Abstract
Background and Aims
The slippery waxy zone in the upper part of pitchers has long been considered the key trapping structure of the Nepenthes carnivorous plants; however, the presence of wax is reported to be variable within and between species of this species-rich genus. This study raises the question of the adaptive significance of the waxy zone and investigates the basis for an ontogenetic cause of its variability and correlation with pitcher shape.
Methods
In Brunei (Borneo) the expression of the waxy zone throughout plant ontogeny was studied in two taxa of the Nepenthes rafflesiana complex, typica and elongata, which differ in pitcher shape and size. We also tested the adaptive significance of this zone by comparing the trapping efficiency and the number of prey captured of wax-bearing and wax-lacking plants.
Key Results
In elongata, the waxy zone is always well expanded and the elongated pitchers change little in form during plant development. Wax efficiently traps experimental ants but the number of captured prey in pitchers is low. In contrast, in typica, the waxy zone is reduced in successively produced pitchers until it is lost at the end of the plant's juvenile stage. The form of pitchers thus changes continuously throughout plant ontogeny, from elongated to ovoid. In typica, the number of captured prey is greater, but the role of wax in trapping is minor compared with that of the digestive liquid, and waxy plants do not show a higher insect retention and prey abundance as compared with non-waxy plants.
Conclusions
The waxy zone is not always a key trapping structure in Nepenthes and can be lost when supplanted by more efficient features. This study points out how pitcher structure is submitted to selection, and that evolutionary changes in developmental mechanisms could play a role in the morphological diversity of Nepenthes.
Key words: Carnivorous plant, developmental evolution, digestive liquid, epicuticular wax, insect trapping, heteroblasty, heterochrony, leaf form, morphological diversity, Nepenthes rafflesiana, ontogenetic change, pitcher plant
INTRODUCTION
Like all carnivorous plants, the Nepenthes pitcher plants have evolved specific adaptations in response to nutrient stress, which characterizes the habitats in which they grow (Juniper et al., 1989; Ellison et al., 2003). They obtain an important part of their nutrients from arthropods they attract, capture and digest in their pitcher-shaped leaves. The genus comprises almost one hundred species, often endemics with a narrow distribution, and is distributed from New Guinea west to Madagascar and from India south to Australia, with two hotspots of diversity in Borneo and Sumatra (Clarke, 1997, 2001). The genus displays a diversity of growth forms, habitats and pitcher shapes that has long fascinated naturalists as well as scientists (Darwin, 1875; Danser, 1928; Clarke, 1997; Cheek and Jebb, 2001). What are the respective roles of the developmental processes and the environmental pressures in the genesis of pitcher diversity? Is pitcher diversity correlated to environmental variation? This could make the Nepenthes genus a spectacular example of adaptive radiation.
According to the classical literature on these carnivorous plants, the pitcher of Nepenthes is composed of three functional parts, the peristome (toothed and nectariferous rim), the slippery/waxy zone and the digestive zone. These parts were held to function, respectively, in attraction, capture/retention and digestion of arthropods (Hooker, 1859; Lloyd, 1942; Juniper et al., 1989; Owen and Lennon, 1999). Until recently, most studies which were aimed at elucidating the trapping mechanism of Nepenthes pitcher plants, focused on the capture and retentive function of the waxy zone (Lloyd, 1942; Juniper et al., 1989; Gaume et al., 2002; Riedel et al., 2003; Gorb et al., 2005). This waxy zone is anatomically different from the digestive zone, which forms the basal part of the pitcher, first because instead of being glandular like the latter, it is composed of modified stomata (Lloyd, 1942; Owen and Lennon, 1999; Gaume et al., 2002). Each of these stomata has a hypertrophied guard cell or ‘lunate cell’, whose convex structure, which does not permit any interlocking with an insect's claw, has been experimentally shown to be part of the trap (Gaume et al. 2002). The waxy zone is also slippery because of its wax coating, which interacts with insect pads. The wax was shown to be primarily responsible for insects falling into the digestive liquid and has been considered the key trapping device of Nepenthes pitcher plants (Juniper and Burras, 1962). It reduces adhesion of insects first because of its rough surface structure that diminishes their points of contact on the pitcher wall and secondly because of the non-cohesive structure of the wax crystals, which detach from the plant and contaminate insects' tarsal pads (Juniper and Buras, 1962; Gaume et al., 2004; Gorb et al., 2005). However, three considerations prompt us to ask whether the role of the waxy zone in the trapping function of Nepenthes pitcher plants has been overestimated. First, several species show interindividual variations for the presence of a waxy zone (Lloyd, 1942), and several other species, such as N. ampullaria and N. bicalcarata (Moran et al., 2003; Bohn and Federle, 2004), are characterized by the complete absence of a waxy zone. Secondly, functional studies investigating the role of plant wax have often been carried out on horticultural hybrids, in which this layer is particularly well expressed (e.g. Riedel et al., 2007). Thirdly, recent studies conducted on the peristome (Bohn and Federle, 2004), the digestive surface (Gorb et al., 2004) and the digestive liquid (Gaume and Forterre, 2007) have shown the role of other pitcher features in the trapping system of some species. For example, in N. bicalcarata and N. rafflesiana, the peristome has been shown to be a highly wettable surface implicated in insect aquaplaning when wetted by rain or nectar secretion (Bohn and Federle, 2004; Bauer et al., 2008), while in N. rafflesiana the highly viscoelastic properties of the digestive liquid were shown to cause retention of insects within the pitcher (Gaume and Forterre, 2007; Di Giusto et al., 2008).
These latter points raise the questions as to whether this waxy zone, generally supposed to be a ‘key trapping device’ in Nepenthes pitcher plants, is always adaptive and whether natural selection could have favoured its disappearance when it no longer enhanced the plant's fitness. The presence of a waxy zone in pitchers is rarely taken into account as a distinctive trait in taxonomic studies (Cheek and Jebb, 2001) but we hypothesized that the study of plasticity in this pitcher developmental sequence is crucial for understanding morphological evolution in Nepenthes.
At the species level, the plant N. rafflesiana Jack is highly polymorphic, with at least 13 types, varying in the shape and size of their pitchers (Phillips and Lamb, 1996; Clarke, 1997; Cheek and Jebb, 2001), four of which are found in Borneo. Among these, two are common in northern Borneo: N. rafflesiana var. typica and N. rafflesiana var. elongata. All their distinctive characteristics are stable in cultivation (Clarke, 1997; L. Gaume, pers. obs.), meaning that these variants are not simply dependent on variations of the environment, but are genetically distinct. The two variants might be taxa in the process of differentiation since, when growing in sympatry, they differ in their timing of flowering (L. Gaume and B. Di Giusto, pers. obs.), creating a partial barrier to gene flow between them. During our field studies in Borneo, we noticed two levels of variation for the presence of a waxy zone in N. rafflesiana. We noticed that the ‘elongated’ pitchers of elongata always bear a waxy zone. In contrast, within populations of typica, some plants bear pitchers with a waxy zone while others do not. These observations mean that intraspecific comparisons are possible, making N. rafflesiana a suitable candidate for investigating the functional role of the waxy zone and the selective advantage it confers.
This study is aimed at characterizing the polymorphism for the waxy zone in two Bornean taxa of the N. rafflesiana complex, testing whether it might have an ontogenetic explanation, whether this zone plays a preponderant role in insect trapping and whether it is an important component of the pitcher shape. The first part of the study compares pitcher size and shape throughout the ontogeny of the plant between typica and elongata. The second part is focused on N. rafflesiana var. typica, which is polymorphic for the waxy layer, and tests whether the waxy zone varies with plant ontogeny and whether it provides substantial benefits to the plant in comparison with other features such as the digestive liquid. For the latter question, waxy and non-waxy plants are compared for their effect on retention of ants and flies and for abundance of prey in traps. The third part of the study compares insect retention ability and abundance of prey in traps between typica and elongata.
MATERIALS AND METHODS
Studied taxa and sites
Nepenthes rafflesiana Jack is a lowland vine characterized, like most Nepenthes species, by a pitcher dimorphism (Cheek and Jebb, 2001), which is well marked in the typical form (Fig. 1). Lower terrestrial pitchers are rounded at their base; bear ‘wings’ and are borne by self-supporting plants. They are attached to the leaf lamina by a straight tendril, which joins the pitcher ventrally at its base. The second type of pitcher is the ‘upper’ or aerial one. Upper pitchers lack wings and are attached dorsally by a coiled tendril, which allows the plant to climb on the surrounding vegetation. These upper pitchers are infundibular (funnel-shaped) and more slender at the base than lower pitchers. Such a swift transition in leaf form characterizes heteroblastic species (Goebel, 1900) such as many vines where it is often associated with the passage from a juvenile stage to a mature, flower-producing stage (Lee and Richards 1991), but not always (Diggle 1999). In N. rafflesiana, the transition from juvenile features to adult features of the vegetative apparatus is tightly correlated with the onset of reproductive maturity (pers. obs.) and it also characterizes the transition from a self-supporting form to a climbing growth form (Clarke, 1997; Cheek and Jebb, 2001).
Fig. 1.
The two main types of N. rafflesiana, typica and elongata in the upper and lower parts of the figure, respectively. (A) Lower pitcher and (B) upper pitcher of typica, the longitudinal section of which is shown in (C). (D) Lower pitcher and (E) upper pitcher of elongata, the longitudinal section of which is shown in (F). Note the presence of a waxy layer in elongata (D, E, long arrow), whose basal limit can be assessed by the presence of a hip (short arrow) on the outer part of the pitcher, and note its absence in typica (C). (F) shows how the lengths of the pitcher (1) and waxy zone (2) were measured.
Nepenthes rafflesiana var. typica G.Beck (but note the taxonomy is controversial), the most common of the Bornean types of N. rafflesiana, grows in northern Borneo, northern Sumatra, Singapore and the Peninsular Malaysia (Clarke, 1997). A combination of visual and olfactory cues accounts for high prey attraction in this type (Moran, 1996; Di Giusto et al., 2008), while the wettable peristome (Bauer et al., 2008) and the viscoelastic digestive liquid (Gaume and Forterre, 2007) have been shown to play an important role in initial fall and retention of insects, respectively. It is abundant in heath, often degraded, forests called ‘kerangas’ usually formed on white acidic sands. The study on N. rafflesiana var. typica was carried out at a site located in a zone of ‘kerangas’ in Brunei (site 1: 4°38′N, 114°30′E) during the dry season (June–July 2003). Typical vegetation of such open ‘kerangas’ includes shrubs from the genera Melastoma and Syzygium, and Gleichenia ferns.
Nepenthes rafflesiana var. elongata Hort. has taller and more slender pitchers (Fig. 1B) and is sparsely distributed throughout the heath and peat swamp forests of Borneo (Clark, 1997) in more closed habitats. This plant does not possess any fragrance detectable by the human nose and attracts significantly less prey than the typical form (Moran, 1996). The experiments that focused on elongata were carried out during the dry season (July 2004) at a second site (site 2: 4°34′N, 114°25′E) in the Badas forest reserve (in a border zone of heath forest and peat swamp forest) where the two taxa were found in sympatry. The comparison of prey contents was done for the two plant taxa during this period at this site. Measurements of the length of pitchers of elongata were conducted at a third site (site 3: 4°35′N, 114°30′E) in wet and semi-closed areas of heath forest in July 2006. The elevation of all these sites was <50 m.
Measurements of pitcher morphology
We used the first mature and functional pitcher located beneath the apex of each studied plant. The pitchers had completed their entire development, their lid was open and they had already captured some insects. Fifty-one lower and 50 upper pitchers of N. rafflesiana var. typica were thus selected at site 1 in July 2003 from 101 plants displaying an extended range of different developmental stages. The plant's height was used as a raw estimate of the development stage or ontogeny of the plant. Twenty-nine lower and 29 upper pitchers of N. rafflesiana var. elongata were selected at site 3 from 58 plants of a similar extended range of developmental stages. The waxy surface was easily detected by the presence of a lighter glossy appearing surface in the inner upper part of the pitcher. If the glossy appearance was weak, the presence of a ‘hip’ or rib (Fig. 1E, small arrows) on the outer part of the pitcher, marking the separation between the basal digestive zone and the upper conductive waxy zone, was used. For each plant, the height of the plant and the lengths of the pitcher and of its waxy zone were measured, as shown in Fig. 1F. For the two types, we took a set of photographs of the studied pitchers and drew some pitchers from these photographs to permit the parallel assessment of the change of both pitcher form and waxy zone over the course of plant ontogeny. The main objectives were to compare the length of pitchers during plant development between the two types as well as the relative length of the waxy zone, expressed as the length of the waxy zone divided by the length of the pitcher. To investigate the relationship between pitcher size and plant size and compare it between the two types, two analyses of covariance (ANCOVAs) were performed, one for plants with lower pitchers and the other one for plants with upper pitchers, using pitcher length as the dependent variable, plant height (log-transformed) as a continuous covariate and identity (typica vs. elongata) as a factor. To investigate the change of the relative length of the waxy zone with plant size and compare it for the two types, two further ANCOVAs were performed, for lower and upper pitchers, respectively.
Retention experiments
The first experiment sought to test whether the presence of the waxy zone confers a benefit to typica in terms of insect retention. As both the peristome and the digestive liquid can play a role in insect trapping, we removed the peristomes of experimental pitchers and tested the effect of wax in both the presence and absence of digestive liquid. In June 2006, we compared the ability of lower pitchers (whose peristome had been removed) with and without a waxy zone to retain ants [Oecophylla smaragdina (approx. 7 mm long) captured in the field] and flies [Drosophila melanogaster (approx. 3·5 mm long) reared in the laboratory]. Ten pitchers with wax and ten pitchers without wax were selected, each from different plants. One fly was drawn into a soft tube and blown onto the pitcher's digestive liquid without direct manipulation. Observations of fly behaviour, including whether the fly escaped or was trapped, were made for 5 min. A second trial was then conducted on the same pitcher. For each of the ten pitchers (plants), the number of escapes could be zero, one or two. The same experiment and analysis were performed with ants. The pitchers were then emptied of their digestive liquids and rinsed with water. The experiment was conducted again with both ants and flies dropped into the empty pitchers. This second experiment was performed to separate the retentive effect of the pitcher liquid from that of the pitcher wall. As the total number of trials per pitcher was constant (i.e. two), we used a Poisson regression model to test the effect of wax (presence or absence), the effect of liquid (presence or absence) and the effect of insect identity (ant O. smaragdina or fly D. melanogaster) on the number of insect escapes.
Two sets of experiments were designed to compare the retentive ability of typica and elongata in 5 min observation sessions. Seven lower and seven upper pitchers of typica were selected on different plants at site 1. The selected lower pitchers were pitchers of diameters comparable with the diameters of upper pitchers, with a waxy zone not exceeding 20 % of the total pitcher length. None of the upper pitchers bore any waxy zone. Two ant species, which are common prey of the two N. rafflesiana types, were selected: Anoplolepis gracilipes (approx. 5 mm long) and Polyrhachis sp. (approx. 1 cm long). The experiment consisted of dropping one ant worker into the pitcher liquid of each type of pitcher and recording its behaviour and escape success, or whether it sank in the pitcher liquid. The experiment was repeated for each ant species so that there were two trials with A. gracilipes and two trials with Polyrhachis sp. per pitcher. A similar experiment but with only one trial per pitcher was conducted on 16 lower pitchers and 18 upper pitchers of different plants of elongata at site 2 with Polyrhachis sp. and 15 lower and 15 upper pitchers of different plants of the same type with A. gracilipes. As the total number of trials per pitcher varied according to the plant type (four for typica and two for elongata), we used a logistic regression to test the effect of plant type (typica or elongata), type of pitcher (lower or upper) and ant species (Polyrhachis sp. or A. gracilipes) on the frequency of ant escapes by pitchers (plants).
Analysis of abundance of prey in pitchers
We collected and preserved (in 75 % ethanol) at site 1 the contents of six waxy lower pitchers and six non-waxy lower pitchers of N. rafflesiana var. typica, whose height had been previously measured. The total number of items of prey was counted. Using an ANCOVA, we tested whether there was an effect of pitcher size and wax presence on the number of items of prey.
We collected at site 2 the contents of ten upper pitchers from ten randomly selected N. rafflesiana var. typica plants and the contents of 14 upper pitchers from 14 randomly selected plants of the waxy elongata. The total number of arthropods was counted and their body length was measured.
Statistical analyses
Statistical analyses were carried out using the software package SAS v.9 (SAS Institute Inc., Cary, NC, USA). For model selection, backward procedures were adopted, starting with the removal of the non-significant highest order interactions. The ANCOVAs were carried out using the GLM procedure. The normal distribution of the residuals was checked using the Shapiro test. The Poisson and logistic regressions were carried out using the GENMOD procedure, with a Poisson and a binomial error distribution, respectively. Correction for overdispersion was applied when necessary using the square root of the ratio of Pearson's χ2 to the associated number of degrees of freedom.
RESULTS
Pitcher differentiation during ontogeny in the two types
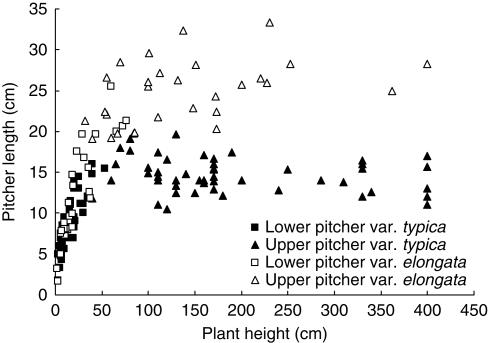
The two types of N. rafflesiana differed in the extent to which pitcher length changed during plant ontogeny. As shown in Fig. 2, the size of pitchers underwent a logarithmic increase with plant ontogeny (significant and positive slope in the ANCOVA performed on log-transformed data, Table 1). The increase was much more pronounced for lower pitchers than for upper ones (comparison of the slopes in Table 1). This increase, though similar for the two types of N. rafflesiana in the beginning (common intercept), was more rapid for elongata than for typica at later stages (significant interaction, two different slopes, Table 1). Pitcher length of typica reached an early plateau and upper pitchers no longer increased in size with plant ontogeny (slope not significantly different from zero, Table 1). In contrast, upper pitchers of elongata continued to undergo a logarithmic increase and their size reached a plateau later in plant ontogeny (Fig. 2, weak slope but significantly different from zero, Table 1). Thus, upper pitchers of N. rafflesiana var. elongata (mean length = 24·8 cm, s.d. = 3·9, n = 50) were on average 1·7 times longer than upper pitchers of var. typica (mean length = 14·6 cm, s.d. = 2·1, n = 29).
Fig. 2.
Pitcher length throughout plant development in N. rafflesiana: comparison between typica and elongata.
Table 1.
Analyses of covariance testing for the effect of (A) plant height (log-transformed) and (B) identity (typica vs. elongata) on pitcher length for lower pitchers (R2 = 0·81, residuals normally distributed: Shapiro statistic W = 0·99, P = 0·78) and upper pitchers (R2 = 0·79, residuals normally distributed: W = 0·98, P = 0·40)
| (A) Plant height | ||||||||
|---|---|---|---|---|---|---|---|---|
| Length of lower pitcher |
Length of upper pitcher |
|||||||
| Covariate | d.f. | SS | F | P | d.f. | SS | F | P |
| Log(plant height) | 1 | 1234 | 273·4 | <0·0001 | 1 | 34·91 | 4·94 | 0·0292 |
| Type | 1 | 10·4 | 2·3 | 0·1333 | 1 | 15 | 2·12 | 0·1491 |
| Log(plant height) × type | 1 | 29·8 | 6·6 | 0·0121 | 1 | 87·13 | 12·34 | 0·0008 |
| (B) identity (typica vs. elongata) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Length of lower pitcher |
Length of upper pitcher |
|||||||
| Parameter | Estimate | s.e. | T | P | Estimate | s.e. | T | P |
| Common intercept | −3·24 | 0·86 | −3·8 | 0·0003 | 15·54 | 2·59 | 5·61 | <0·0001 |
| Slope for N. rafflesiana var. typica | 4·74 | 0·33 | 14·31 | <0·0001 | 0·01 | 0·51 | 0·02 | 0·9834 |
| Slope for N. rafflesiana var. elongata | 5·42 | 0·31 | 17·69 | <0·0001 | 2·16 | 0·54 | 3·97 | <0·0001 |
Ontogenetic loss of wax in typica and associated changes in pitcher form
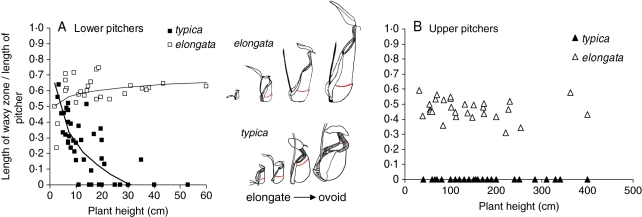
The waxy zone in the elongata form, which is de visu wider, is also longer in relative and absolute length than in the typica form, and this difference explains in part why elongata pitchers are longer. Indeed, the waxy zone occupied, on average, 7 cm and 60 % (minimum–maximum: 25–75 %) of the pitcher length for lower pitchers and 12 cm and 47 % (30–60 %) for upper pitchers. However for the typica form, the waxy zone occupied only a mean of 1·5 cm and 20 % (0–65 %) of the pitcher length for lower pitchers and was systematically absent from the upper pitchers. For lower pitchers, the relative length of the waxy zone was found to be significantly greater in the elongata type than in the typica type (ANCOVA, significant effect of type, Table 2, Fig. 3A) and was shown to vary as a logarithmic function of plant height (ANCOVA, significant effect of log[plant height]) but with either an increasing or a decreasing trend, depending on the plant type (ANCOVA, significant interaction). While the waxy zone tended to increase with plant size for elongata, it was shown to decrease with plant size for typica (Table 2, Fig. 3A). This reduction means that for typica, there is a decrease in the size of the waxy zone during plant ontogeny. For upper pitchers of elongata, the relative length of the waxy zone tended to be constant during plant ontogeny (regression: no significant effect of plant height: F1,27 = 1·88, P = 0·2, Fig. 3B).
Table 2.
Analysis of covariance testing for the effect of (A) plant height (log-transformed) and (B) identity (typica vs. elongata) on relative length of the waxy zone for lower pitchers (R2 = 0·89, residuals normally distributed: Shapiro statistic W = 0·97, P = 0·1)
| (A) Plant height | ||||
|---|---|---|---|---|
| Relative length of the waxy zone |
||||
| Covariate | d.f. | SS | F | P |
| Log(plant height) | 1 | 76·2 | 46·3 | <0·0001 |
| Variety | 1 | 82·9 | 50·3 | <0·0001 |
| Log(plant height) × type | 1 | 281·8 | 171·0 | <0·0001 |
| (B) identity (typica vs. elongata) | ||||
|---|---|---|---|---|
| Relative length of the waxy zone |
||||
| Parameter | Estimate | s.e. | T | P |
| Intercept elongata | 0·47 | 0·06 | 7·64 | <0·0001 |
| Intercept typica | 0·80 | 0·06 | 12·46 | <0·0001 |
| Slope elongata | 0·04 | 0·02 | 2·08 | 0·04 |
| Slope typica | −0·23 | 0·02 | −9·67 | <0·0001 |
Fig. 3.
Relative length of the waxy zone compared between N. rafflesiana var. typica and elongata throughout plant ontogeny for (A) lower pitchers borne by self-supporting plants and (B) upper pitchers borne by climbing plants. Note an ontogenetic loss of the waxy zone (delimited by the red line) in typica and a correlated change in pitcher shape (elongate in young rosette plants to more ovoid in ager but still self-supporting plants).
The presence of a fully developed waxy zone is associated with an elongate form of pitchers such as in lower and upper pitchers of elongata (Fig. 1D–F). The ontogenetic loss of the waxy zone in N. rafflesiana var. typica is correlated with a change in pitcher shape, which tends to be less elongated, and more ovoid for lower pitchers as plant development proceeds (Fig. 3A). Indeed, the small lower pitchers of young rosette plants in the typical form are rounded at the base and elongated in their distal part (corresponding to the waxy zone) with a rib or ‘hip’ separating the two parts (like all the lower pitchers of elongata as shown in Fig. 1D). Lower pitchers, produced by older self-supporting plants, are only rounded, with the hip only visible at the top of the back face of the pitcher just beneath the peristome (Fig. 1A). Upper pitchers, characterizing climbing stages of the plant, are comparatively shorter. They never bear a waxy zone and are infundibular or funnel-shaped as if they even lack the upper part of the digestive zone (Fig. 1B, C). The ontogenetic switch from ‘lower’ to ‘upper’ pitchers is relatively abrupt since transitional pitchers are rarely observed.
Absence of an effect of wax on insect retention and prey abundance in typica
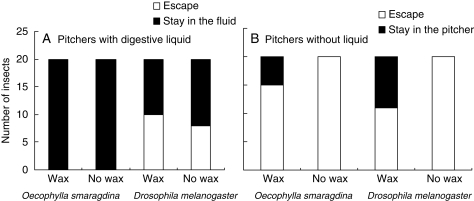
The lower pitchers of N. rafflesiana var. typica are efficient in retaining insects (trapping 100 % of ants and >50 % of flies) but this efficiency cannot be ascribed to an effect of wax. Indeed, the insect bioassays showed no statistically significant effect of pitcher wax on insect retention. The effect of the presence of wax (χ2 = 1·72, P = 0·19, Table 3, Fig. 4) was negligible compared with the effect of the presence of the digestive liquid (χ2 = 46·18, P < 0·0001). The trend of waxy pitchers to retain insects more efficiently compared with non-waxy pitchers was only detectable when the liquid was removed from pitchers (Fig. 4B). Moreover, retention was greatly dependent on insect type, but with contrasting effects according to the presence or absence of the liquid (highly significant interaction insect × liquid, Table 3): retention was more efficient for ants than for flies when the liquid was present (Fig. 4A), but ants were slightly better at escaping than flies when the liquid was absent from pitchers (Fig. 4B).
Table 3.
Poisson regression model testing for the effect of wax (presence vs. absence), digestive liquid (presence vs. absence) and insect identity (ant O. smaragdina vs. fly D. melanogaster) on the retention of insects by lower pitchers of N. rafflesiana var. typica
| Dependent variable: number of insect escapes | |||
|---|---|---|---|
| Covariate | d.f. | χ2 | P |
| Wax | 1 | 1·72 | 0·19 |
| Liquid | 1 | 46·18 | <0·0001 |
| Insect | 1 | 0·24 | 0·6224 |
| Liquid × insect | 1 | 22·85 | <0·0001 |
Fig. 4.
Insect retention in N. rafflesiana var. typica compared for pitcher types (with and without wax) and insect types (ants, Oecophylla, and flies, Drosophila) for lower pitchers (A) with the digestive liquid and (B) emptied.
The observation of insect behaviour partly explains the mechanisms of prey retention. In pitchers with the liquid present, ants could clearly not move their bodies out of the liquid because their legs were retained by viscoelastic filaments. As for the flies, they experienced the same difficulty but sometimes their wings were not completely wetted and they could use them to extract their body and fly away after having groomed themselves for several minutes. In emptied pitchers, the main obstacle to escape comes from the slippery waxy layer, which often makes insects fall several times. The time needed to escape was longer in waxy pitchers than in non-waxy pitchers (significant for flies, Twax = 117·5 ± 149·2 s, Tno wax = 3·6 ± 3·5 s, t-test for unequal variances: t = −2·41, P = 0·04; not significant for ants: Twax = 75·3 ± 91·3 s, Tno wax = 27 ± 38·1 s, t-test for unequal variances: t = –1·54, P = 0·14).
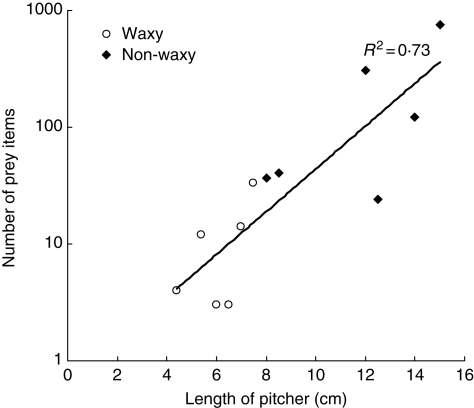
In terms of prey diversity, the lower pitchers of N. rafflesiana var. typica were found to trap mostly ants (= 73·5 ± 33·5 % of the prey, n = 12 pitchers). Other terrestrial arthropods (Orthoptera, Arachnida, Myriapoda and Crustacea) represented a mean of 9 ± 19 % of the total items of prey, while flying insects (Diptera, Lepidoptera, Dictyoptera and Hemiptera) represented a mean of 12·8 ± 27·2 % of the prey. The analyses of prey abundance showed no effect of wax presence on the number of insect items of prey. The total number of items of prey increased as an exponential function of pitcher length (R2 = 0·73, F1,10 = 26·9, P = 0·0004, Fig. 5). There was no effect of wax in the ANCOVA performed on ln[number of items of prey] (same intercept, effect of wax, F2,9 = 0·52, P = 0·49; same slope, wax × pitcher F3,8 = 0·18, P = 0·68 for the waxy and the non-waxy pitchers). This difference means that the presence of wax does not affect the rate of prey capture with increasing pitcher size.
Fig. 5.
Number of items of prey as an exponential function of the length of lower pitchers in N. rafflesiana var. typica. There was no significant difference in the rate of prey capture between waxy pitchers and non-waxy ones, and hence a common regression line was determined.
Better prey retention in the waxy elongata
The insect bioassay carried out on ant species compared retention between types of N. rafflesiana (typica vs. elongata), between pitcher types (lower vs. upper) and between ant species (the large ant Polyrhachis sp. vs. the smaller one A. gracilipes). Retention greatly depended on ant species since 100 % of A. gracilipes were retained within N. rafflesiana pitchers (for both types) compared with only 53 % of Polyrhachis ants (Table 4). Interestingly, 73·5 % of the Polyrhachis ants were retained within pitchers of elongata compared with only 28·5 % in the case of typica. According to the logistic regression performed on the data subset corresponding to the Polyrhachis ants, there was an effect of the Nepenthes type on ant retention (χ2 = 12·9, P = 0·0003) but no significant effect of either pitcher type (χ2 = 1·01, P = 0·31) or interaction between Nepenthes type and pitcher type (χ2 = 0·05, P = 0·83). Although the trend was not significant, for each type, the success of escape was slightly higher in the lower pitchers than in the upper ones (Table 4).
Table 4.
Results of the retention experiment carried out on the two types of N. rafflesiana for the ants Polyrhachis sp. and Anoplolepis gracilipes
|
Polyrhachis sp. |
Anoplolepis gracilipes |
|||
|---|---|---|---|---|
| Escape | Stay in the fluid | Escape | Stay in the fluid | |
| typica | ||||
| Lower pitcher | 11 | 3 | 0 | 15 |
| Upper pitcher | 9 | 5 | 0 | 15 |
| elongata | ||||
| Lower pitcher | 5 | 11 | 0 | 15 |
| Upper pitcher | 4 | 14 | 0 | 15 |
Behavioural observations showed that the small ants, A. gracilipes and O. smaragdina were totally unable to free their bodies from the digestive liquid of typica, as they were retained by viscoelastic filaments. The Polyrhachis ants, which are larger, could apparently generate more muscular force than A. gracilipes to haul themselves up onto the pitcher wall. Once out of the liquid, ants more easily walk on the pitcher wall in typica than in elongata. Indeed, in elongata, ants repeatedly slipped along the waxy walls and succeeded in escaping from the pitchers only after a mean of 12 attempts (minimun = 1, maximum = 30). Moreover, in this latter variety, the ants seemed to be more often trapped by the liquid of upper pitchers than by that of lower pitchers. The ants were observed, on five occasions, to be drawn down abruptly and retained deep within the base of the pitcher.
Higher abundance of prey in pitchers of typica
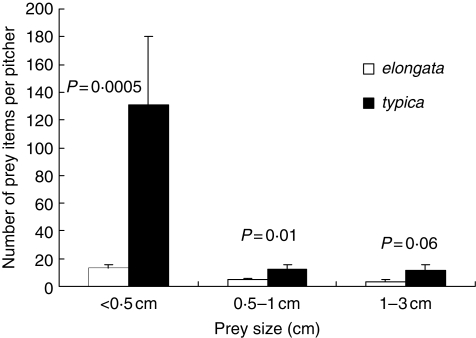
Analysis of prey abundance in the site where the two taxa were found in sympatry showed that the non-waxy upper pitchers of typica trapped on average at least seven times more arthropods than the waxy upper pitchers of elongata (typica, 154 ± 165·4, n = 10; elongata, 21·7 ± 16·5, n = 14; Poisson regression model with correction for overdispersion, effect of variety on total number of items of prey by pitcher, F1,22 = 17·20, P = 0·0004). Both taxa trapped a majority of ants (elongata, 60·4 ± 26·6 %; typica, 74·9 ± 23·1 %). The ranges of prey size for the two taxa were comparable (minimum–maximum prey length for elongata, 0·3–24 mm; typica, 0·2–27 mm), and if elongata seems to trap a greater proportion of larger prey than typica, this is not because it traps a greater number of large items of prey but rather because typica traps a greater number of small items of prey (Fig. 6). Indeed, the trend of typica pitchers to trap more items of prey than elongata pitchers was verified for each category of prey size although it was not significant for the larger categories (Fig. 6; prey <5 mm, F1,22 = 16·41, P = 0·005; 5 mm–1 cm, F1,22 = 6·79, P = 0·01; 1–3 cm, F1,22 = 3·77, P = 0·06). These results suggest that N. rafflesiana var. typica traps more arthropods than N. rafflesiana var. elongata not only in terms of number but also in terms of biomass.
Fig. 6.
Abundance of prey for different categories of prey size compared for upper pitchers of N. rafflesiana var. typica and N. rafflesiana var. elongata at the site where they occur in sympatry.
DISCUSSION
The development of a waxy zone in the pitchers of N. rafflesiana is shown to be variable between and within types, to be greatly dependent on plant ontogeny in typica and to be associated with changes in pitcher form. The elongata type possesses long pitchers with a fully developed waxy zone over the whole course of its ontogeny, i.e. during both the self-supporting and climbing stages. In contrast, for typica, the lower pitchers of self-supporting plants tend to be shorter and more rounded with a progressively smaller waxy zone, and the upper pitchers, characterizing the climbing form of the plant, never bear a waxy zone. In typica, the waxy zone does not seem to confer any significant benefit in terms of trapping efficiency and prey abundance compared with the liquid itself. Comparison of the two taxa showed that the waxy elongata was more effective than its non-waxy sister type in retaining ants within pitchers, but that paradoxically it trapped far less prey. Our results raise the question of the adaptive significance of the waxy zone in Nepenthes and its evolutionary loss in some taxa that have evolved other effective means of insect trapping.
Changes in the development of the waxy zone: a cause of pitcher shape diversity?
Most Nepenthes species have elongated waxy pitchers like those of N. rafflesiana var. elongata, and this has prompted researchers to think that wax plays a major role in insect capture (Juniper and Burras, 1962; Juniper et al., 1989). Quite early on, however, some authors (Lloyd, 1942) noticed that the waxy zone could be variable between individuals of a given species and between different species. Other authors noticed that the relative importance of the digestive and waxy zones may vary according to local populations (Kurata et al., 2004). However, no further work has been carried out to understand such variations. In this study, we show that the polymorphism concerning the waxy zone can be readily explained in the light of plant ontogeny, at least in the case of N. rafflesiana var. typica. Indeed, in this type, when the plant increases in size (and age), it produces pitchers with a reduced waxy zone, and plants taller than 30–40 cm produce pitchers with no waxy zone. This change corresponds to the change in form of pitchers, with waxy pitchers of seedlings having an elongate form like all the pitchers of the waxy elongata, and non-waxy pitchers of larger self-supporting plants having a more rounded form. Our results shows that the change in pitcher form in Nepenthes cannot be reduced to the classically reported leaf dimorphism, which characterizes phase change in numerous vines, but may also occur during the first developmental stage of the plants that have not yet reached sexual maturity, being gradual over the juvenile phase of plant ontogeny.
Morphological changes of pitchers during early developmental stages of plants have already been noticed within several Nepenthes species (Clarke, 2001). We provide an ontogenetic explanation of these leaf-form changes that could apply for other Nepenthes species, which is the progressive deletion of the waxy zone. Why has such a slippery surface, which is usually considered to be a crucial trapping device, been lost during ontogeny in N. rafflesiana var. typica?
Weak fitness effect of the waxy zone in typica and possible causes of its developmental loss
Arthropods are known to provide many essential nutrients, especially nitrogen, required by carnivorous plants (Schultze et al., 1997; Ellison, 2006; Osunkoya et al., 2007), which live in poor soils. Arthropods, ants in particular, were estimated to provide 70 % of the nitrogen used by N. rafflesiana var. typica (Moran and Moran, 1998; Moran et al., 2001). Therefore the abundance of prey captured should be correlated with the fitness of the plant. In N. rafflesiana var. typica, there was no effect of wax presence on insect retention, nor was there any detectable effect of the wax on the number of items of prey trapped. As the peristome was also shown to be the primary, if not the unique, site of initial fall for insects (Bauer et al., 2008), the presence of wax in typica thus probably has a low impact on the plant's fitness. It is therefore not surprising to observe a developmental loss of the waxy zone in this type. The wax layer in Nepenthes, and more precisely the most epicuticular part of the wax layer, is composed mainly of aliphatic compounds dominated by very long chain aldehydes, such as triacontanal or dotriacontanal, which includes 30 or 32 atoms of carbon, respectively (Riedel et al. 2003, 2007). Elaboration of the waxy zone may thus be costly to the plant, and if it does not provide any substantial benefit, it might not be maintained by natural selection. Why does the waxy zone in N. rafflesiana var. typica provide no trapping advantage to the plant? We suggest that the net benefits provided by the waxy zone may have become negligible in comparison with those provided by other more efficient traits of the pitcher.
First, our insect bioassays revealed the importance of the digestive liquid in insect retention and hence confirmed the laboratory results of Gaume and Forterre (2007) on its crucial viscoelastic properties. Moreover, liquid in upper pitchers was shown to be significantly more viscous than that in lower ones (Di Giusto et al., 2008). Therefore, there seem to be contrasting ontogenetic gradients in the viscoelastic property of the liquid and in the slippery property of the pitcher wall. It is possible that in N. rafflesiana var. typica, the evolution of a viscoelastic liquid has replaced the trapping function of the waxy zone and led to its loss. However, the liquid of the waxy elongata also appeared to show retentive properties and, despite the retentive effect of its wax, this type traps less prey than its sister taxon. Hence, the liquid alone cannot have overtaken the trapping function of the plant and explain the high trapping efficiency and ecological success of typica.
Nepenthes rafflesiana var. typica is ecologically successful. In Borneo at least, it is the most abundant type of N. rafflesiana (Clarke, 2001), and its pitchers have been shown to trap far more arthropods than pitchers of elongata. Furthermore, the prey spectrum of typica is among the richest of any Nepenthes (Di Giusto et al., 2008). According to Moran (1996) and Di Giusto et al. (2008), typica displays a sweet fragrance attractive to insects. In contrast, in the field, no odour was detected by smell for either of the two pitcher types in the waxy elongata, and the plant was shown to attract fewer arthropods than the typica form (Moran, 1996). In N. rafflesiana var. typica, the evolution of both greater attractiveness (sweet fragrance) and a more efficient trapping system (viscoelastic liquid) might have favoured the loss of the wax, which had become functionally redundant. Alternatively, greater attractiveness might be a direct consequence of the loss of wax. Indeed, wax, especially in terms of its crystalline structure, hampers the emission of volatile compounds (Jetter, 2006). It is therefore possible that waxy phenotypes are not compatible with those with attractive odours and that only non-waxy mutants would have been able to display insect-attracting fragrance and were thereby favoured by selection.
Therefore, the slippery and waxy pitchers of elongata have a retentive function which is either not sufficient to compensate for its weak power of attraction or, because of the crystalline lipid structure of their wax, prevents efficient olfactory attraction. There is no clear correlation between wax phenotype and plant fitness, and the adaptive significance of the waxy layer could even be questionable for elongata.
To understand the evolutionary pressures that have led to the phenotypic differences between these two taxa, it would first be important to compare their environment in terms of nutrient and prey availability. For example, we dot not know whether nitrogen is more limiting in the open sites typical of the habitat of typica, with an associated decrease of photosynthesis output and carbon gain. However, it would be interesting to investigate the costs and benefits of having waxy phenotypes in the two types of environments characterizing the two types. Preliminary comparative analyses of the prey captured by typica in the open site (site 1) compared with the prey captured in a closer site (site 2), more typical of the habitat of the elongata type, have been performed. They revealed that typica catches more ants and fewer flying insects, such as generalist pollinators, in site 2 where it is found with the elongata type (L. Gaume, unpub. res.). Maybe the colonization of an open habitat with a greater availability of flying insects has favoured, in N. rafflesiana, the evolution of sweet pollinator-attracting odours, which were in this site less costly and more beneficial than wax. The production of wax would also have become more costly in such a habitat because of a lower availability of ants, the main source of nitrogen in these pitcher plants. Alternatively, as waxy plants were shown to be more effective in trapping large ants than non-waxy plants, we can also hypothesize that there are more large ants in deeper forests, which would constitute significant selective pressure for the evolution of well-expressed waxy phenotypes such as in N. rafflesiana var. elongata. However, we showed that elongata did not capture a significantly greater number of larger items of prey than typica. A more detailed analysis of prey identity and prey availability in the sites of each plant type needs to be performed in the future.
Conclusions and evolutionary perspectives
In contrast to what was expected a priori, the slippery waxy zone is shown not to always be a key trapping structure in Nepenthes. It might consequently, by the evolutionary process, have been lost in some species other than N. rafflesiana. Indeed, the most parsimonious assumption is that the waxy trait, which is shared by most of the Nepenthes species, is an ancestral character, at least because the eight most basal species in the phylogeny of Nepenthes, which are not in the same clade (Meimberg and Heubl, 2006), present waxy phenotypes.
Heterochrony, which refers to changes in the rate and timing of growth and development events or patterns, is believed to play a key role in the evolution of morphological diversity (e.g. Gould, 1977; Li and Johnston, 2000). We may have observed evidence of a heterochronic process in the evolutionary diversification of N. rafflesiana. The non-waxy and shortened pitcher shapes in both lower and upper pitchers of N. rafflesiana var. typica would result from such processes of heterochrony. Indeed, if the ancestral character of the waxy trait is confirmed, N. rafflesiana var. elongata would then be more similar to the common ancestor than N. rafflesiana var. typica. For example, let us focus on the juvenile phase of the plant and thereby on the terrestrial pitchers. The ontogenetic loss of the waxy zone in N. rafflesiana var. typica and appearance of a new pitcher shape (ovoid rather than elongate form) produced in the later stages of this developmental phase would represent evidence of a heterochronic process comparable with what was called a peramorphosis (Li and Johnston, 2000). According to this process, only young plants of the derived forms (such as N. rafflesiana var. typica) express the waxy and elongate phenotypes, which are typical of the ancestral forms. Similar events might have occurred several times in the evolution of Nepenthes and heterochrony might provide an explanation for part of the puzzling diversity of Nepenthes pitcher sizes and shapes. Tests of such hypotheses need a careful comparison of plant ontogenies and will become possible when a fully resolved molecular phylogeny of Nepenthes is available.
ACKNOWLEDGEMENTS
We thank L. Lim, D. Marshall, D. Lane and D. Edwards for their administrative help in the Universiti Brunei Darussalam. We are grateful to the Forestry Department of Brunei, which supplied permits to carry out this research in the field. We also greatly acknowledge the help of S. Nyawa from the Brunei Museum and CITES commission and of M. Idris from the National Herbarium of Brunei. P. Heuret, D. McKey and P. Diggle are thanked for their helpful comments on the manuscript. We also acknowledge V. Bonhomme for having quickly provided us with a photograph from his field study.
LITERATURE CITED
- Bauer U, Bohn HF, Federle W. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. Proceedings of the Royal Society B: Biological Science. 2008;275:259–265. doi: 10.1098/rspb.2007.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proceedings of the National Academy of Sciences, USA. 2004;101:14138–14143. doi: 10.1073/pnas.0405885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek M, Jebb M Nepenthaceae. Leiden, The Netherlands: Publication Department of the National Herbarium Nederland; 2001. Flora Malesiana. [Google Scholar]
- Clarke C. Nepenthes of Borneo. Kota Kinabalu: Natural History Publications (Borneo) Sdn. Bhd; 1997. [Google Scholar]
- Clarke C. Nepenthes of Sumatra and Peninsular Malaysia. Kota Kinabalu: Natural History Publications (Borneo) Sdn. Bhd; 2001. [Google Scholar]
- Danser BH. Contributions à l'étude de la flore des Indes néerlandaises. 1928;XV Bulletin du Jardin de Botanique Buitenzorg. [Google Scholar]
- Darwin C. Insectivorous plants. London, UK: John Murray; 1875. [Google Scholar]
- Di Giusto B, Grosbois V, Fargeas E, Marshall D, Gaume L. Contribution of pitcher fragrance and fluid viscosity to high prey diversity in a Nepenthes carnivorous plant from Borneo. Journal of Biosciences. 2008;33:121–136. doi: 10.1007/s12038-008-0028-5. [DOI] [PubMed] [Google Scholar]
- Diggle PK. Heteroblasty and the evolution of flowering phenologies. International Journal of Plant Sciences. 1999;160:S123–S134. doi: 10.1086/314217. [DOI] [PubMed] [Google Scholar]
- Ellison AM. Nutrient limitation and stoichiometry of carnivorous plants. Plant Biology. 2006;8:740–747. doi: 10.1055/s-2006-923956. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ, Brewer JS, et al. The evolutionary ecology of carnivorous plants. Advances in Ecological Research. 2003;33:1–74. [Google Scholar]
- Gaume L, Forterre Y. A viscoelastic deadly trap in Nepenthes pitcher plants. Plos-one. 2007;2(11):e1185. doi: 10.1371/journal.pone.0001185. doi:10·1371/journal.pone. 0001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L, Gorb S, Rowe N. Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytologist. 2002;156:479–489. doi: 10.1046/j.1469-8137.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- Gaume L, Perret P, Gorb E, Gorb S, Labat JJ, Rowe N. How do plant waxes cause flies to slide? Experimental tests of wax-based trapping mechanisms in three pitfall carnivorous plants. Arthropod Structure and Development. 2004;33:103–111. doi: 10.1016/j.asd.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Goebel K. Organography of plants. Part I. General organography. Oxford, UK: Clarendon Press; 1900. [Google Scholar]
- Gorb E, Kastner V, Peressadko A, et al. Structure and properties of the glandular surface in the digestive zone of the pitcher in the carnivorous pitcher plant Nepenthes ventrata and its role in insect trapping and retention. Journal of Experimental Biology. 2004;207:2947–2963. doi: 10.1242/jeb.01128. [DOI] [PubMed] [Google Scholar]
- Gorb E, Haas K, Henrich A, Enders S, Barbakadze N, Gorb S. Composite structure of the crystalline epicuticular wax layer of the slippery zone in the pitchers of the carnivorous plant Nepenthes alata and its effect on insect attachment. Journal of Experimental Biology. 2005;208:4651–4662. doi: 10.1242/jeb.01939. [DOI] [PubMed] [Google Scholar]
- Gould SJ. Ontogeny and phylogeny. Cambridge, MA: Belknap Press of Harvard University Press; 1977. [Google Scholar]
- Hooker JD. On the origin and the development of the pitcher of Nepenthes, with an account of some new Bornean plants of that genus. Transactions of the Linnean Society. 1859;22:415–424. [Google Scholar]
- Jetter R. Examination of the processes involved in the emission of scent volatiles from flowers. In: Dudareva N, Pichersky E, editors. Biology of floral scent. Boca Raton, FL: CRC Press; 2006. pp. 125–144. [Google Scholar]
- Juniper BE, Burras J. How pitcher plants trap insects. New Scientist. 1962;13:75–77. [Google Scholar]
- Juniper BE, Robins RJ, Joel D. The carnivorous plants. London, UK: Academic Press; 1989. [Google Scholar]
- Kurata K, Jaffre T, Setoguchi H. Variation of pitcher morphology within Nepenthes vieillardii Hook. f. (Nepenthaceae) in New Caledonia. Acta Phytotaxonomica et Geobotanica. 2004;55:181–197. [Google Scholar]
- Li P, Johnston M. Heterochrony in plant evolutionary studies through the twentieth century. Botanical Review. 2000;66:57–88. [Google Scholar]
- Lee DW, Richards JH. Heteroblastic development in vines. In: Putz EF, Mooney HA, editors. The biology of vines. Cambridge, UK: Cambridge University Press; 1991. pp. 205–243. [Google Scholar]
- Lloyd FE. The carnivorous plants. Waltham, MA: Chronica Botanica Co; 1942. [Google Scholar]
- Meimberg H, Heubl G. Introduction of a nuclear marker for phylogenetic analysis of Nepenthaceae. Plant Biology. 2006;8:831–840. doi: 10.1055/s-2006-924676. [DOI] [PubMed] [Google Scholar]
- Moran JA. Pitcher dimorphism, prey composition and the mechanism of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. Journal of Ecology. 1996;84:515–525. [Google Scholar]
- Moran JA, Moran AJ. Foliar reflectance and vector analysis reveal nutrient stress in prey-deprived pitcher plants (Nepenthes rafflesiana) International Journal of Plant Sciences. 1998;159:996–1001. [Google Scholar]
- Moran JA, Merbach MA, Livingston NJ, Clarke CM, Booth WE. Termite prey specialization in the pitcher plant Nepenthes albomarginata – evidence from stable isotope analysis. Annals of Botany. 2001;88:307–311. [Google Scholar]
- Moran JA, Clarke CM, Hawkins BJ. From carnivore to detritivore? Isotopic evidence for leaf litter utilization by the tropical pitcher plant Nepenthes ampullaria. International Journal of Plant Science. 2003;164:635–639. [Google Scholar]
- Osunkoya OO, Daud SD, Di Giusto B, Wimmer FL, Holige TM. Construction costs and physico-chemical properties of the assimilatory organs of Nepenthes species in northern Borneo. Annals of Botany. 2007;99:895–906. doi: 10.1093/aob/mcm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen TP, Lennon KA. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae) American Journal of Botany. 1999;86:1382–1390. [PubMed] [Google Scholar]
- Phillipps A, Lamb A. Pitcher plants of Borneo. Kota Kinabalu: Natural History Publications (Borneo) Sdn. Bhd; 1996. [Google Scholar]
- Riedel M, Eichner A, Jetter R. Slippery surfaces of carnivorous plants: composition of epicuticular wax crystals in Nepenthes alata Blanco pitchers. Planta. 2003;218:87–97. doi: 10.1007/s00425-003-1075-7. [DOI] [PubMed] [Google Scholar]
- Riedel M, Eichner A, Meimberg H, Jetter R. Chemical composition of epicuticular wax crystals on the slippery zone of pitchers of five Nepenthes species and hybrids. Planta. 2007;225:1517–1534. doi: 10.1007/s00425-006-0437-3. [DOI] [PubMed] [Google Scholar]
- Schultze W, Schultze ED, Pate JS, Gillison AN. The nitrogen supply from soils and insects during growth of the pitcher plants Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. Oecologia. 1997;112:464–471. doi: 10.1007/s004420050333. [DOI] [PubMed] [Google Scholar]