Abstract
Extended-release products are designed to prolong the absorption of drugs with short half-lives, thereby allowing longer dosing intervals while minimizing fluctuations in serum drug levels. The relationship between serum drug concentration and clinical effects of antiepileptic drugs (AEDs) can be complex and reducing fluctuations in serum drug levels is not equally advantageous for all AEDs. Extended-release formulations have been shown to be particularly valuable for carbamazepine, whereas for other AEDs advantages, other than prolongation of the dosing interval, have not been clearly demonstrated. Differences in bioavailability may exist between extended-release and immediate-release formulations and among different brands of extended-release products. Therefore, when switching from one formulation to another, careful monitoring of clinical response and attention to the need for dose adjustment are warranted.
A major milestone in the treatment of epilepsy occurred in the late 1960s when the measurement of serum levels of antiepileptic drugs (AEDs) was introduced as a tool to tailor dosage to individual needs. Therapeutic drug monitoring has been invaluable in identifying causes for variability in AED levels, which include genetic profile, age, pregnancy, concomitant disease, and drug–drug interactions (1). Routine monitoring of drug levels also led to the realization that the drug concentration in serum fluctuates considerably during a dosing interval for those AEDs that are absorbed and eliminated rapidly. Since the concentration in serum usually correlates with the concentration at the site of action, these fluctuations may result in transient side effects at the time of peak concentration and a potential fall to subtherapeutic levels at the trough (2,3).
An effective strategy to minimize fluctuations in serum drug levels is to reduce the interval between doses. However, taking medications three or four times daily is inconvenient and may adversely affect compliance (4). These drawbacks provide the rationale for developing extended-release formulations, which are intended to ensure relatively stable serum concentrations with less frequent administrations, typically once or twice daily (3). Extended-release formulations are available for several AEDs, and their number is increasing steadily. This article will appraise the scientific rationale for and discuss the value of extended-release AED formulations in the management of seizure disorders.
Is the Serum Level Profile a Faithful Indicator of a Drug's Concentration at the Site of Action?
The scientific prerequisites for developing extended-release formulations of AEDs are that fluctuations in serum drug levels should reflect comparable fluctuations at the site of action and that minimizing such fluctuations is expected to be clinically beneficial. In fact, only a few studies have investigated the relationship between drug concentrations in serum and in the brain over time. This relationship can be complex, partly because transfer across the blood–brain barrier is not equally rapid among AEDs. In a study in dogs, mean half-times to reach equilibrium from blood to CSF were 3–7 min for benzodiazepines and ethosuximide, 12–18 min for phenytoin, phenobarbital, carbamazepine, and valproic acid, and substantially longer at 40–50 min, for primidone and the carbamazepine metabolite, carbamazepine-10,11-epoxide (5). Because of the presence of binding proteins and transporter systems, there also can be important differences in drug concentrations among the blood, the CSF, and the brain as well as within discrete brain areas (6). For example, in a study conducted in rats, Welty et al. found a remarkable dissociation between the concentration of gabapentin in plasma and interstitial brain fluid compared with brain tissue: at 4 h after an intravenous bolus, the interstitial fluid concentration was less than 6% of the plasma concentration, whereas the brain tissue concentration was equal or greater than the plasma concentration (7). Overall, available data suggest that changes in serum AED concentrations are not necessarily mirrored by parallel changes in CSF or brain concentrations and that fluctuations in drug levels in the brain may be less prominent than in blood.
Do Changes in Serum or Brain Concentrations Translate Directly into Changes in Clinical Response?
For a simple and direct relationship to exist between the serum concentration profile of an AED and its effect, at least two conditions must be met: 1) the concentration profile at the site of action mirrors that in serum and 2) the drug action is rapid and reversible, with an intensity of effect proportional to the drug's concentration at the site of action. As discussed, the first of these conditions does not necessarily apply to all AEDs, because passage across the blood–brain barrier may be relatively slow and drug distribution between the blood and discrete brain compartments may be quite complex. The relationship between serum drug concentration and effect, in turn, is dependent on the drug's mechanism of action. In the study by Welty and colleagues, appearance of gabapentin's anticonvulsant effect was found to lag behind both plasma and brain interstitial fluid drug concentrations, and the authors concluded that the effects of the drug are delayed by time-dependent events other than distribution from blood to brain (7). Similar findings have been reported for pregabalin (8). Vigabatrin offers a more striking example of dissociation between the time course of serum drug concentrations and its effect, as it acts by increasing brain GABA levels through irreversible inhibition of GABA transaminase. After a single 50-mg/kg dose in humans, its effect, as assessed by the increase in CSF GABA levels, peaks more than 24 h after the peak concentration in serum and persists for more than 4 days after the drug has been cleared from the body (9,10).
Investigations into the relationship between the serum drug level profile and the time course of antiepileptic effect in humans are scarce because of difficulties in assessing seizure protection at precise time points. However, available evidence suggests that this relationship also can be complex. In an elegant study that used suppression of the photoparoxysmal response as a measure of anticonvulsant effect after single doses of valproate in patients with epilepsy, Rowan and coworkers demonstrated that the effect, on average, appeared 3 h (range, 1–5 h) after the peak serum drug concentration and lasted for up to 5 days, when the drug was no longer detectable in blood (11). While these results may not apply to other clinical settings (e.g., the treatment of status epilepticus), the reported effectiveness in some patients of once daily dosing with immediate-release valproate indicates that the duration of effect can exceed the half-life of the drug (12). The photoparoxysmal model also has been applied to assess the time course of action of newer AEDs, including levetiracetam (13), brivaracetam (14), and carisbamate (15). With these agents, the onset of effect was consistent with the appearance of the drug in serum, but at the highest doses, the response lasted for much longer than the drug's half-life. This observation provided the rationale for selecting a twice-daily dosing scheme in the clinical development of levetiracetam.
It should be noted that a duration of action that is longer than a drug's half-life does not necessarily imply the existence of an indirect or irreversible mechanism of action. When a drug is well tolerated at serum levels far in excess of the minimally effective concentration, intake of large doses at intervals longer than the drug's half-life will ensure sustained therapeutic cover without adverse effects. In fact, multiple daily doses, or extended-release formulations, are most useful in the case of drugs for which high peak serum concentrations are associated with significant adverse effects.
The Rationale for Extended-Release Formulations
For reasons discussed, a short half-life (i.e., ≤8 hours) is not necessarily synonymous with a short duration of action. In fact, even with rapidly distributed and reversibly acting drugs, the duration of action is dependent not only on half-life but also on the size of the dose. Additionally, it may be incorrect to assume that an even serum concentration profile is more beneficial than a profile consisting of peaks and troughs. Only well-controlled studies can determine which frequency of administration is optimal and whether any benefit can be expected from an extended-release formulation.
Perhaps surprisingly, few randomized studies have evaluated different frequencies of administration for AEDs with short half-lives. For tiagabine, which has a half-life of 2–9 h (1), no major differences in efficacy or adverse effects were demonstrated between two and four (16) or between two and three (17) times a day dosing regimens, even though for some patients, twice daily dosing was less well tolerated (18). Similarly, a randomized trial of pregabalin found similar efficacy and tolerability between 300 mg twice daily compared with 200 mg three times a day dosing. (19). Interestingly, for most of the AEDs for which extended-release formulations have been developed, no well-controlled, randomized studies exist that have compared the relative value of different dosing frequencies or the potential advantages of minimizing fluctuations in serum drug concentrations.
Reasons for developing an extended-release formulation are not limited to improving tolerability and, as a result, effectiveness, or to allowing use of longer dosing intervals (2). An additional reason is simply the pharmaceutical industry's desire to extend exclusivity rights for their products. In fact, it is no surprise that many extended-release formulations are introduced at the time exclusivity rights for the immediate-release product approach the expiration date. Regulatory approval for extended-release formulations does not require any comparison of efficacy and safety with the immediate-release product; to the contrary, pharmacokinetic data and, at least in the United States, demonstration of superiority over placebo in a single trial is generally sufficient for marketing approval. This regulatory scenario implies that high-quality data on the comparative efficacy and tolerability of the extended- and immediate-release forms of most AEDs are usually unavailable.
Once Daily Dosing: Advantages and Concerns
Some extended-release products can be taken once daily. In addition to convenience, this regimen may be associated with subtle psychological benefits, particularly for those patients who are seizure-free and perceive each pill-taking act as an unpleasant reminder of their disease. Less frequent dosing also can improve compliance. A systematic review of results from 76 studies found that mean dose-taking compliance increased from 51% with four to 65% with three to 69% with two times a day dosing and to 79% with once-daily dosing, although the differences between once and twice daily or between twice and three times a day dosing were not statistically significant (4).
While compliance is best with one daily dose, the risks of seizure recurrence after missing a dose may also be greatest. The ability of a product to allow omission of a dose without adverse consequences, the so-called “forgiveness period,” can be defined as the post-dose duration of action minus the dosing interval (3). Extended-release products may not sustain effective serum drug levels for much longer than 24 h, and therefore, missing a dose could result in insufficient therapeutic cover for many hours. To what extent these concerns translate into a real risk has not been formally investigated. In a national survey of 661 patients with epilepsy, the odds of experiencing a seizure after a missing dose was highest with a four times a day schedule and lowest with a once-daily schedule (19). However, these findings may have been affected by bias in patients’ recall, and AEDs taken once daily in this survey may have been medications with a long half-life (highly forgiving drugs) rather than extended-release products.
Extended-Release Formulations of Different AEDs: Are They Equally Valuable?
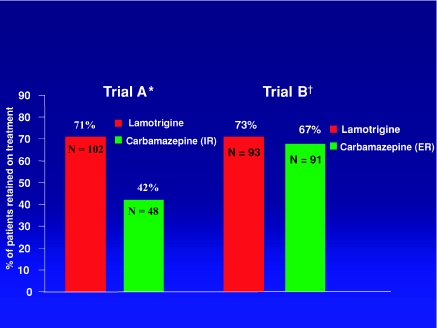
Extended-release products are available or being developed for many AEDs, including phenytoin (20,21), carbamazepine (22,23), valproic acid (24), divalproex sodium (25), lamotrigine (26), gabapentin (27), oxcarbazepine (28), and levetiracetam (29). The characteristics of some of these products have been reviewed (2,3). Among AEDs, carbamazepine stands out because of its relatively narrow therapeutic index and short half-life, particularly in enzyme-induced patients. Serum carbamazepine levels fluctuate considerably, even with multiple daily doses (30,31) and can be associated with transient adverse effects at peak concentrations (31–33). Tolerability and even seizure control have been reported to improve with extended-release carbamazepine, typically given twice daily (34–36), and some of these findings have been confirmed in blinded randomized trials (37,38). The value of extended-release carbamazepine is further suggested by the results of two recent double-blind studies that compared lamotrigine with carbamazepine among elderly patients with new-onset epilepsy (39,40). Carbamazepine was given twice daily in an immediate-release formulation in one trial and an extended-release formulation in the other. While lamotrigine showed identical effectiveness in the two trials, the effectiveness of carbamazepine was greater in the trial in which the extended-release formulation was used, which clearly was a result of superior tolerability (see Figure 1). While there may be other explanations for the improved outcome on extended-release carbamazepine, including a reduced bioavailability compared with the immediate-release product, minimizing fluctuations in serum drug levels is likely to have played an important role (40).
FIGURE 1.
Comparative effectiveness of lamotrigine and carbamazepine (expressed as the percentage of patients retained in the trial at the end of the assessment period) in two randomized, double-blind trials among patients aged 65 years or older with new-onset epilepsy. Duration of follow-up was 24 weeks in trial A and 40 weeks in trial B. The two trials had a very similar design and identical twice-daily dosing regimens, but carbamazepine was administered in an immediate-release (IR) formulation in trial A and in extended-release (ER) formulation in trial B. Abbreviations: IR, immediate release; ER, extended release; N, number of subjects in the intent-to-treat population. References:*39, †40.
Among older generation AEDs, valproic acid and divalproex sodium are often used in extended-release forms, given once or twice daily. In open-label studies, switching from conventional to extended-release formulations of these agents reportedly improved compliance and seizure control (41) or tolerability, particularly for tremor (41,42). In two double-blind randomized trials, however, efficacy and adverse events were not found to differ significantly between extended- and immediate-release formulations (43,44).
For other AEDs, well-controlled trials comparing conventional and extended-release formulations have not been reported. Some formulations offer the advantage of convenience, because they can be given once daily, but efficacy and tolerability in comparison with immediate-release products have not been assessed. Moreover, for some AEDs, immediate-release forms also can be suitable for once-daily dosing, such as lamotrigine, which can be used once daily for adults who are not taking enzyme inducers or are taking inducers but together with valproic acid, or for children taking valproic acid without enzyme inducers.
Conclusions
Extended-release formulations of AEDs with short half-lives represent a useful addition to the therapeutic armamentarium. Compared with immediate-release formulations, these products allow a longer dosing interval, which has the advantage of greater convenience and potentially improved compliance. When these products are used once daily, however, the risk of seizure breakthrough after a missing a dose might also be greater.
There is regrettably, a paucity of studies on the comparative efficacy and tolerability of immediate- and extended-release products of individual AEDs. To date, an improved outcome with use of an extended-release product has been clearly documented only for carbamazepine. Because differences in bioavailability may exist between extended- and immediate-release formulations as well as among different brands of extended-release products, careful monitoring of clinical response and consideration of the possible need for dose adjustments are warranted when switching patients from one formulation to another.
References
- 1.Patsalos PN, Berry DJ, Bourgeois BF, Cloyd JC, Glauser TA, Johannessen SI, Leppik IE, Tomson T, Perucca E. Antiepileptic drugs–best practice guidelines for therapeutic drug monitoring: A position paper by the Subcommission on therapeutic drug monitoring, ILAE Commission on therapeutic strategies. Epilepsia. 2008;49:1239–1276. doi: 10.1111/j.1528-1167.2008.01561.x. [DOI] [PubMed] [Google Scholar]
- 2.Pellock JM, Smith MC, Cloyd JC, Uthman B, Wilder BJ. Extended-release formulations: Simplifying strategies in the management of antiepileptic drug therapy. Epilepsy Behav. 2004;5:301–307. doi: 10.1016/j.yebeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Bialer M. Extended-release formulations for the treatment of epilepsy. CNS Drugs. 2007;21:765–774. doi: 10.2165/00023210-200721090-00005. [DOI] [PubMed] [Google Scholar]
- 4.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 5.Löscher W, Frey HH. Kinetics of penetration of common antiepileptic drugs into cerebrospinal fluid. Epilepsia. 1984;25:346–352. doi: 10.1111/j.1528-1157.1984.tb04199.x. [DOI] [PubMed] [Google Scholar]
- 6.Patel MM, Goyal BR, Bhadada SV, Bhatt JS, Amin AF. Getting into the brain: Approaches to enhance brain drug delivery. CNS Drugs. 2009;23:35–58. doi: 10.2165/0023210-200923010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Welty DF, Schielke GP, Vartanian MG, Taylor CP. Gabapentin anticonvulsant action in rats: Disequilibrium with peak drug concentrations in plasma and brain microdialysate. Epilepsy Res. 1993;16:175–181. doi: 10.1016/0920-1211(93)90078-l. [DOI] [PubMed] [Google Scholar]
- 8.Feng MR, Turluck D, Burleigh J, Lister R, Fan C, Middlebrook A, Taylor C, Su T. Brain microdialysis and PK/PD correlation of pregabalin in rats. Eur J Drug Metab Pharmacokinet. 2001;26:123–128. doi: 10.1007/BF03190385. [DOI] [PubMed] [Google Scholar]
- 9.Richens A. Pharmacology and clinical pharmacology of vigabatrin. J Child Neurol. 1991;2(Suppl):S7–S10. [PubMed] [Google Scholar]
- 10.Ben Menachem E, Persson L, Schechter PJ, Haegele K, Huebert N, Harbenberg J, Dahlgren L, Mumford JP. Effects of single doses of vigabatrin on CSF concentrations of GABA, homocarnosine, homovanillic acid and 5-hydroxyindoleacetic acid in patients with complex partial epilepsy. Epilepsy Res. 1988;2:96–101. doi: 10.1016/0920-1211(88)90025-3. [DOI] [PubMed] [Google Scholar]
- 11.Rowan AJ, Binnie CD, Warfield CA, Meinardi H, Meijer JW. The delayed effect of sodium valproate on the photoconvulsive response in man. Epilepsia. 1979;20:61–68. doi: 10.1111/j.1528-1157.1979.tb04776.x. [DOI] [PubMed] [Google Scholar]
- 12.Covanis A, Jeavons PM. Once-daily sodium valproate in the treatment of epilepsy. Dev Med Child Neurol. 1980;22:202–204. doi: 10.1111/j.1469-8749.1980.tb04328.x. [DOI] [PubMed] [Google Scholar]
- 13.Kasteleijn-Nolst Trenité DG, Marescaux C, Stodieck S, Edelbroek PM, Oosting J. Photosensitive epilepsy: A model to study the effects of antiepileptic drugs. Evaluation of the piracetam analogue, levetiracetam. Epilepsy Res. 1996;25:225–230. doi: 10.1016/s0920-1211(96)00031-9. [DOI] [PubMed] [Google Scholar]
- 14.Kasteleijn-Nolst Trenité DG, Genton P, Parain D, Masnou P, Steinhoff BJ, Jacobs T, Pigeolet E, Stockis A, Hirsch E. Evaluation of brivaracetam, a novel SV2A ligand, in the photosensitivity model. Neurology. 2007;69:1027–1034. doi: 10.1212/01.wnl.0000271385.85302.55. [DOI] [PubMed] [Google Scholar]
- 15.Kasteleijn-Nolst Trenité DG, French JA, Hirsch E, Macher JP, Meyer BU, Grosse PA, Abou-Khalil BW, Rosenfeld WE, van Gerven J, Novak GP, Parmeggiani L, Schmidt B, Gibson D, Guerrini R. Evaluation of carisbamate, a novel antiepileptic drug, in photosensitive patients: An exploratory, placebo-controlled study. Epilepsy Res. 2007;74:193–200. doi: 10.1016/j.eplepsyres.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Sachdeo RC, Leroy RF, Krauss GL, Drake ME, Jr, Green PM, Leppik IE, Shu VS, Ringham GL, Sommerville KW. Tiagabine therapy for complex partial seizuresA dose-frequency study. The Tiagabine Study Group. Arch Neurol. 1997;54:595–601. doi: 10.1001/archneur.1997.00550170069016. [DOI] [PubMed] [Google Scholar]
- 17.Arroyo S, Boothman BR, Brodie MJ, Duncan JS, Duncan R, Nieto M, Calandre EP, Forcadas I, Crawford PM. A randomised open-label study of tiagabine given two or three times daily in refractory epilepsy. Seizure. 2005;14:81–84. doi: 10.1016/j.seizure.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Beydoun A, Uthman BM, Kugler AR, Greiner MJ, Knapp LE, Garofalo EA, Pregabalin 1008–009 Study Group Safety and efficacy of two pregabalin regimens for add-on treatment of partial epilepsy. Neurology. 2005;64:475–480. doi: 10.1212/01.WNL.0000150932.48688.BE. [DOI] [PubMed] [Google Scholar]
- 19.Cramer JA, Glassman M, Rienzi V. The relationship between poor medication compliance and seizures. Epilepsy Behav. 2002;3:338–342. doi: 10.1016/s1525-5050(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 20.Randinitis EJ, Buchanan RA, Kinkel AW. Pharmacokinetic profile of a 300-mg extended phenytoin sodium capsule (Dilantin) formulation. Epilepsia. 1990;31:458–464. doi: 10.1111/j.1528-1157.1990.tb05503.x. [DOI] [PubMed] [Google Scholar]
- 21.Huang M-Y. Individual bioequivalence analysis of Bertek's Phenytek™ (1 × 300 mg extended phenytoin capsules) versus Parke Davis’ Dilantin® Kapseals® (3 × 100 mg) Epilepsia. 2002;43(Suppl. 7):196. abstract 1.287. [Google Scholar]
- 22.Thakker KM, Mangat S, Garnett WR, Levy RH, Kochak GM. Comparative bioavailability and steady state fluctuations of Tegretol commercial and carbamazepine OROS tablets in adult and pediatric epileptic patients. Biopharm Drug Dispos. 1992;13:559–569. doi: 10.1002/bdd.2510130802. [DOI] [PubMed] [Google Scholar]
- 23.Garnett WR, Levy B, McLean AM, Zhang Y, Couch RA, Rudnic EM, Pellock JM, Belendiuk GW. Pharmacokinetic evaluation of twice-daily extended-release carbamazepine (CBZ) and four-times-daily immediate-release CBZ in patients with epilepsy. Epilepsia. 1998;39:274–279. doi: 10.1111/j.1528-1157.1998.tb01372.x. [DOI] [PubMed] [Google Scholar]
- 24.Dulac O, Alvarez JC. Bioequivalence of a new sustained-release formulation of sodium valproate, valproate modified-release granules, compared with existing sustained-release formulations after once- or twice-daily administration. Pharmacotherapy. 2005;25:35–41. doi: 10.1592/phco.25.1.35.55626. [DOI] [PubMed] [Google Scholar]
- 25.Sommerville KW, Dutta S, Biton V, Zhang Y, Cloyd JC, Uthman B. Bioavailability of a divalproex extended-release formulation versus the conventional divalproex formulation in adult patients receiving enzyme-inducing antiepileptic drugs. Clin Drug Investig. 2003;23:661–670. doi: 10.2165/00044011-200323100-00005. [DOI] [PubMed] [Google Scholar]
- 26.Naritoku DK, Warnock CR, Messenheimer JA, Borgohain R, Evers S, Guekht AB, Karlov VA, Lee BI, Pohl LR. Lamotrigine extended-release as adjunctive therapy for partial seizures. Neurology. 2007;69:1610–1618. doi: 10.1212/01.wnl.0000277698.33743.8b. [DOI] [PubMed] [Google Scholar]
- 27.Irving G, Jensen M, Cramer M, Wu J, Chiang YK, Tark M, Wallace M. Efficacy and tolerability of gastric-retentive gabapentin for the treatment of postherpetic neuralgia: results of a double-blind, randomized, placebo-controlled clinical trial. Clin J Pain. 2009;25:185–192. doi: 10.1097/AJP.0b013e3181934276. [DOI] [PubMed] [Google Scholar]
- 28.Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Tomson T, Perucca E. Progress report on new antiepileptic drugs: A summary of the 8th Eilat Conference (EILAT VIII) Epilepsy Res. 2007;73:1–52. doi: 10.1016/j.eplepsyres.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Peltola J, Coetzee C, Jiménez F, Litovchenko T, Ramaratnam S, Zaslavaskiy L, Lu ZS, Sykes DM, Levetiracetam XR N01235 Study Group Once-daily extended-release levetiracetam as adjunctive treatment of partial-onset seizures in patients with epilepsy: A double-blind, randomized, placebo-controlled trial. Epilepsia. 2009;50:406–414. doi: 10.1111/j.1528-1167.2008.01817.x. [DOI] [PubMed] [Google Scholar]
- 30.Höppener RJ, Kuyer A, Meijer JW, Hulsman J. Correlation between daily fluctuations of carbamazepine serum levels and intermittent side effects. Epilepsia. 1980;21:341–350. doi: 10.1111/j.1528-1157.1980.tb04081.x. [DOI] [PubMed] [Google Scholar]
- 31.Johannessen SI, Gerna M, Bakke J, Strandjord RE, Morselli PL. CSF concentrations and serum protein binding of carbamazepine and carbamazepine-10,11-epoxide in epileptic patients. Brit J Clin Pharmacol. 1976;3:575–582. doi: 10.1111/j.1365-2125.1976.tb04878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riva R, Albani F, Ambrosetto G, Contin M, Cortelli P, Perucca E, Baruzzi A. Diurnal fluctuations in free and total steady-state plasma levels of carbamazepine and correlation with intermittent side effects. Epilepsia. 1984;25:476–481. doi: 10.1111/j.1528-1157.1984.tb03446.x. [DOI] [PubMed] [Google Scholar]
- 33.Tomson T. Interdosage fluctuations in plasma carbamazepine concentration determine intermittent side effects. Arch Neurol. 1984;41:830–834. doi: 10.1001/archneur.1984.04050190036011. [DOI] [PubMed] [Google Scholar]
- 34.Eeg-Olofsson O, Nilsson HL, Tonnby B, Arvidsson J, Grahn PA, Gylje H, Larsson C, Norén L. Diurnal variation of carbamazepine and carbamazepine-10,11-epoxide in plasma and saliva in children with epilepsy: A comparison between conventional and slow-release formulations. J Child Neurol. 1990;5:159–165. doi: 10.1177/088307389000500219. [DOI] [PubMed] [Google Scholar]
- 35.Mirza WU, Rak IW, Thadani VM, Cereghino JJ, Garnett WR, Brown LM, Zhang Y, Belendiuk GW. Six-month evaluation of Carbatrol (extended-release carbamazepine) in complex partial seizures. Neurology. 1998;51:1727–1729. doi: 10.1212/wnl.51.6.1727. [DOI] [PubMed] [Google Scholar]
- 36.Miller AD, Krauss GL, Hamzeh FM. Improved CNS tolerability following conversion from immediate- to extended-release carbamazepine. Acta Neurol Scand. 2004;109:374–377. doi: 10.1111/j.1600-0404.2004.00291.x. [DOI] [PubMed] [Google Scholar]
- 37.Canger R, Altamura AC, Belvedere O, Monaco F, Monza GC, Muscas GC, Mutani R, Panetta B, Pisani F, Zaccara G. Conventional vs controlled-release carbamazepine: A multicentre, double-blind, cross-over study. Acta Neurol Scand. 1990;82:9–13. doi: 10.1111/j.1600-0404.1990.tb01579.x. [DOI] [PubMed] [Google Scholar]
- 38.El-Mallakh RS, Salem MR, Chopra AS, Mickus GJ, Penagaluri P. Adverse event load in bipolar participants receiving either carbamazepine immediate-release or extended-release capsules: A blinded, randomized study. Int Clin Psychopharmacol. 2009;24:145–149. doi: 10.1097/YIC.0b013e328329b199. [DOI] [PubMed] [Google Scholar]
- 39.Brodie MJ, Overstall PW, Giorgi L. Multicentre, double-blind, randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy. The UK Lamotrigine Elderly Study Group. Epilepsy Res. 1999;37:81–87. doi: 10.1016/s0920-1211(99)00039-x. [DOI] [PubMed] [Google Scholar]
- 40.Saetre E, Perucca E, Isojärvi J, Gjerstad L, LAM 40089 Study Group An international multicenter randomized double-blind controlled trial of lamotrigine and sustained-release carbamazepine in the treatment of newly diagnosed epilepsy in the elderly. Epilepsia. 2007;48:1292–1302. doi: 10.1111/j.1528-1167.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 41.Doughty J, Baker GA, Jacoby A, Lavaud V. Compliance and satisfaction with switching from an immediate-release to sustained-release formulation of valproate in people with epilepsy. Epilepsy Behav. 2003;4:710–716. doi: 10.1016/j.yebeh.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Zielinski D, Smith MC. The use of extended release formulation of divalproex in patients with epilepsy. Epilepsia. 2001;42(Suppl. 7):92. abstract 1.291. [Google Scholar]
- 43.Herranz JL, Arteaga R, Adín J, Armijo JA. Conventional and sustained-release valproate in children with newly diagnosed epilepsy: A randomized and crossover study comparing clinical effects, patient preference and pharmacokinetics. Eur J Clin Pharmacol. 2006;62:805–815. doi: 10.1007/s00228-006-0175-2. [DOI] [PubMed] [Google Scholar]
- 44.Thibault M, Blume WT, Saint-Hilaire JM, Zakhari R, Sommerville KW. Divalproex extended-release versus the original divalproex tablet: Results of a randomized, crossover study of well-controlled epileptic patients with primary generalized seizures. Epilepsy Res. 2002;50:243–249. doi: 10.1016/s0920-1211(02)00048-7. [DOI] [PubMed] [Google Scholar]