Abstract
The epidermis expresses a number of connexin (Cx) proteins that are implicated in gap junction-mediated cell communication. Distinct dominantly inherited mutations in Cx31 cause the skin disease erythrokeratoderma variabilis (EKV) and hearing loss with or without neuropathy. Functional studies reveal tissue-specific effects of these Cx31 disease-associated mutations. The Cx31 mutants (R42P)Cx31, (C86S)Cx31 and (G12D)Cx31 are associated with EKV and the mutant (66delD)Cx31 with peripheral neuropathy and hearing loss, however the mechanisms of pathogenesis remain to be elucidated. Expression of (R42P)Cx31, (C86S)Cx31 and (G12D)Cx31 in vitro, but not (WT)Cx31 or (66delD)Cx31, cause elevated levels of cell-type specific cell death. Previous studies suggest that Cx-associated cell death may be related to abnormal ‘leaky’ hemichannels but we produced direct evidence against that being the major mechanism. Additionally, our immunocytochemistry showed upregulation of components of the unfolded protein response (UPR) in cells expressing the EKV-associated Cx31 mutants but not (WT)Cx31 or (66delD)Cx31. We conclude that the endoplasmic reticulum (ER) stress leading to the UPR is the main mechanism of mutant Cx31-associated cell death. These results indicate that, in vivo, ER stress may lead to abnormal keratinocyte differentiation and hyperproliferation in EKV patient skin.
INTRODUCTION
Connexins (Cxs) are the major constituents of gap junctions, aqueous pores that connect the cytoplasms of two cells and allow the transfer of ions and small molecules (<1 kDa) between them. Six Cx proteins assemble to form a connexon/hemichannel, which can traffic to the plasma membrane and dock with a hemichannel from an adjacent cell to form an intercellular channel. Hemichannels may also have functional roles at the plasma membrane including paracrine signalling (1–3) and the propagation of calcium waves (4). The importance of gap junction-associated cell communication is indicated by the association of mutations in Cx genes with a range of inherited human disorders (5–7), mouse models (8) and expression and/or localization changes in various disease processes including cancer (9).
The epidermis of skin is predominantly composed of keratinocytes which undergo differentiation, ultimately leading to the formation of the stratum corneum to produce the skin barrier. Cx31 is highly expressed in the stratum granulosum in the upper differentiating layer of the epidermis (10,11) and is also found in peripheral nerves and the cochlea (12). Germline mutations in the GJB3 gene encoding Cx31 are associated with deafness, neuropathy and skin disease, indicating that Cx31 has a role in both epidermal differentiation plus auditory and neuronal function (13). Multiple autosomal dominant [including (R42P)Cx31, (C86S)Cx31 and (G12D)Cx31 (14,15)] and recessive [(L34P)Cx31 (16)] Cx31 mutations are associated with the skin disease erythrokeratoderma variabilis (EKV), a disorder characterized by hyperkeratotic plaques with fixed and transient erythematous patches (17). A dominant neuropathy mutation, (66delD)Cx31, has also been identified in a family with sensorineural hearing loss and peripheral neuropathy (12) and other mutations in Cx31 are associated with non-syndromic hearing loss (18–20).
Previous investigations involving microscopy of EGFP-tagged Cxs expressed in vitro have shown that the EKV mutants and the neuropathy mutant have impaired trafficking to the plasma membrane with a predominantly cytoplasmic localization in contrast to the wild-type which forms aggregates at the plasma membrane and gap junction-like plaques (21). Unlike wild-type or (66delD)Cx31, the expression of the EKV mutants are associated with elevated levels of in vitro cell death (21–23) through a mechanism which remains to be elucidated.
Basal activity of Cx hemichannels is low, with the majority remaining closed most of the time (24), but opening may be induced by stimuli including low extracellular calcium (4,25,26). Open hemichannels can release molecules such as ATP, glutamate and NAD+ and lead to uptake of others (reviewed in 1). Decreased cell viability caused by the expression of some Cx26 and Cx30 disease-associated mutants has been attributed to the presence of hemichannels at the plasma membrane which are ‘leaky’ when cells are incubated in physiological levels of extracellular calcium, with rescue of the phenotype occurring under high levels of calcium (3,27,28). It is suggested that this abnormal hemichannel activity can contribute to the disease phenotype in vivo (29). In contrast, data from another study investigating Cx26 skin and deafness mutants suggest that aberrant hemichannels are not the major mechanism of cell death for these mutants (30).
In this investigation, high levels of cell death were still observed with (R42P)Cx31, (C86S)Cx31 and (G12D)Cx31 when incubated in high extracellular calcium, indicating that hemichannel-mediated cell death is not the major mechanism for these mutants. We describe a novel association of the expression of EKV-associated mutants with upregulation of components of the unfolded protein response (UPR) in vitro. Factors that induce endoplasmic reticulum (ER) stress in cells can lead to the UPR, a cellular process which can induce signalling pathways to cope with stresses, or if this fails, to induce cell death (31). Though the precise molecular mechanism of action of the EKV-associated dominant Cx31 mutations still remains to be elucidated, our data indicate that these mutants induce ER stress, suggesting for the first time the major mechanism of the cell death phenotype of Cx31 skin disease mutants.
RESULTS
Cx31 mutants show defective trafficking and are located on cytoplasmic membranous structures
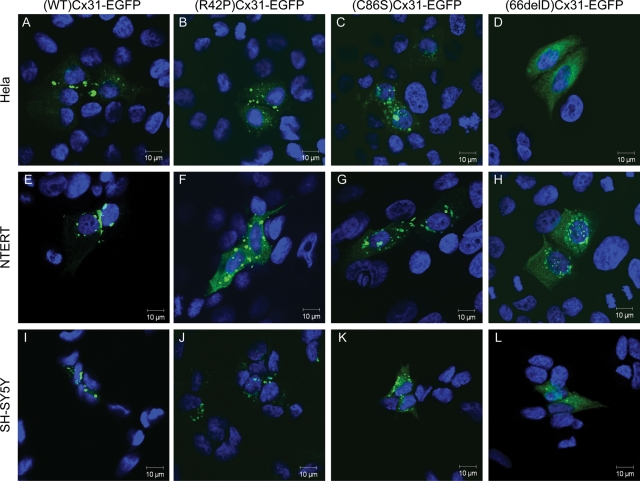
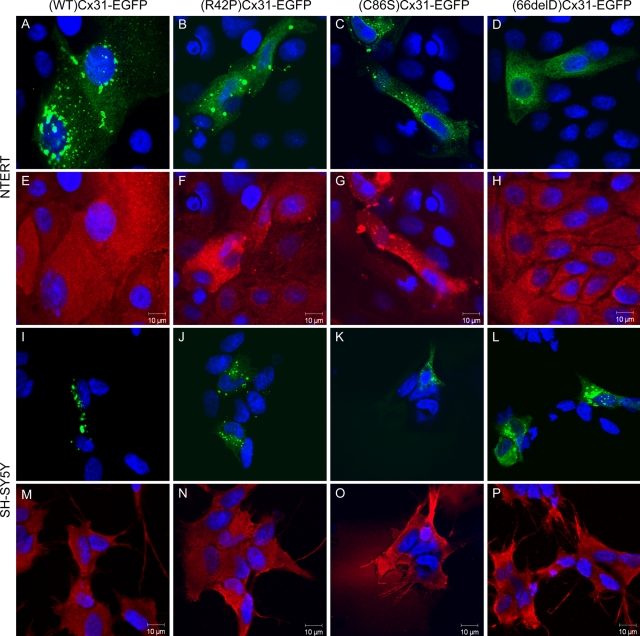
(WT)Cx31-EGFP and the mutants (R42P)Cx31-EGFP, (C86S)Cx31-EGFP and (66delD)Cx31-EGFP [and (G12D)Cx31-EGFP, data not shown] were expressed in HeLa, NTERT and SH-SY5Y cells (Fig. 1). The localization of each specific mutant was comparable in all three cell types. Microscopy confirmed that (WT)Cx31-EGFP traffics to the cell surface membrane forming bright aggregates between cells, indicative of gap junction plaques. In contrast, the four mutants that were tested appear to mistraffic and have a cytoplasmic distribution. Cells expressing each of the mutants lack the gap junction-like aggregates between cells that are found with the wild-type. (G12D)Cx31-EGFP showed a similar behaviour (data not shown). These data are in agreement with the previous investigations performed in HeLa (22,23) and fibroblast and keratinocyte cell lines (21).
Figure 1.
Distinct subcellular localization patterns are observed upon expression of WT, skin disease- and neuropathy-causing Cx31 in HeLa, NTERT and SH-SY5Y cells. In HeLa cells (A–D), bright aggregates are observed at cell–cell boundaries in cells expressing (WT)Cx31-EGFP (A) which are indicative of gap junction plaques. These aggregates are not observed upon overexpression of any mutant, consistent with their inability to traffic properly to the plasma membrane. Cells expressing either of the skin disease mutants (R42P)Cx31-EGFP (B) or (C86S)Cx31-EGFP (C) display larger brighter aggregates than cells expressing the neuropathy mutant (66delD)Cx31-EGFP (D), which show protein residing on smaller diffuse punctate structures (distinct from the diffuse non-punctate EGFP localization (see Supplementary Material, Fig. S1). A similar subcellular localization is observed when the WT and mutant Cx31 constructs are expressed in the immortalized keratinocyte cell line NTERT (E–H) and the neuroblastoma cell line SH-SY5Y (I–L). DAPI-stained nuclei are shown in blue.
Confocal microscopy reveals subtle differences between the different mutant Cx proteins. The EKV-associated mutants are located on large bright aggregates (>3 µm in diameter) in the cytoplasm, in contrast to the neuropathy mutant (66delD)Cx31-EGFP which has a more diffuse localization, residing on smaller punctate structures (<2 µm in diameter). This diffuse punctate localization is distinct from the more homogenous undefined diffuse pattern of EGFP alone (see Supplementary Material, Fig. S1), consistent with a soluble cytosolic protein, suggesting each of the Cx31 mutant proteins reside on cytoplasmic membranous structures.
Additionally, non-EGFP tagged constructs were used to confirm that the differences in trafficking of the different Cx31 proteins was not due to the presence of the EGFP tag (Supplementary Material, Fig. S2).
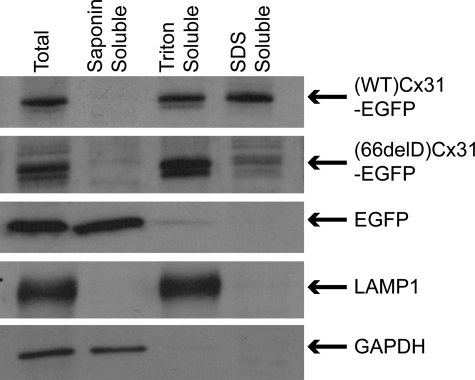
To confirm that the majority of the mutant protein does not form gap junction plaques, sequential detergent-based subcellular fractionation of cell lysates was performed (Fig. 2). This involved solubilizing crude cell lysate in saponin followed by Triton X-100 then SDS to assess each protein's detergent solubility. The Triton-insoluble pool of wild-type Cx31 (indicative of gap junction plaques) was greatly reduced for (66delD)Cx31-EGFP protein, which was present mainly in the Triton-soluble fraction. The absence of saponin-soluble (cytosolic) Cx protein indicates that all Cx proteins tested are present on membranous structures. Identical fractionation of cells expressing skin disease mutants was also performed; however, the low transfection efficiency of these mutants prevented conclusive results from being obtained (data not shown). Additionally, a microscopical approach was used to confirm these results by visualizing any remaining EGFP fluorescence after washing out soluble protein in saponin and/or Triton X-100. This supported (C86S)Cx31-EGFP also being on membranous structures like the other Cx31 proteins (Supplementary Material, Fig. S3).
Figure 2.
The Cx31 mutant 66delD is saponin-insoluble and Triton-soluble, indicating membranous localization. Sequential detergent solubilization of crude lysate of HeLa cells overexpressing (WT)- and (66delD)Cx31-EGFP in saponin followed by Triton X-100 then SDS revealed no Cx31 protein is saponin-soluble which would be indicative of a cytosolic pool (in contrast to the unfused EGFP protein). The presence of (66delD)Cx31-EGFP protein solely in the Triton-soluble fraction indicates that the protein is mainly present on membranous structures. The additional presence of a Triton-insoluble pool of (WT)Cx31-EGFP (present in the SDS-soluble fraction) is indicative of gap junction aggregates. The LAMP1 and GAPDH control proteins are present in the Triton- and saponin-soluble fractions, respectively, as expected.
Aberrant hemichannel activity is not the main mechanism for the cell death phenotype associated with the skin disease mutants
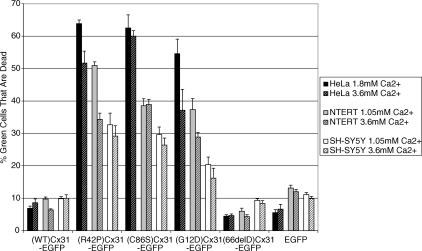
Quantification of cell death by Fluorescence-activated cell sorting (FACS) analysis based on EGFP fluorescence and propidium iodide (PI) uptake showed that expression of (R42P)Cx31-EGFP, (C86S)Cx31-EGFP and (G12D)Cx31-EGFP leads to decreased cell viability, unlike (WT)Cx31-EGFP or (66delD)Cx31-EGFP (Fig. 3). This is in agreement with the previous observations (21,23). There was less death observed in SH-SY5Y cells compared with HeLa and NTERT, suggesting cell-type processing of the mutants.
Figure 3.
FACS quantification of cell death phenotype. Quantification of cell death by FACS showed that the expression of (R42P)Cx31-EGFP, (C86S)Cx31-EGFP and (G12D)Cx31-EGFP leads to decreased cell viability, unlike (WT)Cx31-EGFP or (66delD)Cx31-EGFP. There is less death of SH-SY5Y cells compared with HeLa and NTERT, suggesting cell-type processing of the mutants. Increasing the calcium concentration to 3.6 mM decreased the amount of cell death in some of the samples, however complete rescue was not observed, suggesting that aberrant hemichannels are not the predominant mechanism of cell death for these mutants. Error bars represent SEM.
Previous studies involving expression of specific Cx26 or Cx30 mutants in cell lines/oocytes have suggested that abnormal hemichannels cause cell death due to the observation that increasing extracellular calcium rescued the cell death phenotype (3,27,28). To investigate if aberrant hemichannels lead to the EKV mutant-induced cell death phenotype, transfected cells were maintained in physiological or high calcium and cell death was quantified by FACS analysis of PI uptake (dead cells are permeable to PI). There was little effect on cell viability of SH-SY5Y cells when the extracellular calcium levels were raised for all of the constructs (Fig. 3, hatched bars). There was a small reduction in cell death of HeLa and NTERT cells transfected with (R42P)Cx31-EGFP and (G12D)Cx31-EGFP, and of HeLa cells transfected with (C86S)Cx31-EGFP, when maintained in 3.6 mm calcium compared with those maintained in physiological concentrations of calcium.
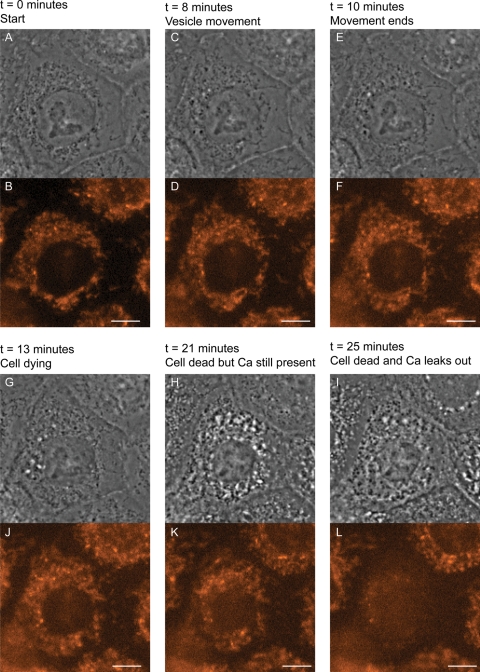
Despite the trend of a small reduction in cell death observed when the extracellular calcium concentration was raised, increases in cell viability did not occur as was reported with Cx26 and Cx30 mutants (3,27,28). In this investigation, elevated levels of cell death were still observed under high-extracellular calcium, suggesting that hemichannel-mediated cell death is not the main mechanism for the Cx31 EKV mutants. In addition, time-lapse imaging of NEB1 keratinocyte cells microinjected with (G12D)Cx31-EGFP and incubated with the calcium-sensitive indicator dye Calcium Orange showed that loss of intracellular calcium occurred only after cell death, indicated by vesicle movement ceasing, rather than a continuous leaking that would be expected if abnormal hemichannels were responsible for cell death (Fig. 4). These data indicate that the major mechanism of cell death associated with these mutants is not due to abnormal hemichannel function.
Figure 4.
Calcium Orange does not leave the cells expressing EKV-associated Cx31, while they are undergoing cell death, eliminating a hemichannel mechanism. Examples of images from time-lapse recording of keratinocytes expressing (G12D)Cx31-EGFP. Although EGFP was also recorded, only phase contrast and Calcium Orange images are presented. Time after microinjection is indicated. Cells were loaded with Calcium Orange 30 min after microinjection and recording started 50 min after microinjection. The cell in the centre of the field died 81 min after microinjection and lost calcium 85 min after microinjection. Scale bars are 10 µm.
Cx31 EKV mutants colocalize with proteasome markers
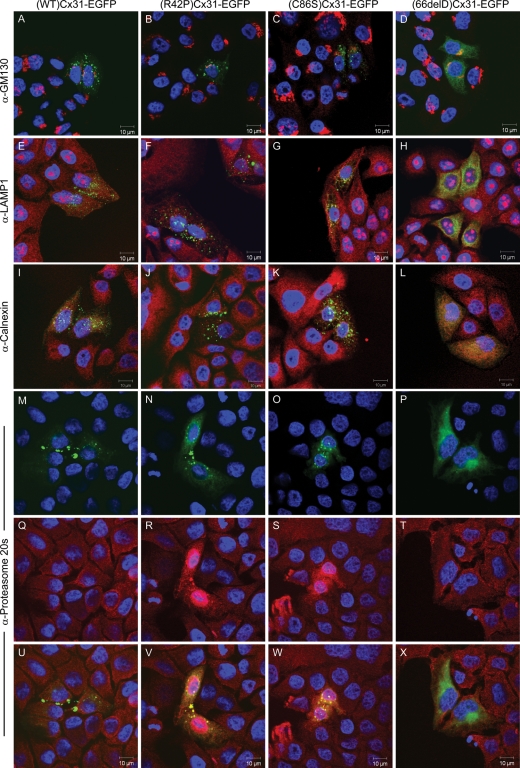
Certain Cx32 mutants have been shown to accumulate in the Golgi apparatus (32) and ER (33). To determine whether the EKV- or neuropathy-associated Cx31 mutants colocalize with the Golgi apparatus, ER or other subcellular compartments known to be involved in Cx assembly and trafficking (reviewed in 34), immunocytochemistry was performed with subcellular markers specific to the Golgi apparatus, ER, lysosome and proteasome (Fig. 5) as well as the endosome, mitochondria and microtubules (data not shown). Although (66delD)Cx31-EGFP staining looks like it is localized to the ER, we did not find any co-localization with any of the ER marker proteins tested [calnexin (Fig. 5), BiP/GRP78 (Fig. 6), KDEL nor GRP94 (data not shown)]. In addition, there was little or no colocalization with the ER and the EKV mutants. Similarly, there was no colocalization of either wild-type or mutant Cx31 protein with the majority of the other subcellular markers tested, indicating that the major pool of each of the mistrafficked mutants does not reside in any of these compartments. The proteasome marker, however, was upregulated in cells expressing the EKV-associated mutants (R42P)Cx31-EGFP and (C86S)Cx31-EGFP with colocalization observed, but not in cells expressing the wild-type or neuropathy-associated mutant (66delD)Cx31-EGFP (Fig. 5).
Figure 5.
Proteasome markers are upregulated in cells expressing the skin disease mutants and colocalize with the mistrafficked protein. Colocalization studies with the EKV- and neuropathy-associated Cx31 mutants and markers against subcellular compartments known to be involved in connexin assembly and trafficking including the Golgi apparatus (A–D), lysosome (E–H) and the ER (I–L) indicates that the major pool of each of the mistrafficked mutants does not reside in any of these compartments. In contrast, the proteasome marker (Q–T) is upregulated in cells overexpressing the (R42P)Cx31-EGFP (N) and (C86S)Cx31-EGFP (O) mutant, and a degree of colocalization is observed (shown in yellow in V and W). This is not observed in cells overexpressing (WT)Cx31-EGFP (M) or (66delD)Cx31-EGFP (P). EGFP-tagged connexin protein is shown in green. The subcellular marker antibody staining is shown in red. DAPI- stained nuclei are shown in blue.
Figure 6.
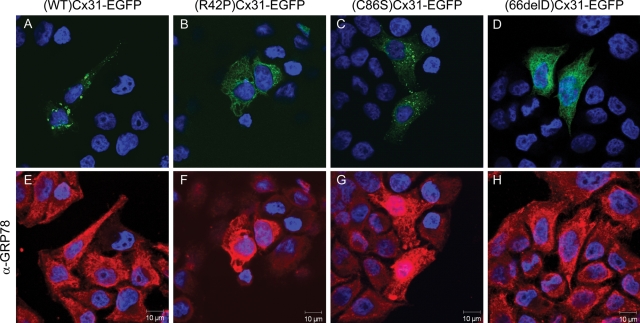
Expression of Cx31 skin disease mutants disrupts the ER marker BiP/GRP78. The ER marker BiP/GRP78 (E–H) is upregulated/disrupted in HeLa cells overexpressing (R42P)Cx31-EGFP (B) and (C86S)Cx31-EGFP (C) but not (WT)Cx31-EGFP (A) or (66delD)Cx31-EGFP (D). Note the exposure settings have been optimized for each field of view, the surrounding non-transfected control cells in (F and G) have a similar level of BiP/GRP78 expression as the cells in (E and H). The KDEL antibody also shows a marked upregulation in cells expressing the (R42P)Cx31-EGFP and (C86S)Cx31-EGFP construct (data not shown). The DAPI-stained nuclei are shown in blue.
Overexpression of skin disease mutants cause upregulation of components of the UPR in vitro
However, similar to the proteasome marker, there was an upregulation of both BiP/GRP78 and proteins containing the KDEL motif in HeLa cells expressing the EKV mutants. The KDEL amino acid motif is present at the C terminus of ER resident proteins including the ER chaperone BiP/GRP78. BiP/GRP78 was also found to be upregulated in cells expressing (R42P)Cx31-EGFP and (C86S)Cx31-EGFP but not the wild-type or neuropathy-associated mutant (66delD)Cx31-EGFP (Fig. 6). Furthermore the dramatic upregulation of BiP/GRP78 is specific to those cell lines where the most cell death is observed [HeLa (Fig. 6) and NTERT (Fig. 7)].
Figure 7.
Expression of either Cx31 skin disease mutant also disrupts the ER marker BiP/GRP78 in NTERT cells but not SH-SY5Y cells. The ER marker BiP/GRP78 (E–H) is upregulated/disrupted in NTERT cells expressing (R42P)Cx31-EGFP (B) and (C86S)Cx31-EGFP (C) but not the (WT)Cx31-EGFP (A) or (66delD)Cx31-EGFP (D). In contrast, BiP/GRP78 (M–P) is unaffected by the expression of any Cx31-EGFP construct (I–L) in SH-SY5Y cells. The DAPI-stained nuclei are shown in blue.
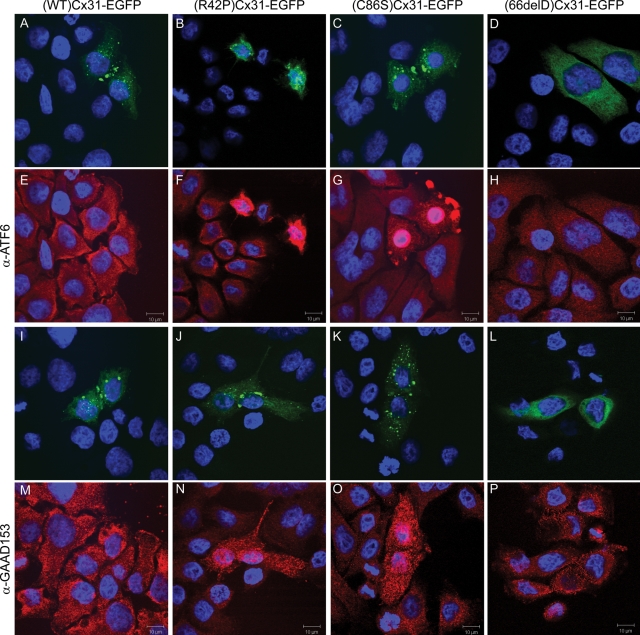
BiP/GRP78 is a component of the UPR, which can be induced by ER stress, and may lead to cell death if the stress is not alleviated (reviewed in 31). For example the neurotoxin 6-OHDA can trigger ER stress inducing the upregulation of UPR markers such as BiP/GRP78 in a dopaminergic cell line (35). To determine whether the expression of EKV-associated mutants affect other components of the UPR as well as BiP/GRP78, immunocytochemistry was performed which showed that ATF6 and the transcription factor CHOP/GAAD153 (Fig. 8) as well as XBP1 (data not shown) were upregulated in HeLa cells expressing the EKV-associated mutant (C86S)Cx31-EGFP but not (WT)Cx31-EGFP. Upon ER stress, ATF6 is cleaved and the N-terminal portion is translocated to the nucleus to initiate transcription of UPR proteins such as ER chaperones and ER-associated degradation components (31,36). An increase in nuclear localization of ATF6 is observed in cells expressing either (C86S)Cx31-EGFP or (R42P)Cx31-EGFP but not (WT)Cx31-EGFP or (66delD)Cx31-EGFP (Fig. 8). Additionally, non-EGFP-tagged constructs were used to confirm that the upregulation/nuclear translocation of BiP/GRP78 and ATF6 was not due to the presence of the EGFP tag (Supplementary Material, Fig. S2). These results demonstrate an association of cell death upon expression of the EKV-associated mutants with UPR components.
Figure 8.
Expression of the Cx31 skin disease mutants upregulate components of the UPR pathway. The UPR components ATF6 (E–H) and CHOP/GAAD153 (M–P) are upregulated in HeLa cells overexpressing (R42P)Cx31-EGFP (B and J) and (C86S)Cx31-EGFP (C and K) but not the (WT)Cx31-EGFP (A and I) nor the (66delD)Cx31-EGFP neuropathy mutant (D and L). Furthermore, the ATF6 staining (F and G) has a more nuclear localization in cells expressing (R42P)Cx31-EGFP (B) and (C86S)Cx31-EGFP (C) compared with untransfected cells and cells expressing (WT)Cx31-EGFP (A) and (66delD)Cx31-EGFP (D), indicating that the cleaved N-terminal portion has been translocated to the nucleus. The DAPI-stained nuclei are shown in blue.
DISCUSSION
Cx31 mutants have cytoplasmic localizations
Previous investigations using EGFP-tagged constructs have shown by microscopy that EKV-associated Cx31 mutants have a predominantly cytoplasmic localization and their expression in vitro decreases cell viability, the mechanism of which was unknown (21,23). However, the neuropathy-associated (66delD)Cx31 also mistraffics but does not increase cell death (21,23). Our data confirm and extend this observation. Confocal microscopy in three different cell lines revealed that the skin disease mutants are characterized by bright cytoplasmic aggregates (>3 µm in diameter) and the neuropathy mutant by smaller punctate structures (<2 µm in diameter), whereas subcellular fractionation demonstrates that both classes are membrane bound. This led us to hypothesise that this difference in intracellular localization could give an indication as to why the two classes of mutants cause different diseases and in vitro cell phenotypes.
The G12S and E208K Cx32 mutants, both linked to X-linked Charcot-Marie-Tooth disease (CMTX), have been found to colocalize with the Golgi apparatus (32) and the ER (33), respectively. We have excluded similar backing-up of mutant Cx31 protein in a range of intermediate transport organelles as well as accumulation in the lysosome. The skin disease mutants were detected in the proteasome, known to be involved in the degradation steps of the turn-over cycle of Cx protein (6,34). In addition, we observed an upregulation of proteasome markers as well as proteins containing the ER resident motif KDEL and the ER chaperone BiP/GRP78.
Aberrant hemichannels are not the main mechanism of EKV-associated mutant cell death
ATP has been hypothesised as a paracrine signalling molecule (1–3). Furthermore, it has been suggested that deregulated release of ATP and other molecules could disrupt paracrine signalling within the skin affecting keratinocyte differentiation and leading to hyperproliferation (3,27–29). The cell death phenotype linked to the expression of hidrotic ectodermal dysplasia Cx30 mutants, G11R and A88V, has been attributed to aberrant hemichannel activity at the plasma membrane of oocytes maintained in low levels of extracellular calcium (3). A similar mechanism has been attributed to expression of keratitis-ichthyosis-deafness syndrome-associated Cx26 mutants G45E and D50N in oocytes (28). Cell death could be rescued by raising the levels of extracellular calcium to close any hemichannels at the cell surface (28). However, another study could not reproduce these findings with (D50N)Cx26-EGFP expression in human cell lines (30), suggesting that other mechanisms may account for a proportion of the observed in vitro human cell death attributable to some Cx mutants.
Quantification of cell death in HeLa cells expressing (R42P)Cx31-EGFP, (C86S)Cx31-EGFP and (G12D)Cx31-EGFP showed that although there was generally some reduction when the calcium levels were raised, levels of cell death still remained high. Time-lapse imaging of keratinocytes microinjected with (G12D)Cx31-EGFP and an indicator dye, Calcium Orange, showed that loss of intracellular calcium occurred only after cell death, indicated by vesicle movement ceasing, rather than a continuous leaking that would be expected if abnormal hemichannels were responsible for cell death. Although we do not exclude the possibility that some hemichannels reach the plasma membrane and exhibit aberrant activity, these data indicate that hemichannel-mediated cell death is not the main mechanism underlying the in vitro cell death phenotype.
Overexpression of the EKV mutants in vitro leads to upregulation of components of the UPR
The accumulation of unfolded proteins in the ER can induce ER stress leading to activation of the UPR pathway, a process which has been linked to the pathogenesis of a variety of disorders including heart disease, neurological diseases such as Alzheimer's disease, diabetes and cancer (reviewed in 31). ER stresses including disrupted redox regulation, glucose deprivation, viral infection, inclusion body diseases and high fat diet can lead to the induction of the UPR, with the objective of restoring normal ER function (31). In mammals, the ER chaperone BiP/GRP78 is bound to inositol-requiring kinase (IRE1α), PERK (PRKR-like ER kinase) and ATF6 (activating transcription factor), however when unfolded proteins accumulate in the ER, these transmembrane ER proteins are activated, signalling through pathways which can lead to the reduction of protein translation and increased the expression of ER chaperone proteins. If these mechanisms fail to control the stress, pathways leading to cell death are activated (31). The UPR has been shown to be activated during normal epidermal keratinocyte differentiation (37), thus deregulated UPR activity may lead to keratinocyte hyperproliferation.
In this study, we have shown that the in vitro expression of (R42P)Cx31-EGFP and (C86S)Cx31-EGFP but not wild-type nor (66delD)Cx31-EGFP was found to correlate with upregulation of the ER resident chaperone BiP/GRP78 and other components of the UPR including ATF6, XBP1 and CHOP/GAAD153. This is the first report of an association of expression of Cx mutants with induction of the UPR, and although members of the UPR have been previously linked with psoriasis and Bowen's disease (37), this is the first study to link EKV with the UPR. There is one report of a recessive Cx mutant that did not appear to cause the nuclear translocation of CHOP/GAAD153 (38), but our study shows that dominant Cx31 mutations associated with increased cell death do upregulate this protein.
It has been hypothesised that Cx mutants lead to pathogenesis by causing defective gap junction formation/regulation, by disrupting other Cxs in the cell, forming aberrant hemichannels or having an abnormal conformational structure (6,39). Our in vitro findings demonstrate that the expression of the skin disease mutants leads to the misregulation of the UPR and that ER homeostasis cannot be restored, leading to elevated levels of cell death. The EKV mutants’ location within the cytoplasm could lead to ER stress, as could the incorrect folding of the protein when transported through the ER. Incorrect protein folding has been implicated in the pathogenesis of Cx32 mutants causing CMTX (40).
Cell death has been linked with in vivo Cx phenotypes particularly certain Cx26 deafness mutations. Cell death is observed in the epithelia supporting the hair cells within the organ of Corti in mice with cell-type specific deletion of Cx26 (41) and, in contrast, delayed cell death is observed in keratinocytes expressing recessive hearing loss-associated Cx26 mutation (42–44). It is not known whether the cell death observed with the Cx31 EKV mutants in vitro also occurs in vivo, and, if so, how this increased level of cell death relates to the disease pathogenesis of EKV. However, abnormal gap junction communication and/or cell death during keratinocyte differentiation has been suggested to lead to hyperproliferation (reviewed in 39) and disease.
In this study, we have elucidated a novel mechanism linking expression of the EKV-associated mutants of Cx31 to the in vitro cell death phenotype by demonstrating an upregulation of the UPR, revealing ER stress as the major mechanism. Furthermore, we have suggested that the cell death phenotype caused by expression of the EKV-associated mutants could explain the in vivo skin disease phenotype by leading to the hyperproliferation that is characteristic of this disease.
MATERIALS AND METHODS
Cells lines, constructs and transfection
HeLa and SH-SY5Y cells were obtained from the American Type Culture Collection. NEB1 and NTERT cells lines were generated as described elsewhere (42,45). All EGFP-tagged DNA constructs were generated as described previously (21). Non-EGFP-tagged Cx31 constructs were generated by excising the EGFP cDNA with XmaI and NotI and replacing it with a duplexed oligo insert containing an in-frame stop codon (sense=CCGGACTAGTCA, antisense=GGCCTGACTAGT). Cells were transiently transfected using Fugene6 (Roche) using the manufacturer's instructions. The percentage of cells expressing the EGFP constructs after 48 h is shown in Supplementary Material, Figure S4.
Antibodies
All antibodies used are available commercially. Antibodies against Calnexin, LAMP1 and GM130 were purchased from BD Biosciences, the Cx31 antibody was purchased from Abnova and antibodies against ATF6, CHOP/GAAD153, GFP, GRP94, KDEL, Proteasome 20s, alpha-tubulin and XBP1 were purchased from Abcam. The GM130 and Proteasome 20s results were confirmed using an anti-giantin antibody (Covance) and an anti-58K protein antibody (Abcam), and an anti-ECM29 antibody (Abcam), respectively (data not shown). The BiP/GRP78 antibody (raised against the N-terminal) was purchased from Sigma (Dorset, UK), and results were confirmed using a C-terminal specific antibody purchased from Sigma and a second C-terminal specific antibody purchased from BD Bioscience (data not shown).
Fluorescent cytochemistry
Cells were seeded onto coverslips the day after transfection, and 24 h later fixed in 4% paraformaldehyde for 30 min. Cells were mounted in Immu-mount (Shandon Thermo, UK) containing 50 ng/ml DAPI. For immunocytochemistry, cells were permeabilized after fixation in 0.1% Triton X-100 for 10 min, blocked in 3% bovine serum albumin (BSA) for 15 min and incubated for 1 h with primary antibody diluted 1/50 (KDEL), 1/100 (LAMP1, GM130, BiP/GRP78), 1/200 (ATF6, Calnexin, CHOP/GAAD153, XBP1) or 1/1000 (alpha-tubulin) in 3% BSA. Cells were washed in PBS, incubated for 1 h with the appropriate Alexa Fluor 488 or Alexa Fluor 546 secondary antibody (Invitrogen) diluted 1/1000 in PBS, before being washed in PBS and mounted as described above. Cells were visualized using a Zeiss META 510 confocal microscope and manipulated using LSM Image Browser and Adobe Photoshop. The exposure settings were optimized for each field of view to illustrate subcellular localization. In the case of upregulation of cellular markers, the unchanged levels of protein in surrounding non-transfected control cells were used for cross-panel comparisons.
Fluorescence-activated cell sorting
Cells were seeded at 30–40% confluency in six-well plates and transfected as described above. Six hours post-transfection cells were either maintained in media containing physiological levels of calcium (1.05 mm for SH-SY5Y and NTERT, 1.8 mm for HeLa) or calcium chloride was added to raise the extracellular calcium levels to 3.6 mm. Twenty-four hours post-transfection, adherent cells were trypsinized and pelleted and combined with pelleted non-adherent (dead) cells. Cells were resuspended in ice-cold PBS/1% FCS and stained with 1 µg/ml PI 5 min prior to cell sorting. Samples were analysed on a BD LSRII and the percentage of EGFP-positive (transfected) cells that were PI positive (dead) was calculated.
Time-lapse imaging of cell death and calcium concentration
NEB1 keratinocytes were seeded into 35 mm glass-bottom dishes, and 2 days later ∼20 cells in an isolated colony were microinjected using an Eppendorf Transjector and micromanipulator with 0.05 mg/ml of (G12D)Cx31-EGFP cDNA construct. After microinjection, cells were incubated in Calcium Orange loading medium [2 µmol Calcium Orange (Molecular Probes) and 0.02% w/v Pluronic F-127 (Molecular Probes)] for 30 min, then normal culture medium for 20 min, before being imaged on an Axiovert 135 TV microscope (Carl Zeiss Ltd.). Acquired sequences were analysed in the AQM6 software by determining time points of cell death defined as cessation of active subcellular vesicle movement, and time points of calcium loss defined as the first dramatic reduction of integrated intracellular fluorescence intensity of Calcium Orange in comparison to uninjected cells.
Detergent fractionation
HeLa cells were transfected as described above and seeded into 90 mm dishes 24 h later; and 48 h post-transfection, cells were washed in ice-cold PBS and scraped into 1 ml ice-cold saponin buffer [0.01% saponin in 10 mm Tris pH7.5, 140 mm NaCl, 5 mm EDTA, 2 mm EGTA plus complete protease inhibitor cocktail (Roche)]. Cells were disrupted with a 21G needle, then centrifuged at 16 000g for 30 min at 4°C. The supernatant was mixed with SDS–PAGE loading buffer (final component concentrations: 100 mm Tris pH 6.8, 4% SDS, 20% glycerol, 0.005% Bromophenol Blue and 2.5% β-mercaptoethanol) and boiled for 5 min, while the pellet was resuspended in ice-cold Triton buffer (1% Triton X-100, other buffer components as saponin buffer), disrupted with a needle and repelleted at 16 000g for 30 min at 4°C. The supernatant was again boiled in SDS loading buffer, while the pellet was solubilized in SDS–PAGE loading buffer by sonicating and boiling. All fractions were analysed by western blotting using standard procedures.
The different solubilities of (WT)Cx31-EGFP and the mutants were verified directly by immunofluorescence. Three low-power fields of view were taken of a near-confluent monolayer of HeLa cells expressing each Cx31 construct after being subjected to either saponin extraction or triton extraction. Buffer components were as above except Triton X-100 was used at 0.1%. Saponin-treated cells were washed for 1 min three times, and Triton X-100-treated cells were washed once for 1 min. The number of green transfected cells were counted in each field of view, and compared with the number of cells in a field that had no detergent treatment.
SUPPLEMENTARY MATERIAL
FUNDING
This work was funded by the Wellcome Trust (D.P.K) and Cancer Research UK (D.Z.). Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust and Barts and The London School of Medicine and Dentistry.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr Wei-Li Di for contributions to preliminary aspects of this study and Rita Cabral for critical reading of the manuscript. We would like to acknowledge the Blizard Advanced Light Microscopy Core Facility within the ICMS.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Saez J.C., Retamal M.A., Basilio D., Bukauskas F.F., Bennett M.V. Connexin-based gap junction hemichannels: gating mechanisms. Biochim. Biophys. Acta. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vuyst E., Decrock E., De Bock M., Yamasaki H., Naus C.C., Evans W.H., Leybaert L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol. Biol. Cell. 2007;18:34–46. doi: 10.1091/mbc.E06-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Essenfelder G.M., Bruzzone R., Lamartine J., Charollais A., Blanchet-Bardon C., Barbe M.T., Meda P., Waksman G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum. Mol. Genet. 2004;13:1703–1714. doi: 10.1093/hmg/ddh191. [DOI] [PubMed] [Google Scholar]
- 4.Stout C.E., Costantin J.L., Naus C.C., Charles A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 5.Aasen T., Kelsell D.P. Connexins in skin biology. In: Harris A., Locke D., editors. Connexins: A Guide; NY. Humana Press Inc; 2008. pp. 307–321. [Google Scholar]
- 6.Laird D.W. Life cycle of connexins in health and disease. Biochem. J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelsell D.P., Dunlop J., Hodgins M.B. Human diseases: clues to cracking the connexin code? Trends Cell Biol. 2001;11:2–6. doi: 10.1016/s0962-8924(00)01866-3. [DOI] [PubMed] [Google Scholar]
- 8.Dobrowolski R., Willecke K. Connexin-caused genetic diseases and corresponding mouse models. Antioxid. Redox Signal. 2009;11:283–295. doi: 10.1089/ars.2008.2128. [DOI] [PubMed] [Google Scholar]
- 9.Laird D.W., Fistouris P., Batist G., Alpert L., Huynh H.T., Carystinos G.D., Alaoui-Jamali M.A. Deficiency of connexin43 gap junctions is an independent marker for breast tumors. Cancer Res. 1999;59:4104–4110. [PubMed] [Google Scholar]
- 10.Common J.E., O'Toole E.A., Leigh I.M., Thomas A., Griffiths W.A., Venning V., Grabczynska S., Peris Z., Kansky A., Kelsell D.P. Clinical and genetic heterogeneity of erythrokeratoderma variabilis. J. Invest. Dermatol. 2005;125:920–927. doi: 10.1111/j.0022-202X.2005.23919.x. [DOI] [PubMed] [Google Scholar]
- 11.Di W.L., Rugg E.L., Leigh I.M., Kelsell D.P. Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J. Invest. Dermatol. 2001;117:958–964. doi: 10.1046/j.0022-202x.2001.01468.x. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Bigas N., Olive M., Rabionet R., Ben-David O., Martinez-Matos J.A., Bravo O., Banchs I., Volpini V., Gasparini P., Avraham K.B., et al. Connexin 31 (GJB3) is expressed in the peripheral and auditory nerves and causes neuropathy and hearing impairment. Hum. Mol. Genet. 2001;10:947–952. doi: 10.1093/hmg/10.9.947. [DOI] [PubMed] [Google Scholar]
- 13.Unsworth H.C., Aasen T., McElwaine S., Kelsell D.P. Tissue-specific effects of wild-type and mutant connexin 31: a role in neurite outgrowth. Hum. Mol. Genet. 2007;16:165–172. doi: 10.1093/hmg/ddl452. [DOI] [PubMed] [Google Scholar]
- 14.Wilgoss A., Leigh I.M., Barnes M.R., Dopping-Hepenstal P., Eady R.A., Walter J.M., Kennedy C.T., Kelsell D.P. Identification of a novel mutation R42P in the gap junction protein beta-3 associated with autosomal dominant erythrokeratoderma variabilis. J. Invest. Dermatol. 1999;113:1119–1122. doi: 10.1046/j.1523-1747.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- 15.Richard G., Smith L.E., Bailey R.A., Itin P., Hohl D., Epstein E.H., Jr, DiGiovanna J.J., Compton J.G., Bale S.J. Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis [see comments] Nat. Genet. 1998;20:366–369. doi: 10.1038/3840. [DOI] [PubMed] [Google Scholar]
- 16.Gottfried I., Landau M., Glaser F., Di W.L., Ophir J., Mevorah B., Ben-Tal N., Kelsell D.P., Avraham K.B. A mutation in GJB3 is associated with recessive erythrokeratodermia variabilis (EKV) and leads to defective trafficking of the connexin 31 protein. Hum. Mol. Genet. 2002;11:1311–1316. doi: 10.1093/hmg/11.11.1311. [DOI] [PubMed] [Google Scholar]
- 17.Kelsell D.P., Di W.L., Houseman M.J. Connexin Mutations in Skin Disease and Hearing Loss. Am. J. Hum. Genet. 2001;68:559–568. doi: 10.1086/318803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X.Z., Xia X.J., Xu L.R., Pandya A., Liang C.Y., Blanton S.H., Brown S.D., Steel K.P., Nance W.E. Mutations in connexin31 underlie recessive as well as dominant non- syndromic hearing loss. Hum. Mol. Genet. 2000;9:63–67. doi: 10.1093/hmg/9.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Xia J.H., Liu C.Y., Tang B.S., Pan Q., Huang L., Dai H.P., Zhang B.R., Xie W., Hu D.X., Zheng D., et al. Mutations in the gene encoding gap junction protein beta-3 associated with autosomal dominant hearing impairment. Nat. Genet. 1998;20:370–373. doi: 10.1038/3845. [DOI] [PubMed] [Google Scholar]
- 20.Liu X.Z., Yuan Y., Yan D., Ding E.H., Ouyang X.M., Fei Y., Tang W., Yuan H., Chang Q., Du L.L., et al. Digenic inheritance of non-syndromic deafness caused by mutations at the gap junction proteins Cx26 and Cx31. Hum. Genet. 2009;125:53–62. doi: 10.1007/s00439-008-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di W.L., Monypenny J., Common J.E., Kennedy C.T., Holland K.A., Leigh I.M., Rugg E.L., Zicha D., Kelsell D.P. Defective trafficking and cell death is characteristic of skin disease-associated connexin 31 mutations. Hum. Mol. Genet. 2002;11:2005–2014. doi: 10.1093/hmg/11.17.2005. [DOI] [PubMed] [Google Scholar]
- 22.Diestel S., Richard G., Doring B., Traub O. Expression of a connexin31 mutation causing erythrokeratodermia variabilis is lethal for HeLa cells. Biochem. Biophys. Res. Commun. 2002;296:721–728. doi: 10.1016/s0006-291x(02)00929-4. [DOI] [PubMed] [Google Scholar]
- 23.He L.Q., Liu Y., Cai F., Tan Z.P., Pan Q., Liang D.S., Long Z.G., Wu L.Q., Huang L.Q., Dai H.P., et al. Intracellular distribution, assembly and effect of disease-associated connexin 31 mutants in HeLa cells. Acta Biochim. Biophys. Sin. (Shanghai) 2005;37:547–554. doi: 10.1111/j.1745-7270.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 24.Stout C., Goodenough D.A., Paul D.L. Connexins: functions without junctions. Curr. Opin. Cell Biol. 2004;16:507–512. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Li H., Liu T.F., Lazrak A., Peracchia C., Goldberg G.S., Lampe P.D., Johnson R.G. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J. Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ripps H., Qian H., Zakevicius J. Pharmacological enhancement of hemi-gap-junctional currents in Xenopus oocytes. J. Neurosci. Methods. 2002;121:81–92. doi: 10.1016/s0165-0270(02)00243-1. [DOI] [PubMed] [Google Scholar]
- 27.Stong B.C., Chang Q., Ahmad S., Lin X. A novel mechanism for connexin 26 mutation linked deafness: cell death caused by leaky gap junction hemichannels. Laryngoscope. 2006;116:2205–2210. doi: 10.1097/01.mlg.0000241944.77192.d2. [DOI] [PubMed] [Google Scholar]
- 28.Gerido D.A., DeRosa A.M., Richard G., White T.W. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am. J. Physiol. Cell Physiol. 2007;293:C337–C345. doi: 10.1152/ajpcell.00626.2006. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.R., Derosa A.M., White T.W. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J. Invest. Dermatol. 2009;129:870–878. doi: 10.1038/jid.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matos T.D., Caria H., Simoes-Teixeira H., Aasen T., Dias O., Andrea M., Kelsell D.P., Fialho G. A novel M163L mutation in connexin 26 causing cell death and associated with autosomal dominant hearing loss. Hear. Res. 2008;240:87–92. doi: 10.1016/j.heares.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Kim I., Xu W., Reed J.C. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 32.Deschenes S.M., Walcott J.L., Wexler T.L., Scherer S.S., Fischbeck K.H. Altered trafficking of mutant connexin32. J. Neurosci. 1997;17:9077–9084. doi: 10.1523/JNEUROSCI.17-23-09077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanSlyke J.K., Deschenes S.M., Musil L.S. Intracellular transport, assembly, and degradation of wild-type and disease-linked mutant gap junction proteins. Mol. Biol. Cell. 2000;11:1933–1946. doi: 10.1091/mbc.11.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He L.Q., Cai F., Liu Y., Liu M.J., Tan Z.P., Pan Q., Fang F.Y., Liang de S., Wu L.Q., Long Z.G., et al. Cx31 is assembled and trafficked to cell surface by ER-Golgi pathway and degraded by proteasomal or lysosomal pathways. Cell Res. 2005;15:455–464. doi: 10.1038/sj.cr.7290314. [DOI] [PubMed] [Google Scholar]
- 35.Holtz W.A., O'Malley K.L. Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J. Biol. Chem. 2003;278:19367–19377. doi: 10.1074/jbc.M211821200. [DOI] [PubMed] [Google Scholar]
- 36.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugiura K., Muro Y., Futamura K., Matsumoto K., Hashimoto N., Nishizawa Y., Nagasaka T., Saito H., Tomita Y., Usukura J. The unfolded protein response is activated in differentiating epidermal keratinocytes. J. Invest. Dermatol. 2009;129:2126–2135. doi: 10.1038/jid.2009.51. [DOI] [PubMed] [Google Scholar]
- 38.Orthmann-Murphy J.L., Enriquez A.D., Abrams C.K., Scherer S.S. Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease. Mol. Cell Neurosci. 2007;34:629–641. doi: 10.1016/j.mcn.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mese G., Richard G., White T.W. Gap junctions: basic structure and function. J. Invest. Dermatol. 2007;127:2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- 40.Vanslyke J.K., Naus C.C., Musil L.S. Conformational maturation and post-ER multisubunit assembly of gap junction proteins. Mol. Biol. Cell. 2009;20:2451–2463. doi: 10.1091/mbc.E09-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen-Salmon M., Ott T., Michel V., Hardelin J.P., Perfettini I., Eybalin M., Wu T., Marcus D.C., Wangemann P., Willecke K., et al. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr. Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Man Y.K., Trolove C., Tattersall D., Thomas A.C., Papakonstantinopoulou A., Patel D., Scott C., Chong J., Jagger D.J., O'Toole E.A., et al. A deafness-associated mutant human connexin 26 improves the epithelial barrier in vitro. J. Membr. Biol. 2007;218:29–37. doi: 10.1007/s00232-007-9025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer C.G., Amedofu G.K., Brandner J.M., Pohland D., Timmann C., Horstmann R.D. Selection for deafness? Nat. Med. 2002;8:1332–1333. doi: 10.1038/nm1202-1332. [DOI] [PubMed] [Google Scholar]
- 44.Common J.E., Di W.L., Davies D., Kelsell D.P. Further evidence for heterozygote advantage of GJB2 deafness mutations: a link with cell survival. J. Med. Genet. 2004;41:573–575. doi: 10.1136/jmg.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morley S.M., Dundas S., James J., Brown R.A., Sexton C., Navsaria H.A., Leigh I.M., Lane E.B. Temperature sensitivity of the keratin cytoskeleton and delayed spreading of keratinocyte lines derived from EBS patients. J. Cell Sci. 1995;108:3463–3471. doi: 10.1242/jcs.108.11.3463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.