, Student’s t-tests revealed something interesting. That is, for the low drug-takers in the SD0 group, there was no difference between active and inactive responding, p > 0.05 (see Figure 8, left panel). This was not the case, however, for the low drug-takers following acute sleep deprivation. Thus, while the non-sleep-deprived low drug-takers failed to exhibit significant goal-directed behavior, just 4 – 8 h of acute sleep deprivation led low drug-takers to make significantly more responses on the active versus the inactive spout, ps < 0.05. Similar goal-directed behavior was seen for the high drug-takers, but this effect was significant across all SD conditions, ps < 0.05, (see Figure 8, right panel). These results demonstrate that acute sleep deprivation focuses the behavior of low drug-taking rats on the active (i.e., drug-associated) spout. A full overview of the results of the current study can be viewed in Table 1.
Table 1.
Behavioral Effects of Acute Sleep Deprivation
| GROUP | GROUP | |||||
|---|---|---|---|---|---|---|
| Low | High | |||||
| SD0 | SD4 | SD8 | SD0 | SD4 | SD8 | |
| Cocaine-Induced Reinstatement | + | − | − | + | + | + |
| PR Infusion # | ↓ | ↓ | ↓ | ↑↑ | ↑ | ↑ |
| Inter-Infusion Interval | ↑ | ↓ | ↓↓ | ↑ | ↓ | ↓ |
| Goal-Directed Behavior | − | + | + | + | + | + |
*** Plus (+) and minus (−) signs indicate the presence or absence of behavior, respectively. Arrows indicate an increase or decrease in the variable in question, as well as the magnitude of that change (e.g., ↑↑ indicates an increase of greater magnitude than↑).
General Discussion
The existence of distinct low and high drug-taking sub-populations of outbred Sprague-Dawley rats has been well documented. Piazza et al. (1989) were the first to characterize individual differences in the vulnerability to self-administer substances of abuse on the basis of locomotor response following placement into a novel environment. Two groups were identified: low responders (who exhibited low levels of locomotor activity) and high responders (who exhibited high levels of locomotor activity). When tested with drug self-administration (i.e., amphetamine or cocaine), the high responders self-administered more drug than did the low responders (Piazza et al., 1989; Piazza et al., 2000) and they exhibited a greater dopamine response in the nucleus accumbens following the administration of cocaine (Hooks et al., 1991). Thus, the high responders were thought to be more sensitive to the rewarding properties of drug compared to the low responders. In accordance, the high responders were initially faster at acquiring drug self-administration than the low responders (i.e., high responders exhibited statistically higher active than inactive responding sooner than low responders). However, that group difference was attenuated as self-administration training progressed and active responding made by the low responders increased (Piazza et al., 1989).
In general, these findings are consistent with our data. Grigson and Twining (2002) reported that outbred male Sprague-Dawley rats given daily saccharin-cocaine (FR10 0.33 mg/infusion 1h) pairings also could be divided into two separate groups: large suppressers and small suppressers. Large suppressors greatly reduced intake of the saccharin cue following saccharin-cocaine pairings, while small suppressors did not. Importantly, when given the opportunity to self-administer cocaine, the rats that most greatly avoided the saccharin cue (i.e., the large suppressers), self-administered three to four times as much cocaine as did the small suppressors. Greater avoidance of the drug-associated taste cue, then, was correlated with greater drug-taking behavior, and some rats were more sensitive than others. Of course, this behavior develops across training trials and, by definition, cannot be used, a priori, to predict who will and will not take drug. Even so, these group differences emerge relatively early and are sustained throughout training. As such, the paradigm is useful for addressing questions related to the acquisition of drug-taking behavior, maintenance of drug-taking behavior, and relapse as well. Finally, it should be noted that, although it is tempting to conclude that the high and low drug-takers in the saccharin-cocaine paradigm (Grigson & Twining, 2002) are the same as those revealed in the present paradigm (cocaine self-administration only), we cannot know this. Thus, we draw conclusions across the two paradigms with some caution.
Once divided on the basis of terminal cocaine intake, the high drug-takers in the present study took more drug, by definition, than did the low drug-takers (~ 20 versus 6 infusions/90 min session, respectively). The high drug-takers also initiated the first infusion more quickly and they made significantly more responses on the active than the inactive spout. The low drug-takers, on the other hand, failed to make significantly more responses on the active than the inactive spout during acquisition. This, however, should not be taken as evidence that the low drug-takers were unmotivated to take drug, because, when shifted to extinction, responses on the previously active spout increased considerably for these low drug-takers. Indeed, during the first five days of extinction, the number of responses made on the previously active spout by the low drug-takers was significantly higher than the number made on the previously inactive spout, and these numbers did not differ from those made by the high drug-takers during the same time period. Thus, during acquisition, high drug-takers are motivated to take a high number of infusions and low drug-takers are motivated to take a low number of infusions.
When tested in a non-sleep-deprived state, both the low and the high drug-takers exhibited marked drug-induced reinstatement, which did not differ between the two groups. Acute sleep deprivation (4 or 8 h) had no significant effect on this behavior in the high drug-takers. All high drug-takers exhibited marked drug-induced reinstatement, regardless of sleep deprivation condition. In the low drug-takers, however, acute sleep deprivation (4 or 8 h) fully prevented drug-seeking behavior following the cocaine challenge. When observing the behavior of the rats, the explanation for these data was obvious. Rather than seek a drug that was not available (the rats received only a single, passive infusion during cocaine-induced reinstatement testing), sleep-deprived low drug-takers chose to sleep. Competing biological motivations, then, lie at the crux of these effects. High drug-takers are highly motivated to seek and take drug. In the case of our high drug-taking rats, the motivation to seek drug overrode the motivation to sleep, even when tested after 4 or 8 h of acute sleep deprivation. Low drug-takers, on the other hand, do not possess the same high level of motivation for drug. Thus, in the absence of drug, the motivation to sleep overpowered the motivation to seek drug. This general pattern of behavior is interesting when considering the behavior of low drug-takers in the saccharin-cocaine paradigm (Grigson & Twining, 2002). In that case, when saccharin predicted the opportunity to self-administer drug, thirsty low drug-takers continued to consume a great deal of the saccharin cue while thirsty high drug-takers did not. Low drug-takers, then, may be less responsive to drug and more responsive to alternative natural rewards (like fluid or sweets when thirsty or hungry, or sleep when sleep-deprived). Perhaps similar individual differences in the motivation for drug also affect choice when involving other competing motivations, such as caring for one’s offspring (Seip & Morrell, 2007).
Although the cocaine-induced reinstatement paradigm was informative, it does not mimic relapse situations encountered by human addicts. That is, when human addicts relapse, the drug is either readily available or it can be acquired. Thus, we re-evaluated the impact of acute sleep deprivation on responding when drug was present using a PR schedule of reinforcement. As was evident with FR testing during acquisition, the high drug-takers worked harder on the PR schedule for drug than did the low drug-takers. In general, despite the increased workload, both the high and the low drug-takers maintained the same number of infusions that they were accustomed to self-administering when working on the FR schedules of reinforcement (i.e., ~ 20 vs. 6 infusions/session, respectively). Interestingly, the low drug-takers maintained this infusion number on the PR schedule even when sleep-deprived. This finding suggests that acute sleep deprivation did not increase break point (i.e., did not increase the apparent perceived incentive value of the drug) for the low drug-takers, and the ratio requirement was, presumably, not a strain for these rats. The high drug-takers, on the other hand, tended to exhibit lower break points when sleep-deprived. This trend toward a decrease in break point could indicate that acute sleep deprivation decreased the perceived value of the cocaine reward for the high drug-takers or, alternatively, that the drive for sleep simply interfered with completing the higher ratio requirements.
We suspect the latter because, on the whole, the evidence suggests that acute sleep deprivation increases, rather than decreases, the drive to self-administer cocaine. First, all sleep-deprived rats (low and high drug-takers) self-administered cocaine at a faster rate than did their non-sleep-deprived counterparts, as indicated by a significant decrease in inter-infusion interval. Of course, by definition, inter-infusion intervals increase as a function of the number of infusions self-administered across the PR test (i.e., as the number of infusions being self-administered increases, more licks are required/infusion and, thus, more time is required to reach the next infusion). Even so, the facilitative effect of sleep deprivation on inter-infusion interval cannot be fully accounted for by mere differences in the number of infusions administered, because inter-infusion intervals were shorter for sleep-deprived low and high drug-takers even though the total number of infusions obtained was not significantly reduced relative to non-sleep-deprived controls. Second, acute sleep deprivation also served to focus drug-seeking behavior when responding for drug on the PR schedule. Of particular interest is the finding that 4 or 8 h of acute sleep deprivation led to marked goal-directed behavior in the low drug-taking population of rats. In accordance, other data have linked an increase in goal-directed behavior to an increase in motivation (Kuntz et al., 2008). Therefore, the increases in the motivation to self-administer drug, resulting from acute sleep deprivation, evident in both low and high drug-taking rats in the present study, appear to reflect an increase in drive (i.e., as evidenced by an increase in rate of infusion among low and high drug-takers, and an increase in the goal-directed nature of the drug-seeking behavior exhibited by the low drug-takers) rather than an increase in the perceived incentive value of the drug (i.e., as evidenced by a failure to significantly alter PR responding for drug). Again, competing biological motivations appear to elucidate these effects. When drug is available, the motivation to sleep appears to drive the motivation to take drug at a faster rate and to seek drug more efficiently (i.e., the motivation to sleep and the motivation to seek and take drug coexist, and the animals strive to satisfy the need to take drug so that the need to sleep can then also be satisfied).
The facilitative effect of sleep deprivation on drug-seeking and drug-taking behavior, in the presence of the drug, is consistent with other data showing a sleep-deprivation-induced increase in responding for a reward. Specifically, sleep deprivation also increased the rate of responding for rewarding electrical brain stimulation and lowered the threshold for intracranial self-stimulation (Steiner & Ellman, 1972). In the present case, however, it appeared that the increase in the rate of responding for drug related not only to the drive for the drug, but also to the drive to sleep. An interesting study of night eating syndrome in humans conducted by O’Reardon et al. (2006) may shed some light on these results. Night eating syndrome is an eating disorder characterized by nocturnal awakenings, during which the individual engages in ingestive behaviors. This syndrome may relate to the drug-seeking behaviors we observed in sleep-deprived rats, since it too involves competing biological motivations between the drive to eat and the drive to sleep. Thus, in the case of night eating syndrome, the motivation or desire to eat is so powerful that individuals awaken during the night in order to satisfy their hunger. When individuals suffering from this disorder were asked to keep a diary of the times they woke during the night to eat, and their associated feelings, many subjects failed to report nighttime eating episodes or to record how they were feeling because they began eating almost immediately after awakening and, thereafter, quickly returned to sleep. In essence, the patients did not have time to record their data because they were racing to eat and then rapidly returned to sleep. Similar pressures may have dictated the behavior of our sleep-deprived rats that very rapidly took the “requisite” amount of drug and then promptly fell asleep.
Finally, while acute sleep deprivation may be responsible for the effects discussed here, the possibility must be considered that these effects may not be specific to sleep deprivation, per se. Thus, it may be the case that they are a more general consequence of the creation of a biological state of deprivation. In fact, it has been shown that acute food deprivation induces reinstatement of heroin and cocaine seeking in rats (Shalev et al., 2000a; Shalev et al., 2000b) and, also, that chronic food restriction augments the central rewarding effects of cocaine in rats (Carr et al., 2000). Thus, rats in need of food, water, or salt, for example, also may rapidly take drug before turning to satisfy the alternative need state. This more general hypothesis remains to be tested.
As previously stated, 17% of Americans meet the diagnostic criteria for some form of substance dependence (Anthony and Helzer, 1991) and 90% of these individuals will relapse even after prolonged periods of abstinence (DeJong, 1994). Like substance abuse and addiction, sleep deprivation also is ubiquitous in our society. Sixty-five percent of Americans report experiencing symptoms of a sleep problem several nights per week (National Sleep Foundation, 2008 Sleep in America Poll). Finally, the problems associated with each of these conditions are greatly exacerbated when these two diagnoses intersect. For example, in the rat population, high responders in the locomotor task (i.e., those likely to be high drug-takers) exhibited greater amounts of wakefulness and less slow wave sleep compared to low responders (Bouyer et al., 1998). Similarly, abstinence and withdrawal in humans are associated with difficulty sleeping and frank insomnia (Malcolm et al., 2007) and it is now clear that sleep deprivation causes relapse in humans (Brower, 2001; Clark et al., 1998; Foster and Peters, 1999; Gillin et al., 1994). Along with these reports, the present data show that even acute sleep deprivation markedly increases the rate of drug-taking in low and high drug-takers and the sharpening of goal-directed behavior in the rats that, otherwise, would not exhibit goal-directed behavior (i.e., in rats with a history of low drug-taking). Given that humans suffer primarily from chronic, rather than acute, sleep deprivation, future studies must examine the effect of both acute and chronic sleep deprivation on acquisition, maintenance, and reinstatement of drug-seeking and drug-taking behavior, and must begin to examine the underlying neural correlates. In addition, it will be important to assess the impact of both acute and chronic sleep deprivation using a CNS depressant, such as an opiate (e.g., heroin or morphine). Finally, given the prevalence of chronic sleep deprivation in the adolescent population (Carskadon, 1990) and the adolescent’s propensity for drug-taking behavior, future studies also must examine the impact of chronic sleep deprivation on both acquisition and reinstatement of drug-taking behavior in the adolescent rat.
Acknowledgments
The authors would like to thank Dr. Xiaorui Tang for producing the mathematical equations used to describe the bimodal distribution shown in Figure 1, as well as the National Institute on Drug Abuse for generously supplying the cocaine HCl used in these studies. This work was supported by grants DA009815 and DA023315 from the National Institutes of Health and a grant from the PA State Tobacco Settlement Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony JC, Helzer JE. Syndromes of drug abuse and dependence. In: Robins LN, Regier DA, editors. Psychiatric Disorders in America. New York: Free Press; 1991. pp. 116–154. [Google Scholar]
- Bouyer JJ, Vallée M, Deminière JM, Le Moal M, Mayo W. Reaction of sleep-wakefulness cycle to stress is related to differences in hypothalamo-pituitary-adrenal axis reactivity in rat. Brain Research. 1998;804(1):114–124. doi: 10.1016/s0006-8993(98)00670-2. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Research & Health. 2001;25(2):110–125. [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Medicine Reviews. 2003;7(6):523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EAR, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. American Journal of Psychiatry. 2001;158(3):399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Kim GY, Cabeza de Vaca S. Chronic food restriction in rats augments the central rewarding effect of cocaine and the delta1 opioid agonist, DPDPE, but not the delta2 agonist, deltorphin-II. Psychopharmacology. 2000;152(2):200–207. doi: 10.1007/s002130000523. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17(1):5–12. [PubMed] [Google Scholar]
- Cirelli C, Pompeiano M, Tononi G. Sleep deprivation and c-fos expression in the rat brain. Journal of Sleep Research. 1995;4(2):92–106. doi: 10.1111/j.1365-2869.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Shaw PJ, Rechtschaffen A, Tononi G. No evidence of brain cell degeneration after long-term sleep deprivation in rats. Brain Research. 1999;840(1–2):184–193. doi: 10.1016/s0006-8993(99)01768-0. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. Journal of Neuroscience. 2000;20(24):9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CP, Gillin JC, Golshan S, Demodena A, Smith TL, Danowski S, Irwin M, Schuckit M. Increased REM sleep density at admission predicts relapse by three months in primary alcoholics with a lifetime diagnosis of secondary depression. Biological Psychiatry. 1998;43(8):601–607. doi: 10.1016/s0006-3223(97)00457-5. [DOI] [PubMed] [Google Scholar]
- DeJong W. Relapse prevention: An emerging technology for promoting long-term abstinence. International Journal of the Addictions. 1994;29(6):681–705. doi: 10.3109/10826089409047904. [DOI] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: An opportunity for prevention? JAMA. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Foster JH, Marshall EJ. Predictors of relapse to heavy drinking in alcohol dependent subjects following alcohol detoxification: The role of quality of life measures, ethnicity, social class, cigarette and drug use. Addiction Biology. 1998;3(3):333–343. doi: 10.1080/13556219872146. [DOI] [PubMed] [Google Scholar]
- Foster JH, Peters TJ. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcoholism: Clinical & Experimental Research. 1999;23(6):1044–1051. [PubMed] [Google Scholar]
- Gillin JC. Are sleep disturbances risk factors for anxiety, depressive and addictive disorders? ACTA Psychiatrica Scandinavica. 1998;98 Suppl. 393:39–43. doi: 10.1111/j.1600-0447.1998.tb05965.x. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Butters N, Demodena A, Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Archives of General Psychiatry. 1994;51(3):189–197. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- Girardin J-L, Kripke DF, Ancoli-Isreal S. Sleep and quality of well-being. Sleep. 2000;23(8):1–5. [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: A model of drug-induced devaluation of natural rewards. Behavioral Neuroscience. 2002;116(2):321–333. [PubMed] [Google Scholar]
- Hohagen F, Rink K, Käppler C, Schramm E, Riemann D, Weyerer S, Berger M. Prevalence and treatment of insomnia in general practice: A longitudinal study. European Archives of Psychiatry & Clinical Neuroscience. 1993;242(6):329–336. doi: 10.1007/BF02190245. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9(2):121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Kuntz KL, Twining RC, Baldwin AE, Vrana KE, Grigson PS. Heroin self-administration: I. Incubation of goal-directed behavior in rats. Pharmacology, Biochemistry, & Behavior. 2008;90(3):344–348. doi: 10.1016/j.pbb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Grigson PS. Brief access to sweets protect against relapse to cocaine-seeking. Brain Research. 2005;1049(1):128–131. doi: 10.1016/j.brainres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Longo LP, Johnson B. Treatment of insomnia in substance abusing patients. Psychiatric Annals. 1998;28(3):154–159. [Google Scholar]
- Malcolm R, Myrick LH, Veatch LM, Boyle E, Randall PK. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: A randomized, double blind, controlled comparison of lorazepam vs gabapentin. Journal of Clinical Sleep Medicine. 2007;3(1):24–32. [PubMed] [Google Scholar]
- O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278(5335):66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- O'Reardon JP, Allison KC, Martino NS, Lundgren JD, Heo M, Stunkard AJ. A randomized, placebo-controlled trial of sertraline in the treatment of night eating syndrome. American Journal of Psychiatry. 2006;163(5):893–898. doi: 10.1176/ajp.2006.163.5.893. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière J, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245(4925):1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerability phenotype predisposed to addiction. Journal of Neuroscience. 2000;20(11):4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: Place preference and locomotor analyses in the lactating female rat. Psychopharmacology. 2007;194(3):309–319. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: Studies on the generality of the effect. Psychopharmacology. 2000a;150(3):337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shalev U, Marinelli M, Baumann MH, Piazza P, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology. 2000b;168(1–2):170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- Steiner SS, Ellman SJ. Relation between REM sleep and intracranial self-stimulation. Science. 1972;177(54):1122–1124. doi: 10.1126/science.177.4054.1122. [DOI] [PubMed] [Google Scholar]
- Teplin D, Raz B, Daiter J, Varenbut M, Tyrrell M. Screening for substance use patterns among patients referred for a variety of sleep complaints. American Journal of Drug and Alcohol Abuse. 2006;32(1):111–120. doi: 10.1080/00952990500328695. [DOI] [PubMed] [Google Scholar]
- Tononi G, Pompeiano M, Cirelli C. The locus coeruleus and immediate-early genes in spontaneous and forced wakefulness. Brain Research Bulletin. 1994;35(5–6):589–596. doi: 10.1016/0361-9230(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30(12):1631–1642. doi: 10.1093/sleep/30.12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57(5):774–785. doi: 10.1016/j.neuron.2008.01.024. www.nida.nih.gov, www.nimh.nih.gov, www.sleepfoundation.org. [DOI] [PubMed] [Google Scholar]