Abstract
Background
Salt stress has become a major threat to plant growth and productivity. Arbuscular mycorrhizal fungi colonize plant root systems and modulate plant growth in various ways.
Scope
This review addresses the significance of arbuscular mycorrhiza in alleviation of salt stress and their beneficial effects on plant growth and productivity. It also focuses on recent progress in unravelling biochemical, physiological and molecular mechanisms in mycorrhizal plants to alleviate salt stress.
Conclusions
The role of arbuscular mycorrhizal fungi in alleviating salt stress is well documented. This paper reviews the mechanisms arbuscular mycorrhizal fungi employ to enhance the salt tolerance of host plants such as enhanced nutrient acquisition (P, N, Mg and Ca), maintenance of the K+ : Na+ ratio, biochemical changes (accumulation of proline, betaines, polyamines, carbohydrates and antioxidants), physiological changes (photosynthetic efficiency, relative permeability, water status, abscissic acid accumulation, nodulation and nitrogen fixation), molecular changes (the expression of genes: PIP, Na+/H+ antiporters, Lsnced, Lslea and LsP5CS) and ultra-structural changes. Theis review identifies certain lesser explored areas such as molecular and ultra-structural changes where further research is needed for better understanding of symbiosis with reference to salt stress for optimum usage of this technology in the field on a large scale. This review paper gives useful benchmark information for the development and prioritization of future research programmes.
Key words: Arbuscular mycorrhizal fungi, salt stress, PIP, Na+/H+ antiporters, nutrient uptake, soil salinity
INTRODUCTION
Salinization of soil is a serious problem and is increasing steadily in many parts of the world, in particular in arid and semi-arid areas (Giri et al., 2003; Al-Karaki, 2006). Saline soils occupy 7 % of the earth's land surface (Ruiz-Lozano et al., 2001) and increased salinization of arable land will result in to 50 % land loss by the middle of the 21st century (Wang et al., 2003). At present, out of 1·5 billion hectares of cultivated land around the world, about 77 million hectares (5 %) is affected by excess salt content (Sheng et al., 2008). High levels of salinity (>4 dS m−1 or >0·1 % soil content; Richards,1954; Juniper and Abbott, 1993) in soils is mainly due to the soluble salts in irrigation water and fertilizers used in agriculture (Abrol, 1986; Copeman et al., 1996; Al-Karaki, 2000), low precipitation and high temperature in these regions and over-exploitation of available water resources (e.g. ground water) (Cantrell and Linderman, 2001; Al-Karaki, 2006; Mouk and Ishii, 2006).
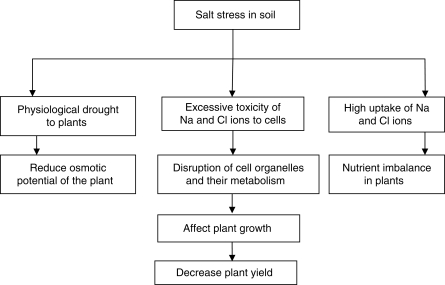
The significance of soil salinity for agricultural yield is enormous (Tester and Davenport, 2003) as it affects the establishment, growth and development of plants leading to huge losses in productivity (Giri et al., 2003; Mathur et al., 2007). The direct effects of salt on plant growth may involve: (a) reduction in the osmotic potential of the soil solution that reduces the amount of water available to the plant causing physiological drought – to counteract this problem plants must maintain lower internal osmotic potentials in order to prevent water movement from roots into the plant soil (Feng et al., 2002; Jahromi et al., 2008); (b) toxicity of excessive Na+ and Cl− ions towards the cell – the toxic effects include disruption to the structure of enzymes and other macromolecules, damage to cell organelles and plasma membrane, disruption of photosynthesis, respiration and protein synthesis (Juniper and Abbott, 1993; Feng et al., 2002); and (c) nutrient imbalance in the plant caused by nutrient uptake and/or transport to the shoot leading to ion deficiencies (Marschner, 1995; Adiku et al., 2001; Fig. 1).
Fig. 1.
Effect of salt stress on plants. Salt stress causes physiological drought to plants, imbalance in nutrient composition and excessive toxicity due to Na and Cl ions thereby leading to reduction in osmotic potential of plants, disruption of cell organelles and their metabolism. These ultimately affect plant growth and reduce the yield.
To deal with saline soils and minimize crop loss, scientists have searched for new salt-tolerant crop plants (Gallagher, 1985; Glenn and O'Leary, 1985), and developed salt-tolerant crops through breeding (Cuartero and Fernandez-Munoz, 1999). To tackle the detrimental effects of salinity, scientists are also in the process of engineering plants genetically using different genes (Zhang and Blumwald, 2001; Sanan-Mishra et al., 2005; Tang et al., 2005; Wu et al., 2005; Wei-Feng et al., 2008). Wu et al. (2005) obtained a salt-tolerant perennial rye-grass (Lolium perenne) by transformaton with a rice vacuolar membrane Na+/H+ antiporter gene (OsNHX1) via an Agrobacterium-mediated method: the salt tolerance of perennial rye grass was improved by overexpression of the OsNHX1 gene. Sanan-Mishra et al. (2005) reported that PDH45 (pea DNA helicase 45) mRNA is induced in pea seedlings in response to high salt, and its overexpression in tobacco plants, driven by a constitutive cauliflower mosaic virus 35S promoter, confers salinity tolerance. Recently, Wei-Feng et al. (2008) have reported that a transgenic Arabidopsis thaliana carrying a peroximal-type ascorbate peroxidase (pAPX) gene from barley was found to be more salt tolerant than the wild type. Leaching of excessive salts or desalinizing seawater for use in irrigation (Muralev et al., 1997) are other methods employed to combat salt stress. Though successful, these approaches are costly and beyond the economic means of developing nations (Cantrell and Linderman, 2001).
Plants, in their natural environment are colonized both by external and internal microorganisms. Some microorganisms, particularly beneficial bacteria and fungi can improve plant performance under stress environments and, consequently, enhance yield (Brown, 1974; Levy et al., 1983; Creus et al., 1998). Arbuscular mycorrhizal fungi (AMF) are associated with the roots of over 80 % terrestrial plant species (Smith and Read, 1997; Hejiden et al., 1998) including halophytes, hydrophytes and xerophytes. In this respect, biological processes such as mycorrhizal application to alleviate salt stress would be a better option. AMF have been shown to promote plant growth and salinity tolerance by many researchers. They promote salinity tolerance by employing various mechanisms, such as enhancing nutrient acquisition (Al-Karaki and Al-Raddad, 1997), producing plant growth hormones, improving rhizospheric and soil conditions (Lindermann, 1994), altering the physiological and biochemical properties of the host (Smith and Read, 1995) and defending roots against soil-borne pathogens (Dehne, 1982). In addition, AMF can improve host physiological processes like water absorption capacity of plants by increasing root hydraulic conductivity and favourably adjusting the osmotic balance and composition of carbohydrates (Rosendahl and Rosendahl, 1991; Al-Karaki and Clark, 1998; Ruiz-Lozano and Azcón, 2000; Ruiz-Lozano, 2003). This may lead to increased plant growth and subsequent dilution of toxic ion effect (Juniper and Abbott, 1993). These benefits of AMF have prompted it to be a suitable candidate for bio-amelioration of saline soils.
This review will cover the occurrence of AMF in saline soils and the effect of salinity on the arbuscular mycorrhizal (AM) fungus: colonization, hyphal length and sporulation both in vivo and in vitro. It will also cover literature relating to the alleviation of salt stress by AMF and its beneficial effects on growth, changes in biochemical, physiological and molecular mechanisms used by host plants to alleviate salt stress. The article has identified certain areas where more investigations are required in order to gain a whole understanding of the different mechanisms by which AMF symbiosis protects the plants against salt stress.
AMF IN SALINE SOILS
AMF have been known to occur naturally in saline environments (Khan, 1974; Allen and Cunningam, 1983; Pond et al., 1984; Rozema et al., 1986; Sengupta and Chaudhuri, 1990; Carvalho et al., 2001; Hilderbrandt et al., 2001; Harisnaut et al., 2003; Yamato et al., 2008), despite the low mycorrhizal affinity of the halophytes (Brundrett, 1991). The average density of spores in saline areas is reported to be low by some researchers (Barrow et al., 1997; Carvalho et al., 2001) but not others (Khan, 1974; Bhaskaran and Selvaraj, 1997; Aliasgharzadeh et al., 2001; Landwehr et al., 2002). Aliasgharzadeh et al. (2001) observed that the most predominant species of AMF in the severely saline soils of the Tabriz plains [with an electrical conductivity (ECe) of 162 dS m−1] were Glomus intraradices, G. versiform and G. etunicatum. The authors also found that the number of AMF spores did not significantly decrease with soil salinity and reported a relatively high spore number (mean of 100 per 10 g soil). The higher fungal spore density in saline soils may be due to the fact that sporulation is stimulated under salt stress (Tressner and Hayes, 1971) which means that AMF may produce spores at low root-colonization levels in severe saline conditions (Aliasgharzadeh et al., 2001). This is in contrast to other reports on saline soils where low or even zero spore population was found in soils with ECe approx. 45 dS m−1 (Hirrel et al., 1978; Kim and Weber, 1985; Barrow et al., 1997). McMillen et al. (1998) reported that the spore germination and hyphal growth of AMF were inhibited by salt (150 mm NaCl). This may again cause the accumulation of spores in saline soil (Aliasgharzadeh et al., 2001). F.Y. Wang et al. (2004), while investigating the relationship between the distribution of AMF in the rhizosphere of different wild plants in the Yellow River Delta (ECe approx. 40·2 dS m−1), observed a total of 33 species representing three genera of AMF, including two species of Archaeospora, seven in Acaulospora and 24 in Glomus. They also found most spores at a depth of 0–40 cm. There were significant differences between different depths. The number of AMF spores decreased with increasing soil depth in the rhizosphere. Ho (1987), had reported the same while studying AMF of halophytic grasses in the Alvord Desert of Oregon.
The occurrence of AMF in salt-marsh plants has also been reported by many authors (Khan, 1974; Rozema et al., 1986; Sengupta and Chaudhuri, 1990; Carvalho et al., 2001; Hilderbrandt et al., 2001). Findings by Landwehr et al. (2002) reported abundant occurrence of AMF spores in extremely alkaline soils of pH values up to 11, independently of the soil type and irrespective of NaCl, Na2CO3, Na 2SO4 or CaSO4 salt types, though the degree of colonization varied from one individual to the next. Recently, Wilde et al. (2009) evaluated the distribution of AMF spores in two salt-marshes – Terschelling, The Netherlands (an almost natural site on the Atlantic Coast, with an ECe of 6·2–19·0 mS cm−1) and at Schreyahn, Germany (of anthropogenic origin due to potash mining, ECe 6·3–20·1 mS cm−1) – and reported that the distribution of AMF spores was unlikely to follow the salt gradient at both sites. They also reported that morphological analyses of spores from soil samples at both sites showed a higher AMF biodiversity, while the overall biodiversity of the AMF based on sequence analysis was comparably low in roots at both sites. There is correlation between AMF spore population and different edaphoclimatic factors. A positive correlation between spore density and soil pH and organic carbon has been reported by some authors (Ho, 1987; Mohammad et al., 2003; Mathur et al., 2007). A negative correlation was observed with available soil P and Na. Aliasgharzadeh et al. (2001) had reported negative correlation between spore density and available soil Mg, Ca, Cl, clay, electrical conductivity, SO4 and the sodium absorption ratio and a positive correlation with sandy soil. Saint-Etienne et al. (2006) reported significantly negative correlations between salt levels and mycorrhizal soil infectivity (measured in most-probable number values), i.e. when the salinity of soil increased from 5 to 22 %, the infectivity level decreased from 301 to 20 most probable number per 100 g of soil.
AMF are also known to colonize halophytes and such a finding was reported as early as 1928 (Mason, 1928) and later by various authors (Khan, 1974; Hoefnagels et al., 1993; Brown and Bledsoe, 1996). Halophytes, the salt-tolerant, salt-loving or saltwater plants can increase total plant growth within a range of 5–20 dS m−1 ECe followed by a decrease in growth (O'Leary, 1995; Marcum, 2002). The extent of benefits derived by AM halophytes has been addressed by some authors (Allen and Cunningham, 1983; Rozema et al., 1986; Baker et al., 1995). Allen and Cunningham (1983) observed that, in saline soils, AM plants of Distichlis spicata had similar or lower biomass than non-AM plants. Further, under salt-stress conditions, a beneficial effect of AMF symbiosis was observed on the water status, accumulation of osmolytes and growth of Phragmites australis plants (Al-Garni, 2006). An improved water relationship was also reported in mycorrhizal Aster tripolium plants in saline soil (Rozema et al., 1986). Johnson-Green et al. (2001) proposed that AMF could tolerate 50 mg total salts ml−1 soil water. They suggested that the mycorrhizal benefit to halophytes might occur primarily through improved mineral content, rather than through increased biomass.
In most of the studies mentioned above, identification of the AMF spores was carried out. In the earlier studies, identification was based mainly on the morphological criteria. Complementary to morphology based identification methods, use of molecular techniques such as polymerase chain reaction and restriction fragment length polymorphism for identification of AMF has been on the rise. Many authors have employed molecular techniques for identification of AMF spores (Landwehr et al., 2002; Regvar et al., 2003; Wilde et al., 2009). The molecular identification techniques can overcome some of the pitfalls in morphology based identification; however, it is not advisable to rely solely on molecular identification techniques as the gene content of the different nuclei within an AMF individual is to some extent variable (Lloyd-MacGilip et al., 1996; Sanders et al., 1996). Pringle et al. (2000) reported that the sequences of the ITS region within a single spore can be more variable than between spores of a single isolate. According to Antoniolli et al. (2000), the divergence of the ITS region within a single spore can be between 2·4 % and 5·7 %. Therefore, to define a species, any characterization of a microbe by sequencing of ribosomal genes needs to be complemented by morphological and/or physiological characterization (Wilde et al., 2009).
The AMF most commonly observed in saline soils are Glomus spp. (Allen and Cunningam, 1983; Ho, 1987; F. Y. Wang et al., 2004). Studies employing molecular biological techniques revealed that 80 %, on average, of these spores belonged to one single species, Glomus geosporum (Wilde et al., 2009). However, this finding does not necessarily indicate that Glomus geosporum confers salt tolerance on plants and enables them to grow under salt-stress conditions. Fuzy et al. (2008) reported that an isolate of G. geosporum from the inland salt-marsh at D-Jerxheim near Braunschweig consistently failed to give positive plant growth-promoting effects when diverse plants were grown with different NaCl concentrations under greenhouse conditions. Tian et al. (2004) observed that G. mosseae isolated from saline soil had a lower capacity to alleviate saline stress in cotton than the one isolated from non-saline soil. Recently, Porras-Soriano et al. (2009) tested the efficacy of three species of AMF – Glomus mosseae, G. intraradices and G. claroideum – to alleviate salt stress in olive trees under nursery conditions. The authors observed that G. mosseae was the most efficient fungus in terms of olive tree performance and particularly in the protection offered against the detrimental effects of salinity. These findings suggest that the capability of AMF in protecting plants from the detrimental effects of salt stress may depend on the behaviour of each species.
Effect of salinity on colonization, spore germination and hyphal growth
Salinity, not only affects the host plant but also the AMF. It can hamper colonization capacity, spore germination and growth of hyphae of the fungus. Several researchers have reported the negative effects of salinity on the fungus (Hirrel, 1981; Estaun, 1989; McMillen et al., 1998; Jahromi et al., 2008). Colonization of plant roots by some AMF is reduced in the presence of NaCl (Hirrel and Gerdemann, 1980; Ojala et al., 1983; Menconi et al., 1995; Poss et al., 1985; Rozema et al., 1986; Duke et al., 1986; Giri et al., 2007; Juniper and Abbott, 2006; Sheng et al., 2008) probably due to the direct effect of NaCl on the fungi (Juniper and Abbott, 2006) indicating that salinity suppresses the formation of arbuscular mycorrhiza (Tian et al., 2004; Sheng et al., 2008). Before primary colonization of a plant root by an AMF can commence, fungal propagules in the soil must become hydrated and activated and produce a germ tube. One or more hyphae then extend through the soil and encounter a receptive root. Hyphae elongate 20 times more slowly in the absence of host roots than in their presence (Becard and Piche, 1989). AMF respond to host exudates with extensive hyphal growth and branching (Giovannetti et al., 1996). Despite the high mycorrhizal growth in the presence of roots, hyphae do not always appear to exhibit ‘directional growth’ toward the roots until they are very close to the host (Mosse and Hepper, 1975). Once, contact occurs, branching on the root surface takes place. However, hyphal branching does not occur on a non-host root, thereby suggesting that directional attraction may not be a general phenomenon, but may be more characteristic of the specific host tested (Vierheilig et al., 1998). The relative timing of these stages is highly dependent on the characteristics of the individual fungus. The extent to which colonization by AMF is reduced in the presence of NaCl is dependent on the timing of the observation, such that there is more inhibition in the early than in the later stages of the symbiosis (McMillen et al., 1998). It is, therefore, likely that the inhibition in these cases was due to an effect on primary rather than secondary infection (Wilson, 1984).
Besides, the AMF symbiosis with plant roots may also depend on various factors including the topographical or biochemical signals on the root surface (Gadkar et al., 2001) and phenology of host plants (Wilson and Hartnett, 1998; Carvalho et al., 2001). Carvalho et al. (2001) reported that the highest levels of AM colonization in Aster tripolium and Inula crithmoides corresponded, in both species, to the period of highest plant growth and the flowering period – summer and autumn, respectively. The varying levels of AM colonization may also be related to the different behaviour of each AM fungal species, even in similar ecosystems (Klironomos et al., 1993) or to the influence of different environmental conditions (Carvalho et al., 2001).
Contrary to the reports above, a few studies reported that AMF colonization is not reduced in the presence of NaCl (Levy et al., 1983; Hartmond et al., 1987). Increased AMF sporulation and colonization under salt-stress conditions has also been reported (Aliasgharzadeh et al., 2001). Recently, Yamato et al. (2008) reported that colonization rates were not reduced in all AMF present in coastal vegetation on Okinawa Island, Japan even when treated with high salinity of 200 mm. This discrepancy in the results invites researchers to look out for salt-tolerant species of AMF.
In the presence of NaCl, germination of spores is delayed rather than prevented (Cantrell and Lindermann, 2001; Juniper and Abbott, 2006). It has been reported that preinoculation of a transplant with AMF bypasses the inhibitory effects that salt could have on spore germination (Cantrell and Lindermann, 2001; Al-Karaki, 2006).
The rate of germination and maximum germination of AMF spores may also depend on the salt type. According to Juniper and Abbott (1993), the different salts NaNO3 and Na2SO4 with similar osmotic potentials (−0·48 and −0·43 MPa, respectively) impart differential effects on the rate and maximum germination of spores. They attributed the difference to a higher concentration of Na+ in the latter. They also reported that NaCl allows a faster rate and maximum germination as compared with KCl with similar osmotic potentials. The percentage of AM colonization observed in a variety of host plants at varied levels of salinity is presented in Table 1.
Table 1.
Studies on percentage of AMF colonization in root under salinity stress
| Range of salinity* | Plant | Fungus | % root colonization in AM plants | References |
|---|---|---|---|---|
| 1·4–7·4 dS m−1 | Lycopersicon esculentum | Glomus mosseae | 49·6–36 | Al-Karaki (2000) |
| 2 –12 dS m−1 | Lactuca sativa | Mixture of Glomus, Acaulospora and Entrosphora spp. procured from (a) saline playa or (b) non-saline vegetable farm | (a) 43·0–26·2 (b) 34·8–29·9 |
Cantrell and Linderman (2001) |
| 2–12 dS m−1 | Allium cepa | Mixture of Glomus, Acaulospora and Entrosphora spp. procured from (a) saline playa or (b) non-saline vegetable farm | (a) 61·7–38·8 (b) 28·8–18·0 |
Cantrell and Linderman (2001) |
| 0–13·19 dS m−1 (0–100 mm) | Zea mays | Glomus mosseae | 70–80 | Feng et al. (2002) |
| 0–6·10 dS m−1 (0–3 g kg−1) | Gossypium arboreum | Glomus mosseae: (a) GM1 from non-saline soils; (b) GM2 from saline soils | (a) 38 ± 3 to 15 ± 2 (b) 46 ± 5 to 21 ± 3 |
Tian et al. (2004) |
| 40·2 dS m−1 | Tamarix chinensis, Phragmites communis, Suaeda glauca, Aeluropus littoralis var. sinensis and Cirsium setosum (in Yellow River Delta, China) | Mixture of Archaeospora, Acaulospora and Glomus | 0·2–9·5 | F. Y. Wang et al. (2004) |
* The range of salinity within brackets is the actual salt concentrations used by the authors.
The trend to decreasing hyphal length as soil salt concentration increases has been reported (Cantrell and Lindermann, 2001; Juniper and Abbott, 2006). These reports suggest that hyphal growth of the fungi can be taken as being more sensitive to NaCl than spore germination, which is delayed but not necessarily reduced.
The relative tolerance of different types of the same fungal genus can vary as is reported in the case of Glomus sp. Propagules of Glomus sp. within the colonized root pieces grow in 300 mm NaCl, but the spores of the same fungi extracted from the soil did not. This may indicate a difference in the energy status between them, or differences in the amount of water and energy required to initiate germination (Juniper and Abbott, 2006). Tommerup (1984) indicated that initial hyphal extensions have higher requirements for water than the intermediate stages for activation and germ-tube production. Therefore it may be possible that the germination rate of a prehydrated spore will have a greater percentage than the non-prehydrated spore in a saline environment (Juniper and Abbott, 2006). This contrasting result invites further studies in this aspect.
In a sole attempt, Jahromi et al. (2008) studied the effect of salinity on the AM fungus, Glomus intraradices, in vitro. They observed that there was no significant difference in hyphal length and branched absorbing structures (BAS) between control (no salt) and 50 mm NaCl, though there was a significant decrease in hyphal length and the number of BAS at 100 mm NaCl. It was also reported that salinity in the medium reduces the number of spores produced by Glomus intraradices. This decline suggests that if salinity persists, there can be a reduction in plant colonization by reducing the ability of inoculum (i.e. spores). An increase in salinity will reduce the hyphal length which will inhibit the colonization and symbiotic capability of AMF. BAS are believed to be associated with spore formation. Bago et al. (1998) had also shown that BAS can gradually form spores in their ramification. So, the reduction in the number of BAS can also reduce sporulation further.
ARBUSCULAR MYCORRHIZA AND SALT-STRESS AMELIORATION
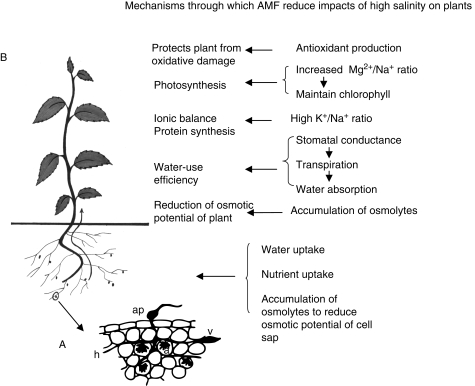
Several studies investigating the role of AMF in protection against salt stress have demonstrated that the symbiosis often results in increased nutrient uptake, accumulation of an osmoregulator, an increase in photosynthetic rate and water-use efficiency, suggesting that salt-stress alleviation by AMF results from a combination of nutritional, biochemical and physiological effects (Fig. 2). Studies carried out so far have suggested several mechanisms by which AM symbiosis can alleviate salt stress in host plants. The various mechanisms employed are discussed in the following sections.
Fig. 2.
The intricate functioning of arbuscular mycorrhizal (AM) fungi in ameliorating salt stress in plants. In AM symbiosis, the fungus forms an appressorium (ap) on the root surface and enters the root cortex by extending its hyphae (h). The hyphae form arbuscules (a) and vesicles (v) in the cortex. Salinity deprives plants of the basic requirements of water and nutrients, causing physiological drought and a decrease in osmotic potential accompanied by nutrient deficiency, rendering plants weak and unproductive. Arbuscular mycorrhiza help plants in salt stress by improving water and nutrient uptake: a decrease in osmotic potential is countered by increasing accumulation of osmolytes, and water-use efficiency, photosynthesis and antioxidant production (to scavenge ROS) is more efficient in salt-stressed plants in the presence of AMF (see text).
Plant growth and biomass
Under salt stress, plant growth and biomass suffered a setback. The reasons may be the non-availability of nutrients and the expenditure of energy to counteract the toxic effects of NaCl. However, mycorrhization was found to increase the fitness of the host plant by enhancing its growth and biomass. Several researchers have reported that AMF-inoculated plants grow better than non-inoculated plants under salt stress (Al-Karaki, 2000; Cantrell and Linderman, 2001; Giri et al., 2003; Sannazzaro et al., 2007; Zuccarini and Okurowska, 2008). It has been reported that mycorrhizal Acacia nilotica seedlings had higher root and shoot dry weight than the non-mycorrhizal seedlings (Giri et al., 2007). Al-Karaki (2000) observed a higher shoot and root dry weight, fresh fruit yield, fruit weight and fruit number in a mycorrhizal tomato plant than in a non-mycorrhizal tomato plant. Colla et al. (2008) reported improved growth, yield, water status, nutrient content and quality of fruits of Cucurbita pepo plants colonized by Glomus intraradices when exposed to salinity stress. Enhanced growth of AM plants has been partly attributed to mycorrhizically mediated enhanced nutrient acquisition, especially better P nutrition (Plenchette and Duponnis, 2005; Sharifi et al., 2007).
Nutrient uptake
AMF have been shown to have a positive influence on the composition of mineral nutrients (especially poor mobility nutrients such as phosphorus) of plants grown in salt-stress conditions (Al-Karaki and Clark, 1998) by enhancing and/or selective uptake of nutrients. This is primarily regulated by the supply of nutrients to the root system (Giri and Mukerji, 2004) and increased transport (absorption and/or translocation) by AMF (Al-Karaki, 2000; Sharifi et al., 2007). Mycorrhizal dependency increases with increasing salt concentrations (Giri and Mukerji, 2004). It is found to vary with the isolates of fungus and species of plant (Tian et al., 2004). A number of studies have shown the effect of salinity on the nutrient uptake of mycorrhizal plants (Table 2). The impact of mycorrhizal fungi on different mineral nutrients is given below.
Table 2.
Some examples of increased/decreased nutrient uptake in AM plants under salinity stress
| Nutrient | Range of salinity* | Plant | Fungus | Effect | References |
|---|---|---|---|---|---|
| Phosphorus | 0–24·6 dS m−1 (0–200 mm) | Glycine max | Glomus etunicatum | Increase | Sharifi et al. (2007) |
| 1·2–9·5 dS m−1 | Acacia nilotica | Glomus fasciculatum | Increase | Giri et al. (2007) | |
| 0–19·12 dS m−1 (0–150 mm) | Citrus karma | Mixed inoculum of Glomus sp. and Gigaspora sp. | Increase | Murkute et al. (2006) | |
| 0–6·10 dS m−1 (0–3 g kg−1) | Gossypium arboreum | Glomus mosseae | Increase | Tian et al. (2004) | |
| 3–10 dS m−1 (0·3–1·0 S m−1) | Acacia auriculiformis | Glomus macrocarpum and Glomus fasciculatum | Increase | Giri et al. (2003) | |
| 0–13·19 dS m−1 (0–100 mm) | Zea mays | Glomus mosseae | Increase | Feng et al. (2002) | |
| 1·4–7·4 dS m−1 | Lycopersicon esculentum | Glomus mosseae | Increase | Al-Karaki (2000) | |
| Nitrogen | 4–8 dS m−1 | Cajanus cajan | Glomus mosseae | Increase | Garg and Manchanda (2008) |
| 0–19·12 dS m−1 (0–150 mm) | Citrus karma | Mixed inoculum of Glomus sp. and Gigaspora sp. | Increase | Murkute et al. (2006) | |
| 15·8 dS m−1 (1·58 S m−1) | Sesbania aegyptiaca | Glomus macrocarpum | Increase | Giri and Mukerji (2004) | |
| Potassium | 0–7·56 dS m−1 (0–3 g L−1) | Ocimum basilicum | Glomus intraradices | Increase | Zuccarini and Okurowska (2008) |
| 0–24·6 dS m−1 (0–200 mm) | Glycine max | Glomus etunicatum | Increase | Sharifi et al. (2007) | |
| Calcium | 0–24·6 dS m−1 (0–200 mm) | Glycine max | Glomus etunicatum | Increase | Sharifi et al. (2007) |
| 0·72–7·39 dS m−1 | Musa sp. | Glomus clarum | Increase | Yano-Melo et al. (2003) | |
| Magnesium | 15·8 dS m−1 (1·58 S m−1) | Sesbania aegyptiaca | Glomus macrocarpum | Increase | Giri and Mukerji (2004) |
| Sodium | 1·2–9·5 dS m−1 | Acacia nilotica | Glomus fasciculatum | Increase | Giri et al. (2007) |
| 0–6·10 dS m−1 (0–3 g kg−1) | Gossypium arboreum | Glomus mosseae | Increase | Tian et al. (2004) | |
| 0·12 S m−1 | Acacia auriculiformis | Glomus macrocarpum and Glomus fasciculatum | Increase | Giri et al. (2003) | |
| 0–7·56 dS m−1 (0–3 g L−1) | Ocimum basilicum | Glomus intraradices | Decrease | Zuccarini and Okurowska (2008) | |
| 0–24·6 dS m−1 (0–200 mm) | Glycine max | Glomus etunicatum | Decrease | Sharifi et al. (2007) | |
| 1·4–7·4 dS m−1 | Lycopersicon esculentum | Glomus mosseae | Decrease | Al-Karaki (2000) | |
| Chloride | 0–6·10 dS m−1 (0–3 g kg−1) | Gossypium arboreum | Glomus mosseae | Increase | Tian et al. (2004) |
| 0–7·56 dS m−1 (0– g L−1) | Ocimum basilicum | Glomus intraradices | Decrease | Zuccarini and Okurowska (2008) | |
| Copper | 1·2–9·5 dS m−1 | Acacia nilotica | Glomus fasciculatum | Increase | Giri et al. (2007) |
| 1·4–7·4 dS m−1 | Lycopersicon esculentum | Glomus mosseae | Decrease | Al-Karaki (2000) | |
| Zinc | 0–24·6 dS m−1 (0–200 mm) | Glycine max | Glomus etunicatum | Increase | Sharifi et al. (2007) |
| 1·4–7·4 dS m−1 | Lycopersicon esculentum | Glomus mosseae | Decrease | Al-Karaki (2000) |
* The range of salinity within brackets is the actual salt concentrations used by the authors.
Phosphorus
Soil salinity significantly reduces the absorption of mineral nutrients, especially phosphorus (P), because phosphate ions precipitate with Ca2+, Mg2+ and Zn2+ ions in salt-stressed soils and become unavailable to plants (Azcón-Aguilar et al., 1979). Therefore, P solubilization or fertilization is necessary for plant growth which may be helpful in mitigating salt stress by overcoming P-binding capacity of the soil (Cantrell and Lindermann, 2001). Mycorrhizal inoculation can increase P concentration in plants by enhancing its uptake facilitated by the extensive hyphae of the fungus which allows them to explore more soil volume than the non-mycorrhizal plants (Ruiz-Lozano and Azcón, 2000). It is estimated that external hyphae deliver up to 80 % of a plant's P requirements (Matamoros et al., 1999). Studies have shown higher P content (1. 2, 1·2, 0·9 and 0·6 %) in mycorrhizal than non-mycorrhizal Acacia nilotica plants (0·6, 0·5, 0·2 and 0·1 %) in saline soils at varied levels of soil salinity (1·2, 4, 6·5 and 9·5 dS m−1), respectively (Giri et al., 2007). Recently, Shokri and Maadi (2009) recorded that phosphorus concentration in Trifolium alexandrium plants was reduced with increasing levels of salinity. They observed that as salinity of soil increased (2·2, 5 and 10 dS m−1), the concentrations of P decreased (0·41, 0·36 and 0·28 mg g−1, respectively) in shoots of non-mycorrhizal Trifolium alexandrium plants while the P concentrations were relatively higher (0·68, 0·62 and 0·47 mg g−1, respectively) in their mycorrhizal counterparts. The higher P concentrations in mycorrhizal plants at all salinity levels suggest that AMF increased P uptake in plants under saline conditions. Improved P nutrition in AM-inoculated plants may improve their growth rate, increase antioxidant production and enhanced nodulation and nitrogen fixation in legumes (Feng et al., 2002; Alguacil et al., 2003; Garg and Manchanda, 2008). Enhanced uptake of P by AMF in plants grown under saline conditions may reduce the negative effects of Na+ and Cl− ions by maintaining vacuolar membrane integrity, which facilitates compartmentalization within vacuoles and selective ion intake (Rinaldelli and Mancuso, 1996), thereby preventing ions from interfering in metabolic pathways of growth (Cantrell and Lindermann, 2001).
Nitrogen
Salinity interferes with nitrogen (N) acquisition and utilization by influencing different stages of N metabolism, such as, NO3− uptake and reduction and protein synthesis (Aslam et al., 1984, Frechill et al., 2001). Application of AMF can help in better assimilation of nitrogen in the host plant. Giri and Mukerji (2004) recorded higher accumulation of N in shoots of mycorrhizal Sesbania grandiflora and S. aegyptiaca than non-mycorrhizal control plants. The extra-radical mycelia take up inorganic nitrogen from the soil in the form of nitrate and assimilated it via nitrate reductase, located in the arbuscule-containing cells (Kaldorf et al., 1998) and the GS-GOGAT cycle leading to the formation of arginine. Arginine, so formed, is transported from the extra-radical to the intra-radical mycelia where it is catabolized again producing amongst other substances ammonia, which equilibrates with ammonium according to the pH. These processes are consistent with increased expression of enzymes involved in primary nitrogen fixation in the extra-radical mycelia, whereas enzymes involved in arginine catabolism are up-regulated in the intra-radical mycelia. However, little is known about the transfer of nitrogen from the fungus to the plant and the authors have proposed the involvement of ammonium transporters (Govindarajulu et al., 2005). Cliquet and Stewart (1993) observed increased N uptake in an AM plant due to a change in N metabolism brought about by changes in the enzymes associated with N metabolism (Mathur and Vyas, 1996). Studies have reported that improved N nutrition may help to reduce the toxic effects of Na ions by reducing its uptake and this may indirectly help in maintaining the chlorophyll content of the plant. The form of available N (NO3− or NH4+) strongly influences Na+ accumulation (Giri and Mukerji, 2004). However, the exact mechanisms used by AMF to uptake N under salt-stress conditions are not clearly understood.
K+ : Na+ ratio
When Na+ or salt concentration in the soil is high, plants tend to take up more Na+ resulting in decreased K+ uptake. Na+ ions compete with K+ for binding sites essential for various cellular functions. Potassium plays a key role in plant metabolism. It activates a range of enzymes, and plays an important role in stomatal movements and protein synthesis. High concentrations of K+ are required in protein synthesis as K+ is used in the binding of tRNA to the ribosomes (Blaha et al., 2000). These functions cannot be replaced by Na+ ions (Giri et al., 2007); a higher Na+ : K+ ratio generated due to salinity disrupts the ionic balance in the cytoplasm, consequently disrupting various metabolic pathways (Giri et al., 2007). Mycorrhizal colonization of a plant with AMF can reverse the effect of salinity on K+ and Na+ nutrition. Mycorrhizal colonization can enhance K+ absorption under saline conditions (Alguacil et al., 2003; Giri et al., 2007; Sharifi et al., 2007; Zuccarini and Okurowska, 2008) while preventing Na+ translocation to shoot tissues. Na+ uptake may also be influenced by the synthesis and storage of polyphosphate (Olrovich and Ashford, 1993) as well as by other cations, particularly K (Giri et al., 2003). The uptake of K+ increased in shoot tissues of mycorrhizal plants even at a high salinity level (9·5 dS m−1). This increases the K+ : Na+ ratio in roots and shoots of AM plants (Giri et al., 2007). It is accomplished by regulating the expression and activity of K+ and Na+ transporters and of H+ pumps that generate the driving force to transport ions (Parida and Das, 2005). The Na+/H+ antiporter catalyses the transfer of Na+ out of the cytoplasm into either vacuoles or apoplasm (Ouziad et al., 2006). The higher K+ : Na+ ratio helps to prevent the disruption of various K-mediated enzymatic processes and inhibition of protein synthesis. High K+ : Na+ ratios are also beneficial in influencing the ionic balance of the cytoplasm or Na+ efflux from plants (Allen and Cunningham, 1983; Founoune et al., 2002; Colla et al., 2008). Lower Na+ in the mycorrhizal plants than non-AM plants may also be explained by the dilution effect due to growth enhancement (Al-Karaki, 2000, 2006).
There are contrasting reports that AMF sometimes enhance Na+ uptake (Allen and Cunningham, 1983), while others suggest that AMF-colonized plants have lower levels of Na+ (Dixon et al., 1993; Sharifi et al., 2007; Zuccarini and Okurowska, 2008). The concentration of Na+ increased in AM plants with increasing salinity levels up to a certain level, and subsequently decreased at higher salinity. This suggests that AMF induce a buffering effect on the uptake of Na+ when the content of Na+ is within the permissible limit (Allen and Cunningham, 1983). This also indicates the possibility of a regulatory mechanism operating in the plant to contain Na+ ions. P uptake by mycorrhizal plants also decreased in an excessive salt concentration (9·5 dS m−1) due to the toxic effect of Na+ on fungal development (Giri et al., 2007), suggesting that mycorrhizal responses were only significant up to a certain level of salinity (4·7 dS m−1; Al-Karaki, 2000).
Chloride ions
Root cells take up Cl− from the soil solution through H+/Cl− symporters at low [Cl−]ext, and also through anion channels under saline conditions. To reach the xylem and then the shoot, Cl−traverses the root by a symplastic pathway and is released from cells within the stele through specific anion channels. At high salinity, Cl− accumulation increases greatly, though it remains constant in the roots (White and Broadley, 2002). The high tissue Cl− concentrations can be toxic to crop plants and may restrict agriculture in saline regions (Xu et al., 2000). This problem can be tackled to some extent by the application of arbuscular mycorrhiza, which can reduce the uptake of Cl− ions (Zuccarini and Okurowska, 2008). The Cl− ions can be compartmentalized in vacuolar membranes, thereby preventing them from interfering with the metabolic pathways in the plant (Cantrell and Lindermann, 2001). However, there are reports of increased Cl− accumulation due to mycorrhizal colonization, the reason of which may be due to the carbon drain imposed by mycorrhizal hyphae on plants, which enhances the translocation of highly mobile anions like Cl− from the soil (Bulwada et al., 1983; Graham and Syversten, 1984).
Calcium
Calcium acts as a second messenger and during salt stress the Ca2+ concentration is increased to transduce signals. Studies revealed that mycorrhization strongly affects Ca2+ in the plant. Cantrell and Linderman (2001) reported increased Ca2+ uptake in mycorrhizal lettuce. A higher Ca2+ concentration in mycorrhizal than in non-mycorrhizal banana plants was reported by Yano-Melo et al. (2003). High Ca2+ has a beneficial effect on toxic effects of NaCl by facilitating higher K+/Na+ selectivity leading to salt adaptation (Cramer et al., 1985; Rabie and Almadini, 2005). Moreover, high Ca2+ was also found to enhance colonization and sporulation of AMF (Jarstfer et al., 1998). However, in contrast to the reports above, Giri et al. (2003) reported that Ca2+ concentration remains unchanged in shoot tissues of mycorrhizal and non-mycorrhizal Acacia auriculiformis plants. This suggests that AMF may not be so important to the nutrients moving to plant roots by mass flow as compared with nutrients moving by diffusion (Tinker, 1975). Rhodes and Gerdemann (1978) indicated that Ca2+ is not translocated to onion roots through mycorrhizal hyphae as readily and efficiently as P. Moreover, AM inoculation depressed the Ca : P ratio by increased production of oxalate in the mycorrhizosphere, which is able to scavenge Ca2+ from the solution (Azcón and Barea, 1992).
Magnesium
Biosynthesis of chlorophyll is impeded by salt stress, which prevents light harvesting and causes impairment of photosynthesis. Mycorrhiza, by improving Mg2+ can support a higher chlorophyll concentration (Giri et al., 2003). This suggests that salt interfers less with chlorophyll synthesis in mycorrhizal than non-mycorrhizal plants (Giri and Mukerji, 2004). Effective Mg2+-uptake helps by increasing the chlorophyll concentration and hence improving photosynthetic efficiency and plant growth. Some examples of increased or reduced nutrient uptake in AM plants under salinity are enlisted in Table 2.
Biochemical changes
As soil dries out and soil water potential becomes more negative, plants must decrease their water potential to maintain a favourable gradient for water flow from soil into roots. To achieve such an effect, plants develop a plethora of mechanisms, the most important being osmotic adjustment or osmoregulation, which may require a reduction in the plant osmotic potential which is mitigated by active accumulation of organic ions or solutes (Morgan, 1984; Hoekstra et al., 2001). A number of nitrogen-containing compounds accumulate in plants exposed to saline stress. The most commmon of these include amino acids, amide and proteins; also quaternary ammonium compounds (betaines) and polyamines (Rabie and Almadini, 2005). These compounds are generally present in low concentrations when the plant is not under salt stress (Feng et al., 2002). The specific nitrogen-containing compounds that accumulate in saline environments vary with plant species (Rabie and Almadini, 2005). Osmoregulation allows cells to maintain turgor and turgor-dependent processes including cellular expansion and growth, stomatal opening and photosynthesis, while keeping a gradient of water potential favourable to water entering the plant. Table 3 lists the effects of salinity on various parameters on AM plants.
Table 3.
Some examples of increased/decreased content of the indicated compounds in AM plants compared with non-AM plants under saline conditions
| Compound | Range of salinity* | Plant | Fungus | Effect | References |
|---|---|---|---|---|---|
| Proline | 0–24·6 dS m−1 (0–200 mm) | Glycine max | Glomus etunicatum | Increase | Sharifi et al. (2007) |
| 0–6 dS m−1 | Vicia faba | Glomus clarum | Decrease | Rabie and Almadini (2005) | |
| Polyamines | 0–24·6 dS m−1 (0–200 mm) | Lotus glaber | Glomus intraradices | Increase | Sannazzaro et al. (2007) |
| Betaines | 0–34·5 dS m−1 (0–300 mm) | Phragmites australis | Glomus fasciculatum | Increase | Al-Garni (2006) |
| Carbohydrates | 0–34·5 dS m−1 (0–300 mm) | Phragmites australis | Glomus fasciculatum | Increase | Al-Garni (2006) |
| 0–24·6 dS m−1 (0–200 mm) | Glycine max | Glomus etunicatum | Decrease | Sharifi et al. (2007) | |
| Antioxidants | 0–13·19 dS m−1 (0–100 mm) | Glycine max | Glomus etunicatum | Increase | Ghorbanli et al. (2004) |
| 4–8 dS m−1 | Cajanus cajan | Glomus mosseae | Increase | Garg and Manchanda (2002) | |
| Abscissic acid | 0–24·6 dS m−1 (0–200 mm) | Lotus glaber | Glomus intraradices | Increase | Sannazzaro et al. (2007) |
| 0–13·19 dS m−1 (0–100 mm) | Lactuca sativa | Glomus intraradices | Decrease | Jahromi et al. (2008) | |
| Chlorophyll | 15·8 dS m−1 (1·58 S m−1) | Sesbania aegyptiaca and Sesbania grandiflora | Glomus macrocarpum | Increase | Giri and Mukerji (2004) |
| 0–4·24 dS m−1 (0–2 g kg−1) | Zea mays | Glomus mosseae | Increase | Sheng et al. (2008) | |
| 0·84–5·8 dS m−1 | Lactuca sativa | Mixture of Glomus mosseae, Glomus intraradices and Glomus coronatum | Increase | Zuccarini (2007) | |
| Chlorophyll fluorescence | 0–7·56 dS m−1 (0–3 g L−1) | Ocimum basilicum | Glomus intraradices | Increase | Zuccarini and Okurowska (2008) |
* The range of salinity within brackets is the actual salt concentrations used by the authors.
Proline
Accumulation of amino acid proline is one of the most frequently reported modifications induced by water and salt stress in plants. Under saline conditions, many plants accumulate proline as a non-toxic and protective osmolyte to maintain osmotic balance under low water potentials (Stewart and Lee, 1974; Jain et al., 2001; Parida et al., 2002; Ashraf and Foolad, 2007; Sannazzaro et al., 2007). It also acts as a reservoir of energy and nitrogen for utilization during salt stress (Goas et al., 1982). Proline accumulation has been found to increase when the plant is colonized by AMF. Mycorrhizal mung bean (Vigna radiata) plants were reported to have a higher proline content than non-mycorrhizal plants at 12·5 and 25 mm NaCl at 40 and 60 d after sowing (Jindal et al., 1993). Sharifi et al. (2007) also reported a higher proline concentration in AM soybean than the non-AM plants at different salinity levels (0, 50, 100, 150 and 200 mm NaCl). They also observed that in AM plants, a higher level of proline concentration is found in roots than shoots. This may be due to the fact that the roots are the primary sites of water absorption and, therefore, must maintain osmotic balance between water-absorbing root cells and the external media. However, in contrast to the reports above, Rabie and Almadini (2005) reported that non-AM Vicia faba plants accumulated more proline than AM plants at various salinity levels (0–6 dS m−1). S. Wang et al. (2004) suggested that proline accumulation in plants may be a symptom of stress in less-salt-tolerant species and its contribution to osmotic adjustment was apparently negligible as compared with potassium ions. The accumulation of proline may also be due to salinity and not necessarily by mycorrhizal colonization as reported by Sannazzaro et al. (2006). High levels of proline are known to accumulate in Lotus glaber in response to salinity (Maiale et al., 2004) but, so far there are no reports regarding the influence of AMF on proline accumulation in this plant. The inconsistency in these reports needs to be elucidated to determine the salt-tolerance mechanisms operating in different plant systems.
Betaines
Accumulation of betaines in plants under salt stress is a common occurrence. Betaines are quaternary ammonium compounds which are N-methylated derivatives of amino acids. Once formed, they are seldom metabolized (Grattan and Grieve, 1985; Duke et al., 1986). This can therefore be used as an effective indicator of salt stress (Duke et al., 1986). Betaines are not merely non-toxic cellular osmolytes but they can also stabilize the structures and activities of enzymes and protein complexes and maintain the integrity of membranes against the damaging effects of excessive salt (Gorham, 1995). Accumulation of betaines under salt stress is found to increase when the plant is colonized by AMF. It was found that at higher salinity levels the glycine betaine content of AM plants was about 2-fold greater than that of non-AM plants (Al-Garni, 2006).
Polyamines
Free polyamines are small organic cations that are necessary for eukaryotic cell growth. There are three main polyamines in plants: putrescine (Put), spermidine (Spd) and spermine (Spm). Spd and Spm are synthesized from Put by successive addition of aminopropyl groups and Put is synthesized directly from ornithine via ornithine decarboxylase or indirectly following decarboxylation of arginine by arginine decarboxylase. These cations are thought to play an important role in plant responses to a wide array of environmental stressors such as salinity (Delauney and Verma, 1993; Krishnamurthy and Bhagwat, 1989), high osmolarity (Besford et al., 1993) and anti-oxidative stress (Langebartels et al., 1991; Kurepa et al., 1998). They have been proposed as candidates for regulation of root development under saline situations (Couée et al., 2004). Under saline conditions, free polyamine pools are reduced. However, inoculation of host plants with AMF increases free polyamine concentrations. Sannazzaro et al. (2007) reported an increase in total free polyamine pools in Lotus glaber plants colonized by Glomus intraradices. They observed variations in individual polyamines in response to salinity and mycorrhization depending on the plant genotype and organ (root/shoot) considered. Mycorrhizal, salt-tolerant genotypes of Lotus glaber plants showed higher levels of root Spm than non-AM plants. Mycorrhizal salt-sensitive Lotus glaber plants under salinity showed higher root Spm, lower shoot and root Put and lower Spd levels than the corresponding non-AM plants. It may be inferred that modulation of polyamine pools can be one of the mechanisms used by AMF to improve plant adaptation to saline soils (Sannazzaro et al., 2007).
Carbohydrates
Several studies report the accumulation of soluble sugars to adjust the osmotic potential of plants during salt stress – a means of lowering the osmotic potential of the plant which constitutes an important plant protection mechanism against stress (Thanna and Nawar, 1994). The soluble sugar content of Phragmites australis was significantly increased by increasing concentrations of NaCl (Al-Garni, 2006). The increase in total carbohydrates is found to be positively correlated with mycorrhization of the host plant as reported by Thomson et al. (1990). Al-Garni (2006) reported that Phragmites australis plants colonized by Glomus fasciculatum had higher levels of soluble sugars than those non-mycorrhizal plants. Porcel and Ruiz-Lozano (2004) also reported increased sugar concentrations in soybean roots colonized by Glomus intraradices. The positive correlation between sugar content and mycorrhization is due to the sink effect of the fungus demanding sugars from the shoot tissues (Augé, 2000). The processes involved in the development of mycorrhiza frequently lead to increased rates of photosynthesis and of carbon compounds to the root systems of host plants (Finlay and Söderström, 1992). The increased sugar accumulation may also be due to hydrolysis of starch to sugars in the seedlings inoculated with mycorrhiza (Nemec, 1981). Feng et al. (2002) studied the prevalence of correlation between P concentration and sugar accumulation in host plants under saline conditions. They reported that in spite of similar P concentrations, the soluble sugar concentration in roots of mycorrhizal plants was higher than that of the non-mycorrhizal maize plants. This suggests that the higher soluble sugar concentration in mycorrhizal roots is due to AMF colonization and not due to an improvement in the P status of the plants. Trehalose, a non-reducing disaccharide, is the main storage carbohydrate in AMF and has been found to play an important role as an abiotic stress protectant stabilizing dehydrated enzymes and membranes and protecting biological structures from desiccation damage. It is present in the extra-radical mycelium as well as in spores of AMF (Becard et al., 1991). In higher vascular plants, trehalose is a rare sugar, but it gets induced on AMF colonization of plant roots (Hoekstra et al., 1992; Schubert et al., 1992) and thus may help in protecting plants against salt stress. Recently, Ocon et al. (2007) made an attempt to decipher the effect of salt stress on trehalose content and metabolism in the extra-radical hyphae of Glomus intraradices and its possible role in protecting plants against abiotic stresses. They did not observe any change in trehalose content in Glomus intraradices when treated with 0·5 m NaCl. However, moderate transient activations of trehalose-6-phosphate phosphatase (required for the conversion of trehalose-6-phosphate to trehalose and orthophosphate) and neutral trehalase (required for the breakdown of trehalose into glucose) activities not associated with any transcriptional change were observed. Studying the accumulation of trehalose in extra-radical hyphae and mycorrhizal roots is of interest and it might provide a powerful insight into the response of AMF to stress conditions and trehalose being exploited as a stress-protective agent. Trehalose metabolism has been found to play a significant role in adaptation to hyperosmotic conditions of symbiotic bacteria (Lopez et al., 2008). This strengthens the hypothesis that there is a role for this molecule in imparting tolerance against salt stress. Therefore, investigations are required to evaluate the potential of trehalose in protecting the cell from salt stress.
Conversely, some authors reported negative correlations between AMF colonization and sugar accumulation in host plants. Pearson and Schweiger (1993) reported a reduction in carbohydrate concentration with an increase in the percentage of root colonization. Sharifi et al. (2007) observed no role of soluble carbohydrates in responses of AM (colonized by Glomus etunicatum) soybean plants to salinity.
Antioxidants
Activated oxygen species such as singlet oxygen, superoxide anion (O2•−), hydrogen peroxide and hydroxyl radical (•OH) are inevitable by-products of the interaction between oxygen and electrons leaked from the electron transport chains in chloroplast and mitochondria during normal aerobic metabolism (Scandalios, 1993; Asada, 1999; Møller, 2001). All activated oxygen species can react with DNA, proteins and lipids (Fridovich, 1986) and in the absence of the protective mechanism; they can damage cell structure and function (Alguacil et al., 2003). Thus plants have protective mechanisms to escape from oxidative damage (Jiang and Zhang, 2002; Núñez et al., 2003; Yamane et al., 2004) involving antioxidant molecules and enzymes (Jiang and Zhang, 2002; Núñez et al., 2003). A correlation between antioxidant capacity and NaCl tolerance has been demonstrated in several plant species (Gossett et al., 1994; Benavides et al., 2000; Núñez et al., 2003). Plants with high concentrations of antioxidants have been reported to have greater resistance to oxidative damage (Spychalla and Desbough, 1990; Dionisio-Sese and Tobita, 1998; Jiang and Zhang, 2002). Antioxidants include superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APOX), glutathione reductase (Alguacil et al., 2003; Ghorbanli et al., 2004; Yamane et al., 2004; Wu et al., 2006), dehydroascorbate reductase (Ghorbanli et al., 2004, Wu et al., 2006), monodehydroascorbate reductase (Ghorbanli et al., 2004), guaiacol peroxidase, oxidized glutathione (Wu et al., 2006), glutathione peroxidase, and the enzymes involved in the ascorbate–glutathione cycle (Alguacil et al., 2003). The non-enzymatic compounds which scavenge activated oxygen species include carotenoids, glutathione, tocopherols and ascorbic acid (Alguacil et al., 2003; Wu et al., 2006).
Several studies suggested that AM symbiosis helps plants to alleviate salt stress by enhancing the activities of antioxidant enzymes (Alguacil et al., 2003; Harisnaut et al., 2003; Zhong Qun et al., 2007). Several researchers have observed higher antioxidant enzyme activity in mycorrhizal plants than the non-mycorrhizal plants. Ghorbanli et al. (2004) have shown higher activities of SOD, peroxidase, APOX activities in mycorrhizal soybean plants than in non-mycorrhizal plants. However, the activities of CAT and polyphenol peroxidase remain unchanged in both the plants. The higher activities of peroxidase and polyphenol peroxidase (a component of the plant defence mechanism against pathogens) in mycorrhizal plants have been reported by Mathur and Vyas (1996). Alguacil et al. (2003) also reported enhanced activities of CAT, APOX and SOD in Olea europaea and Retama sphaerocarpa. The increased SOD will help detoxify superoxide to hydrogen peroxide (Smirnoff, 1993). The hydrogen peroxide generated will be detoxified by CAT and peroxidase. APOX is also reported to be involved in detoxification of hydrogen peroxide produced in the chloroplasts of stressed host plants (Lopez et al., 1996; Benavides et al., 2000). The elevated levels of glutathione reductase activity may serve to ensure the availability of NADP+ to accept electrons derived from photosynthetic electron transport, thereby directing electrons away from oxygen and minimizing the production of O2•− (Gamble and Burke, 1984; Menconi et al., 1995).
The plants possess higher antioxidant enzyme activities as a result of mycorrhizal colonization but the response of the individual enzymes varies with respect to the host plant and the fungal species. This variation may also depend on the micronutrients available to some of the enzymes, e.g. CAT, APOX and SOD are metalloenzymes (Alguacil et al., 2003) whose activities depend on availability. The activity of these metalloenzymes can be determined by the availability of the metals they utilize. Both excess and deficiency of micronutrients can modulate the expression of these metalloenzymes. For example, the presence of iron can enhance the activities of CAT and APX in Nicotiana plumbaginifolia (Kamfenkel et al., 1995). The increase in Fe, Cu, Zn and Mn in shoots of plants inoculated with mycorrhiza could be involved in the increase in total SOD activity observed in mycorrhizal plants. These data indicate that mycorrhiza-induced activities of several antioxidant enzymes may be related to enhanced growth and acquisition of P or N in the plants (Alguacil et al., 2003). However, the role of AMF on the status of non-enzymatic antioxidants such as carotenoids, tocopherols and ascorbic acid of the host plant has not been reported. Therefore, this aspect requires an in-depth investigation.
Physiological changes
Salt stress can affect the plant by disrupting its physiological mechanisms such as decreasing photosynthetic efficiency, gas exchange, membrane disruption, water status, etc. There is evidence demonstrating that AM symbiosis can alleviate such effects by employing various mechanisms which are discussed below.
Chlorophyll content
Increasing salinity causes a reduction in chlorophyll content (Sheng et al., 2008) due to suppression of specific enzymes that are responsible for the synthesis of photosynthetic pigments (Murkute et al., 2006). A reduction in the uptake of minerals (e.g. Mg) needed for chlorophyll biosynthesis also reduces the chlorophyll concentration in the leaf (El-Desouky and Atawia, 1998). A higher chlorophyll content in leaves of mycorrhizal plants under saline conditions has been observed by various authors (Giri and Mukerji, 2004; Sannazzaro et al., 2006; Zuccarini, 2007; Colla et al., 2008; Sheng et al., 2008). This suggests that salt interfers less with chlorophyll synthesis in mycorrhizal than in non-mycorrhizal plants (Giri and Mukerji, 2004). In the presence of mycorrhiza, the antagonistic effect of Na+ on Mg2+ uptake is counterbalanced and suppressed (Giri et al., 2003). Inoculated plants under salt stress reach levels of photosynthetic capacity (estimated by chlorophyll content) even superior to those of non-stressed plants, showing that in this respect, mycorrhization is capable of fully counterbalancing stress (Zuccarini, 2007).
Chlorophyll fluorescence
Chlorophyll fluorescence is a measure of photosynthetic efficiency. It is calculated as the ratio between variable and maximum fluorescence (Fv/Fm) (Sheng et al., 2008; Zuccarini and Okurowska, 2008). The ratio Fv : Fm measures the capacity of the primary photochemistry of PSII which itself is particularly sensitive to a variety of environmental stress-inducing factors (Figueroa et al., 1997). Salt stress could destroy the PSII reaction centre and disrupt electron transport in the photosynthetic apparatus of the plants. This toxic influence of salinity on the PSII reaction centre could be mitigated by AM symbiosis (Sheng et al., 2008). The ratio Fv : Fm in the leaves of mycorrhizal plants was significantly higher than that in non-AM plants (Sheng et al., 2008; Zuccarini and Okurowska, 2008). Mycorrhiza-inoculated plants also showed higher non-photochemical quenching than the uninoculated plants (Sheng et al., 2008). An increase in non-photochemical quenching can occur as a result of processes that protect the leaf from light-induced damage (Maxwell and Johnson, 2000). AM symbiosis also triggers the regulation of energy bifurcation between photochemical and non-photochemical events (Sheng et al., 2008). Studies that showed a higher or lower content of the various compounds in AM plants compared with non-AM plants are listed in Table 3.
Relative permeability
Arbuscular mycorrhizal fungal inoculation of host plants enables plants to maintain a higher electrolyte concentration than the non-mycorrhizal plants by maintaining improved integrity and stability of the membrane (Feng et al., 2002; Garg and Manchanda, 2008; Kaya et al., 2009). Consequently, electrical conductivity of mycorrhizal roots was found to be higher than the non-mycorrhizal roots (Garg and Manchanda, 2008). The mycorrhizal Cajanus cajan roots showed a higher relative permeability than the non-mycorrhizal plants at different levels of soil salinity (4, 6 and 8 dS m−1 ECe; Garg and Manchanda, 2008). Kaya et al. (2009) reported that the electrolyte leakage in leaves of Capsicum annum treated with 50 mm and 100 mm concentrations of NaCl were 31·66 and 42·45, respectively while the AMF-inoculated plants had a relatively lower electrolyte leakage of 26·87 and 30·98, respectively, This suggests that mycorrhizal plants had a much lower root plasma membrane electrolyte permeability than the non-mycorrhizal plants. The increased membrane stability has been attributed to mycorrhiza-mediated enhanced P uptake and increased antioxidant production (Feng et al., 2002).
Abscissic acid (ABA) content
It has been documented that mycorrhization can alter the ABA levels in the host plant (Duan et al., 1996; Ludwig-Muller, 2000; Estrada-Luna and Davies, 2003). Sannazzaro et al. (2007) reported higher ABA levels in Lotus glaber plants colonized by Glomus intraradices than non-colonized plants. They suggested that ABA regulates free spermine pools in the shoots and the increased spermine content in the mycorrhizal plant may be due to increased ABA content. However, in contrast to this report, Jahromi et al. (2008) reported lower ABA levels in lettuce plants colonized by Glomus intraradices than in the non-AM plants, indicating that AM plants are less strained by imposed salinity stress than non-AM plants and, hence, accumulated less ABA. Comparing the above observations, it might be suggested that the effect of AMF species on ABA content varies with the host plants.
Water status
Plants in saline soils are subjected to physiological drought as Na+ and Cl− ions bind water that is needed to be mobilized by the plants (Fuzy et al., 2008). Studies have indicated that colonization by AMF can help plants in such situations. Many authors have reported that plants inoculated with AMF maintain a relatively higher water content compared with uninoculated plants (Colla et al., 2008; Jahromi et al., 2008; Sheng et al., 2008). This is facilitated by the improved hydraulic conductivity of the root at low water potential (Kapoor et al., 2008). The improved root conductance is associated with a longer root and an altered root system morphology induced by AMF (Dehne, 1982, Kothari et al., 1990). AM plants are found to exhibit a higher stomatal conductance thereby increasing the demand for transpiration (Duan et al., 1996; Ruiz-Lozano et al., 1996; Dell'Amico et al., 2002; Jahromi et al., 2008; Sheng et al., 2008). Mycorrhizal plants are also shown to possess a lower osmotic potential which is maintained by fungal accumulating solutes, consequently resulting in improved plant osmotic adjustment. Lower water saturation deficit and higher turgor potential in AM plants also improves the water status of the plant (Al-Garni, 2006; Sheng et al., 2008). All these improved parameters facilitated by mycorrhizal colonization enable host plants to use water more efficiently (Graham and Syversten, 1984) allowing them to maintain a lower intercellular carbon dioxide concentration. As a consequence, the gas exchange capacity increases in the mycorrhizal plants.
Nodulation and nitrogen fixation
Nodules, formed through symbiosis with nitrogen-fixing bacteria are considered a soft target for salt stress and their occurrence decreases due to salt stress (Harisnaut et al., 2003; Rabie and Almadini, 2005; Garg and Manchanda, 2008). This is likely due to premature nodule senescence triggered by salt stress (Gogorcente et al., 1997; Gonzalez et al., 1998; Matamoros et al., 1999) which causes an acceleration of lytic activities, formation of green pigments from leghaemoglobin (Sarath et al., 1986) and loss of nitrogen fixation (Delgado et al., 1994). Application of AMF can counteract the harmful effects of salinity on nodulation and nitrogen fixation in legumes. There are reports that AM symbiosis could alleviate drought stress-induced premature nodule senescence (Ruiz-Lozano et al., 2001; Porcel et al., 2003). Giri and Mukerji (2004) reported a strong effect of mycorrhizal inoculation on nodule formation under salt stress. Colonization of a legume by AMF can increase the number of nodules (Giri and Mukerji, 2004; Rabie and Almadini, 2005; Garg and Manchanda, 2008). This may indicate a positive influence of AMF on legume–nitrogen-fixing bacteria symbiosis. Higher leghaemoglobin content was observed in mycorrhizal plants. The leghaemoglobin content was determined by estimating the change of colour in the nodule from pink to brownish pink due to synthesis of green pigments from leghaemoglobin. This greening of nodule was observed much earlier in non-AM plants (8 weeks) than AM plants (10 weeks; Garg and Manchanda, 2008). Mycorrhizal plants also possess a higher nitrogenase activity. All these parameters contribute to the higher nitrogen-fixing ability of AM plants. This increased nitrogenase activity and nitrogen fixation in AM plants as opposed to non-AM plants has been attributed to relief from P stress, which is beneficial for the functioning of the nitrogenase enzyme of the bacterial symbionts and possibly due to uptake of some essential micro-nutrients which results in either improved growth of plants (Founoune et al., 2002) or vice versa (Rabie and Almadini, 2005). Therefore it may be suggested that mycorrhizal and nodule symbioses often act synergistically on infection rate, mineral nutrition and plant growth (Patreze and Cordeiro, 2004; Rabie, 2005) which supports the need for both N and P and increased tolerance of plants to salinity stress (Rabie and Almadini, 2005).
Molecular changes
Studies describing the effects of AMF on molecular responses to saline conditions are very rare due to the complex nature of the trait. The fact that the factors conferring salt tolerance to plants are generally encoded by complex gene families hampers any study evaluating the impact of mycorrhizal colonization on expression of genes with products involved in salt tolerance with the need to track numerous variables (Ouziad et al., 2006). The heterokaryotic and obligate nature of AMF also substantiates the difficulty. However, molecular studies on salt tolerance by AMF are gaining a fast momentum as scientists are trying to unravel the molecular mechanisms of AM symbiosis to gain a whole understanding of the alleviation of salt stress by AM symbiosis.
In the few molecular studies done so far, the focus has been on the expression studies of a few proteins, including Na+/H+ antiporters, Δ1-pyrroline-5-carboxylate synthetase (LsP5CS), late embryogenesis abundant protein (LsLea) and ABA (Lsnced).
Aquaporins belong to the major intrinsic protein (MIP) family of transmembrane channels, which permit the selective membrane passage of water (and a few other compounds) but not of H+ and other ions (Weig et al., 1997; Chen et al., 2001; Hill et al., 2004) through the plasma lemma (by PIPs) and the tonoplast (by TIPs). It has been reported that abiotic factors such as drought and salt stress influence aquaporin expression most probably via phytohormones like ABA and giberrellic acid (Mariaux et al., 1998; Siefritz et al., 2001). Expression analysis of aquaporin genes in salt-stressed AM plants revealed contrasting results. Data from Ouziad et al. (2006) showed that continuous salt treatment in AM Lycopersicon esculentum down-regulated the amount of LeTIP and LePIP1 transcripts by roughly 20 % but not of LePIP2 transcripts. They also observed that the effect on transcript formation of aquaporin was more drastic after AMF colonization than after salinity. In the communication, AMF significantly reduced the mRNA transcripts of LePIP1 and LeTIP but not of LePIP2 in non-treated controls and salt-stressed roots. In contrast to the report of Ouziad et al. (2006), Aroca et al. (2007) reported that salt treatment (0·12–3·06 dS m−1) induces a higher expression of all PvPIP genes in AM Phaseolus vulgaris plants than non-AM plants except the PvPIP1;2 gene which reduced its expression in AM root after salt treatment, while maintaining its expression in non-AM roots. They also reported an increase in PIP1 protein in AM plants. Likewise, Jahromi et al. (2008) reported that under salt-stress conditions (0–100 mm NaCl), mycorrhizal lettuce plants maintained the expression of the LsPIP2 gene and up-regulation of LsPIP1 gene mainly at 100 mM NaCl. The enhanced expression of the PIP1 gene and abundance of its protein could contribute to regulating root water permeability and, consequently, to better tolerance of the osmotic stress generated by salinity (Aroca et al., 2007; Jahromi et al., 2008). These results point to the possibility that AMF differentially exerts control on the expression of these genes (Ouziad et al., 2006) and that each PIP gene analysed may have a different function and regulation in AM symbiosis. How much this contrasting result reflects biological or technical differences remains to be evaluated.
Ouziad et al. (2006) also evaluated the expression of two Na+/H+ antiporters – LeNHX1 and LeNHX2 – in dependence on salt and mycorrhizal colonization and reported that no significant alterations are observed under these conditions.
The expression of genes encoding Δ1-pyrroline-5-carboxylate synthetase (LsP5CS), late embryogenesis abundant protein (LsLea) and ABA (Lsnced) were determined following varied salt treatments (0–100 mm NaCl) on Lactuca sativa plants colonized by Glomus intraradices (Jahromi et al., 2008). The PC5S enzyme catalyses the rate-limiting step in the biosynthesis of proline (Kishor et al., 1995), an osmoregulator in plants. Late embryogenesis abundant proteins act as stress markers. They also possess chaperone-like activity, thus having a protective role during osmotic stress. Lsnced encodes for 9-cis-epoxycarotenoid dioxygenase, a key enzyme in the biosynthesis of the stress hormone ABA. ABA promotes stomatal closure to minimize transpirational water loss. It also mitigates stress damage through the activation of many stress-responsive genes, which collectively increase plant stress tolerance (Bray, 2002). They reported a higher expression of genes LsP5CS and Lsnced in non-AM plants than AM plants at 50 mm NaCl, though at 100 mm, the levels were similar. The LsLea gene was found to express under conditions of salt stress and the induction of this gene was found to be lower in AM plants than non-AM plants. The lower expression of this gene suggests that AM plants suffer less stress than non-AM plants, which may be due to a primary salt avoidance mechanism such as Na+ and Cl− accumulation (Giri et al., 2003; Al-Karaki, 2006).
Ultra-structural changes
Salinity is reported to bring about drastic ultra-structural changes in plants (Yamane et al., 2004; Mahmoodzadeh, 2008). Salt stress caused an increase in membrane surface and quantity of vesicles in root cells of Sorghum (Koyro, 1997). Thickened cell wall, increased frequency of plasmodesmata and vacuolization of cytoplasm were reported in the shoot apical meristem of canola (Mahmoodzadeh, 2008) due to salt stress. Yamane et al. (2004) observed disruptions in thyllakoid and thyllakoid membrane, while Sun et al. (2004) reported induction of ovule abortion in Arabidopsis thaliana due to dissipation of mitochondrial membrane potential (Hauser et al., 2006). Subsequently, cells in the gametophyte accumulate reactive oxygen species which gradually leads to programmed cell death (Hauser et al., 2006). The aborting gametophytes develop concentric rings of endoplasmic reticulum (autophagic bodies) surrounding chloroplasts, mitochondria, micro-bodies and cytoplasm. The cytoplasmic contents and organelles were invaginated into the vacuole. However, the formation of autophagic bodies and vacuoles is not found in every aborting ovule suggesting that these events are not related to programmed cell death (Hauser et al., 2006). Up till now; there have been no published reports on the effect of AM in plants under this aspect of salt stress. Since, AMF inoculation can increase antioxidant activities in plants, it may be suggested that AMF can be applied to counteract the activities of reactive oxygen species and alleviate salt stress. Unfortunately, the role of AMF in this aspect has not yet been deciphered. Therefore, this aspect seeks more attention from the researchers to unveil the mechanism of salt-stress alleviation by AMF.
FUTURE PERSPECTIVES
While research over many years has broadened our understanding of the multi-complex processes directing plant–mycorrhiza symbiosis in ameliorating salt stress in plants, yet, there are several aspects that need to be addressed. Investigation of these issues in the future will lead to a better understanding of the process.
Our knowledge of the molecular mechanisms governing the process of salt amelioration by AMF in plants is limited to partial understanding of only a few genes. The role of salt overly sensitive (SOS) genes with respect to mycorrhizal application needs to be uncovered. Identification of the genes involved in production of various antioxidants and enzymes controlling the synthesis of various osmoregulators will provide further insights into the molecular basis of the mechanism.
The transporter systems operating in the symbiosis need to be elucidated. Various techniques such as PIXE (proton-induced X-ray emission) may also be employed for studying the localization of various micro- and macro-nutrients in the plant as well as the fungus.
The ultrastructural aspects of AM plants under salinity have remained untouched till now. Demonstrating the role of AMF in this aspect may contribute significantly to our understanding of the mechanism.
In vitro studies of the fungus have just begun to explore morphology under salt stress. Such studies need to be directed towards biochemical, physiological and molecular mechanisms and signalling systems operating in the fungus to confer tolerance of salt stress in plants.
ACKNOWLEDGEMENTS
Heikham Evelin thanks the Council of Scientific and Industrial Research (CSIR), New Delhi, India for financial support. The authors thank Mr Ajay Bahadur Singh for his assistance during the preparation of the manuscript.
LITERATURE CITED
- Abrol JP. Fuel and forage production from salt affected wasteland in India. Reclamation and Revegetation Research. 1986;5:65–74. [Google Scholar]
- Adiku G, Renger M, Wessolek G, Facklam M, Hech-Bischoltz C. Simulation of dry matter production and seed yield of common beans under varying soil water and salinity conditions. Agricultural Water Management. 2001;47:55–68. [Google Scholar]
- Al-Garni SMS. Increasing NaCl-salt tolerance of a halophytic plant Phragmites australis by mycorrhizal symbiosis. American-Eurasian Journal of Agricultural and Environmental Science. 2006;1:119–126. [Google Scholar]
- Alguacil MM, Hernandez JA, Caravaca F, Portillo B, Roldan A. Antioxidant enzyme activities in shoots from three mycorrhizal shrub species afforested in a degraded semi-arid soil. Physiologia Plantarum. 2003;118:562–570. [Google Scholar]
- Aliasgharzadeh N, Saleh Rastin N, Towfighi H, Alizadeh A. Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil. Mycorrhiza. 2001;11:119–122. doi: 10.1007/s005720100113. [DOI] [PubMed] [Google Scholar]
- Al-Karaki GN. Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza. 2000;10:51–54. [Google Scholar]
- Al-Karaki GN. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Scientia Horticulturae. 2006;109:1–7. [Google Scholar]
- Al-Karaki GN, Al-Raddad A. Effect of arbuscular mycorrhizal fungi and drought stress on growth and nutrient uptake of two wheat genotypes differing in drought resistance. Mycorrhiza. 1997;7:83–88. [Google Scholar]
- Al-Karaki GN, Clark RB. Growth, mineral acquisition and water use by mycorrhizal wheat grown under water stress. Journal of Plant Nutrition. 1998;21:263–276. [Google Scholar]
- Allen EB, Cunningham GL. Effects of vesicular-arbuscular mycorrhizae on Distichlis spicata under three salinity levels. New Phytologist. 1983;93:227–236. [Google Scholar]
- Antoniolli ZI, Schachtman K, Ophel-Keller K, Smith SE. Variation on rDNA ITS sequences in Glomus mosseae and Gigaspora margarita from a permanent pasture. Mycologia Research. 2000;104:708–715. [Google Scholar]
- Aroca R, Porcel R, Ruiz-Lozano JM. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytologist. 2007;173:808–816. doi: 10.1111/j.1469-8137.2006.01961.x. [DOI] [PubMed] [Google Scholar]
- Asada K. The water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:609–931. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany. 2007;59:207–216. [Google Scholar]
- Aslam M, Huffaker RC, Rais DW. Early effects of salinity on nitrate assimilation in barley seedlings. Plant Physiology. 1984;76:321–325. doi: 10.1104/pp.76.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augé RM. Stomatal behaviour of arbuscular mycorrhizal plants. In: Kapulnik Y, Douds DD, editors. Arbuscular mycorrhizas: physiology and functions. Drodrecht: Kluwer Academic Publishers; 2000. pp. 201–237. [Google Scholar]
- Azcón R, Barea JM. The effect of vesicular arbuscular mycorrhizae in decreasing Ca acquisition by alfalfa plants in calcareous soils. Biology and Fertility of Soils. 1992;13:155–159. [Google Scholar]
- Azcón-Aguilar C, Azcón R, Barea JM. Endomycorrhizal fungi and Rhizobium as biological fertilizers for Medicago sativa in normal cultivation. Nature. 1979;279:325–327. [Google Scholar]
- Bago B, Azcón-Aguilar C, Goulet A, Piche Y. Branched absorbing structure (BAS), a feature of the extraradical mycelium of symbiotic arbuscular fungi. New Phytologist. 1998;139:375–388. [Google Scholar]
- Baker A, Sprent JI, Wilson J. Effects of sodium chloride and mycorrhizal infection on the growth and nitrogen fixation of Prosopis juliflora. Symbiosis. 1995;19:39–51. [Google Scholar]
- Barrow JR, Havstad KM, McCaslin BD. Fungal root endophytes in four wing saltbrush, Atriplex canescens, on arid rangeland of southwestern USA. Arid Soil Research and Rehabilitation. 1997;11:177–185. [Google Scholar]
- Becard G, Piche Y. Fungal growth stimulation by CO2 and root exudates in vesicular-arbuscular mycorrhizal simbiosis. Applied Environmental Microbiology. 1989;55:2320–2325. doi: 10.1128/aem.55.9.2320-2325.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becard G, Doner LW, Rolin DB, Douds DD, Pfeffer PE. Identification and quantification of trehalose in vesicular arbuscular mycorrhizal fungi in vivo 13C NMR and HPLC analyses. New Phytologist. 1991;108:547–552. [Google Scholar]
- Benavides MP, Marconi PL, Gallego SM, Comba ME, Tomaro ML. Relationship between antioxidant defense systems and salt tolerance in Solanum tuberosum. Australian Journal of Plant Physiology. 2000;27:273–278. [Google Scholar]
- Besford RT, Richardson CM, Campos JL, Tiburico AF. Effect of polyamines on stabilization of molecular complexes in thyllakoid membranes of osmotically stressed oat leaves. Planta. 1993;189:201–206. [Google Scholar]
- Bhaskaran C, Selvaraj T. Seasonal incidence and distribution of VAM fungi in native saline soils. Journal of Environmental Biology. 1997;18:209–212. [Google Scholar]
- Blaha G, Stelzl U, Spahn CMT, Agrawal RK, Frank J, Nierhaus KH. Preparation of functional ribosomal complexes and effect of buffer conditions on tRNA positions observed by cryoelectron microscopy. Methods in Enzymology. 2000;317:292–309. doi: 10.1016/s0076-6879(00)17021-1. [DOI] [PubMed] [Google Scholar]
- Bray EA. Abscissic acid regulation of gene expression during water-deficit stress in the era of Arabidopsis genome. Plant, Cell and Environment. 2002;25:153–161. doi: 10.1046/j.1365-3040.2002.00746.x. [DOI] [PubMed] [Google Scholar]
- Brown ME. Seed and root bacterization. Annual Review of Phytopathology. 1974;12:181–197. [Google Scholar]
- Brown AM, Bledsoe C. Spatial and temporal dynamics of mycorrhizas in Jaumea carnosa, a tidal saltmarsh halophyte. Journal of Ecology. 1996;84:703–715. [Google Scholar]
- Brundrett M. Mycorrhizas in natural ecosystem. Advances in Ecological Research. 1991;21:300–313. [Google Scholar]
- Bulwada JG, Stribley DP, Tinker PB. Increased uptake of chloride and bromide by plants infected with vesicular-arbuscular mycorrhizas. New Phytologist. 1983;93:217–225. [Google Scholar]
- Cantrell IC, Linderman RG. Preinoculation of lettuce and onion with VA mycorrhizal fungi reduces deleterious effects of soil salinity. Plant and Soil. 2001;233:269–281. [Google Scholar]
- Carvalho LM, Correia PH, Martins-Loucao A. Arbuscular mycorrhizal fungal propagules in a salt marsh. Mycorrhiza. 2001;14:165–170. doi: 10.1007/s00572-003-0247-4. [DOI] [PubMed] [Google Scholar]
- Chen GP, Wilson ID, Kim SH, Greison D. Inhibiting expression of a tomato-ripening associated membrane protein increases organic acids and reduces sugar levels of fruit. Planta. 2001;212:799–807. doi: 10.1007/s004250000431. [DOI] [PubMed] [Google Scholar]
- Cliquet JB, Stewart GR. Ammonia assimilation in Zea mays L. infected with a vesicular arbuscular mycorrhizal fungus Glomus fasciculatum. Plant Physiology. 1993;101:865–871. doi: 10.1104/pp.101.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colla G, Rouphael Y, Cardarelli M, Tullio M, Rivera CM, Rea E. Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentration. Biology and Fertility of Soils. 2008;44:501–509. [Google Scholar]
- Copeman RH, Martin CA, Stutz JC. Tomato growth in response to salinity and mycorrhizal fungi from saline or nonsaline soils. Horticultural Science. 1996;31:341–344. [Google Scholar]
- Couée I, Hummel I, Sulmon C, Gowsbet G, El Armani A. Involvement of polyamines in root development. Plant Cell, Tissue and Organ Culture. 2004;76:1–10. [Google Scholar]
- Cramer GR, Lauchli A, Polito VS. Displacement of Ca2+ by Na+ from the plasmalemma of root cells: a primary response to salt stress? Plant Physiology. 1985;79:207–277. doi: 10.1104/pp.79.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creus CM, Sueldo RJ, Barassi CA. Water relations in Azospirillum inoculated wheat seedlings under osmotic stress. Canadian Journal of Botany. 1998;76:238–244. [Google Scholar]
- Cuartero J, Fernandez-Munoz Effects of salinity on tomato. Scientia Horticulturae. 1999;78:83–125. [Google Scholar]
- Dehne HW. Interaction between vesicular-arbuscular mycorrhizal fungi and plant pathogens. Phytopathology. 1982;72:1115–1119. [Google Scholar]
- Delauney AJ, Verma DPS. Proline biosynthesis and osmoregulation in plants. The Plant Journal. 1993;4:215–223. [Google Scholar]
- Delgado MJ, Ligero F, Lluch C. Effects of salt stress on growth and nitrogen fixation by pea, faba-bean, common bean, and soybean plants. Soil Biology and Biochemistry. 1994;26:371–376. [Google Scholar]
- Dell'Amico J, Torrecillas A, Rodriguez P, Morte A, Sanchez-Blanco M. Responses of tomato plants associated with the arbuscular mycorrhizal fungus Glomus clarum during drought and recovery. Journal of Agricultural Science. 2002;138:387–393. [Google Scholar]
- Dionisio-Sese ML, Tobita S. Antioxidant response of rice seedlings to salinity stress. Plant Science. 1998;135:1–9. [Google Scholar]
- Dixon RK, Garg VK, Rao MV. Inoculation of Leucaena and Prosopis seedlings with Glomus and Rhizobium species in saline soil: rhizosphere relations and seedlings growth. Arid Soil Research and Rehabilitation. 1993;7:133–144. [Google Scholar]
- Duan X, Newman DS, Reibee JM, Green CD, Saxton AM, Augé RM. Mycorrhizal influence on hydraulic and hormonal factors implicated in the control of stomatal conductance during drought. Journal of Experimental Botany. 1996;47:1541–1550. [Google Scholar]
- Duke ER, Johnson CR, Koch KE. Accumulation of phosphorus, dry matter and betaine during NaCl stress of split-root citrus seedlings colonized with vesicular-arbuscular mycorrhizal fungi on zero, one or two halves. New Phytologist. 1986;104:583–590. doi: 10.1111/j.1469-8137.1986.tb00658.x. [DOI] [PubMed] [Google Scholar]
- El-Desouky SA, Atawia AAR. Growth perfomance of citrus rootstocks under saline conditions. Alexandria Journal of Agricultural Research. 1998;43:231–254. [Google Scholar]
- Estaun MV. Effect of sodium chloride and mannitol on germination and hyphal growth of the vesicular-arbuscular mycorrhizal fungus Glomus mosseae. Agriculture, Ecosystems and Environment. 1989;29:123–129. [Google Scholar]
- Estrada-Luna AA, Davies FT. Arbuscular mycorrhizal fungi influence water relations, gas exchange, abscissic acid and growth of micropropagated Chile ancho pepper (Capsicum annuum) plantlets during acclimatization and post-acclimatization. Journal of Plant Physiology. 2003;160:1073–1083. doi: 10.1078/0176-1617-00989. [DOI] [PubMed] [Google Scholar]
- Feng G, Zhang FS, Li Xl, Tian CY, Tang C, Rengel Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza. 2002;12:185–190. doi: 10.1007/s00572-002-0170-0. [DOI] [PubMed] [Google Scholar]
- Figueroa ME, Frenandez-Baco L, Luque T, Davy AJ. Chlorophyll fluorescence, stress and survival in populations of Mediterranean grassland species. Journal of Vegetation Science. 1997;8:881–888. [Google Scholar]
- Finlay R, Söderström B. Mycorrhiza and carbon flow to the soil. In: Allen MF, editor. Mycorrhizal functioning: an integrative plant–fungal process. New York, NY: Chapman and Hall; 1992. pp. 134–162. [Google Scholar]
- Founoune H, Duponnis R, Ba AM, Ei Bouami F. Influence of the dual arbuscular endomycorrhizal/ectomycorrhizal symbiosis on the growth of Acacia holosericea (A. Cunn. ex G. Don) in glasshouse conditions. Annals of Forest Science. 2002;59:93–98. [Google Scholar]
- Frechill S, Lasa B, Ibarretxe L, Lamsfus C, Aparicio Trejo P. Pea response to saline stress is affected by the source of nitrogen nutrition (ammonium or nitrate) Plant Growth Regulators. 2001;35:171–179. [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Archives of Biochemistry and Biophysics. 1986;247:1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Fuzy A, Biro B, Toth T, Hildebrandt U, Bothe H. Drought, but not salinity, determines the apparent effectiveness of halophytes colonized by arbuscular mycorrhizal fungi. Journal of Plant Physiology. 2008;165:1181–1192. doi: 10.1016/j.jplph.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Gadkar V, David-Schwartz R, Kunik T, Kapilnik Y. Arbuscular mycorrhizal fungal colonization: factors involved in host recognition. Plant Physiology. 2001;127:1493–1499. [PMC free article] [PubMed] [Google Scholar]
- Gallagher JK. Halophytic crops for cultivation at seawater salinity. Plant and Soil. 1985;89:323–336. [Google Scholar]
- Gamble P, Burke JJ. Effect of water stress on the chloroplast antioxidant system. I. Alteration in glutathione reductase activity. Plant Physiology. 1984;76:615–621. doi: 10.1104/pp.76.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg N, Manchanda G. Effect of arbuscular mycorrhizal inoculation of salt-induced nodule senescence in Cajanus cajan (pigeonpea) Journal of Plant Growth Regulators. 2008;27:115–124. [Google Scholar]
- Ghorbanli M, Ebrahimzadeh H, Sharifi M. Effects of NaCl and mycorrhizal fungi on antioxidative enzymes in soybean. Biologia Plantarum. 2004;48:575–581. [Google Scholar]
- Giri B, Mukerji KG. Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandiflora under field conditions: evidence for reduced sodium and improved magnesium uptake. Mycorrhiza. 2004;14:307–312. doi: 10.1007/s00572-003-0274-1. [DOI] [PubMed] [Google Scholar]
- Giri B, Kapoor R, Mukerji KG. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass and mineral nutrition of Acacia auriculiformis. Biology and Fertility of Soils. 2003;38:170–175. [Google Scholar]
- Giri B, Kapoor R, Mukerji KG. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum, may be partly related to elevated K+/Na+ ratios in root and shoot tissues. Microbial Ecology. 2007;54:753–760. doi: 10.1007/s00248-007-9239-9. [DOI] [PubMed] [Google Scholar]
- Giovannetti M, Sbrana C, Citernesi AS, Avio L. Analysis of factors involved in fungal recognition responses to host-derived signals by arbuscular mycorrhizal fungi. New Phytologist. 1996;133:65–71. [Google Scholar]
- Glenn EP, O'Leary JW. Productivity and irrigation requirements of halophytes grown with seawater in the Sonoran desert. Journal of Arid Environments. 1985;9:81–91. [Google Scholar]
- Goas G, Goas M, Larher F. Accumulation of free proline and glycine betaine in Aster tripolium subjected to a saline shock: a kinetic study related to light period. Physiologia Plantarum. 1982;55:383–388. [Google Scholar]
- Gogorcente Y, Gordon AJ, Escuredo PR, et al. N2 fixation, carbon metabolism, and oxidative damage in nodules of dark-stressed common bean plants. Plant Physiology. 1997;113:1193–1201. doi: 10.1104/pp.113.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EM, Aparicio-Tejo PM, Gordon AJ, Minchin FR, Royuela M, Arrese-Igor C. Water deficit effects on carbon and nitrogen metabolism of pea nodules. Journal of Experimental Botany. 1998;49:1705–1714. [Google Scholar]
- Gorham J. Betaines in higher plants – biosynthesis and role in stress metabolism. In: Wallgrove RM, editor. Amino acids and their derivatives in higher plants. Cambridge: Cambridge University Press; 1995. pp. 171–203. [Google Scholar]
- Gossett DR, Milhollon EP, Lucas MC. Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivar of cotton. Crop Science. 1994;34:706–714. [Google Scholar]
- Govindarajulu M, Pfeffer PE, Jin HR, et al. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature. 2005;435:819–823. doi: 10.1038/nature03610. [DOI] [PubMed] [Google Scholar]
- Graham JH, Syversten JP. Influence of vesicular arbuscular mycorrhiza on the hydraulic conductivity of roots of two Citrus rootstocks. New Phytologist. 1984;97:277–284. [Google Scholar]
- Grattan SR, Grieve CM. Betaine status in wheat in relation to nitrogen stress and to transient salinity stress. Plant and Soil. 1985;85:3–9. [Google Scholar]
- Harisnaut P, Poonsopa D, Roengmongkol K, Charoensataporn R. Salinity effects on antioxidant enzymes in mulberry cultivar. Science Asia. 2003;29:109–113. [Google Scholar]
- Hartmond U, Schaesberg NV, Graham JH, Syversten JP. Salinity and flooding stress effects on mycorrhizal and non-mycorrhizal citrus rootstock seedlings. Plant and Soil. 1987;104:37–43. [Google Scholar]
- Hauser BA, Oppenheimer KSDG, Sage TL. Changes in mitochondrial membrane potential and accumulation of reactive oxygen species precede ultra structural changes during ovule abortion. Planta. 2006;223:492–499. doi: 10.1007/s00425-005-0107-x. [DOI] [PubMed] [Google Scholar]
- Hejiden JN, Klironomos M, Ursic P, et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature. 1998;396:69–72. [Google Scholar]
- Hilderbrandt U, Janetta K, Ouziad F, Renne B, Nawrath K, Bothe H. Arbuscular mycorrhizal colonization of halophytes in Central European salt marshes. Mycorrhiza. 2001;10:175–183. [Google Scholar]
- Hill AE, Shachar-Hill B, Shachar-Hill Y. What are aquaporins for? Journal of Membrane Biology. 2004;197:1–32. doi: 10.1007/s00232-003-0639-6. [DOI] [PubMed] [Google Scholar]
- Hirrel MC. The effect of sodium and chloride salts on the germination of Gigaspora margarita. Mycologia. 1981;73:610–617. [Google Scholar]
- Hirrel MC, Gerdemann JW. Improved growth of onion and bell pepper in saline soils by two vesicular-arbuscular mycorrhizal fungi. Soil Science Society of America Journal. 1980;44:654–665. [Google Scholar]
- Hirrel MC, Mehravaran H, Gerdemann JW. Vesicular-arbuscular mycorrhiza in the Chenopodiaceae and Cruciferae: do they occur? Canadian Journal of Botany. 1978;56:2813–2817. [Google Scholar]
- Ho I. Vesicular-arbuscular mycorrhizae of halophytic grasses in the Alvord desert of Oregon. Northwest Science. 1987;61:148–151. [Google Scholar]
- Hoefnagels MH, Broome SW, Shafer SR. Vesicular-arbuscular mycorrhizae in salt marshes in north Carolina. Estuaries. 1993;16:851–858. [Google Scholar]
- Hoekstra FA, Crow JH, Crowe LM, Van Roekel T, Vermeer T. Do phospholipids and sucrose determine membrane phase transitions in dehydrating pollen species? Plant, Cell and Environment. 1992;15:601–606. [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J. Mechanisms of plant desiccation tolerance. Trends in Plant Sciences. 2001;6:431–438. doi: 10.1016/s1360-1385(01)02052-0. [DOI] [PubMed] [Google Scholar]
- Jahromi F, Aroca R, Porcel R, Ruiz-Lozano JM. Influence of salinity on the in vitro development of Glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microbial Ecology. 2008;55:45–53. doi: 10.1007/s00248-007-9249-7. [DOI] [PubMed] [Google Scholar]
- Jain M, Mathur G, Koul S, Sarin NB. Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.) Plant Cell Reports. 2001;20:463–468. [Google Scholar]
- Jarstfer AG, Farmer-Koppenol P, Sylvia DM. Tissue magnesium and calcium affect mycorrhiza development and fungal reproduction. Mycorrhiza. 1998;7:237–242. doi: 10.1007/s005720050186. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Water stress-induced abscissic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize-leaves. Journal of Experimental Botany. 2002;53:2401–2410. doi: 10.1093/jxb/erf090. [DOI] [PubMed] [Google Scholar]
- Jindal V, Atwal A, Sekhon BS, Singh R. Effect of vesicular-arbuscular mycorrhizae on metabolism of moong plants under NaCl salinity. Plant Physiology and Biochemistry. 1993;3:475–481. [Google Scholar]
- Johnson-Green P, Kenkel NC, Booth T. Soil salinity and arbuscular mycorrhizal colonization of Puccinellia nuttalliana. Mycological Research. 2001;105:1094–1110. [Google Scholar]
- Juniper S, Abbott LK. Vesicular-arbuscular mycorrhizas and soil salinity. Mycorrhiza. 1993;4:45–57. doi: 10.1007/s00572-006-0046-9. [DOI] [PubMed] [Google Scholar]
- Juniper S, Abbott LK. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza. 2006;16:371–379. doi: 10.1007/s00572-006-0046-9. [DOI] [PubMed] [Google Scholar]
- Kamfenkel K, Van Montagu M, Inze D. Effects of iron on Nicotiana plumbaginifolia plants: implication to oxidative stress. Plant Physiology. 1995;107:725–737. doi: 10.1104/pp.107.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor R, Sharma D, Bhatnagar AK. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Scientia Horticulturae. 2008;116:227–239. [Google Scholar]
- Kaldorf M, Schemelzer E, Bothe H. Expression of maize and fungal nitrate reductase in arbuscular mycorhiza. Molecular Plant–Microbe Interactions. 1998;11:439–448. doi: 10.1094/MPMI.1998.11.6.439. [DOI] [PubMed] [Google Scholar]
- Kaya C, Ashraf M, Sonmez O, Aydemir S, Tuna AL, Cullu MA. The influence of arbuscular mycorrhizal colonization on key growth parameters and fruit yield of pepper plants grown at high salinity. Scientia Horticulturae. 2009;121:1–6. [Google Scholar]
- Khan AG. The occurrence of mycorrhizas in halophytes, hydrophytes and xerophytes, of endogone spores in adjacent soils. Journal of General Microbiology. 1974;81:7–14. [Google Scholar]
- Kim CK, Weber DJ. Distribution of VA mycorrhiza on halophytes on inland sea playas. Plant and Soil. 1985;83:207–214. [Google Scholar]
- Kishor PB, Hong Z, Miao GH, Hu CA, Verma DPS. Over expression of Δ′-pyrroline-5-carboxilate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiology. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klironomos JN, Moutoglis P, Kendrick B, Widden P. A comparison of spatial heterogeneity of vesicular arbuscular mycorrhizal fungi in two maple-forest soils. Canadian Journal of Botany. 1993;71:1472–1480. [Google Scholar]
- Kothari SK, Marschner H, George E. Effect of VA mycorrhizal fungi and rhizosphere microorganism on root and shoot morphology, growth and water relations of maize. New Phytologist. 1990;116:303–311. [Google Scholar]
- Koyro HW. Ultrastructural changes in root cells of Sorghum plants (Sorghum bicolor × S. sudanensis cv. Sweet Siuox) induced by NaCl. Journal of Experimental Botany. 1997;48:693–706. [Google Scholar]
- Krishnamurthy R, Bhagwat KA. Polyamines as modulators of salt tolerance in rice cultivars. Plant Physiology. 1989;91:500–504. doi: 10.1104/pp.91.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurepa J, Smalle J, Montagu MV, Inzé́ D. Polyamines and paraquat toxicity in Arabidopsis thaliana. Plant Cell Physiology. 1998;39:987–992. doi: 10.1093/oxfordjournals.pcp.a029463. [DOI] [PubMed] [Google Scholar]
- Landwehr M, Hilderbrandt U, Wilde P, et al. The arbuscular mycorrhizal fungus Glomus geosporum in Europaen saline, sodic and gypsum soils. Mycorrhiza. 2002;12:199–211. doi: 10.1007/s00572-002-0172-y. [DOI] [PubMed] [Google Scholar]
- Langebartels C, Kerner K, Leonardi S, et al. Biochemical plant responses to ozone: differential induction of polyamine and ethylene biosynthesis in tobacco. Plant Physiology. 1991;95:882–889. doi: 10.1104/pp.95.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y, Dodd J, Krikun J. Effect of irrigation water salinity and rootstock on the vertical distribution of vesicular-arbuscular mycorrhiza in citrus roots. New Phytologist. 1983;95:397–403. [Google Scholar]
- Lindermann RG. Role of VAM in biocontrol. In: Pfleger FL, Linderman RG, editors. Mycorrhizae and plant health. St. Paul: American Phytopathological Society; 1994. pp. 1–26. [Google Scholar]
- Lloyd-MacGilp SA, Chambers SM, Dodd JC, Fitter AH, Walker C, Young JPW. Diversity of the ribosomal internal transcribed spacers within and among isolates of Glomus mosseae and related mycorrhizal fungi. New Phytologist. 1996;133:103–111. [Google Scholar]
- Lopez F, Vansuyt G, Casse-Delbart F, Fourcroy P. Ascorbate peroxidase activity, not the mRNA level, is enhanced in salt stressed Raphanus sativus plants. Physiology Plantarum. 1996;97:13–20. [Google Scholar]
- Lopez M, Tejera NA, Iribarne C, Lluch C, Herrera-Cervera J. Trehalose and trehalase in root nodulae of Medicago truncatula and Phaseolus vulgaris in response to salt stress. Physiologia Plantarum. 2008;134:575–582. doi: 10.1111/j.1399-3054.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J. Indole-3-butyric acid in plant growth and development. Plant Growth Regulators. 2000;32:219–230. [Google Scholar]
- Mahmoodzadeh H. Ultrastructural changes in shoot apical meristem of canola (Brassica napus cv. Symbol) treated with sodium chloride. Pakistan Journal of Biological Sciences. 2008;11:1161–1164. doi: 10.3923/pjbs.2008.1161.1164. [DOI] [PubMed] [Google Scholar]
- Maiale S, Sanchez DH, Guirado A, Vidal A, Ruiz OA. Spermine accumulation under salt stress. Journal of Plant Physiology. 2004;161:35–42. doi: 10.1078/0176-1617-01167. [DOI] [PubMed] [Google Scholar]
- Marcum KB. Growth and physiological adaptations of grasses to salinity stress. In: Pessaraki M, editor. Handbook of plant and crop physiology. New York, NY: Marcel Dekker; 2002. pp. 623–636. [Google Scholar]
- Mariaux JB, Bockel C, Salamini F, Bartels D. Dessication and abscissic acid responsive genes encoding major intrinsic proteins (MIPs) from the resurrection plant Crateriostigma plantsgineum. Plant Molecular Biology. 1998;38:1089–1099. doi: 10.1023/a:1006013130681. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. New York, NY: Academic Press; 1995. [Google Scholar]
- Mason E. Note on the presence of mycorrhizae in the roots of salt-marsh plants. New Phytologist. 1928;27:193–195. [Google Scholar]
- Matamoros MA, Baird LM, Escuredo PR, et al. Stress-induced legume root nodule senescence: physiological, biochemical and structural alterations. Plant Physiology. 1999;121:97–111. doi: 10.1104/pp.121.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur N, Vyas A. Biochemical changes in Ziziphus xyloropus by VA mycorrhizae. Botanical Bulletin of Academia Sinica. 1996;37:209–212. [Google Scholar]
- Mathur N, Singh J, Bohra S, Vyas A. Arbuscular mycorrhizal status of medicinal halophytes in saline areas of Indian Thar Desert. International Journal of Soil Science. 2007;2:119–127. [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- McMillen B, Juniper S, Abbott LK. Inhibition of hyphal growth of a vesicular-arbuscular mycorrhizal fungus in soil containing sodium chloride limits the spread of infection from spores. Soil Biology and Biochemistry. 1998;30:1639–1646. [Google Scholar]
- Menconi M, Sgherri CLM, Pinzino C, Navari-izzo F. Activated oxygen species production and detoxification in wheat plants subjected to a water deficit programme. Journal of Experimental Botany. 1995;46:1123–1130. [Google Scholar]
- Mohammad MJ, Hamad SR, Malkani HI. Population of arbuscular mycorrhizal fungi in semi-arid environment of Jordan as influenced by biotic and abiotic factors. Journal of Arid Environments. 2003;53:409–417. [Google Scholar]
- Møller IM. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:561–591. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]
- Morgan JM. Osmoregulation and water stress in higher plants. Annual Review of Plant Physiology. 1984;33:299–319. [Google Scholar]
- Mosse B, Hepper CM. Vesicular-arbuscular mycorrhizal infections in root organ cultures. Physiology and Plant Pathology. 1975;5:215–233. [Google Scholar]
- Mouk BO, Ishii T. Effect of arbuscular mycorrhizal fungi on tree growth and nutrient uptake of Sclerocarya birrea under water stress, salt stress and flooding. Journal of the Japanese Society for Horticultural Science. 2006;75:26–31. [Google Scholar]
- Muralev E, Nazarenko PI, Poplavskij VM, Kuznetsov IA. Proceedings of a Symposium in Taejon, Republic of Korea. Vienna, Austria: International Atomic Energy Agency; 1997. Seawater desalination. In: Nuclear desalinization of seawater; pp. 355–366. [Google Scholar]
- Murkute AA, Sharma S, Singh SK. Studies on salt stress tolerance of citrus rootstock genotypes with arbuscular mycorrhizal fungi. Horticultural Science. 2006;33:70–76. [Google Scholar]
- Nemec S. Histochemical characterization of Glomus etunicatum infection of Citrus limon roots. Canadian Journal of Botany. 1981;59:609–617. [Google Scholar]
- Núñez M, Mazzafera P, Mazorra LM, Siquera WJ, Zullo MAT. Influence of a brassinosteroid analogue on antioxidant enzymes in rice grown in culture medium with NaCl. Biologia Plantarum. 2003;47:67–70. [Google Scholar]
- Ocon A, Hampp R, Requena N. Trehalose turnover during abiotic stress in arbuscular mycorrhizal fungi. New Phytologist. 2007;174:879–891. doi: 10.1111/j.1469-8137.2007.02048.x. [DOI] [PubMed] [Google Scholar]
- Ojala JC, Jarrell WM, Menge JA, Johnson ELV. Influence of mycorrhizal fungi on the mineral nutrition and yield of onion in saline soil. Agronomy Journal. 1983;75:255–259. [Google Scholar]
- O'Leary JW. Adaptive components of salt tolerance. In: Pessaraki M, editor. Handbook of plant and crop physiology. New York, NY: Marcel Dekker; 1995. pp. 577–585. [Google Scholar]
- Olrovich DA, Ashford AE. Polyphosphate granules are artifact of specimen preparation in the ectomycorrhizal fungus Pisolithus tinctorius. Protoplasma. 1993;173:91–102. [Google Scholar]
- Ouziad F, Wilde P, Schmelzer E, Hilderbrandt U, Bothe H. Analysis of expression of aquaporins and Na+/H+ transporters in tomato colonized by arbuscular mycorrhizal fungi and affected by salt stress. Environment and Experimental Botany. 2006;57:177–186. [Google Scholar]
- Parida A, Das AB, Das P. NaCl stress causes changes in photo-synthetic pigments, proteins and other metabolic components in the leaves of a tree mangrove, Bruguiera parviflora, in hydroponic cultures. Journal of Plant Biology. 2002;45:28–36. [Google Scholar]
- Parida SK, Das AB. Salt tolerance and salinity effects on plants. Ecotoxicology and Environment Safety. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Patreze CM, Cordeiro L. Nitrogen fixing and vesicular arbuscular mycorrhizal symbioses in some tropical legume trees of tribe Mimosaceae. Forest Ecology and Management. 2004;196:275–285. [Google Scholar]
- Pearson JN, Schweiger P. Scutellospora calospora (Nicol. and Gerd.) Walker & Sanders associated with subterranean clover: dynamics of colonization, sporulation and soluble carbohydrates. New Phytologist. 1993;124:215–219. doi: 10.1111/j.1469-8137.1993.tb03810.x. [DOI] [PubMed] [Google Scholar]
- Plenchette C, Dupponois R. Growth response of the salt brush Atriplex numularia L. to inoculation with the arbuscular mycorrhizal fungus Glomus intraradices. Journal of Arid Environments. 2005;61:535–540. [Google Scholar]
- Pond EC, Menge JA, Jarrell WM. Improved growth of tomato in salinized soil by vesicular arbuscular mycorrhizal fungi collected from saline sites. Mycologia. 1984;76:74–84. [Google Scholar]
- Porcel R, Ruiz-Lozano JM. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation and oxidative stress in soybean plants subjected to drought stress. Journal of Experimental Botany. 2004;55:1743–1750. doi: 10.1093/jxb/erh188. [DOI] [PubMed] [Google Scholar]
- Porcel R, Barea JM, Ruiz-Lozano JM. Antioxidative activities in mycorrhizal soybean plants under drought stress and their possible relationship to the process of nodule senescence. New Phytologist. 2003;157:135–143. doi: 10.1046/j.1469-8137.2003.00658.x. [DOI] [PubMed] [Google Scholar]
- Porras-Soriano A, Soriano-Martin ML, Porras-Piedra A, Azcón R. Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. Journal of Plant Physiology. 2009 doi: 10.1016/j.jplph.2009.02.010. doi: 10.1016/j.jplph.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Poss JA, Pond EC, Menge JA, Jarrell WM. Effect of salinity on mycorrhizal onion and tomato in soil with and without additional phosphate. Plant and Soil. 1985;88:307–319. [Google Scholar]
- Pringle A, Moncalvo JM, Vilgalys R. High levels of variation in ribosomal DNA sequences within and among spores of a natural population of the arbuscular mycorrhizal fungus Acaulospora collosica. Mycologia. 2000;92:259–268. [Google Scholar]
- Rabie GH. Influence of VA-mycorrhizal fungi and kinetin on the response of mungbean plants to irrigation with seawater. Mycorrhiza. 2005;15:225–230. doi: 10.1007/s00572-004-0345-y. [DOI] [PubMed] [Google Scholar]
- Rabie GH, Almadini AM. Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. African Journal of Biotechnology. 2005;4:210–222. [Google Scholar]
- Regvar M, Vogel K, Irgel N, et al. Colonization of pennycress (Thlaspi spp.) of the Brassicaceae by arbuscular mycorrhizal fungi. Journal of Plant Physiology. 2003;160:615–626. doi: 10.1078/0176-1617-00988. [DOI] [PubMed] [Google Scholar]
- Rhodes LH, Gerdemann JW. Translocation of calcium and phosphate by external hyphae of VA mycorrhiza. Soil Science. 1978;126:125–126. [Google Scholar]
- Richards LA, editor. Diagnosis and improvement of saline and alkali soils. Washington, DC: United States Department of Agriculture; 1954. pp. 4–18. Handbook no. 60. [Google Scholar]
- Rinaldelli E, Mancuso S. Response of young mycorrhizal and non mycorrhizal plants of olive tree (Olea europaea L.) to saline conditions. 1. Short term electro physiological and long term vegetative salt effects. Advances in Horticultural Science. 1996;10:126–134. [Google Scholar]
- Rosendahl CN, Rosendahl S. Influence of vesicular-arbuscular mycorrhizal fungi (Glomus spp.) on the response of cucumber (Cucumis sativus L.) to salt stress. Environmental and Experimental Botany. 1991;31:313–318. [Google Scholar]
- Rozema J, Arp W, Van Diggelen J, Van Esbroek M, Broekman R, Punte H. Occurrence and ecological significance of vesicular-arbuscular mycorrhiza in the salt marsh environment. Acta Botanica Neerlandica. 1986;35:457–467. [Google Scholar]
- Ruiz-Lozano JM. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress: new perspectives for molecular studies. Mycorrhiza. 2003;13:309–317. doi: 10.1007/s00572-003-0237-6. [DOI] [PubMed] [Google Scholar]
- Ruiz-Lozano JM, Azcón R. Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp from saline soils and Glomus deserticola under salinity. Mycorrhiza. 2000;10:137–143. [Google Scholar]
- Ruiz-Lozano JM, Azcón R, Goméz M. Alleviation of salt stress by arbuscular-mycorrhizal Glomus species in Lactuca sativa plants. Physiologia Plantarum. 1996;98:767–772. [Google Scholar]
- Ruiz-Lozano JM, Collados C, Barea JM, Azcón R. Arbuscular mycorrhizal symbiosis can alleviate drought induced nodule senescence in soybean plants. Plant Physiology. 2001;82:346–350. [Google Scholar]
- Saint-Etienne L, Paul S, Imbert D, et al. Arbuscular mycorrhizal soil infectivity in a stand of the wetland tree Pterocarpus officinalis along a salinity gradient. Forest Ecology and Management. 2006;232:86–89. [Google Scholar]
- Sanan-Mishra N, Pham XH, Sopori SK, Tuteja N. Pea DNA helicase 45 overexpression in tobacco confers high salinity tolerance without affecting yield. Proceedings of National Academy of Sciences of the USA. 2005;102:509–514. doi: 10.1073/pnas.0406485102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders IR, Clapp JP, Wiekman A. The genetic diversity of arbuscular mycorrhizal fungi in natural ecosystems – a key to understanding the ecology and functioning of the mycorrhizal symbiosis. New Phytologist. 1996;133:123–134. [Google Scholar]
- Sannazzaro AI, Ruiz OA, Albetró EO, Menéndez AB. Alleviation of salt stress in Lotus glaber by Glomus intraradies. Plant and Soil. 2006;285:279–287. [Google Scholar]
- Sannazzaro AI, Echeverria M, Albertó EO, Ruiz OA, Menéndez AB. Modulation of polyamine balance in Lotus glaber by salinity and arbuscular mycorrhiza. Plant Physiology and Biochemistry. 2007;45:39–46. doi: 10.1016/j.plaphy.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Sarath G, Pleiffer NE, Sodhi CS, Wagner FW. Bacteroids are stable during dark-induced senescence of soybean root nodules. Plant Physiology. 1986;82:346–350. doi: 10.1104/pp.82.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. Oxygen stress and superoxide dismutases. Plant Physiology. 1993;101:7–12. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert A, Wyss P, Wiekman A. Occurrence of trehalose in vesicular-arbuscular mycorrhizal fungi and in mycorrhizal roots. Journal of Plant Physiology. 1992;140:41–45. [Google Scholar]
- Sengupta A, Chaudhuri S. Vesicular arbuscular mycorrhiza (VAM) in pioneer salt marsh plants of the Ganges river delta in West Bengal (India) Plant and Soil. 1990;122::111–113. [Google Scholar]
- Sharifi M, Ghorbanli M, Ebrahimzadeh H. Improved growth of salinity-stressed soybean after inoculation with pre-treated mycorrhizal fungi. Journal of Plant Physiology. 2007;164:1144–1151. doi: 10.1016/j.jplph.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tang M, Chan H, Yang B, Zhang F, Huang Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza. 2008;18:287–296. doi: 10.1007/s00572-008-0180-7. [DOI] [PubMed] [Google Scholar]
- Shokri S, Maadi B. Effects of arbuscular mycorrhizal fungus on the mineral nutrition and yield of Trifolium alexandrium plants under salinity stress. Journal of Agronomy. 2009;8:79–83. [Google Scholar]
- Siefritz F, Biela A, Eckert M, Otto B, Uehlein N, Kaldenhoff R. The tobacco plasma membrane aquaporin NtAQP1. Journal of Experimental Botany. 2001;52:1953–1957. doi: 10.1093/jexbot/52.363.1953. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and dessication. New Phytologist. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. New York, NY: Academic Press; 1995. pp. 105–160. [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal symbiosis. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Spychalla JP, Desbough SL. Superoxide dismutase, catalase, and alpha-tocopherol content of stored potato tubers. Plant Physiology. 1990;94:1214–1218. doi: 10.1104/pp.94.3.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CR, Lee JA. The rate of proline accumulation in halophytes. Planta. 1974;120:279–289. doi: 10.1007/BF00390296. [DOI] [PubMed] [Google Scholar]
- Sun K, Hunt K, Hauser BA. Ovule abortion in Arabidopsis triggered by stress. Plant Physiology. 2004;135:2358–2367. doi: 10.1104/pp.104.043091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Peng X, Newton RJ. Enhanced salt tolerance in transgenic loblolly pine simultaneously expressing two genes encoding mannitol-1-phosphate dehydrogenase and glucitol-6-phosphate dehydrogenase. Plant Physiology and Biochemistry. 2005;43:139–146. doi: 10.1016/j.plaphy.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Annals of Botany. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanna E, Nawar A. Salinity and mycorrhizal association in relation to carbohydrate status, leaf chlorophyll and activity of peroxidase and polyphenol oxidase enzymes in sour orange seedlings. Alexandria Journal of Agricultural Research. 1994;39:263–280. [Google Scholar]
- Thomson BD, Robson AD, Abbott LK. Mycorrhizas formed by Gigaspora calospora and Glomus fasciculatum on subterranean clover in relation to soluble carbohydrates in roots. New Phytologist. 1990;114:217–225. [Google Scholar]
- Tian CY, Feng G, Li XL, Zhang FS. Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline on salinity tolerance of plants. Applied Soil Ecology. 2004;26:143–148. [Google Scholar]
- Tinker PB. Soil chemistry of phosphorus and mycorrhizal effects on plant growth. In: Sanders FE, Mosse B, Tinker PB, editors. Endomycorrhizas. London: Academic Press; 1975. pp. 353–371. [Google Scholar]
- Tommerup IC. Effect of soil water potential on spore germination by vesicular-arbuscular mycorrhizal fungi. Transitions of British Mycological Society. 1984;83:193–202. [Google Scholar]
- Tressner HD, Hayes JA. Sodium chloride tolerance of terrestrial fungi. Applied Microbiology. 1971;22:210–213. doi: 10.1128/am.22.2.210-213.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierheilig H, Alt-Hug M, Streitwolf-Engel R, Mader P, Wiekman A. Studies on the attractional effect of root exudates on hyphal growth of an arbuscular mycorrhizal fungus in a soil compartment-membrane system. Plant Soil. 1998;203:137–144. [Google Scholar]
- Wang FY, Liu RJ, Lin XG, Zhou JM. Arbuscular mycorrhizal status of wild plants in saline-alkaline soils of the Yellow River Delta. Mycorrhiza. 2004;14:133–137. doi: 10.1007/s00572-003-0248-3. [DOI] [PubMed] [Google Scholar]
- Wang S, Wan C, Wang Y, et al. The characteristics of Na+, K+ and free proline distribution in several drought-resistance plants of the Alxa Desert, China. Journal of Arid Environment. 2004;56::525–539. [Google Scholar]
- Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: toward genetic engineering for stress tolerance. Planta. 2003;218:1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- Wei-Feng XU, Wei-Ming SHI, Ueda A, Takabe T. Mechanisms of salt tolerance in transgenic Arabidopsis thaliana carrying a peroxisomal ascorbate peroxidase gene from barley. Pedosphere. 2008;4:486–495. [Google Scholar]
- Weig A, Deswarte C, Chrispeels MJ. The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiology. 1997;114:1347–1357. doi: 10.1104/pp.114.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde P, Manal A, Stodden M, Sieverding E, Hilderbrandt U, Bothe H. Biodiversity of arbuscular mycorrhizal fungi in roots and soils of two salt marshes. Environmental Microbiology. 2009;11:1548–1561. doi: 10.1111/j.1462-2920.2009.01882.x. [DOI] [PubMed] [Google Scholar]
- Wilson JN. Comparative development of infection by three vesicular-arbuscular mycorrhizal fungi. New Phytologist. 1984;97:413–426. [Google Scholar]
- Wilson GWT, Harnett DC. Interspecific variation in plant response to mycorrhizal colonization in tall grass prairie. American Journal of Botany. 1998;85:1732–1738. [PubMed] [Google Scholar]
- White PJ, Broadley MR. Chloride in soils and its uptake and movement within the plant: a review. Annals of Botany. 2001;88:967–988. [Google Scholar]
- Wu QS, Zou YN, Xia RX. Effects of water stress and arbuscular mycorrhizal fungi on reactive oxygen metabolism and antioxidant production by citrus (Citrus tangerine) roots. European Journal of Soil Biology. 2006;42:166–172. [Google Scholar]
- Wu YY, Chen QJ, Chen M, Chen J, Wang XC. Salt-tolerant transgenic perennial ryegrass (Lolium perenne L.) obtained by Agrobacterium tumefasciens-mediated transformation of the vacuolar Na+/H+ antiporter gene. Plant Science. 2005;169:65–73. [Google Scholar]
- Xu G, Magen H, Tarchitzky J, Kafkaki U. Advances in chloride nutrition. Advances in Agronomy. 2000;68:96–150. [Google Scholar]
- Yamane K, Rahman Md S, Kawasaki M, Taniguchi M, Miyake H. Pretreatment with a low concentration of methyl viologen decreases the effects of salt stress on chloroplast ultrastructure in rice leaves (Oryza sativa L.) Plant Production Science. 2004;7:435–441. [Google Scholar]
- Yamato M, Ikeda S, Iwase K. Community of arbuscular mycorrhizal fungi in coastal vegetation on Okinawa Island and effect of the isolated fungi on growth of sorghum under salt-treated conditions. Mycorrhiza. 2008;18:241–249. doi: 10.1007/s00572-008-0177-2. [DOI] [PubMed] [Google Scholar]
- Yano-Melo AM, Saggin OJ, Maia LC. Tolerance of mycorrhized banana (Musa sp. cv. Pacovan) plantlets to saline stress. Agriculture, Ecosystems and Environments. 2003;95:343–348. [Google Scholar]
- Zhang HX, Blumwald E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nature Biotechnology. 2001;19:765–768. doi: 10.1038/90824. [DOI] [PubMed] [Google Scholar]
- Zhong Qun H, Chao Xing H, Zhibin Z, Zhirong Z, Huai Song W. Changes in antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCl stress. Colloids and Surfaces B: Biointerfaces. 2007;59:128–133. doi: 10.1016/j.colsurfb.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Zuccarini P. Mycorrhizal infection ameliorates chlorophyll content and nutrient uptake of lettuce exposed to saline irrigation. Plant, Soil and Environment. 2007;53:283–289. [Google Scholar]
- Zuccarini P, Okurowska P. Effects of mycorrhizal colonization and fertilization on growth and photosynthesis of sweet basil under salt stress. Journal of Plant Nutrition. 2008;31:497–513. [Google Scholar]