Abstract
Background and Aims
The polygalacturonase (PG) gene family has been found to be enriched in pollen of several species; however, little is currently known about the function of the PG gene in pollen development. To investigate the exact role that the PG gene has played in pollen development and about this family in general, one putative PG gene, Brassica campestris Male Fertility 9 (BcMF9), was isolated from Chinese cabbage (Brassica campestris ssp. chinensis, syn. B. rapa ssp. chinensis) and characterized.
Methods
RT-PCR, northern blotting and in situ hybridization were used to analyse the expression pattern of BcMF9, and antisense RNA technology was applied to study the function of this gene.
Key Results
BcMF9 is expressed in particular in the tapetum and microspore during the late stages of pollen development. Antisense RNA transgenic plants that displayed decreased expression of BcMF9 showed pollen morphological defects that resulted in reduced pollen germination efficiency. Transmission electron microscopy revealed that the homogeneous pectic exintine layer of pollen facing the exterior was over-developed and predominantly occupied the intine, reversing the normal proportional distribution of the internal endintine layer and the external exintine in transgenic pollen. Inhibition of BcMF9 also resulted in break-up of the previously formed tectum and baculae from the beginning of the binucleate stage, as a result of premature degradation of tapetum.
Conclusions
Several lines of evidence, including patterns of BcMF9 expression and phenotypic defects, suggest a sporophytic role in exine patterning, and a gametophytic mode of action of BcMF9 in intine formation. BcMF9 might act as a co-ordinator in the late stages of tapetum degeneration, and subsequently in the regulation of wall material secretion and, in turn, exine formation. BcMF9 might also play a role in intine formation, possibly via regulation of the dynamic metabolism of pectin.
Key words: Brassica campestris, Chinese cabbage, exine, intine, PG, pollen wall, polygalacturonase, BcMF9
INTRODUCTION
Studies of the Arabidopsis pollen transcriptome and pollen gene expression in other organisms have concluded that the reduced transcriptome of pollen accumulated a restricted and unique set of genes, including the extensive representation of gene families and genes related to specific pathways (Becker et al., 2003; Lee and Lee, 2003; Honys and Twell, 2004; Pina et al., 2005). For example, several members of the ‘classical’ arabinogalactan gene family were detected exclusively in pollen, and have been indicated to play roles in pollen tube nutrition, pollen tube guidance and other processes (Cheung and Wu, 1999; Pereira et al., 2006). Similarly, members of another large family, the polygalacturonase (PG) gene family, were also found to be expressed in pollen and/or anthers of several species, including maize, tobacco (Nicotiana tabacum), cotton, Brassica napus and Arabidopsis (Allen and Lonsdale, 1993; Robert et al., 1993; John and Petersen, 1994; Tebbutt et al., 1994; Hadfield et al., 1998; Honys and Twell, 2003).
PG is a hydrolase and loosening enzyme involved in the degradation of pectin and disintegration of the cell wall. During anther and pollen development, many tissue types, including pollen mother cells, microspores, tapetum and septum, undergo cell-wall modification (Neelam and Sexton, 1995; Owen and Makaroff, 1995). Thus, PG may play multiple roles in pollen and/or anther development. One of the pollen-associated PG genes that has been well described is QRT3 in Arabidopsis. QRT3 is specifically and transiently expressed in the tapetum during the phase when microspores separate from their meiotic siblings. Mutation of QRT3 results in failed pollen mother cell-wall degradation and subsequent tetrad separation. It is presumed that QRT3 protein plays a direct and specific role in the degradation of the pectic polysaccharides of the pollen mother cell wall (Rhee et al., 2003). Their study excluded, there is little available evidence to support the importance and elaborate upon the actual roles of PGs in pollen development. Most of current research on pollen and/or anther PG or PG genes has focused on gene cloning and expression pattern analysis. Other than QRT3, most of the PG genes were found to be expressed during the late stages of pollen development. These genes were considered to be necessary for pollen maturation and pollen tube growth (Allen and Lonsdale, 1993; Robert et al., 1993; John and Petersen, 1994; Tebbutt et al., 1994; Honys and Twell, 2003), but little direct evidence has been provided.
By performing genome-wide transcriptional profiling on the flower buds of the male sterile mutant bcms (Brassica campestris male sterile) and wild-type Chinese cabbage (Brassica campestris ssp. chinensis, syn. B. rapa ssp. chinensis) using cDNA–amplified fragment length polymorphism (cDNA-AFLP) technology, three transcript-derived fragments (TDFs) which specifically accumulated in the wild-type flower buds showed high similarities to sequences of PG genes (Wang et al., 2005; Huang et al., 2008). The corresponding genes of two of the three TDFs, BcMF2 (Brassica campestris Male Fertility 2) and BcMF6, have been cloned and their function analysed by antisense RNA technology. BcMF2 was shown to be associated with intine development (Huang et al., 2009), and BcMF6 might play a role in pollen maturation and pollen tube growth (Zhang et al., 2008). To expand our knowledge of the PG gene family in pollen and/or anther, the full-length sequence of the third TDF, BcMF9, was cloned and its function in pollen and anther development was investigated. BcMF9 has its own nucleotide and amino-acid sequence separate from BcMF2 and BcMF6. It is expressed in particular in the tapetum and microspore during the late stages of pollen development, and inhibition of its expression resulted in abnormal exine and intine development, which may suggest an important role for BcMF9 in pollen wall formation. The results presented here provide additional experimental evidence for the roles and pattern of action of PG genes in pollen and/or anther.
MATERIALS AND METHODS
Plant material
The bcms mutant of Chinese cabbage (B. campestris ssp. chinensis Makino, syn. B. rapa ssp. chinensis) was found in the field in the 1980s. It was maintained by crossing it with its wild-type ancestor. The plants were then cultivated in the experimental farm of Zhejiang University.
DNA, RNA extraction and cDNA synthesis
DNA was extracted from leaves according to the procedures described by Cao et al. (1995). Total RNA was extracted from different tissues using Trizol reagent (Gibco, Berlin, Germany), while poly-(A)+RNA was isolated from total RNA with an Oligotex mRNA Mini Kit (Qiagen, Hilden, Germany). Single-strand cDNA and double-strand cDNA were then synthesized using a SMART cDNA Library Construction Kit (Clontech, Mountain View, CA, USA) according to the manufacturer's instructions.
Isolation and sequencing of fragments
The band of interest from the cDNA-AFLP was cut with a surgical blade and incubated overnight at 37 °C in 100 µL of TE (10 mm Tris, pH 7·5, and 1 mm EDTA, pH 8·0). The AFLP fragment was recovered though PCR under the same conditions used in preamplification. The reamplified cDNA was subcloned using the pGEM-Teasy vector system (Promega, Madison, WI, USA) and sequenced using an ABI Prism 3730 sequencer by Invitrogen Biotech Co., Ltd (Shanghai, China). The full-length cDNA was amplified by rapid amplification of cDNA ends PCR (RACE-PCR), and the genomic DNA sequence was amplified using the gene-specific primer set designed based the full-length cDNA.
RT-PCR analysis and in situ hybridization
Two rounds of reverse transcriptase–PCR (RT-PCR) were conducted with two independently isolated total RNA samples as templates to investigate the expression pattern of BcMF9. RT-PCR was performed for 18, 23, 28, 33 and 38 cycles to determine the linearity of the PCR; 28 cycles was ultimately determined to be optimal. A 350-bp Actin-1 fragment was amplified under the same RT-PCR conditions using the primer pair 5′-TCTCTATGCCAGTG GTCGTA-3′ and 5′-CCTCAGGACAACGGAATC-3′ as a positive control. The gene specificity of the RT-PCR products was then confirmed by sequencing.
Flower buds at different developmental stages were used for in situ hybridization. Samples were fixed in 4 % formaldehyde PBS solution with 0·1 % Triton-X-100 and 0·1 % Tween-20, serially dehydrated, cleared with dimethylbenzene and embedded in paraffin. Sections (8 µm) of flower buds were hybridized to specific digoxigenin-labelled RNA probes (Roche, Branchburg, NJ, USA). Templates for the BcMF9-specific probes were obtained from amplification with specific primers. The sense and antisense probes were synthesized using an SP6/T7 transcription kit (Roche).
Antisense RNA construction and plant transformation
The oligonucleotide primers 5′-ACAGGATCCGAGCTTTTACCGTTTTTA-3′ (forward, with a BamHI restriction endonuclease site) and 5′-GCCTCTAGATGGCATCTATTG AAGATA-3′ (reverse, with an XbaI restriction endonuclease site) were used to amplify the 462-bp partial cDNA sequence of BcMF9. This sequence, which had maximal divergence between the three PG genes isolated from Chinese cabbage, was then cloned in the antisense orientation into the binary vector pBI121 with constitutive promoter CaMV35S. Restriction digestion, PCR and DNA sequencing confirmed the correct orientation of the fragment. The positive antisense BcMF9 RNA construct was then transferred into the fertile Chinese cabbage mediated by Agrobacterium tumefaciens as described by Yu et al. (2004).
Southern hybridization and northern blotting
Southern hybridization was performed according to Sambrook et al. (1989) to confirm the integration of the antisense RNA fragment of BcMF9 into the transgenic plant genome. The DNA probes were prepared by labelling the NPTII gene with α-32P using a Random Primer DNA Labeling Kit ver. 2 (Takara Bio Inc., Dalian, China) according to the manufacturer's instructions.
Northern blotting of total RNA from flower buds or flowers was performed to detect expression of BcMF9 in the transgenic or control plants. Total RNA (20 µg) was separated by electrophoresis on a 1·2 % formaldehyde agarose gel, followed by blotting onto a nylon membrane (Hybond-N+, Amersham Pharmacia Uppsala, Sweden), and probed via α-32P-labelling as described above. Hybridization was performed as previously described (Cho and Kende, 1997). The Southern and northern hybridized membranes were scanned via a Typhoon 9210 scanner (Amersham).
Microscopy and pollen germination observation
For in vitro pollen germination, pollen grains were collected and cultured in culture medium (15 % sucrose, w/v; 0·4 mmol L−1 HBO3; 0·4 mmol L−1 Ca(NO3)2; 0·1 % agar, w/v). Pollen grains spread on the agar plates were cultured immediately at 20–25 °C and 100 % relative humidity for 4 h. Germinating pollen grains were counted under a microscope. At least three independent plants from each selected antisense BcMF9 transgenic line and the wild-type control line were examined and five flowers from each plant were tested. From each culture, at least 300 pollen grains were examined to calculate an average germination rate.
For scanning electron microscopy (SEM), individual pollen grains from either control or antisense BcMF9 transgenic plants were mounted on scanning electron microscopy (Philips XL-40) stubs and coated with palladium–gold using standard techniques and vacuum desiccation. Digital images were then taken.
For transmission electron microscopy (TEM), anthers were fixed with 2·5 % glutaraldehyde (containing 0·01 % Tween-20) overnight, rinsed in 0·1 m phosphate buffer and then transferred to 1 % osmic acid for 1 h. Specimens were washed again in phosphate buffer as above, dehydrated through a graded ethanol series (to 80 %). Samples were then embedded in Spurr's resin, and ultrathin sections were stained with uranyl acetate and lead citrate before being viewed in a JEM-1230 electron microscope operating at 80 kV.
RESULTS
BcMF9 possesses features of PG in its amino-acid sequence
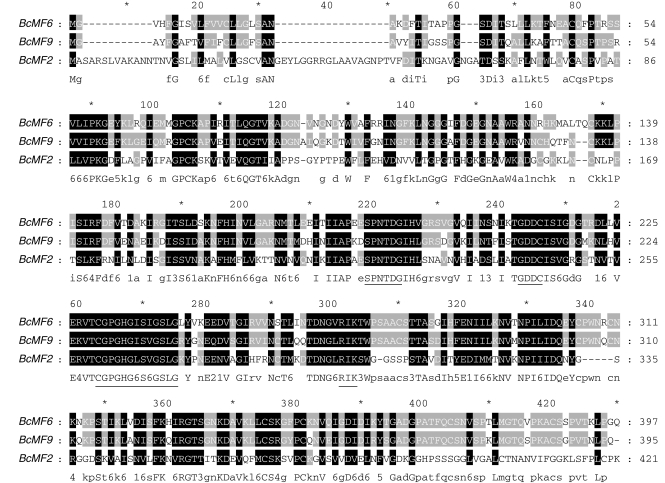
The corresponding full-length cDNA of BcMF9 was obtained by RACE-PCR and the DNA sequence was then amplified with the gene-specific primers designed according to the full-length cDNA sequence. The sequence was 1594 bp in length and included two introns and three exons (GenBank accession no. EF101564). The sequence for BcMF9 was different from those of BcMF2 and BcMF6, the latter both having three introns and four exons in their sequences (Wang et al., 2005; Zhang et al., 2007). The cDNA of BcMF9 has a 1188-bp-long open reading frame encoding a 395-amino-acid protein with a calculated molecular mass of 42·4 kDa and a predicted pI of 7·97. A hydrophobic signal sequence is predicted at the N-terminus between amino-acid residue Met1 and Ala22. A homology search using the Sequence Retrieval System against the Swiss-Prot/TrEMBL database revealed that BcMF9 showed 86 % identity and 94 % similarity to the putative pollen-expressed PGA3 from Arabidopsis on the amino-acid level. Scanning of the deduced BcMF9 protein against protein profile databases found one typical PG active position and four highly conserved domains of the plant PGs (Torki et al., 2000; Athanasiou et al., 2003), which was similar to BcMF2 and BcMF6 (Fig. 1). However, no high overall sequence identity was identified among the three proteins. BcMF9 showed only 35 and 72·9 % identity to BcMF2 and BcMF6 respectively, and that between BcMF2 and BcMF6 was even lower (33 %). These results suggest that BcMF9 is a typical PG gene but different from BcMF2 and BcMF6 already characterized in Chinese cabbage.
Fig. 1.
The deduced amino-acid sequences of BcMF9 and comparison with those of BcMF2 and BcMF6 in Chinese cabbage. Underlined sequences indicate the polygalacturonase active domain conserved in plant PGs.
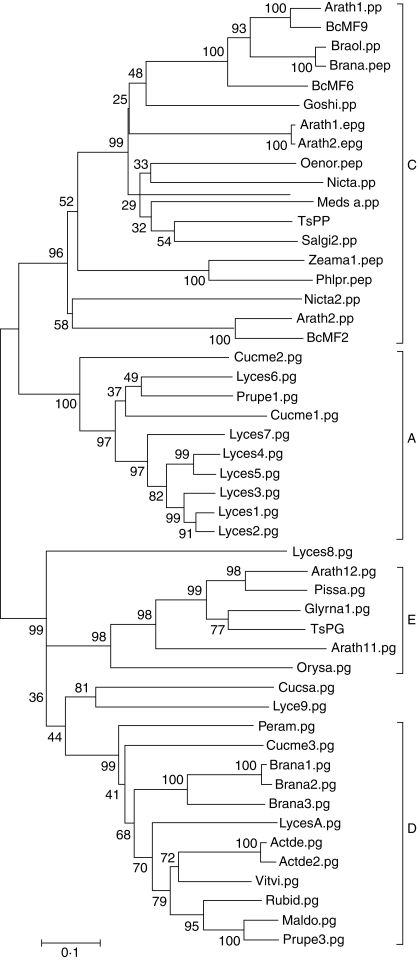
The deduced amino-acid sequence of BcMF9 was compared with other published plant PGs (Supplementary Data Table S1, available online), and a phylogenetic tree based on these sequences was reconstructed. The tree, which was similar to ones previously reconstructed for plant PGs (Torki et al., 2000; Athanasiou et al., 2003), has four main clades (Fig. 2). BcMF9, together with BcMF2 and BcMF6, belonged to a separate clade C, on which the corresponding genes of PGs were all expected to be expressed in pollen (Torki et al., 2000), such as the Arabidopsis pollen-specific exo-PG gene Arath1.pp, Arabidopsis flower-expressed Arath1.epg and Arath2.epg, Brassica napus pollen-specific Brana. pep (Sta 44-4), and tobacco sperm cell-expressed Nicta. pp and Nicta2. pp. Classification of BcMF9 to clade C suggests a pollen-expressed pattern for this gene.
Fig. 2.
Maximum-parsimony molecular phylogenetic trees based on the amino-acid sequence of plant PGs. Numbers at nodes represent bootstrap confidence values based on 1000 replicates.
BcMF9 is expressed in developing tapetal cells and microspore
The expression pattern of BcMF9 in different tissues was tested by RT-PCR and northern blotting. Flower buds of five different sizes, open flowers, siliques, scape and leaves of wild-type Chinese cabbage were selected. The developmental stage of the different-sized flower buds was determined by cytological observation (Huang et al., 2008). Flower buds with a diameter equivalent to or less than 1·0 mm (stage I flower buds) corresponded to the onset of microspore mother cell formation or the period leading up it. Flower buds with a diameter of about 1·6 mm (stage II) represented the meiosis period. Flower buds with a diameter of about 2·2 mm (stage III) corresponded to the tetrad period. Flower buds with a diameter of 2·8 mm (stage IV flower buds) represented the uninucleate pollen period. Finally, flower buds with the largest diameter were opening flower buds (stage V). At this point, opened anthers and a pollen-spreading appearance could be observed.
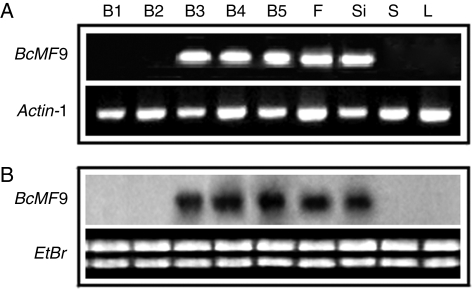
RT-PCR results were consistent with those of northern blotting analysis. Expression of BcMF9 was first detected in the flower buds at stage III, which corresponds to the tetrad period; a high level of expression was maintained throughout stage IV and stage V flower buds, which contain uninucleate pollen and mature pollen, respectively. This signal was also detected in open flowers and siliques. Remarkably, transcript of BcMF9 was not detected in scapes or leaves or stage II or stage I flower buds prior to meiosis (Fig. 3).
Fig. 3.
Spatial and temporal expression pattern of BcMF9 in Chinese cabbage based on RT-PCR and northern blotting analysis. (A) RT-PCR analysis of the expression pattern of BcMF9; (B) Northern blotting analysis of the expression pattern of BcMF9. B1 − B5, flower buds of wild-type plants at stage I − V respectively; F, Si, S and L indicate flowers, siliques, scape and leaves, respectively. Transcripts for the ubiquitously expressed Actin-1 gene were used as controls.
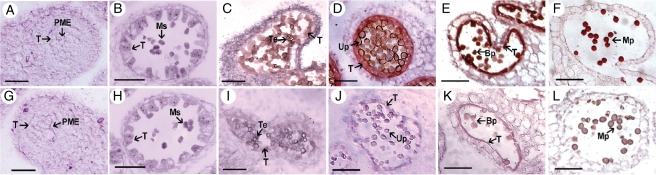
To determine further the cellular location of BcMF9 mRNA during pollen and anther development, in situ hybridization was performed on transverse sections of flower buds at different developmental stages. Particular hybridization signals were first detected in the tapetal cells and microspore tetrads of the anther (Fig. 4C). The signals became stronger gradually from the uninucleate pollen stage to pollen maturity until the tapetum disappeared completely (Fig. 4D–F). No expression signal was observed in the anthers prior to meiosis (Fig. 4A, B), as well as in the sense controls (Fig. 4G–L).
Fig. 4.
Analysis of the expression pattern of BcMF9 in Chinese cabbage using in situ hybridization. (A − F) Sections of anthers at the pollen mother cell, meiosis, tetrads, uninucleate, binucleate and mature pollen stage, respectively, hybridized with a BcMF9 antisense probe. (G − L) Section of anthers at the pollen mother cell, meiosis, tetrads, uninucleate, binucleate and mature pollen stage, respectively, hybridized with a BcMF9 sense probe as a negative control. Abbreviations: Bp, binucleate pollen; Mp, mature pollen; Ms, microspore; PME, pollen mother cell; Te, tetrad; T, tapetum; Up, uninucleate pollen. Scale bars = 20 µm.
Antisense transformed plants with inhibited BcMF9 expression had lower rates of fruit set by self-pollination
To investigate the function of BcMF9 in the development of pollen or anther, antisense RNA technology was used specifically to inhibit BcMF9 expression in Chinese cabbage with the constitutive promoter CaMV35S. Although the activity of the CaMV35S promoter in pollen and anther was controversial in some species such as tobacco and Arabidopsis (Wilkinson et al., 1997), it could be expressed normally in Brassica pollen (Robert and Hong, 1999; Cao et al., 2006; Zhang et al., 2008; Huang et al., 2009). To ensure exact gene silencing, the antisense RNA expression vectors were constructed with partial cDNA sequences specific to BcMF9 but having the lowest identities to those of BcMF2 and BcMF6 formerly isolated from Chinese cabbage. The agrobacterium-mediated method was used to introduce expression vector to a normal fertile plant. After kanamycin screening and Southern hybridization analysis, 11 transformed lines were obtained (35S-bcmf9).
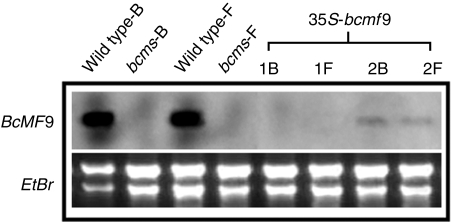
Northern blotting was used to detect the expression of BcMF9 in transgenic plants 35S-bcmf9. The results revealed that BcMF9 expression in flower buds at stage III–V and open flowers of transgenic plants was strongly inhibited. Nine 35S-bcmf9 lines had no expression signals detected both in the flower buds and in open flowers. The other two lines had a very weak hybridization signal detected in the two tissues, which was significantly lower than the expression in normal plants. In contrast, high expression levels of BcMF9 were detected in the flower buds and open flowers of wild-type plants. As expected, no hybridization signal was detected in the flower buds and open flowers of the bcms mutant (Fig. 5). These results verified that the antisense gene effectively inhibited gene expression.
Fig. 5.
Detection of the expression of BcMF9 in antisense gene transgenic Chinese cabbage by northern blotting. Wild type-B, wild-type flower buds; bcms-B, bcms mutant flower buds; Wild type-F, wild-type flowers; bcms-F, bcms mutant flowers; 1B and 1F, flower buds and flowers, respectively, from one transformed 35S-bcmf9 line, which served as a example to show lack of expression of BcMF9; 2B and 2F, flower buds and flowers, respectively, from another transformed 35S-bcmf9 line, which served as a example to show weak BcMF9 expression.
All the transgenic plants showed normal vegetative growth; they flowered normally, and had normal sepal, petal, gynoecium, anther and other floral organs. However, compared with the wild-type plants, fewer pollen grains in the anthers were found in nine transformed lines which lacked BcMF9 expression (as shown by northern blotting). This phenotype, however, was not immediately obvious in the remaining transgenic lines that showed weak BcMF9 expression. Three 35S-bcmf9 lines displaying this apparent phenotype were chosen for further investigation. Through self-pollination during the budding period, these transgenic plants were able to bear fruit pods when pollinated with sufficient pollen grains. However, transgenic self-pollinated fruit pods were approx. 50 % smaller than those of the wild-type plants. The fruit pods of wild-type plants were full and each pod was able to produce about 15 seeds, whereas the fruit pods of transgenic plants were able to produce only 1–5 seeds. A test-cross was therefore performed, pollinating transgenic plants with the normal pollens of a wild-type plant. The resulting fruit pod showed no difference in fruit size or seed number in comparison with self-pollinated fruit pods of non-trangenic plants. These facts suggest that female fertility and ovule development of these transgenic plants were normal, such that the decline in fertility should be attributed to the abnormality in male organs. This phenotype was very similar to that found in the antisense BcMF2 transgenic plant which had exhibited BcMF2 expression (Huang et al., 2009).
Germination in vitro forms burst pollen tubes in antisense BcMF9 transformed plants
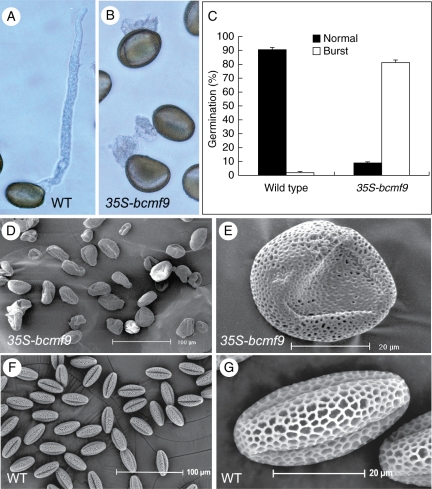
To investigate the cause for the low rate of fruit-set by self-pollination in transgenic plant 35S-bcmf9, an in vitro germination test was performed. It was found that 10 % of the transgenic pollen did not germinate at all after culture for 4 h, and only about 8 % of the transgenic pollen germination was normal, while the remaining 82 % was able to germinate but burst in the tip when the pollen tube had just appeared from the pollen surface. By contrast, the germination rate of the wild-type plant pollen reached 90 % under the same conditions. Burst pollen tubes were also found in the wild-type plants, but at only about 2 % (Fig. 6A–C). For the bulk of the vascular bundles in the large, thick pistils of Chinese cabbage, which can also be stained with aniline blue, no good results were obtained in the in vivo pollen tube growth test. Nonetheless, the much lower fruit-set rate for transgenic plants as compared with wild-type plants following self-fertilization may provide indirect but persuasive evidence for abnormality in the in vivo pollen tube elongation of these transgenic plants. A likely scenario is that the seeds harvested from transgenic plants were pollinated by pollen grains with normal pollen tube growth. By contrast, burst pollen tubes could not reach the ovules.
Fig. 6.
In vitro germination and scanning electron micrographs of pollen from antisense BcMF9 transgenic Chinese cabbage. (A, B) In vitro germination showed the normal pollen tube growth of wild-type plants (WT) and the burst pollen tube of transgenic 35S-bcmf9 plants after culture for 4 h. (C) Comparison of the pollen germination percentage between wild-type and transgenic 35S-bcmf9 plants after culture for 4 h. (D, E) Deformities in pollen grains of 35S-bcmf9. (F, G) Normal pollen grains of wild-type plants (WT).
Deformity of antisense BcMF9 transformed plants in pollen grains with abnormal pollen wall development
In order to explore the induction of abnormal pollen tube growth as a result of the inhibition of BcMF9 expression in the transgenic 35S-bcmf9 plants, aniline blue staining and DAPI staining were used to check the structure of transgenic pollen. However, no abnormality was detected; pollen structure such as callose and karyokinesis was normal (data not shown). Pollen morphology of transgenic plants was also investigated via SEM, which revealed that that nearly 100 % of the 35S-bcmf9 pollen had deformities. Compared with the normal ovular shape of pollen grains, with three evenly distributed germinal furrows (Fig. 6F, G), the pollen grains of transgenic plants were irregular in shape with a disordered number and location of germinal furrows (Fig. 6D, E).
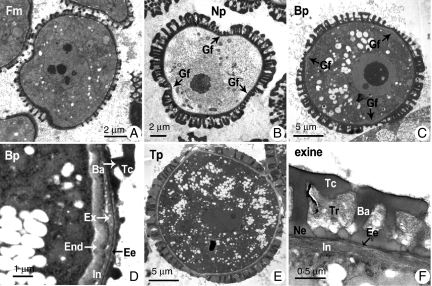
TEM was then used to observe anther development in transgenic plants. Prior to development of binucleate pollen, there was no obvious difference detected between the transgenic pollen (Fig. 8A) and wild-type control pollen (Fig. 7A). Furthermore, the cell nuclei exhibited normal division and differentiation, and the cell organelles formed normally during the entire pollen development process; all of the distinct developmental stages, including uninucleate, binucleate and trinucleate, could be observed (Fig. 8B, C, E). However, pollen grains cut in various directions revealed that shape was somewhat different from that of normal control pollen, and this difference could be detected at the early uninucleate microspore stage. In normal control pollen, at the free microspore stage, the exine began to take shape, exhibiting clear tectum and baculae layers; however, within the regions of the three prospective germinal furrows, exine deposition did not occur (Fig. 7A). At the uninucleate stage, the exine underwent further thickening, with the regions of the three germinal furrows depressed towards the interior, leading to a cloverleaf-like pattern of the microspore (Fig. 7B). The exine of the transgenic pollen showed a normal origin and thickening, the germinal furrows were indicated by a regional lack of exine (Fig. 8A, B), but the cloverleaf pattern was replaced by an irregular shape caused by the disordered distribution of germinal furrows. The most typical situation illustrating the abnormal germinal furrow was in transverse sections cut perpendicular to the germinal furrow (Fig. 8B). In wild-type control pollen, the intine layers began to form at the late uninucleate stage (data not shown), and underwent a subsequent thickening during the binucleate stage (Fig. 7C, D), then completed its formation at the trinucleate stage (Fig. 7E, F). This development process was also displayed in the transgenic pollen. However, the extent of intine thickening, especially within the germinal furrow region, was much greater than that of the normal control pollen (Fig. 8C, D). Moreover, coverage of the germinal furrow region appeared to become wider, resulting in the uneven distribution of the germinal furrows. At the binucleate stage, this disordered distribution became more evident, creating the appearance of only two germination furrows in most of the transgenic pollen grains. The most typical example was that the germinal furrow accounted for almost half of the pollen circumference (Fig. 8C). Furthermore, at the binucleate stage, the intine within the germinal furrow region in the transgenic pollen appeared to be smooth, homogeneous and granular (Fig. 8C, D). By contrast, the intine of the control pollen within the same region was wavy in appearance, exhibiting a visible internal microfibrillar endintine and an external granular exintine (Fig. 7C, D). Outside the germinal furrow region, the demarcation of endintine and exintine layers was not obvious in transgenic and wild-type pollen. However, the intine was more microfibrillar in the control pollen (Fig. 7F) but more granular in the transgenic pollen (Fig. 8F). In other words, the homonymous granular exintine layer facing the exterior predominantly occupied the intine of the transgenic pollen, and the microfibrillar endintine layer facing the interior predominantly occupied that of the control pollen. This unusual intine shape and structure was observed in about 50 % of pollen from the transgenic plants.
Fig. 8.
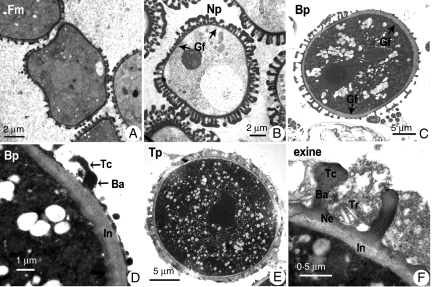
Transmission electron micrographs of pollen development of the antisense BcMF9 transgenic Chinese cabbage 35S-bcmf9. (A) Free microspores; (B) loss of the cloverleaf pattern of the uninucleate microspore; (C) intine, especially within the germinal furrow region, in binucleate pollen is thickened greatly with smooth and granular composition; (D) no multilamellar layer is seen in the binucleate pollen; (E) tectum and baculae of the trinucleate pollen are broken, resulting in overflowing tryphine; (F) magnified image showing the pollen wall of the trinucleate pollen. Abbreviations: Ba, baculae; Bp, binucleate pollen; Gf, germinal furrow; Fm, free microspore; In, intine; Ne, nexine; Tc, tectum; Tp, trinucleate pollen; Tr, tryphine; Np, uninucleate pollen.
Fig. 7.
Transmission electron micrographs of pollen development of the wild-type control Chinese cabbage. (A) Free microspores; (B) uninucleate microspore showing cloverleaf pattern; (C) binucleate pollen showing integrated exine and wavy intine; (D) binucleate pollen showing visible internal microfibrillar endintine and external granular exintine; (E) trinucleate pollen showing a white to grey matrix designated tryphine filling the gaps between the intact tectum and baculae; (F) magnified image of the pollen wall of trinucleate pollen. Abbreviations: Ba, baculae; Bp, binucleate pollen; End, endintine; Ex, exintine; Gf, germinal furrow; Fm, free microspore; In, intine; Ee, endexine; Ne, nexine; Np, uninucleate pollen; Tc, tectum; Tp, trinucleate pollen; Tr, tryphine.
In addition to the abnormalities found in intine formation, aberrations in exine patterning were also detected in the transgenic pollen. One such was the disappearance of the endexine, the inner part of the exine, which becomes visible between the foot layer and the exintine after the binucleate pollen stage (Figs 7D, F and 8D, F). The second was the break of the previously formed tectum and baculae from the beginning of the binucleate stage (Fig. 8C, D). At the trinuleate stage, most of the tectum and baculae had fallen off, leaving just the foot layer on the surface of the pollen grain. The break in tectum and baculae subsequently resulted in overflow of the latest-formed tryphine, which was derived from the remnant of tapetum, filling the gaps within the exine layer and coating the pollen grains (Fig. 8E, F). By contrast, in the wild-type control pollen, gaps within the intact tectum and baculae were filled with a white to grey matrix designated tryphine (Fig. 7E, F). Unlike the intine development defect, the aberration in exine was observed in all transgenic pollen grains.
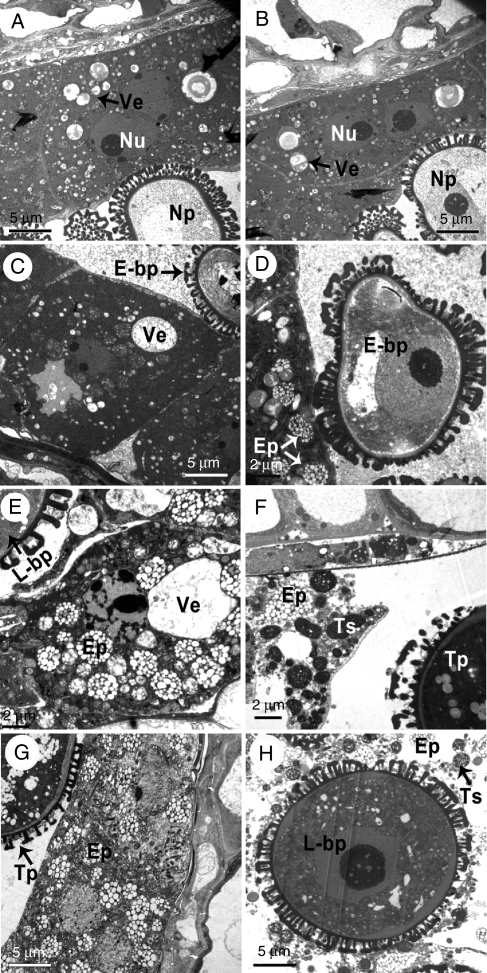
TEM was also used to observe tapetum development in the transgenic 35S-bcmf9 plants. No obvious defect in morphology or organellar distribution was detected, but the degradation process of tapetum apperared to be accelerated, which was similar to the situation observed in the antisense BcMF2 transgenic plant (Huang et al., 2009). Prior to the uninucleate pollen stage, tapetum development of the transgenic plants was not significantly different from that of the wild-type plants (Fig. 9A, B). However, at the early binucleate stage, the boundaries between tapetal cells became unclear and a large number of elaioplasts emerged in the tapetum of the transgenic plants, which is an indicator of imminent tapetum degradation (Fig. 9D). During the late binucleate and trinucleate pollen stage, the tapetum underwent serious degradation and fell off (Fig. 9F). The anther locule was full of shedding materials such as elaioplasts and tapetosomes (Fig. 9H). However, in the anthers of control plants, integral tapetal cells could be observed at the early binucleate stage (Fig. 9C). A large amount of elaioplasts began to appear at the late binucleate stage (Fig. 9E), and the tapetum at the trinucleate stage was still relatively complete (Fig. 9G). This premature degradation of tapetum was observed in all transgenic pollen grains.
Fig. 9.
Transmission electron micrographs of tepetum development of antisense BcMF9 transgenic Chinese cabbage 35S-bcmf9. (A) Tapetum of wild-type plants at uninucleate stage; (B) tapetum of 35S-bcmf9 at uninucleate stage showing no difference to that of wild-type plants; (C) tapetum of wild-type plants at the early binucleate stage showing integral cells with a comparatively dense cytoplasm; (D) numerous elaioplasts appear in the 35S-bcmf9 tapetum at the early binucleate stage; (E) elaioplasts begin to appear in the wild-type tapetum at the late binucleate stage; (F) tapetum of 35S-bcmf9 largely degenerates at the late binucleate stage; (G) tapetum of wild-type plants at the trinucleate stage is still relatively complete; (H) anther locule of 35S-bcmf9 full of shedding materials at the late binucleate stage. Abbreviations: E-bp, early binucleate pollen; Ep, elaioplast; L-bp, late binucleate pollen; Np, uninucleate pollen; Nu, nuclei; Tp, trinucleate pollen; Ts, tapetosome; Ve, vacuole.
Pollen and tapetum development of the F2 progeny of a wild-type and transgenic cross was also observed. Pollen from these F2 plants exhibited an unusual intine and normal intine in a 3 : 1 ratio (P > 0·05), and abnormal exine and wild-type exine in an approximately 1 : 1 ratio (P > 0·05). It was also found that half of the anther from these F2 plants showed premature tapetum degradation (data not shown). These results indicate that the antisense BcMF9 phenotype is stable and heritable in the transgenic line, and the segregation patterns also demonstrate that the antisense BcMF9 is active in the heterozygous condition.
DISCUSSION
BcMF9 is a new putative PG gene preferentially expressed in tapetum and pollen in Chinese cabbage
The BcMF9 gene isolated from the flower buds of Chinese cabbage has a typical PG structure character in its deduced amino-acid sequence, similar to the PG genes BcMF2 and BcMF6. However, overall sequence similarities between BcMF9 and the other two PG genes were not high, and therefore it could be considered a new putative PG gene expressed in Chinese cabbage. The high sequence similarity between BcMF9 and the Arabidopsis pollen-specific PG gene PGA3 suggest that BcMF9 may be a homologue of PGA3. Expression pattern analysis showed that BcMF9 was expressed preferentially in the developing tapetum and pollen from the tetrad stage onward. Most pollen PG genes have been found to be expressed at the late stage of pollen development (Robert et al., 1993; John and Petersen, 1994; Tebbutt et al., 1994; Honys and Twell, 2003). Thus, pollen PG genes are considered to be necessary for the maturation of pollen or pollen tube growth (Allen and Lonsdale, 1993; Honys and Twell, 2003). The expression of BcMF9 in late developmental pollen suggest its similar role to other pollen PG genes. However, its non-pollen-specific features also indicates its possible association with tapetum development.
BcMF9 may be a new gene taking part in pollen wall development
Antisense RNA technology was used to study the function of BcMF9. Transformed lines with small amounts of pollen grains but normal vegetative growth and normal floral organ morphology were chosen for further investigation. The first aberration detected in the transgenic plants due to the inhibition of BcMF9 was pollen tube burst in the tip and subsequent decreased fertility. Pollen tube bursting in the tip has previously been reported in several studies. For example, functional interruption of VANGUARD1 (VGD1), a gene encoding a pectin methylesterase protein in Arabidopsis, resulted in the frequent bursting of pollen tubes and retarded growth of the pollen tube in the style and transmitting tract. It was speculated that VGD1 was required for growth of the pollen tube, possibly via modifying the cell wall and enhancing the interaction of the pollen tube with the female style and transmitting tract tissues (Jiang et al., 2005). Silencing of the tapetum-specific zinc finger gene TAZ1 by co-suppression in petunia (Petunia hybrida) also resulted in burst tips of the surviving pollen grains when germinated under partial in vivo conditions. TAZ1, which belongs to the 5-enolpyruvylshikimate-3-phosphate synthase promoter binding factor family of TFIIIA-type zinc finger transcription factors, may take a role in flavonol accumulation and pollen wall formation (Kapoor et al., 2002). Thus, the silencing or suppression of different genes could lead to burst pollen tubes. Interestingly, however, most of these genes share a common feature that is then acting in pollen wall development. It appears that pollen tube bursting is one of the consequences of the defects in pollen wall development.
In trinucleate pollen species, although there is species-specific variation, the pollen wall is generally composed of the outer exine and inner intine. The exine, facing the exterior, comprises two layers: the innermost featureless nexine (consisting of endexine and foot layer) and the outer sculpted sexine (including baculae and tectum layer; Shukla et al., 1998). The intine is the innermost layer located adjacent to the pollen plasma membrane, which is a highly ordered complex of polysaccharides and structural proteins similar to other of plant cell walls (Owen and Makaroff, 1995). The intine also consists of two layers: a granular exintine with pectin and protein inclusions towards the exterior, and a microfibrillar cellulosic endintine towards the interior. Both of the layers of the pollen wall are important to pollen hydration, activation, germination and pollen tube growth (Blackmore and Barnes, 1990; Owen and Makaroff, 1995).
The most dramatic aberrations in transgenic 35S-bcmf9 plants (detected by SEM) were pollen deformities characterized by the disordered location of germinal furrows. TEM revealed that pollen grains underwent an abnormal thickening in the intine region. The external exintine layer occupied most of the intine, thus reversing the typical proportions of internal endintine and external exintine layers. Furthermore, inhibition of BcMF9 also resulted in aberrations in exine formation, including endexine hypoplasia and breakage of the previously formed tectum and baculae at late developmental stages.
Aberrations in exine formation have been found in several Arabidopsis mutants, for example ms2 (Aarts et al., 1997), dex1 (Paxson-Sowders et al., 1997, 2001), nef1 (Ariizumi et al., 2004), tde1 (Ariizumi et al., 2008), kaonashi (Suzuki et al., 2008) and rpg1 (Guan et al., 2008). Some of the exine mutants such as calS5 (Dong et al., 2005; Nishikawa et al., 2005) affect callose accumulation, and one of them, flp1, is defective in wax biosynthesis. flp1 shows an excess of tryphine covering the exine baculae. Although baculae are formed and sporopollenin polymerizes in this mutant, sculpturing of the exine is irregular with patchy architecture, and some parts of the exine are broken apart (Ariizumi et al., 2003). The phenotypic character observed in transgenic 35S-bcmf9 plants shows similarities to those of flp1. However, the FLP1 gene was demonstrated to be involved in the synthesis of tryphine and sporopollenin; the sequence of this gene is very different from that of BcMF9. Therefore, BcMF9 is neither an identical replica to FLP1 nor its homologue in Arabidopsis.
Two mutants, ms33 (Fei and Sawhney, 2001) and ms1 (Ariizumi et al., 2005; Vizcay-Barrena and Wilson, 2006), defective both in exine and in intine have also been characterized in Arabidopsis. In ms33 pollen, the intine is precociously formed, and the tryphine lacks electron-lucent globuli. The defects in exine and intine development subsequently affect pollen grain desiccation and therefore pollen viability (Fei and Sawhney, 2001). Otherwise, ms1 has only a preliminary intine structure, and exine formation is abnormal, with initial deposition of primary exine occurring but with only limited sporopollenin deposition (Ariizumi et al., 2005; Vizcay-Barrena and Wilson, 2006).
In transgenic 35S-bcmf9 plants, primary exine deposition was normal, as the early developmental stages prior to binucleate pollen showed no difference with those of control plants. The formation of tectum and baculae as well as deposition of tryphine occurred. However, during the tryphine deposition process, the previously formed tectum and baculae began to break apart and ultimately resulted in the overflow of tryphine. From these observations, although 35S-bcmf9 plants possess similarities with former mutants with defective pollen wall, they also have their own distinctness. The BcMF9 gene can therefore be considered as a new gene playing roles in pollen wall formation.
Possible model for BcMF9 in pollen wall formation
It is known that the exine is sporophytic in origin (Owen and Makaroff, 1995), and exine defects are mainly caused by impaired development and/or function of tapetal cells (Aarts et al., 1997; Paxson-Sowders et al., 2001; Ariizumi et al., 2003, 2004, 2008; Dong et al., 2005; Nishikawa et al., 2005; Guan et al., 2008). For example, the exine structure defect in the ms1 mutant resulted from the failure of the normal passage of pollen wall materials in vesicles through the tapetum for secretion into the locule (Yang et al., 2007). In the present study, an exine patterning defect in antisense BcMF9 transgenic plants was also shown to be under sporophytic control, as indicated by the presence of defective exine in all pollen grains from transgenic plants and half of normal pollen from the F2 progeny of a test cross. Meanwhile, the expression characteristics of BcMF9 and the phenotype caused by its inhibition indicated that, like other cases, BcMF9 might originally affect tapetum development and consequently influence exine formation. BcMF9 was first expressed in tapetal cells at the tetrads stage, during which the tapetum has begun to release the material required for pollen wall formation into the anther locule. In the transgenic plants with inhibited BcMF9 expression, anther tapetum development occurred normally before the uninucleate stage; prior to this stage, the secretion from tapetum had been deposited on the pollen surface and formed the exine frame, and normal tectum and baculae developed. However, just after the uninucleate stage, tapetum degeneration accelerated, ultimately leading to abnormal exine formation. The ultimate contribution of tapetum to the formation of the pollen wall is release of cell remnants, which are essential for completion of the extracellular sculpting of the pollen grains into the anther locule (Papini et al., 1999). The breakdown of normal tapetum development in the anther of transgenic plants may alter the wall material secretion process. The resulting metabolism products may decompose the pollenkitt, and in turn, destroy the previously formed exine framework, resulting in the fall off of tectum and baculae. As a consequence, pollen grains deform as the exine collapses as a result of the tectum failing and therefore the tryphine layer does not remain intact. It seems likely that inhibition of BcMF9 expression might facilitate the degeneration of tapetum and ultimately affect exine formation. A possible role for BcMF9 could be as a co-ordinator in the events of latest tapetum degeneration and secretion.
Whereas the exine is sporophytic, the intine is secreted by the microspore (gametophytic origin) around the ring-vacuolated microspore (late uninucleate) stage (Owen and Makaroff, 1995). In the present study, an intine defect was observed in about half of the pollen isolated from transgenic plants and in only one-quarter from the F2 progeny of a test cross, thus appearing to be under gametophytic control. A similar mode of both sporophytic and gametophytic control can be found in the recently described AtbZIP34, a transcription factor that was also expressed in gametophytic and surrounding sporophytic tissues during flower development. The AtbZIP34 expression pattern together with the phenotypic defects observed suggest a sporophytic role in exine patterning, and a sporophytic and/or gametophytic mode of action of AtbZIP34 in several metabolic pathways (Gibalova et al., 2009).
The intine of transgenic 35S-bcmf9 plants underwent abnormal thickening and homogenization after the late uninucleate stage. The homologous granular intine in 35S-bcmf9 pollen may be mainly occupied by the exintine layer which is composed of pectin and various proteins and appeared to be granular. The microfibrillar endintine, which consisted of cellulose, accounts for a large proportion of the intine in control pollen. It is noticeable that BcMF9 encodes a typical PG protein, which is a hydrolase enzyme in the degradation of pectin. Inhibition of BcMF9 may result in the decline of PG activity and subsequent abnormal metabolism of pectin during intine formation. Thus, abnormal thickening of the exintine filled with pectin occurred in the transgenic pollen. However, further experiments, such as determination of the activity of PG enzyme, are required to address this hypothesis.
Members of pollen or anther PG gene family may be involved in pollen wall formation but may behave differently
Three putative pollen development-associated PG genes (BcMF2, BcMF6 and BcMF9) have been isolated from Chinese cabbage, implying that there is not just one polygalacturonase gene expressed in Chinese cabbage pollen or anther. These results are in accordance with data previously published for Arabidopsis, maize and tobacco, in which several members of the polygalacturonase gene family were detected exclusively in pollen (Allen and Lonsdale, 1993; Honys and Twell, 2003).
The function of BcMF2 and BcMF6 in pollen development of Chinese cabbage has previously been investigated. BcMF2′ expression was found to be specific in tapetum and pollen after the tetrad stage of anther development, which was very similar to that of BcMF9. Functional analysis revealed that there were deformities in the antisense BcMF2 transgenic mature pollen grains, such as abnormal location of germinal furrows. In addition, the same situation of abnormal intine formation due to the over-development of the granular exintine has also been observed in transgenic plants with BcMF2 inhibition. However, instead of the pollen tube burst observed in antisense BcMF9 transgenic pollen, inhibition of BcMF2 resulted in a halted growth and formation of a balloon-like swelling structure in the pollen tube tip. Although premature degradation of tapetum was also found, exine formation was normal (Huang et al., 2009). From these results, BcMF2 and BcMF9 may play a similar role in intine formation. It is known that pollen grain aperture, exintine in particular, is rich in pectins (Geitmann et al., 1995; Aouali et al., 2001; Suarez-Cervera et al., 2002; Parre and Geitmann, 2005). Inhibition of BcMF2 or BcMF9 may result in a decline of PG activity and subsequent abnormal dynamic metabolism of pectin during intine formation. Pectin was also the major component at the growing apex of the pollen tube (Parre and Geitmann, 2005). As the cell wall of the primary pollen tube is a continuation of the intine layer (Stone et al., 2004), we conclude that either pollen tube burst or pollen tube swelling is a consequence of the variation in intine development. Burst or swelling may differ regarding the degree of influence resulting from a change in the metabolism of pectin. A large influence led to bursting of the pollen tube as soon as it emerged, while a lesser degree of influence initiated growth of the pollen tube but stopped it with a swelling at the tip. By contrast, BcMF9 was also involved in exine formation, but BcMF2 was not. Thus, the available results suggest a more diverse role for BcMF9 than for BcMF2 in pollen wall formation.
Regarding BcMF6, we found that antisense transgenic plants developed a smaller floral organ with a thin anther and less pollen; about 50 % of the transgenic pollen had deformities and could not germinate normally, and the process of microsporogenesis was held up with disrupted microspores maturation and failed separation of pollen grains (Zhang et al., 2008). These findings suggested a similar role of BcMF6 in pollen tube growth and pollen wall formation, but also suggested a unique role for it in pollen maturation.
Inhibition of expression of any of three PG genes by an antisense vector resulted in premature degeneration of tapetum (Huang et al., 2009; Zhang et al., 2008). These results implied that the BcMF2, BcMF6 and BcMF9 protein might be secreted from tapetum and released to the developing microspores to play a role in pollen development and maturation. However, the different genes may behave differently.
These results, together with sequence conservation in the different pollen PG genes, suggest that the three genes are all derived from a single primordial gene that has been repeatedly modified during evolution to perform various functions. Further investigation on these putative PG genes in Chinese cabbage pollen is in progress. We look forward to a better understanding of these pollen and/or anther PG genes.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Chinese Natural Science Foundation (nos. 30671426 and 30800697).
LITERATURE CITED
- Aarts MG, Hodge R, Kalantidis K, et al. The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. The Plant Journal. 1997;12:615–623. doi: 10.1046/j.1365-313x.1997.00615.x. [DOI] [PubMed] [Google Scholar]
- Allen RL, Lonsdale DM. Molecular characterization of one of the maize polygalacturonase gene family members which are expressed during late pollen development. The Plant Journal. 1993;3:261–271. doi: 10.1111/j.1365-313x.1993.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Aouali N, Laporte P, Clement C. Pectin secretion and distribution in the anther during pollen development in Lilium. Planta. 2001;213:71–79. doi: 10.1007/s004250000469. [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Hatakeyama K, Hinata K, et al. A novel male-sterile mutant of Arabidopsis thaliana, faceless pollen-1, produces pollen with a smooth surface and an acetolysis-sensitive exine. Plant Molecular Biology. 2003;53:107–116. doi: 10.1023/B:PLAN.0000009269.97773.70. [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Hatakeyama K, Hinata K, et al. Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. The Plant Journal. 2004;39:170–181. doi: 10.1111/j.1365-313X.2004.02118.x. [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Hatakeyama K, Hinata K, et al. The HKM gene, which is identical to the MS1 gene of Arabidopsis thaliana, is essential for primexine formation and exine pattern formation. Sexual Plant Reproduction. 2005;18:1–7. [Google Scholar]
- Ariizumi T, Kawanabe T, Hatakeyama K, et al. Ultrastructural characterization of exine development of the transient defective exine 1 mutant suggests the existence of a factor involved in constructing reticulate exine architecture from sporopollenin aggregates. Plant & Cell Physiology. 2008;49:58–67. doi: 10.1093/pcp/pcm167. [DOI] [PubMed] [Google Scholar]
- Athanasiou A, Khosravi D, Tamari F, Shore JS. Characterization and localization of short-specific polygalacturonase in distylous Turnera subulata (Turneraceae) American Journal of Botany. 2003;90:675–682. doi: 10.3732/ajb.90.5.675. [DOI] [PubMed] [Google Scholar]
- Becker J, Boavida LC, Carneiro J, Haury M, Feijó JA. Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiology. 2003;133:713–725. doi: 10.1104/pp.103.028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore S, Barnes SH. Pollen wall development in angiosperms. In: Blackmore S, Knox RB, editors. Microspores evolution and ontogeny. San Diego: Academic Press; 1990. pp. 173–192. [Google Scholar]
- Cao J, Cao S, Yi Q. RAPD analysis on genomic DNA of Chinese cabbage and the other groups of Brassica. Acta Horticulture Sinica. 1995;22:47–52. [Google Scholar]
- Cao J, Yu X, Ye W, Lu G, Xiang X. Functional analysis of a novel male fertility CYP86MF gene in Chinese cabbage (Brassica campestris L. ssp. chinensis Makino) Plant Cell Reports. 2006;24:715–723. doi: 10.1007/s00299-005-0020-6. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM. Arabinogalactan proteins in plant sexual reproduction. Protoplasma. 1999;208:87–98. [Google Scholar]
- Cheung AY, Wu HM. Expression of expansin genes is correlated with growth in deepwater rice. The Plant Cell. 1997;9:1661–1671. doi: 10.1105/tpc.9.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DP. Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. The Plant Journal. 2005;42:315–328. doi: 10.1111/j.1365-313X.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- Fei HM, Sawhney VK. Ultrastructural characterization of male sterile33 (ms33) mutant in Arabidopsis affected in pollen desiccation and maturation. Canadian Journal of Botany. 2001;79:118–129. [Google Scholar]
- Geitmann A, Hudak J, Vennigerholz F, Walles B. Immunogold localization of pectin and callose in pollen grains and pollen tubes of Brugmansia suaveolens–implication for the self-incompatibility reaction. Journal of Plant Physiology. 1995;147:225–235. [Google Scholar]
- Gibalova A, Renak D, Matczuk K, et al. AtbZIP34 is required for Arabidopsis pollen wall patterning and the control of several metabolic pathways in developing pollen. Plant Molecular Biology. 2009;70:581–601. doi: 10.1007/s11103-009-9493-y. [DOI] [PubMed] [Google Scholar]
- Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN. RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiology. 2008;147:852–863. doi: 10.1104/pp.108.118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield KA, Rose JKC, Yaver DS, Berka RM, Bennett AB. Polygalacturonase gene expression in ripe melon fruit supports a role for polygalacturonase in ripening-associated pectin disassembly. Plant Physiology. 1998;117:363–373. doi: 10.1104/pp.117.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D. Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiology. 2003;132:640–652. doi: 10.1104/pp.103.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biology. 2004;5:R85. doi: 10.1186/gb-2004-5-11-r85. doi:10.1186/gb-2004-5-11-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Cao J, Ye W, Liu T, Jiang L, Ye Y. Transcriptional differences between the male-sterile mutant bcms and wild-type Brassica campestris ssp.chinensis reveal genes related to pollen development. Plant Biology. 2008;10:342–355. doi: 10.1111/j.1438-8677.2008.00039.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Cao J, Zhang A, Ye Y, Zhang Y, Liu T. The polygalacturonase gene BcMF2 from Brassica campestris is associated with intine development. Journal of Experimental Botany. 2009;60:301–313. doi: 10.1093/jxb/ern295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Yang S-L, Xie L-F, et al. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. The Plant Cell. 2005;17:584–596. doi: 10.1105/tpc.104.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John ME, Petersen MW. Cotton (Gossypium hirsutum L.) pollen-specific polygalacturonase mRNA: tissue and temporal specificity of its promoter in transgenic tobacco. Plant Molecular Biology. 1994;26:1989–1993. doi: 10.1007/BF00019509. [DOI] [PubMed] [Google Scholar]
- Kapoor S, Kobayashi A, Takatsuji H. Silencing of the tapetum-specific zinc finger gene TAZ1 causes premature degeneration of tapetum and pollen abortion in petunia. The Plant Cell. 2002;14:2353–2367. doi: 10.1105/tpc.003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Lee DH. Use of serial analysis of gene expression technology to reveal changes in gene expression in Arabidopsis pollen undergoing cold stress. Plant Physiology. 2003;132:517–529. doi: 10.1104/pp.103.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelam A, Sexton R. Cellulase (endo-1, 4 glucanase) and cell wall breakdown during anther development in the sweet pea (Lathyrus odoratus L.): isolation and characterization of partial cDNA clones. Journal of Plant Physiology. 1995;146:622–628. [Google Scholar]
- Nishikawa S, Zinkl GM, Swanson RJ, Maruyama D, Preuss D. Callose (β-1, 3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biology. 2005;5:22. doi: 10.1186/1471-2229-5-22. doi:10.1186/1471-2229-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen HA, Makaroff CA. Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. ecotype Wassilewskija (Brassicaceae) Protoplasma. 1995;185:7–21. [Google Scholar]
- Papini A, Mosti S, Brighigna L. Programmed-cell-death events during tapetum development of angiosperms. Protoplasma. 1999;207:213–221. [Google Scholar]
- Parre E, Geitmann A. Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta. 2005;220:582–592. doi: 10.1007/s00425-004-1368-5. [DOI] [PubMed] [Google Scholar]
- Paxson-Sowders DM, Owen HA, Makaroff CA. A comparative ultrastructural analysis of exine pattern development in wild type Arabidopsis and a mutant defective in pattern formation. Protoplasma. 1997;198:53–65. [Google Scholar]
- Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA. DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiology. 2001;127:1739–1749. [PMC free article] [PubMed] [Google Scholar]
- Pereira LG, Coimbra S, Oliveira H, Monteiro L, Sottomayor M. Expression of arabinogalactan protein genes in pollen tubes of Arabidopsis thaliana. Planta. 2006;223:374–380. doi: 10.1007/s00425-005-0137-4. [DOI] [PubMed] [Google Scholar]
- Pina C, Pinto F, Feijó J, Becker J. Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiology. 2005;138:744–756. doi: 10.1104/pp.104.057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SY, Osborne E, Poindexter PD, Somerville CR. Microspore separation in the quartet 3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiology. 2003;133:1170–1180. doi: 10.1104/pp.103.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert L, Hong HP. Brassica sp. gene promoter highly expressed during tapetum development. 1999 United States Patent 5919919. [Google Scholar]
- Robert LS, Allard S, Gerster JL, Cass L, Simmonds J. Isolation and characterization of a polygalacturonase gene highly expressed in Brassica napus pollen. Plant Molecular Biology. 1993;23:1273–1278. doi: 10.1007/BF00042360. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shukla AK, Vijayaraghavan MR, Chaudhry B. Biology of pollen. New Delhi: APH Publishing Corporation; 1998. [Google Scholar]
- Stone LM, Seaton KA, Kuo J, McComb JA. Fast pollen tube growth in Conospermum species. Annals of Botany. 2004;93:369–378. doi: 10.1093/aob/mch050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Cervera M, Arcalis E, Le Thomas A, Seoane-Camba JA. Pectin distribution pattern in the apertural intine of Euphorbia peplus L. (Euphorbiaceae) pollen. Sexual Plant Reproduction. 2002;14:291–298. [Google Scholar]
- Suzuki T, Masaoka K, Nishi M, Nakamura K, Ishiguro S. Identification of kaonashi mutants showing abnormal pollen exine structure in Arabidopsis thaliana. Plant & Cell Physiology. 2008;49:1465–1477. doi: 10.1093/pcp/pcn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbutt SJ, Rogers HJ, Lonsdale DM. Characterization of a tobacco gene encoding a pollen-specific polygalacturonase. Plant Molecular Biology. 1994;25:283–297. doi: 10.1007/BF00023244. [DOI] [PubMed] [Google Scholar]
- Torki M, Mandaron P, Mache R, Falconet D. Characterization of a ubiquitous expressed gene family encoding polygalacturonase in Arabidopsis thaliana. Gene. 2000;242:427–436. doi: 10.1016/s0378-1119(99)00497-7. [DOI] [PubMed] [Google Scholar]
- Vizcay-Barrena G, Wilson ZA. Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. Journal of Experimental Botany. 2006;57:2709–2717. doi: 10.1093/jxb/erl032. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ye W, Cao J, Yu X, Xiang X, Lu G. Cloning and characterization of the microspore development related gene BcMF2 in Chinese cabbage pak-choi (Brassica campestris L. ssp. chinensis Makino) Journal of Integrative Plant Biology. 2005;47:863–872. [Google Scholar]
- Wilkinson JE, Twell D, Lindsey K. Activities of CaMV 35S and nos promoters in pollen: implications for field release of transgenic plants. Journal of Experimental Botany. 1997;48:265–275. [Google Scholar]
- Yang C, Vizcay-Barrena G, Conner K., Wilson ZA. MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell. 2007;19:3530–3548. doi: 10.1105/tpc.107.054981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Cao J, Ye W, Wang Y. Construction of an antisense CYP86MF gene plasmid vector and production of a male-sterile Chinese cabbage transformant by the pollen-tube method. Journal of horticulture and biotechnology. 2004;79:833–839. [Google Scholar]
- Zhang Q, Huang L, Cao J. Cloning, characterization and expression of a polygalacturonase gene BcMF6 from Chinese cabbage-pak-choi (Brassica campestris L. ssp. chinensis Makino) Acta Horticulture Sinica. 2007;1:117–124. [Google Scholar]
- Zhang Q, Huang L, Liu T, Yu X, Cao J. Functional analysis of a pollen-expressed polygalacturonase gene. BcMF6 in Chinese cabbage (Brassica campestris L. ssp. chinensis. Makino) Plant Cell Reports. 2008;27:1207–1215. doi: 10.1007/s00299-008-0541-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.