Abstract
Background and Aims
Reduction in female fitness in large clones can occur as a result of increased geitonogamous self-fertilization and its influence through inbreeding depression. This possibility was investigated in the self-compatible, bee-pollinated perennial herb Aconitum kusnezoffii which varies in clone size.
Methods
Field investigations were conducted on pollinator behaviour, flowering phenology and variation in seed set. The effects of self-pollination following controlled self- and cross-pollination were also examined. Selfing rates of differently sized clones were assessed using allozyme markers.
Key Results
High rates of geitonogamous pollination were associated with large display size. Female fitness at the ramet level decreased with clone size. Fruit and seed set under cross-pollination were significantly higher than those under self-pollination. The pre-dispersal inbreeding depression was estimated as 0·502 based on the difference in seed set per flower between self- and cross-pollinated flowers. Selfing rates of differently sized clones did not differ.
Conclusions
It is concluded that in A. kusnezoffii the negative effects of self-pollination causing reduced female fertility with clone size arise primarily from a strong early-acting inbreeding depression leading to the abortion of selfed embryos prior to seed maturation.
Key words: Early-acting inbreeding depression, Aconitum kusnezoffii, clone size, female reproductive success, geitonogamy
INTRODUCTION
Clonal growth can influence the number and spatial distribution of flowers and this can have profound consequences for pollen dispersal and mating opportunities (Charpentier, 2002; Barrett, 2003). Large genets with clumped architecture can incur a high rate of pollen transfer between flowers of the same plant, referred to as geitonogamous self-pollination (Handel, 1985; De Jong et al., 1993; Harder and Barrett, 1995). In self-compatible species geitonogamy can result in mating costs associated with selfing and inbreeding depression, and in self-incompatible plants it can reduce fitness through pollen discounting (Harder and Barrett, 1995).
The high rate of geitonogamy expected for large clones leads to the prediction that maternal success should be affected by clone size, especially by the number of flowering ramets. Unfortunately, as mentioned by Routley et al. (2004), the relationship between clone size and geitonogamy is poorly understood. To date, only a handful of studies have addressed the influence of clone size on maternal (Handel, 1985; Wilcock and Jennings, 1999; Wolf et al., 2000; Charpentier, 2002; Routley et al., 2004; Tarasjev, 2005) and/or paternal success (Routley et al., 2004). Most of these studies observed negative relationships between female reproductive success by ramets and clone size, with the exception of domesticated apple Malus × domestica (Routley et al., 2004), for which self-incompatibility prevents self-fertilization.
Several explanations have been invoked to explain the observed reduction in female reproductive success in large clones. First, large clones with larger floral display may receive more pollinator visits at the genet level, but the number of visits per flower and stigmatic pollen load may be lower because of a large number of flowers, reducing receipt of pollen (De Jong et al., 1992; Wilcock and Jennings, 1999; Wolf et al., 2000; Wang et al., 2005). Secondly, resource competition among ramets might be more intense in large than in small clones, especially in plants with a clumped architecture. Because female reproduction is often limited by available resources (Burd, 1994; Liao et al., 2006), reproductive success per flower might decline with flower number. Thirdly, because larger clones display more flowers, individual flowers are more likely to be surrounded by inflorescences of the same genet, resulting in proportionally more geitonogamous pollination. In self-compatible species, this should result in a higher proportion of selfed zygotes and greater risk of reductions in maternal fitness through inbreeding depression (Charlesworth and Charlesworth, 1987; Husband and Schemske, 1996; Eckert, 2000; Charpentier, 2002). Variations in the magnitude of inbreeding depression have been observed among life stages and populations (Husband and Schemske, 1996; Goodwillie and Knight, 2006). Early-acting inbreeding depression resulting from abortion of homozygous offspring during embryo development because of the presence of deleterious recessive alleles (Seavey and Bawa, 1986; Krebs and Hancock, 1990; Husband and Schemske, 1996) could be an important factor reducing fruit/seed set (Charlesworth and Charlesworth, 1987; Guillaume and Jacquemart, 1999; Mahy and Jacquemart, 1999; Araujo et al., 2007). The incidence of early-acting inbreeding depression can be evaluated by comparing embryo abortion following controlled selfing and crossing. Fourthly, in self-incompatible species large clones may be more susceptible to ‘pollen clogging’, whereby self-pollen restricts access of cross-pollen to the stigma surface or self-pollen tubes interfere with cross-pollen tube growth in the style or ovary (Barrett, 2002). Several investigations have provided experimental evidence for the inhibitory effects of self-pollen on female fertility (Bertin and Sullivan, 1988; Galen et al., 1989; Waser and Price, 1991; Lloyd and Wells, 1992; Broyles and Wyatt, 1993; Barrett, 2002; Kawagoe and Suzuki, 2005).
Despite the importance of clonal growth in perennial plants, few studies have assessed the causes and consequences of clone size on maternal reproductive success. Here, these issues are investigated in the perennial herb Aconitum kusnezoffii (Ranunculaceae), which has a clumped clonal architecture. First, patterns of fruit and seed set were correlated with the number of flowering ramets per genet in four natural populations. The role of pollen limitation was then assessed based on per-flower pollinator visitation and stigmatic pollen loads. The resource limitation hypothesis was evaluated by quantifying raceme size and ramet size in large and small clones. The effects of self- and cross-pollination on maternal success were also examined to test the self-incompatibility. Finally, to determine the role of early-acting inbreeding depression in maternal success, we evaluated seed set following crossing and selfing, and estimated the selfing rates of clones of different sizes using allozyme markers
MATERIALS AND METHODS
Study species and sites
Aconitum kusnezoffii Rchb. is a bee-pollinated protandrous herb. It grows clonally via root tubers, resulting in a clumped architecture. Clones occupy different local patches, as confirmed by allozyme analysis. We used six polymorphic loci (Aat, Skd-1, Skd-2, Pgd, Est and Gdh) in five enzyme systems to estimate the clonal architecture. By randomly choosing ten patches each with >20 ramets and scoring the genotypes, it was found that all ramets within a patch had the same multilocus genotypes across the six polymorphic loci, so genets are easily distinguished under field conditions. Here, we use the number of flowering ramets of a genet as a reasonably accurate proxy for clone size. We conducted field studies in four populations in Xiaolongmen National Forest Park, west Beijing, China (Table 1). Few fruit matured in the lateral racemes during either 2006 or 2007, so we focused on terminal racemes in this study.
Table 1.
The four populations investigated in this study and the types of experimental manipulations performed
| Population ID | Location | Altitude (m) | Community type | Investigation performed* |
|---|---|---|---|---|
| 1 | 39°57′32·1″N, 115°27′03·8″E | 1034 | Grassland dominated by Artemisia dubia | E, P, R, S, Po |
| 2 | 39°57′43·7″N, 115°26′32·1″E | 1091 | Deciduous forest dominated by Juglans mandshurica | E |
| 3 | 39°57′18·6″N, 115°25′15·7″E | 1368 | Deciduous forest dominated by Populus cathayana | E |
| 4 | 39°58′05·5″N, 115°25′48·0″E | 1188 | Deciduous forest dominated by Populus cathayana | E, H, Po |
*E, effects of clone size on maternal success; P, pollen limitation test; R, resource limitation test; S, selfing rate variation; H, hand-pollination experiments; Po, pollinator observations.
Flowering phenology
In 2006, one ramet from each of nine putative genets was sampled to quantify the flowering phenology in population 4. Every sampled ramet had a terminal raceme with more than eight flowers. The flowers on the nine terminal racemes were checked daily from 12 August to early September. We measured the duration of male function of each open flower based on pollen exposure and of female function based on stigma receptivity. Male and female functions of a flower are considered as overlapping if the flower presented some pollen exposure while its stigmas were receptive. Finally, floral longevity was defined as the duration from first anther dehiscence to the end of stigma receptivity. Stigma receptivity was determined using the MTT method (Dafni, 1992). During 2008, five genets were chosen to monitor flower phenology in population 4. We checked all flowers on terminal and lateral racemes for each genet from 11 August to early September.
Pollinator observations
Observations of pollinators were recorded to determine whether their behaviour could cause geitonogamy. Bombus ignites Smith was the principal pollinator and foraged primarily on nectar. Voucher specimens of pollinators are stored at Beijing Normal University. On 27 August, 2006, two vicinal clones, the larger with eight flowering ramets and the smaller with only one ramet, were chosen for the observation of pollinators in population 1. Pollinators were observed from 8:00 to 17:00. Visit frequency is presented as the number of events per 30-min period. On 27 August, 2007, pollinators were observed for eight clones of different sizes from 9:00 to 11:00 in population 1 and for eight clones from 14:00 to 16:00 in population 4. Visit frequency is presented as the total number of events. The number of opening flowers was counted, and the visiting time of Bombus ignites and the numbers of ramets and flowers visited per foraging bout were recorded. Stigmas were also collected from two flowers in each of the 43 clones with different numbers of flowering ramets in population 1, and counted the stigmatic pollen load to determine whether maternal success was limited by the number of pollen grains on stigmas.
Clone size and female reproductive success
The number of flowering ramets per clone was recorded to estimate clone size in four populations, with 60 clones in each population. For each flowering ramet of a sampled clone, the flowers of the terminal raceme were counted. Four weeks after the flowering season, fruits were collected from the terminal inflorescences of all marked ramets and the seeds were counted. Three fruits were selected from the top, middle, and bottom of each inflorescence. For each fruit, we counted the number of developed and undeveloped seeds (aborted embryos and unfertilized ovules are visually discernible) to determine the seed set of each ramet. Each of these values was then averaged across all ramets within a clone for statistical analysis.
Female reproductive success was estimated by: fruit set, the number of fruits produced divided by the number of flowers originally present in the terminal raceme; seed set, the number of seeds per fruit divided by the sum of seeds, aborted embryos and undeveloped ovules; and seed set per flower, calculated as fruit set × seed set. The influence of clone size on female reproductive success was analysed by regressing fruit set, seed set, and seed set per flower against the number of flowering ramets for individual populations, respectively, using a linear regression model in SPSS (Release 13·0).
Resource availability related to clone size
Sixty differently sized clones were randomly sampled from population 1. For each clone the number of flowers in the terminal raceme and the height of all the ramets were measured and these values were averaged at the ramet level within clones. These values were then used to test whether the resources available to each ramet decreased with increasing clone size.
Effects of self pollination
To assess the effects of self-pollination on fruit and seed production, hand-pollination experiments were performed in population 4. We randomly selected 90 terminal racemes and randomly classified them into three groups (30 per group) to conduct the following three pollination treatments: cross-pollination with cross-pollen from at least three other genets; self-pollination; and natural pollination. All flowers on a raceme experienced the same pollination treatment. Raceme size varied from three to 18 flowers, with an average of 8·9 ± 0·40. All sampled flower buds in the cross-pollination treatment were bagged and emasculated, but buds were only bagged in self-pollination treatment, without emasculation. From the beginning of the flowering season, all flowers were checked every day. Once the stigma was receptive, the flower was marked and pollinated with more of the appropriate pollen grains than needed to fertilize all ovules. Three days after all flowers on a raceme had been pollinated, the bag was removed. Four weeks after hand pollination, all developed fruits were collected and counted. Female reproductive success in our study represents fruit set, seed set, and seed set per flower.
The effect of self-pollination on female reproductive success was tested with one-way analysis of variance (ANOVA) using SPSS version 13·0. The Tamhane test was used to perform multiple comparisons. The values of all the three variables were arcsine square-root transformed (Sokal and Rohlf, 1981).
Pollen tube growth of self- and cross-pollination
To examine self-compatibility, in vivo pollen tube growth of self- and cross-pollination was observed using fluorescence microscopy. Both treatments involved six flowers on each of six inflorescences from six individuals, which were emasculated before dehiscence and then bagged. After their stigmas became receptive, the flowers were unbagged, hand-pollinated and then rebagged. For each treatment, one flower from each was removed 2, 4, 8, 12, 24 and 48 h after pollination, and its pistil was fixed in FAA for 24 h and then stored in 70 % ethanol in 1·5 mL microcentrifuge tubes at 4 °C. Pistils were cleared in 8 mol/L NaOH for 12 h and rinsed with tap water for 1–2 h, after which they were stained with 0·1 % (w/v) aniline blue in 0·1 mol/L potassium acetate as described in Dafni (1992). The growth rate of pollen tubes (v) was measured by the length of the pollen tube (Lpt), divided by the length of the style (Ls), i.e. v = Lpt/Ls.
Selfing rate variation among genets and inbreeding depression
At the end of the growth season, 22 clones from population 1 were sampled. We randomly sampled 72 out of all matured seeds from each maternal plant to assess the selfing rate at the genet level. The seeds were placed separately into a cooled mortar and homogenized with a pestle in 150 μL of 0·1 m Tris–HCl extraction buffer consisting of 1 mm tetrasodium salt (EDTA), 10 mm potassium chloride (KCl), 10 mm magnesium chloride (MgCl2), 5 % (w/v) sucrose and 14 % (w/v) polyvinylpyrolidine-40 (PVP-40), all dissolved overnight in 0·1 m Tris–HCl buffer (pH 7·0), with 1 % (v/v) β-mercaptoethanol added just before use (Soltis et al., 1983). Using a Tris–glycine buffer system (pH 8·3), vertical slab polyacrylamide gel electrophoresis was used to separate allozyme bands. Gels were run under constant current (200 V) until a bromophenol blue marker reached the bottom of the gel. Then gels were stained for enzyme activity following recipes described by Wendel and Weeden (1989) and Wang (1996). Four variable loci were resolved for three enzyme systems: aspartate aminotransferase (Aat; EC 2·6·1·1), shikimate dehydrogenase (Skd; EC 1·1·1·25) and 6-phosphogluconate dehydrogenase (Pgd; EC 1·1·1·44), and genotypes were inferred based on the segregation patterns characteristic of either dimeric or monomeric co-dominant enzymes. We detected two alleles at the Aat locus (with relative frequencies of 0·962 and 0·038), two alleles at Skd-1 (0·974 and 0·026), three alleles at Skd-2 (0·938, 0·061 and 0·001) and three alleles at Pgd (0·253, 0·629 and 0·119).
We jointly estimated single- and multilocus selfing rates for population 1, with the expectation–maximization method, using the program MLTRWIN (Ritland, 2002). Post-dispersal inbreeding depression (δ) was estimated based on the inbreeding coefficient of parents and offspring and the selfing rate of maternal families following Ritland (1990). The single- and multilocus selfing rates of each genet were then estimated, specifying the outcrossing rate at the population level as the initial tm value that allowed iterations to start. Standard deviations were based on 1000 bootstrap values, using individual seeds within families as the unit of resampling.
Pre-dispersal inbreeding depression was estimated as 1 – (ws/wx), where ws was the product of fruit and seed set for self-pollinated flowers and wx was the same for cross-pollinated flowers. Given estimates of pre-dispersal inbreeding depression (E) and post-dispersal inbreeding depression (L), lifetime inbreeding depression equals 1 – (1 – E)(1 – L).
RESULTS
Flowering phenology
During 2006 flowering commenced on 12 August and ceased in early September. Every flowering ramet had a terminal raceme with 2–34 hermaphroditic flowers. The mean (± s.e.) floral longevity was 6·3 ± 0·09 d. Flowers are protandrous, with 4·8 ± 0·09 d of pollen exposure, followed by 1·7 ± 0·06 d of stigma receptivity. Sixteen of the 136 flowers investigated had a 1 d overlap between male and female function. During 2008, the flowering time is almost same as the observation in 2006, beginning on 11 August and ceasing in early September. The mean floral longevity was 6·6 ± 0·74 d, first with 5·3 ± 0·7 d of pollen exposure, and then 1·3 ± 0·54 d of stigma receptivity. Only eight of the 344 flowers investigated had a 1 d overlap between male and female function. The flowering period for each genet involved two phases; flowers on terminal racemes bloomed first and those on lateral racemes flowered after the flowers on terminal racemes withered. Flowering progressed acropetally within inflorescences.
Pollinator visitation
Bombus ignites was the principal pollinator: while foraging primarily on nectar it contacted the sexual organs and carried pollen. Other flower visitors included Apis mellifera and Episyrphus balteatus; both only collected pollen and are unlikely to function as pollinators because they did not contact stigmas.
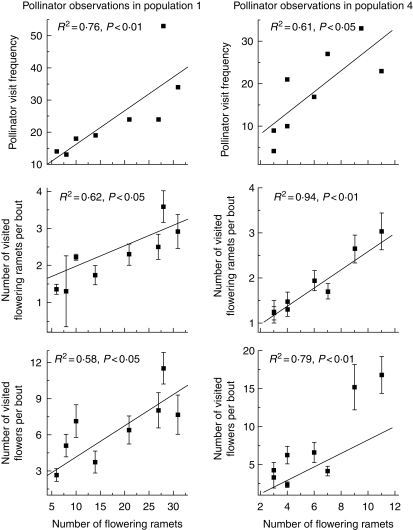
The rate of pollinator visitation varied positively with display size of A. kusnezoffii. Bombus ignites visited large clones more frequently (6·0 ± 0·62 visits per 30 min) than small clones with a single flowering ramet (1·7 ± 0·27 visits per 30 min), during 2006. In the large clones with eight flowering ramets, bees visited 2·4 ± 0·16 ramets and 7·7 ± 0·67 flowers per foraging bout, whereas they visited only 3·0 ± 0·32 flowers in the small clones. During 2007, pollinator visit frequency, the numbers of flowering ramets and flowers visited by B. ignites per foraging bout varied positively with the number of flowering ramets (Fig. 1). However, per-flower pollinator visits did not differ between large and small clones (P > 0·05).
Fig. 1.
The effects of the number of flowering ramets on pollinator visit frequencies, the number of flowering ramets visited by pollinators per foraging bout and the flowers visited per bout for populations 1 and 4.
Effects of clone size on maternal success
The estimated clone size (the number of flowering ramets) varied from one to 28 across the four populations. A total of 182 of 240 marked clones (76 %) were harvested, and the rest were excluded from analysis because of senescence or herbivory.
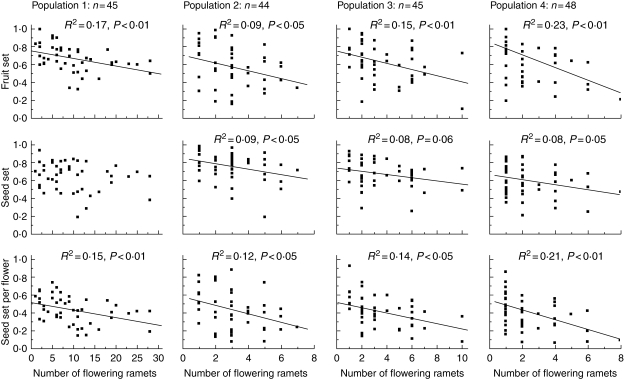
Fruit set and seed set per flower varied negatively with clone size in all four populations. Seed set was not consistent across the four populations, decreasing with increase of clone size in populations 2, 3 and 4, while not varying significantly with clone size in population 1. Generally, larger clones had lower fruit set, seed set, and seed set per flower (Fig. 2).
Fig. 2.
The effects of the number of flowering ramets on female reproductive success (fruit set, seed set, and seed set per flower) in four populations of Aconitum kusnezoffii.
Pollen and resource limitation
The stigmatic pollen loads collected from 43 clones ranged from one to 111 and did not vary significantly with the number of flowering ramets (P > 0·05). If female reproductive success per flower is limited by resource availability, then both the ramet height and the number of flowers in a ramet should decline in large clones as a result of more intense competition among ramets. However, the results indicate that neither raceme size (P > 0·05) nor ramet height (P > 0·05) varied significantly with the number of flowering ramets.
Effects of self-pollination
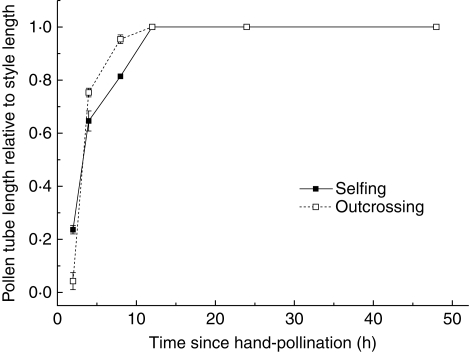
Because of the senescence of the inflorescence or possibly herbivory, only 55 (61 %) of the initial 90 hand pollination experimental ramets were sampled. The results indicate seed set was 0·465 ± 0·029 in self-pollination and 0·810 ± 0·020 in cross-pollination. Self-pollen significantly reduced the seed set by 43 % after cross-pollination (P < 0·05). Results of pollen tube growth from controlled pollinations demonstrate that A. kusnezoffii is self-compatible. Both self- and cross-pollen grains germinated quickly upon arrival on stigmas and all can grow to the ovary after 12 h, with similar pollen tube growth rates (Fig. 3).
Fig. 3.
Growth of self- and cross-pollen tubes, as indicated. The growth rate of pollen tubes was measured by the length of the pollen tube divided by the length of the style.
Selfing rates and inbreeding depression
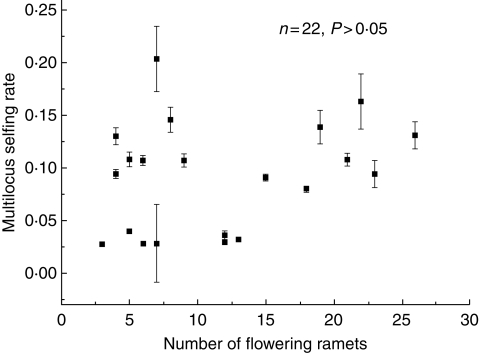
The estimated mean multilocus selfing rate was 0·145 ± 0·016 for population 1. At the genet level, the selfing rates varied from 0·028 ± 0·000 to 0·204 ± 0·031; however, they did not vary significantly with clone size (P > 0·05), so genets with more flowering ramets did not exhibit higher selfing rates (Fig. 4). However, we can note that selfing rates <0·05 occur only in plants with <15 flowering ramets, suggesting a role for clone size and geitonogamy in the mating system of A. kusnezoffii.
Fig. 4.
Variation in the multilocus selfing rate for genets with contrasting numbers of flowering ramets.
Both pre- and post-dispersal inbreeding depression were estimated. Based on the product of fruit set and seed set, pre-dispersal inbreeding depression was 0·502. Post-dispersal inbreeding depression was 0·876 ± 0·299. As a result, lifetime inbreeding depression was estimated to be 0·938.
DISCUSSION
Our aim was to estimate the effects of the number of flowering ramets on maternal success in clonal A. kusnezoffii and to determine the mechanisms underlying this pattern. The negative effects of floral display size on female reproductive success were apparent, with reductions in fruit set, seed set, and seed set per flower in large clones as measured by the number of flowering ramets. Our pollinator visitation results (Fig. 1) indicate that larger clones may suffer higher geitonogamous pollination. Seed set following self-pollination (0·465 ± 0·029) was significantly lower than after crossing (0·810 ± 0·020). As A. kusnezoffii is self-compatible, such results suggest early-acting inbreeding depression. This depression was estimated as 0·502, so most of the selfed embryos were aborted during the stage of seed maturation. As a consequence, the selfing rates of differently sized clones estimated at the seed stage would be much lower than the primary selfing rate, ranging from 0·028 ± 0·000 to 0·204 ± 0·031, and higher selfing rates in genets with more flowering ramets were not exhibited.
Effect of pollen and/or resource limitation on maternal success
Reduced female success by large clones has been reported previously (Eriksson and Bremer, 1993; Free, 1993; Wilcock and Jennings, 1999; Wolf et al., 2000; Tarasjev, 2005). Our results also show such a reduction, with fruit set, seed set, and seed set per flower decreasing with increasing clone size across all four populations of A. kusnezoffii (Fig. 2).
Pollen limitation is often proposed to account for reduced female fitness with increasing clone size (Haig and Westoby, 1988; Burd, 1994). In animal-pollinated plants, pollen quantity may be reduced as a result of fewer pollinator visits or less pollen delivered per visit (Ashman et al., 2004). Since neither per-flower pollinator visits nor stigmatic pollen loads changed with the number of flowering ramets, pollen limitation appears unlikely for A. kusnezoffii.
Resource competition among ramets may also lead to reduced maternal success per flower with increasing clone size. Larger clones, especially in clonal plants with a clumped architecture, would be expected to allocate fewer resources per ramet, and this effect may reduce female reproductive success per flower by resource limitation. If so, larger clones should have fewer flowers at the ramet level and smaller ramets than smaller clones if the amount of available resources does not increase proportionally with the increase of the number of flowering ramets. In fact, neither the flower number per ramet nor the ramet height differed with clone size, suggesting that the resource limitation hypothesis may not be relevant. A plausible explanation for the absence of resource limitation is that larger clones may be able to ‘forage’ in a wider area (Magyar et al., 2007), thus reducing competition among the ramets.
Negative effects of self-pollination and early-acting inbreeding depression
Inbreeding depression is another potential consequence of geitonogamous pollination within clones. This trend depends on flowering phenology and pollinator foraging behaviour (Goulson, 2000; Ishii and Sakai, 2001). Aconitum kusnezoffii is dichogamous, but the flowers on terminal racemes bloom asynchronously within ramets, and synchronously among different ramets. These factors allow the possibility of geitonogamous pollination within and among ramets, although autogamy is uncommon because there is little overlap between the male and female functions within a single hermaphroditic flower. Pollinators usually visited more ramets and more flowers per foraging bout within larger clones (Fig. 1), which is not only indirect evidence of the occurrence of geitonogamy within a clone but also supports the view that larger clones may have a higher rate of geitonogamy. As a consequence, the stigmas in larger clones may receive pollen grains containing a larger percentage of self-pollen than do those in smaller clones.
Geitonogamy is considered to be a negative but unavoidable consequence of cross-pollination when more than one flower opens simultaneously on individual plants (Lloyd, 1992; Jarne and Charlesworth, 1993). It is expected to cause severe male and female fitness reduction through higher selfing rates and consequent inbreeding depression (Charlesworth and Charlesworth, 1987; Eckert and Barrett, 1994; Husband and Schemske, 1996; Charpentier, 2002). Our results demonstrate the impairment of fruit and seed production under self-pollination compared with that under cross-pollination in A. kusnezoffii. Similar results were observed for clonal Iris pumila (Tarasjev, 2005) and Asclepias speciosa (Finer and Morgan, 2003).
Such negative effects of self-pollination on female function might be attributed to the consequences of geitonogamous pollination, the selfing rate and the subsequent inbreeding depression (Charlesworth and Charlesworth, 1987; Husband and Schemske, 1996; Charpentier, 2002). In self-compatible species, larger clones with proportionally more geitonogamous pollination may exhibit higher embryo selfing rates and result in a reduction in fruit and/or seed production through the expression of deleterious recessive alleles under mixed mating systems (Eckert and Barrett, 1994). We calculated the selfing rates of 22 genets by detecting the genotypic variations in their seeds, and found that the selfing rates did not correlate with clone size (Fig. 4), whereas the stigmas in the larger clones may have received pollen grains with a greater percentage of self-pollen than did those in the smaller clones. Given that self-pollen does grow into the ovary (Fig. 3), it is highly likely that a proportion of the selfed ovules were aborted in the early stage of seed maturation in A. kusnezoffii.
Nonetheless, it is important to note that late-acting ovarian self-incompatibility (OSI), the combination of self-pollen tube entry into the ovary followed by little or no seed set after self-pollinations, could also lead to a reduction in female fertility within large clones. However, OSI has not been reported in Aconitum species, and phylogenetic analysis indicates that self-incompatibility in Ranunculaceae is mostly of stylar inhibition (Allen and Hiscock, 2008). In A. kusnezoffii, both self- and cross-pollen tubes could enter into ovaries, with a similar pollen tube growth speed (Fig. 3), suggesting that stylar inhibition is absent. Moreover, self-compatibility was found in some congeneric species, including A. lycoctonum (Utelli and Roy, 2000) and A. gymnandrum (Zhang et al., 2006). Taken together, we may reach a tentative conclusion that early-acting inbreeding depression, rather than OSI, is the most probable explanation for the reduction in female function within large clones in A. kusnezoffii. More studies are clearly needed to obtain a definite resolution of this issue.
ACKNOWLEDGEMENTS
We thank Spencer Barrett and Lawrence Harder for their useful comments and guidance in the planning of this study and linguistic help with the English of this manuscript, anonymous reviewers for their comments, and Chuan Ni for his assistance in the field. This work was supported by the National Natural Science Foundation of China (30430160).
LITERATURE CITED
- Allen AM, Hiscock SJ. Evolution and phylogeny of self-incompatibility systems in angiosperms. In: Franklin-Tong VE, editor. Self-incompatibility in flowering plants. Berlin: Springer; 2008. pp. 73–101. [Google Scholar]
- Araujo ACG, Falcao R, Carneiro VTD. Seed abortion in the sexual counterpart of Brachiaria brizantha apomicts (Poaceae) Sexual Plant Reproduction. 2007;20:109–121. [Google Scholar]
- Ashman TL, Knight TM, Steets JA, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Barrett SCH. Sexual interference of the floral kind. Heredity. 2002;88:154–159. doi: 10.1038/sj.hdy.6800020. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. Mating strategies in flowering plants: the outcrossing–selfing paradigm and beyond. Philosophical Transactions of the Royal Society B: Biological Sceince. 2003;358:991–1004. doi: 10.1098/rstb.2003.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin RI, Sullivan M. Pollen interference and cryptic self-fertility in Campsis radicans. American Journal of Botany. 1988;75:1140–1147. [Google Scholar]
- Broyles SB, Wyatt R. The consequences of self-pollination in Ascelpias exaltata, a self-incompatible milkweed. American Journal of Botany. 1993;80:41–44. [PubMed] [Google Scholar]
- Burd M. Bateman principle and plant reproduction: the role of pollen limitation in fruit and seed set. Botanical Review. 1994;60:83–139. [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Charpentier A. Consequences of clonal growth for plant mating. Evolutionary Ecology. 2002;15:521–530. [Google Scholar]
- Dafni A. Pollination ecology: a practical approach. Oxford: Oxford University Press; 1992. [Google Scholar]
- De Jong TJ, Waser NM, Price MV, Ring RM. Plant size, geitonogamy and seed set in Ipomopsis aggregata. Oecologia. 1992;89:310–315. doi: 10.1007/BF00317407. [DOI] [PubMed] [Google Scholar]
- De Jong TJ, Waser NM, Klinkhamer PGL. Geitonogamy: the neglected side of selfing. Trends in Ecology and Evolution. 1993;8:321–325. doi: 10.1016/0169-5347(93)90239-L. [DOI] [PubMed] [Google Scholar]
- Eckert CG. Contributions of autogamy and geitonogamy to self-fertilization in a mass-flowering, clonal plant. Ecology. 2000;81:532–542. [Google Scholar]
- Eckert CG, Barrett SCH. Inbreeding depression in partially self-fertilizing Decodon verticillatus (Lythraceae): population genetic and experimental analyses. Evolution. 1994;48:952–964. doi: 10.1111/j.1558-5646.1994.tb05285.x. [DOI] [PubMed] [Google Scholar]
- Eriksson O, Bremer B. Genet dynamics of the clonal plant Rubus saxatilis. Journal of Ecology. 1993;81:533–542. [Google Scholar]
- Finer MS, Morgan MT. Effects of natural rates of geitonogamy on fruit set in Asclepias speciosa (Apocynaceae): evidence favoring the plant's dilemma. American Journal of Botany. 2003;90:1746–1750. doi: 10.3732/ajb.90.12.1746. [DOI] [PubMed] [Google Scholar]
- Free JB. Insect pollination of crops. San Diego, California: Academic Press; 1993. [Google Scholar]
- Galen C, Gregory T, Galloway LF. Costs of self-pollination in a self-incompatible plant, Polemonium viscosum. American Journal of Botany. 1989;76:1675–1680. [Google Scholar]
- Goodwillie C, Knight MC. Inbreeding depression and mixed mating in Leptosiphon jepsonii: a comparison of three populations. Annals of Botany. 2006;98:351–360. doi: 10.1093/aob/mcl105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D. Why do pollinators visit proportionally fewer flowers in large patches? Oikos. 2000;91:485–492. [Google Scholar]
- Guillaume P, Jacquemart AL. Early-inbreeding depression in Vaccinium myrtillus and V. vitis-idaea. Protoplasma. 1999;208:107–114. [Google Scholar]
- Haig D, Westoby M. On limits to seed production. American Naturalist. 1988;131:757–759. [Google Scholar]
- Handel SN. The intrusion of clonal growth patterns on plant breeding systems. American Naturalist. 1985;125:367–384. [Google Scholar]
- Harder LD, Barrett SCH. Mating cost of large floral displays in hermaphrodite plants. Nature. 1995;373:512–515. [Google Scholar]
- Husband BC, Schemske DW. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution. 1996;50:54–70. doi: 10.1111/j.1558-5646.1996.tb04472.x. [DOI] [PubMed] [Google Scholar]
- Ishii HS, Sakai S. Effects of display size and position on individual floral longevity in racemes of Narthecium asiaticum (Liliaceae) Functional Ecology. 2001;15:396–405. [Google Scholar]
- Jarne P, Charlesworth D. The evolution of the selfing rate in functionally hermaphroditic plants and animals. Annual Review of Ecology and Systematics. 1993;24:441–466. [Google Scholar]
- Kawagoe T, Suzuki N. Self-pollen on a stigma interferes with outcrossed seed production in a self-incompatible monoecious plant, Akebia quinata (Lardizabalaceae) Functional Ecology. 2005;19:49–54. [Google Scholar]
- Krebs SL, Hancock JF. Early-acting inbreeding depression and reproductive success in the highbush blueberry, Vaccinium corymbosum. Theoretical and Applied Genetics. 1990;79:825–832. doi: 10.1007/BF00224252. [DOI] [PubMed] [Google Scholar]
- Liao WJ, Song QF, Zhang DY. Pollen and resource limitation in Veratrum nigrum L. (Liliaceae), an andromonoecious herb. Journal of Integrative Plant Biology. 2006;48:1401–1408. [Google Scholar]
- Lloyd DG. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences. 1992;153:370–380. [Google Scholar]
- Lloyd DG, Wells MS. Reproductive biology of a primitive angiosperm, Pseudowintera colorata (Winteraceae) and the evolution of pollination systems in the Anthophyta. Plant Systematics and Evolution. 1992;181:77–95. [Google Scholar]
- Magyar G, Kun A, Oborny B, Stuefer JF. Importance of plasticity and decision-making strategies for plant resource acquisition in spatio-temporally variable environments. New Phytologist. 2007;174:182–193. doi: 10.1111/j.1469-8137.2007.01969.x. [DOI] [PubMed] [Google Scholar]
- Mahy G, Jacquemart AL. Early inbreeding depression and pollen competition in Calluna vulgaris (L.) Hull. Annals of Botany. 1999;83:697–704. [Google Scholar]
- Ritland K. Inferences about inbreeding depression based on changes of the inbreeding coefficient. Evolution. 1990;44:1230–1241. doi: 10.1111/j.1558-5646.1990.tb05227.x. [DOI] [PubMed] [Google Scholar]
- Ritland K. Extensions of models for the estimation of mating systems using n independent loci. Heredity. 2002;88:221–228. doi: 10.1038/sj.hdy.6800029. [DOI] [PubMed] [Google Scholar]
- Routley MB, Kron P, Husband BC. The consequences of clone size for paternal and maternal success in domestic apple (Malus × domestica) American Journal of Botany. 2004;91:1326–1332. doi: 10.3732/ajb.91.9.1326. [DOI] [PubMed] [Google Scholar]
- Seavey SR, Bawa KS. Late-acting self-incompatibility in angiosperms. Botanical Review. 1986;52:195–219. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. San Francisco: W.H. Freeman; 1981. [Google Scholar]
- Soltis DE, Haufler CH, Darrow DC, Gastony GJ. Starch gel electrophoresis of ferns: a compilation of grinding buffers, gel and electrode buffers, and staining schedules. American Fern Journal. 1983;73:9–27. [Google Scholar]
- Tarasjev A. Impact of genet size and flowering stage on fruit set in Iris pumila L. clones in wild. Acta Oecologica. 2005;27:93–98. [Google Scholar]
- Utelli AB, Roy BA. Pollinator abundance and behavior on Aconitum lycoctonum (Ranunculaceae): an analysis of the quantity and quality components of pollination. Oikos. 2000;89:461–470. [Google Scholar]
- Wang Y, Wang QF, Guo YH, Barrett SCH. Reproductive consequences of interactions between clonal growth and sexual reproduction in Nymphoides peltata: a distylous aquatic plant. New Phytologist. 2005;165:329–336. doi: 10.1111/j.1469-8137.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- Wang ZR. Plant allozyme analysis. Beijing: Academic Press; 1996. [Google Scholar]
- Waser NM, Price MV. Reproductive costs of self-pollination in Ipomopsis aggregata (Polemoniaceae) American Journal of Botany. 1991;78:1036–1043. [Google Scholar]
- Wendel JF, Weeden NF. Visualization and interpretation of plant isozymes. In: Soltis DE, Soltis PS, editors. Isozyme in plant biology. Oregon: Dioscorides Press; 1989. pp. 5–45. [Google Scholar]
- Wilcock CC, Jennings SB. Partner limitation and restoration of sexual reproduction in the clonal dwarf shrub Linnaea borealis L. (Caprifoliaceae) Protoplasma. 1999;208:76–86. [Google Scholar]
- Wolf AT, Harrison SP, Hamrick JL. Influence of habitat patchiness on genetic diversity and spatial structure of a serpentine endemic plant. Conservation Biology. 2000;14:454–463. [Google Scholar]
- Zhang TF, Duan YW, Liu JQ. Pollination ecology of Aconitum gymnandrum (Ranunculaceae) at two sites with different altitudes. Acta Phytotaxonomica Sinica. 2006;44:362–370. [Google Scholar]