Abstract
Background and Aims
In hypocotyls of flax (Linum usitatissimum) cadmium-induced reorientation of growth (i.e. an increase in expansion and a decrease in elongation) coincides with marked changes in the methylesterification and cross-linking of homogalacturonans within various cell-wall (CW) domains. The aim of the present study was to examine the involvement of pectin methylesterase (PME) and peroxidase (PER) in this cadmium-induced CW remodelling.
Methods
CW proteins were extracted from hypocotyls of 10- and 18-d-old flax that had been treated or not treated with 0·5 mm Cd(NO3)2. PME and PER expression within these extracts was detected by LC/MS, by isoelectric focusing and enzyme activity assays. Transcript expression by RT-PCR of known flax PME and PER genes was also measured in corresponding samples.
Key Results
In cadmium-treated seedlings, PME activity increased as compared with controls, particularly at day 10. The increased activity of PME was accompanied by increased abundance of both a basic protein isoform (B2) and a particular transcript (Lupme5). In contrast, induction of PER activity by cadmium was highest at day 18. Among the four reported PER genes, Flxper1 and 3 increased in abundance in the presence of cadmium at day 18.
Conclusions
The temporal regulation of Lupme and Flxper genes and of their respective enzyme activities fits the previously reported cadmium-induced structural changes of homogalacturonans within the CWs. After PME-catalysed de-esterification of homogalacturonans, their cross-linking would depend on the activity of PERs interacting with calcium-dimerized blocks and reinforce the cell cohesion during the cadmium-induced swelling.
Keywords: Cadmium, cell wall, homogalacturonan, Linum usitatissimum, pectin methylesterase, peroxidase
INTRODUCTION
The control of cell wall (CW) extensibility is one of the ways in which plant growth is directed. CW extension is driven by turgor pressure but is governed by the mechanical behaviour of the CW itself (Jarvis and McCann, 2000). To support ongoing growth, the CW structure must incorporate new materials and also undergo remodelling. Among CW remodelling enzymes, peroxidase (PER) and pectin methylesterase (PME) are of particular importance. Increased PER activity resulting in the stiffening and lignification of CW has been reported in many plant systems in association with reduced growth rate (Cosgrove, 1997). Also, the relationship between (a) growth rate and (b) the degree of methylesterification of homogalacturonans (HGAs) as well as PME activity has been demonstrated in many species (e.g. Yamaoka and Chiba, 1983; Knox et al., 1990; Kim and Carpita, 1992; Goldberg et al., 1996; Femenia et al., 1998) including Arabidopsis thaliana hypocotyls (Derbyshire et al., 2007) and developing wood cells of poplar (Siedlecka et al., 2008).
The main phenotypic symptoms of cadmium (Cd) toxicity consist of an overall reduction of plant growth and acceleration of senescence; however, its impact on the CW ultrastructure has been examined in only a few studies (e.g. Barceló et al., 1988; Douchiche et al., 2007). Most of the studies on PER and PME in Cd-treated seedlings have been carried out in root, i.e. the organ which is in closest contact with the metal (e.g. Pan et al., 2004). A few papers have considered the action of PER in Cd-growth regulation (e.g. Chaoui and Ferjani, 2004; Verma et al., 2008). More generally, an increase in PER activity and gene expression has been reported in plants exposed to biotic and abiotic stresses (reviewed by Passardi et al., 2005). Interestingly, in Lygodium japonicum treated with copper, uronic acid-rich pectic polymers became highly bound to the other CW components (Konno et al., 2005), which might be due to the activity of Class III PERs.
It is well-established that heavy metals are polyvalent cations that strongly bind to the negatively charged pectins of the CW, with a net impact of their charge density and the selectivity for the cations (Demarty et al., 1984). Schmohl and Horst (2000) observed a close positive relationship between pectin content and aluminium (Al) accumulation in Zea mays. Moreover, a close negative correlation was found between the degree of methylesterification of pectins and Al concentration in the CW of the maize root apex (Eticha et al., 2005). The authors observed that the root pectins of a sensitive cultivar were characterized by a lower degree of methylesterification (higher JIM5 and LM7 epi-fluorescence); conversely, the resistant-cultivar CW exhibited a high level of JIM7 epi-fluorescence, indicating the presence of high methylesterified HGAs. Transgenic potato mutants overexpressing PME proved to be more sensitive to Al than the wild type (Schmohl et al., 2000). In rice root apex, a higher proportion of JIM5 epitopes was localized in the sensitive cultivar while an increased PME activity was measured in the presence of Al in both sensitive and tolerant cultivars (Yang et al., 2008). A fragment highly homologous to Arabidopsis thaliana PME (At3g59010) was isolated from a tolerant Thlaspi caerulescens ecotype exposed to Zn (Hassinen et al., 2007).
In the course of studies on the variation in pectin structure during the development of hypocotyls in flax (Linum usitatissimum) in various light conditions (Morvan et al., 1991a, b; Burel et al., 1994; Jauneau et al., 1994, 1997; Rihouey et al., 1995a, b; Alexandre et al., 1997; His et al., 1997; Andeme-Onzighi et al., 2000), it was reported that the growth rate was related not only with the degree of methylesterification of HGAs but also with its linkage within different CW domains. The treatment of seedlings with Cd appeared to be an interesting way to modify hypocotyl development, specifically to reduce elongation and on the other hand to enhance the expansion of tissues. Indeed, marked changes in length and diameter of the hypocotyl, CW thickening, structure and distribution of HGAs were observed within various CW domains (Douchiche et al., 2007). We hypothesized that PME might be involved in the opposite redistribution of high methylesterified HGAs (containing epitopes recognized by JIM7 antibody; Knox et al., 1990) and low methylesterified HGAs (containing epitopes recognized by JIM5 antibody; VandenBosch et al., 1989). Based on biochemical data that revealed a Cd-induced increase of the covalent cross-linking of HGAs in the CW of cortical tissues, an increase in PER activity was also hypothesized.
The aim of the present paper was therefore to examine whether the Cd-induced structural changes in HGAs might be related to the regulation of ionically CW-bound PME and PER enzymes. The PME activity and the expression of three specific Lupme genes were compared in Cd-treated and control seedlings. These Lupme genes (Lupme1, AF355056; Lupme3, AF188895; Lupme5, AF355057) were previously identified from RNA isolated from hypocotyls of flax seedlings grown under light (Lacoux et al., 2003; Al-Qsous et al., 2004; Carpentier, 2005). Also the expression and activity of CW PER isoforms were characterized in relation to four previously identified flax Flxper genes (Omann et al., 1994; Omann and Tyson, 1996). These analyses were performed when seedlings were 10 and 18 d old, as these two time points define characteristic stages of hypocotyl development: the former corresponding to the end of elongation phase (Morvan et al., 1991a; Al-Qsous et al., 2004) and the latter covering the expansion phase including the enlargement of secondary tissues and the thickening of their secondary wall (Morvan et al., 1991b; Douchiche et al., 2007; Roach and Deyholos, 2008).
MATERIALS AND METHODS
Preparation of CWs and protein extraction
Plants of flax (Linum usitatissimum L. ‘Hermes’, a gift of the ‘Cooperative Terre de Lin’, Normandy, France) were sown, as previously reported, on culture medium containing either 2·6 mm Ca(NO3)2 or 0·5 mm Cd(NO3)2 and 2·1 mm Ca(NO3)2 (Douchiche et al., 2007) and were sampled when they were 10 or 18 d old.
The middle parts of the hypocotyl, i.e. the segment 5 mm (in the presence of Cd) or 10 mm (for the control) above the collar and below the apex, were excised and ground in liquid nitrogen. An aliquot was frozen at −80 °C for RNA extraction and the remaining powder was treated overnight with Triton X100 (0·1 % in 1 mm CaCl2) and extensively washed with 1 mm CaCl2 (Gaffé et al., 1992). Ionically bound proteins were collected in 3 m LiCl after overnight incubation of the isolated CW in 1 mm CaCl2. An aliquot was stored at 4 °C for further measurements of enzymatic activities (without loss of activity for several weeks). The remaining proteins were extensively dialysed against H2O in dialysis tubing (Spectrapor 3-3500 C.O., Spectrum Labs, Rancho Dominguez, CA, USA) for electrophoretic experiments. All operations were run at 4 °C. The protein content of each extract was estimated using bovine serum albumin as standard, according to the micro-Bradford procedure (Bradford, 1976).
Pectin methylesterase (PME) activity
PME (EC 3·1·1·11) activity was measured at 25 °C, pH 7·5, using 0·2 % pectins diluted in 0·1 m salt, as previously reported (Gaffé et al., 1992). The amount of methanol produced during the reaction was controlled using the alcohol oxidase method of Klavons and Bennet (1986).
PER activity
Haem peroxidase (EC 1·11·1·7) assays were performed spectrophotometrically at 470 nm using 10−4 m H2O2 − 0·01 m gaïacol as the substrate, diluted in 0·05 m Tris maleate buffer (pH 7 and containing 1 mm CaCl2). The mixture (1·5 mL) was incubated for 10 min at 25 °C and the change in optical density was continuously recorded after the addition of 25 µL H2O2 (Burel et al., 1994).
Electrophoretic procedure
SDS–PAGE was performed in 12 % gel polyacrylamide in a mini-protean II system (Biorad, Hercules, CA, USA), according to Laemmli (1970). Electrophoresis was run for 1–1·5 h (from 60 to 220 V). Polypeptides were detected using silver or Coomassie brillant blue R250 staining. For silver stain, each band of gel was placed in 50 % ethanol/10 % acetic acid for 2 h. The gel was then transferred to 50 % ethanol for three changes of 20 min each, incubated in 0·02 % sodium thiosulfate and briefly rinsed in deionized water. Next the gel was placed in 0·1 % AgNO3 and 0·28 ‰ formaldehyde for 30 min, rinsed with deionized water and developed in 1·2 % Na2CO3, 0·037 ‰ formaldehyde solution. The reaction was stopped by washing the gel in 2 % acetic acid.
IEF gel electrophoresis (Multiphor II, LKB-Pharmacia, Saint-Quentin-en-Yvelines, France) was performed within a 3·5–10·0 pH range as previously described (Mareck et al., 1995). An aliquot (10 µL containing 0·1 PER to 0·5 PME nkatal enzyme activity) was loaded to the wells at 3 cm from the anode side. After electrophoresis, the pH gradient was determined using a contact electrode.
Gel staining procedures to reveal PME and PER activities
PME activity was visualized using the citrus pectin–agar technique and ruthenium red staining (Gaffé et al., 1992). PER activity was revealed directly on the IEF gel using H2O2 − gaïacol or H2O2–chloronaphthol, and staining was further stabilized with Coomassie blue solution (Burel et al., 1994; Bruyant et al., 1996).
Identification of peptides
After SDS-PAGE using 20–60 µg of protein, the polypeptides were visualized by R250 Coomassie blue, excised and tryptically digested with an automatic digester (MULTIPROBE II, Perkin-Elmer, Waltham, MA, USA) according to the protocol previously described (Collet et al., 2008). After freeze-drying, the peptide extracts were resuspended in 8 µL of 0·05 % trifluoroacetic acid/0·5 % acetonitrile. The samples were analysed by nano LC/MS/MS (Vilain et al., 2004). A 5-μL aliquot of the sample was injected onto an Ultimate nanoLC system (Dionex-LC Packings, Voisins le Bretonneux, France). Peptides were enriched and desalted on an RP-C18 trap column, and separated on a 75-μm ID*15 cm C18 column. A 45-min linear gradient (10–45 % acetonitrile in 2·2 % formic acid) at a flow rate of 200 nL min−1 was used. The eluent was analysed on a Q-TRAP System (Applied Biosystems, Courtaboeuf, France) equipped with a nanospray source. For protein identification, MS/MS peak lists were extracted and compared with the NCBInr protein database restricted to Viridiplantae, using the MASCOT MS/MS Ions Search toll (http://www.matrixscience.com/cgi/search_form.pl?FORMVER=2&SEARCH=MIS).
Reverse transcription and analysis of gene expression by PCR
Total RNA was prepared using an RNA extraction kit (Nucleospin RNA plant, Macherey-Nagel, Düren, Germany) and DNase I (Promega, Madison, WI, USA) from 200–400 mg of hypocotyls. Two micrograms of total RNA were reverse-transcribed to cDNA using an oligo-(dT) of 17 bp, 30 units of the AMV reverse-transcriptase (Promega) and 40 units of protector RNasin inhibitor (Promega). The resulting cDNA was then diluted to 100 ng L−1 for use in PCR reactions. The absence of genomic DNA contamination was tested performing two PCR reactions: one with the diluted first-strand cDNA and the other with total RNA products that were not treated with AMV reverse-transcriptase.
Semi-quantitative RT-PCR was performed in a final volume of 20 µL with 1·2 µL of the diluted cDNA, 0·5 units of GoTaq Polymerase (Promega), 5 mm dNTPs, and 10 pmol of each gene-specific primer (Table 1). After an initial denaturation step of 4 min at 94 °C, the following thermal amplification was used: 45 s at 94 °C, 1 min at the specific annealing temperature and 30 s at 72 °C. The number of cycles was adjusted for each gene studied to obtain a detectable signal without reaching saturation. Because a flax actin gene showed some variation in its expression under Cd treatment, elongation factor LuEF1α was used as the constitutive control (Roach and Deyholos, 2008). Eighteen microlitres of the amplification products were loaded on a 1·2 % (w/v) agarose gel stained with ethidium bromide and run at 100 V for 30 min.
Table 1.
Experimental conditions of PCR: specific primers, annealing temperature (T), total number of amplification cycles (N) used for semi-quantitative PCR analysis of gene expression and corresponding size of the amplified fragments (S)
| Gene (accession number) | Primer sequence (5′ → 3′) |
T (°C) | N | S (bp) | |
|---|---|---|---|---|---|
| LuEF1α | Sense | TTGGATACAACCCCGACAAAA | 60 | 31 | 100 |
| Anti-sense | GGGCCCTTGTACCAGTCAAG | ||||
| Lupmet | Sense | GGRCCATCRAARCACCARGC | 55 | 30 | 158 |
| Anti-sense | AAGATGARGTCGACKGTSCC | ||||
| Lupme1 (AF355056) | Sense | CATCAACCCCAACTTCCTCCTC | 55 | 35 | 223 |
| Anti-sense | CCCACCACCGCAGTTTTAATATC | ||||
| Lupme3 (AF188895) | Sense | ACCGGTCCATGTATCGC | 55 | 32 | 178 |
| Anti-sense | TGACGTGCTCATTGTCTC | ||||
| Lupme5 (AF355057) | Sense | CGCCGCCGTTTTATCC | 53 | 35 | 176 |
| Anti-sense | CGCACAGCTCGATGCA | ||||
| Flxper1 (L07554) | Sense | TACTTCACCAATCTCCAGACC | 58 | 29 | 101 |
| Anti-sense | GCGAACCTGTTGACGAGCTC | ||||
| Flxper2 (L24120) | Sense | CCACTCCGACCAAGTCCTGTT | 58 | 32 | 133 |
| Anti-sense | CAGTGAGGGGCTTGATGTCT | ||||
| Flxper3 (U59284) | Sense | CGACAACAAGTATTATGTTGACTTG | 60 | 30 | 185 |
| Anti-sense | ACCGGTCAACACGCTTATCTGC | ||||
| Flxper4 (AF049881) | Sense | TACCAGGATCTGGTGGCGAG | 58 | 35 | 98 |
| Anti-sense | TTGTTGCTGTAAGTCCTCACC | ||||
A theoretical total Lupmet was obtained using degenerate primers designated as the most common sequences within the three Lupme genes.
RESULTS
Ionically bound proteins
The total amount of ionically bound proteins (specifically eluted with LiCl) was in the same range (1–2·5 mg g−1 dry CW) as that previously determined when eluting with NaCl (Alexandre et al., 1997). In control plants, the protein yield decreased with age mainly due to the CW mass increase (Table 2). In 10-d Cd-treated hypocotyl, the yield was similar to the 10-d control despite of the greater CW mass in Cd, indicating that Cd treatment induced a parallel increase of both CW polysaccharides (which consisted of the main component of CW) and proteins. Then in 18-d Cd-treated seedlings, the protein yield and CW mass remained about constant as compared with 10-d Cd-treated seedlings.
Table 2.
Pectin methylesterase and peroxidase in vitro activities from cell-wall ionically bound proteins of hypocotyl
| 10-d hypocotyl |
18-d hypocotyl |
|||
|---|---|---|---|---|
| − Cd | + Cd | − Cd | + Cd | |
| Cell-wall yield (%) | 3·4 ± 0·3 | 3·9 ± 0·4 | 4·2 ± 0·4 | 4·1 ± 0·5 |
| Cell-wall proteins (mg g−1 dry CW) | 2·4 ± 0·2 | 2·1 ± 0·2 | 1·0 ± 0·1 | 2·5 ± 0·2 |
| PME (μkatal g−1 dry CW) | 1·0 ± 0·1 | 1·6 ± 0·1 | 0·6 ± 0·1 | 1·0 ± 0·1 |
| Peroxidase (nkatal g−1 dry CW) | 70 ± 6 | 76 ± 8 | 120 ± 10 | 186 ± 14 |
Seedlings were grown in the presence (+ Cd) or absence (− Cd) of cadmium and harvested at 10 and 18 d (10 d and 18 d, respectively). Data represent the means ± s.d. (n = 3). Note the highest value of PME activity at 10 d while the peroxidase activity increased at 18 d. The CW % was calculated as the mass of dry CW (g) per 100 g of fresh hypocotyls.
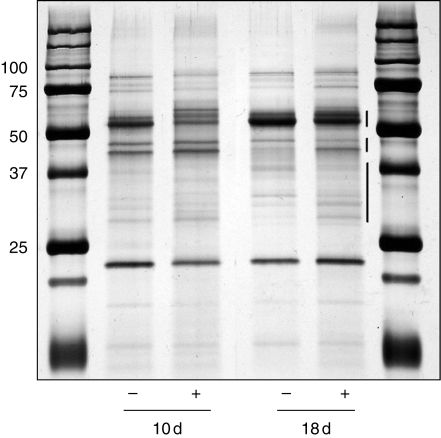
The SDS–PAGE silver-stained protein pattern was strongly affected by the presence of Cd (Fig. 1). The most obvious changes were detected in the range of 50–60 kDa at 10 d and within a couple of bands between 42 and 48 kDa at 18 d. Other minor varying bands were in the range of 30–36 kDa. All of these silver-stained bands were also labelled by anti-fucose/xylose antibodies (data not shown) and principally contained, according to Lerouge et al. (1998), proteins that were CW specific.
Fig. 1.
SDS–PAGE of the cell-wall ionically bound proteins of the hypocotyl. Seedlings were grown in the presence (+) or absence (−) of cadmium and harvested at 10 and 18 d old, and 1 µg of proteins from − Cd and + Cd were loaded per well. This figure is representative of three SDS–PAGE repeats. Note the high variability in the distribution of the silver-stained polypeptides especially in the ranges of 60 kDa, 43–50 kDa and 30–37 kDa.
Among the scored proteins, the highest number of peptides identified by LC/MS/MS analysis derived from the Lu-EP1 secreted protein (Table 3), a protein commonly found among ionically CW-bound proteins of flax. Other peptides of interest in the present study originated from pectin esterase, PME and PER. In the presence of Cd, a high number of FlxPER3 peptides specifically appeared with very high score of confidence. Also, four peptides specific to LuPME5 were clearly identified in Cd-treated hypocotyls.
Table 3.
Identification of peptides by LC/MS/MS from LiCl-extracted cell-wall proteins of flax hypocotyl
| Mass range (kDa) | Protein identification | Species | Protein accession | Theoretical mass (kDa) | % coverage | Score | Peptide no. | Notes* |
|---|---|---|---|---|---|---|---|---|
| Control seedlings | ||||||||
| 55–60 | Unnamed protein product | Vitis vinifera | gi|27463662 | 60·188 | 7 | 206 | 3 | a |
| Pectinesterase | Solanum lycopersicum | gi|1944575 | 56·206 | 4 | 111 | 2 | ||
| 50–55 | Secreted glycoprotein (LuEP1) | Linum usitatissimum | gi|27463662 | 47·906 | 12 | 353 | 6 | |
| Os01g0312500 | Oryza sativa | gi|115436216 | 46·120 | 2 | 61 | 1 | b | |
| 34–36 | Secreted glycoprotein (LuEP1) | Linum usitatissimum | gi|27463662 | 47·906 | 1 | 188 | 4 | |
| Putative pectinesterase | Arabidopsis thaliana | gi|22531132 | 60·013 | 7 | 138 | 3 | ||
| Peroxidase (FlxPER3) | Linum usitatissimum | gi|1389835 | 38·171 | 1 | 134 | 3 | ||
| Anionic peroxidase (fragments) | Glycine max | gi|415474 | 31 | 63 | 1 | |||
| 30–34 | Secreted glycoprotein (LuEP1) | Linum usitatissimum | gi|27463662 | 47·906 | 22 | 487 | 8 | |
| Unnamed protein product | Vitis vinifera | gi|157340696 | 60·188 | 8 | 211 | 4 | c | |
| Peroxidase (FlxPER3) | Linum usitatissimum | gi|1389835 | 38·171 | 5 | 92 | 3 | ||
| Cd-treated seedlings | ||||||||
| 55–60 | Secreted glycoprotein (LuEP1) | Linum usitatissimum | gi|27463662 | 47·906 | 17 | 345 | 7 | |
| 50–55 | Secreted glycoprotein (LuEP1) | Linum usitatissimum | gi|27463662 | 47·906 | 26 | 521 | 9 | |
| 34–36 | Secreted glycoprotein (LuEP1) | Linum usitatissimum | gi|27463662 | 47·906 | 7 | 168 | 4 | |
| Peroxidase (FlxPER3) | Linum usitatissimum | gi|1389835 | 38·171 | 33 | 533 | 10 | ||
| 30–34 | Pectinesterase | Solanum lycopersicum | gi|1944575 | 56·206 | 4 | 131 | 2 | |
| Peroxidase (FlxPER3) | Linum usitatissimum | gi|1389835 | 38·171 | 15 | 293 | 5 | ||
| Putative pectin methylesterase (LuPME5) | Linum usitatissimum | gi|14582867 | 59·932 | 8 | 230 | 4 | ||
Proteins (60 µg from a mix of 10- and 18-d-old seedlings grown with or without Cd) were submitted to SDS–PAGE; after coloration with Comassie blue, the bands were excised and tryptically digested. The mass range of the band was estimated from the marker position. Theorical mass corresponded from the amino acid sequence without glycan.
* Notes corresponded to BLAST result: ‘a’, multicopper oxidase, putative (Ricinus communis), pectinesterase (Annona cherimola), sks5 (SKU5 Similar 5); copper ion binding/oxidoreductase (arabidopsis); ‘b’, pectin methylesterase (Musa acuminata) AAA group, pectinesterase-3 precursor, putative (Ricinus communis); ‘c’, pectinesterase (Annona cherimola), multicopper oxidase, putative (Ricinus communis).
PME activity
Whatever the growth conditions, the activity of ionically bound PME in flax hypocotyl was estimated within the range of 0·5–1·7 µkatal g−1 of dry CW and there was a decrease of about 40 % at 18 d as compared with 10 d (Table 2). The decrease in control seedlings was mainly related to the increase of the CW mass. Importantly, in the presence of Cd, PME activity increased relative to controls at both 10 d and 18 d. However, when expressed as specific activity, the PME level increased (by 81 %) with Cd at 10 d while it slightly decreased at 18 d. This result indicates that the impact of Cd on the PME activity enhancement mainly occurred at 10 d.
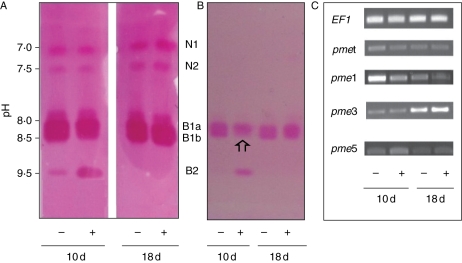
After isofocusing, up to five isoforms of PME were detected with isoelectric points from 7 to >9·5: namely neutral (N1 and N2), moderately basic (containing B1a and B1b) and basic (B2) forms (Fig. 2A, B). As previously reported, B1a and B1b were the main isoforms carrying PME activity (Alexandre et al., 1997); the former being found mainly in the inner tissues while the latter was rather specific to the cortical tissues. Interestingly, a decrease of ruthenium-red-coloured B1b in the presence of Cd was observed in the pectin–agarose gel mainly at 10 d (Fig. 2B). Such behaviour was barely observed on the acrylamide IEF gel, due to the highly saturated staining (Fig. 2A). On the other hand, the B2 isoform was described as a highly regulated protein since, within a same region of the hypocotyl, it could be alternatively up- or down-regulated over the period 3–10 d of growth (Al-Qsous et al., 2004). In the present study, at 10 d, when cell elongation had just ceased and cell expansion was in progress, the staining of the B2 isoform markedly increased when the plants were grown in the presence of Cd. Conversely, at 18 d, whatever the conditions, the B2 isoform could be hardly detected.
Fig. 2.
Pectin methylesterase isoenzymes ionically bound to the cell wall of the hypocotyl. Seedlings were grown in the presence (+) or absence (−) of cadmium and harvested at 10 and 18 d old. (A) IEF and (B) transfer of the gel onto a pectin–agarose gel. PME activity was revealed by ruthenium-red staining de-esterified homogalacturonans. A similar amount of PME (0·5 nkatal) activity was loaded per well. The pH values measured with a surface electrode are given on the left. Note the decrease of B1b (arrow) which paralleled the increased of B2 at day 10. (C) Expression of Lupme genes compared with the elongation factor gene LuEF1 α (EF1) as seen by PCR. All gel figures were representative of three to five repeats. N and B are neutral and basic isoforms, respectively.
Altogether, the data indicated that the Cd-induced increase of PME activity at 10 d originated, at least partly, from a B2 isoform. The decrease in PME activity between 10 d and 18 d must therefore originate at least in part from a turnover of the B2 isoform.
Expression of Lupme genes
In flax, only three genes encoding PME, named Lupme1, 3 and 5, have thus far been found to be expressed in the hypocotyl. This appears to be a relatively low number compared with the 30 putative PME genes that have been reported to be expressed in whole arabidopsis seedlings (Bosh and Hepler, 2005). However, the post-germinative hypocotyl is an unusual organ with almost no cell division, which might explain the low number of expressed PME. Indeed, in hypocotyls (etiolated) of arabidopsis only six PME proteins were identified (Irshad et al., 2008). Among them, AtPME2 and 3 (Micheli et al., 1998), shared the highest identity with LuPME3 and 5.
In order to assess a role for these specific Lupme genes in the regulation of the methylesterification of pectins during the Cd-mediated axis reorientation of flax hypocotyl, their mRNA expression was analysed using three specific primers. The expression of Lupme1, 3 and 5 was compared with that of a theoretical total Lupme (indexed with t), as obtained when using degenerate primers designated as the most common sequences within these three genes, and being, according to Markovic and Janecek (2004), characteristic of PME signature.
Figure 2C indicates that the expression of Lupmet was the highest at 10 d in control seedlings. A similar expression pattern was found with Lupme1, although much more contrasted. On the other hand, the expression of Lupme3 was maximal at 18 d (with or without Cd), while that of Lupme5 appeared relatively low whatever the day of culture of the control seedlings. When the seedlings had been grown in the presence of Cd, the expression of Lupme1 decreased, while that of Lupme5 was enhanced and that of Lupme3 was unaffected. The up-regulation of Lupme5 was maximal at 10 d.
According to Al-Qsous et al. (2004), the gene Lupme5 codes for the B2 isoform. Consequently, at 10 d in the presence of Cd, the increase in B2 activity was correlated with an induction of Lupme5 transcripts. At 18 d, the decline of B2 isoform was related to both the under regulation of Lupme5 and to the turnover of the protein.
PER activity
CW PER activity was estimated in the range of 64–200 nkatal g−1 dry CW, the activity being much higher at 18 d (Table 2). In the presence of Cd, the activity was stimulated particularly at 18 d.
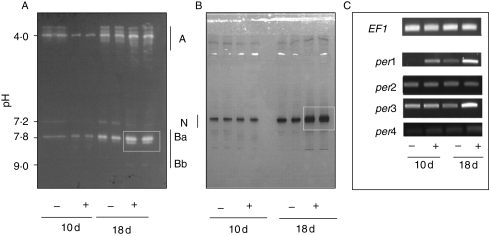
After isofocussing electrophoresis and activity staining either in the presence of gaïacol (Fig. 3A) or chloronaphthol (Fig. 3B), three main groups of PER isoforms (based on pI) were evident. From 16 isoforms that have been attributed to normal or stressed flax cells and plants (Bruyant et al., 1996; Gerhardt and Fieldes, 1999), four acidic bands (out of six), one neutral band (out of the four specifically identified in flax-cell culture medium) and three basic forms (out of six) could be detected. The anionic forms (A) and one or two low-basic forms (Ba) were revealed with gaïacol while the neutral band (N) and the faint basic one (Bb) were most reactive with chloronaphthol. In control seedlings, the staining was generally low at 10 d (apart from the main acidic one) while the main bands A, N and Ba were clearly contrasted at 18 d.
Fig. 3.
Peroxidase isoenzymes ionically bound to the cell wall of the hypocotyl. Seedlings were grown in the presence (+) or absence (−) of cadmium and harvested at 10 and 18 d old. (A, B) After IEF, the peroxidase activity was revealed in the presence of gaïacol (A) or chloronaphthol (B). A similar amount of PER (0·1 nkatal) activity was loaded per well, each sample being repeated in two successive wells. The pH values measured with a surface electrode are given on the left. Note the increase with age and Cd of the isoforms N and Ba. (C) Expression of Flxper genes compared with elongation factor gene LuEF1 α (EF1) as seen by PCR. All gel figures were representative of three to five repeats. A, N and B are acidic, neutral and basic isoforms, respectively.
When the seedlings were grown in the presence of Cd, the staining of the two isoforms Ba and N was particularly reinforced at 18 d. The main anionic form (around pH 4) was Cd-inhibited at 10 d and Cd-stimulated at 18 d. The basic form Bb was detected mainly at 18 d with Cd.
Expression of PER genes
To date, four sequences have been reported for flax PER genes, Flxper1–4 (Omann et al., 1994; Omann and Tyson, 1996). Flxper1 encodes an anionic polypeptide specifically expressed in stem, possibly associated with membranes. Flxper2 is a pathogenesis-related protein. No additional functional information is available about Flxper3 and 4. In arabidopsis, the genome contains an estimated 73 genes encoding Class III PERs, of which, only nine were identified within CW of etiolated hypocotyl (Irshad et al., 2008); Flxper1 shares 54–55 % identity with At3g32980, At3g49110 and At3g49120 while Flxper3 presents 64 % identity with At1g71695.
Using specific primers to identify Flxper1–4 cDNA, differential expression was found between 10 d and 18 d and in the presence of Cd (Fig. 3C). Despite the use of several primers, Flxper4 expression could be hardly detected. The signal intensity increased slightly at 18 d when the plantlets had been grown in the presence of Cd. Flxper2 expression was relatively constant whatever the growth conditions. Flxper3 expression was the highest at 10 d in control seedlings and highly stimulated in the presence of Cd but at 18 d. Flxper1 was the most variable gene being low-expressed in control seedlings and stimulated in the presence of Cd.
Overall, Flxper1 and 3 were particularly up-regulated in the presence of Cd at 18 d . In the same conditions, the two major isoforms were Ba and N. Both sets of data fit with the PER activity data. Interestingly, the highest number of peptides identified in the presence of Cd corresponded to FlxPER3 (Table 3).
DISCUSSION
Two main conclusions can be drawn from the present PME data. Firstly, a decrease of activity was observed with increasing hypocotyl age, which paralleled the decreased abundance of both Lupme1 and 5 and the IEF isoform B2. Isoform B2 protein has been reported to be encoded by Lupme5 (Al-Qsous et al., 2004). Hence the specific decline of the isoform B2 with age and the expansion of the organ was due to a high protein turnover and, to some extent, to a decrease in transcript expression of Lupme5. Conversely, Lupme3, which was shown to be specific to vascular tissue in the hypocotyl (Roger et al., 2001), increased in abundance with age. On the other hand, the decreased abundance of Lupme1 could not yet be related to a particular PME. Studying the PME activity pattern in flax hypocotyl, Alexandre et al. (1997) located the B2 isoform in cortical tissues. In the future it would be worthwhile to separate cortical and vascular tissues in order to check whether the 40 % decrease of PME was specific to cortical tissues.
Secondly, a significant impact of Cd on PME activity has been shown through an enhancement occurring mainly at 10 d and that was partly maintained at 18 d. At 10 d, an over-regulation of Lupme5 and a specific increase in B2 isoform were also noted. Al-Qsous et al. (2004) underlined the high spatio-temporal variability of Lupme5 expression during the elongation of the hypocotyl, relating the over-regulation of the gene to cell maturation. They also noted a particular behaviour at 10 d, with a new increase of Lupme5 in the hypocotyl basal part where tissues are extensively expansing, while almost no expression was detected in the middle part. In the presence of Cd, B2 isoform was detected in the middle part of the hypocotyl at 10 d. Thus, the impact of Cd would not only increase the hypocotyl expansion level (Douchiche et al., 2007), but also accelerate the expansion initiation. Further kinetics data are needed to check whether and when such a process occurs in the middle part of an untreated hypocotyl.
Below, it is discussed whether the variation of B2 observed here was related to the Cd-induced modifications of HGA structure reported by Douchiche et al. (2007). A sharp decrease in JIM7 gold-labelling (specific to high methylesterified HGAs) was observed by Douchiche (2006) in the junctions of cortical tissues, not only at 18 d but also at 10 d. As the impact of Cd on B2 isoform is maximum at 10 d, it is hypothesized that part of the Cd-induced de-esterification of HGAs observed in these domains was catalysed by this particular activity.
In the context of metal stress, it is interesting to note the 60 % identity of Lupme5 with the At3g14310 PME gene whose expression was up-regulated in the presence of Hg (Heidenreich et al., 2001). Moreover, B2 had been measured in abundance in the wall of cell suspension and callus of flax, which might also be related to stress impact (Gaffé et al., 1992).
According to the nomenclature of Markovic and Janecek (2004) the gene Lupme5 is of type I, with a PME inhibitor domain in the N-terminal part. Following Al-Qsous et al. (2004), B2 could represent the so-called mature enzyme (34–37 kDa) while one B1 isoform could be the pro-protein (60 kDa after SDS–PAGE). The two isoforms B1b and B2 being localized in cortical tissues (Alexandre et al., 1997), the Cd-related increase of B2 activity at 10 d might be due to the partial proteolysis of B1b whose decrease was observed at 10 d in pectin–agarose. Actually, four peptides specific to the LuPME5 sequence were identified from SDS–PAGE electrophoresis, in the range of 30–34 kDa, specifically from the Cd-treated hypocotyl. A few peptidases were found to be up-regulated in arabidopsis roots exposed to Cd (Roth et al., 2006) or Al stress (Kumari et al., 2008). No peptidase sequence was identified after SDS–PAGE. Microarray experiments are under way (M. J. Roach and M. K. Deyholos, University of Alberta, Canada, pers. comm.) which might provide evidence of the up-regulation of such an endopeptidase.
However, this hypothesis does not fit the conclusion of Wolf et al. (2009), indicating that maturation of type I PMEs inside the secretory pathway would be required for their secretion in the CW. Alternatively, the isoform B1b could be encoded by another gene to that of Lupme5 and could have another role to that of B2 in seedling response to Cd at 10 d, possibly in the opposite redistribution of high-methylesterified and low-methylesterified HGAs in the epidermis (Douchiche et al., 2007). Interestingly, the strong decrease in the JIM5 labelling (specific to low methylesterified HGAs) occurred specifically at 10 d in the inner part of the external tangential wall of the epidermis (Douchiche, 2006). The data indicated a local reduction in PME activity, which can be possibly related to the decrease in cortical isoform B1b. No antibodies specific to this isoform which would allow this hypothesis to be checked are available yet.
In this study, Class III PERs were also investigated because their functions in plants have been identified to be important in cell maturation and tissue differentiation (Gaspar et al., 1985; Passardi et al., 2004). Goldberg et al. (1986) observed active PERs in the epidermis at the end of the elongation phase.
The present data in flax pointed out that PER activity increased not only with age but also in the presence of Cd. Also, Gerhardt and Fieldes (1999) related the effect of Cd on PER activity and isoform distribution to seedling ageing. The relationship of CW PER to abiotic stress has been reported in plants other than flax, although the isoform pattern has not often been described (McDougall, 1993; Lin and Kao, 1999; Lee et al., 2007; Jouili et al., 2008; Verma et al., 2008). In arabidopsis, 14 of the 73 genes encoding PER of class III are related to metal stresses (Cosio and Dunand, 2009). Among them, the gene At3g49120 was also identified in arabidopsis hypocotyl CW (Irshad et al., 2008) and shares 55 % identity with Flxper1. On the other hand, Flxper1–4 have <40 % identity with two PERs (At5g64100 and At4g30170) which were responsive to Al in the root (Kumari et al., 2008).
The increase in PER activity that occurred at 18 d was accompanied by increased transcript abundance of Flxper1 and 3 as well as an enhancement of Ba and N isoforms. This warrants a consideration of their possible role in Cd-induced cross-linking of HGAs which was biochemically shown to occur in the cortical tissue (Douchiche et al., 2007). The PER temporal regulation appeared complementary to that of PME, suggesting a cross-linking of HGAs by PER within the CW once the redistribution of the methylester of HGAs had been set by PME. Calcium-bridged HGAs have been reported to bind a particular class III PER and to initiate polysaccharide covalent cross-linking (Penel and Greppin, 1996). According to the authors, calcium pectates do not behave like a simple cation exchanger retaining basic isoforms since the most basic ones of zucchini do not bind them. Experiments should be run with the Cd-induced N and Ba isoforms in order to test the hypothesis. On the other hand, Carpin et al. (2001) show that the binding of a soluble anionic PERs resulted from electrostatic interactions between the positive charges of arginines and the negative charges of the pectates, possibly reinforced by calcium bridges between both anionic moities. Although the main anionic isoform of flax hypocotyl appeared relatively constant whatever the samples, we cannot exclude that Cd-modulated minor anionic isoforms might be involved in such interactions with calcium pectates.
As far as the genes are concerned, it was found that Flxper1 and 3 shared significant identities (55 and 64 %, respectively) with two arabidopsis genes (At3g32980 and At1g71695, respectively), known to bind calcium-bridged HGAs (Dunand et al., 2002; Shah et al., 2004). Interestingly, the highest number of peptides identified in the LiCl extract collected from Cd-treated seedlings corresponded to Flxper3. None of these sequences has definitively been correlated with specific isozymes yet.
Altogether, the present data support the hypotheses raised by Douchiche et al. (2007) on the role of PME and PER in the Cd-induced alterations of structural features of HGAs in flax hypocotyl. The pectin remodellings, both in charges and in extractibility, would reinforce the cell cohesion during the Cd-induced swelling and maintain the toxic cation within the most external CW domains. Interestingly, the cell machinery mobilized in these particular remodellings resembles the one usually involved for its expansion, which consists of one adaptative response to the metallic stress.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Michael Deyholos, University of Alberta (Canada) for reading the manuscript, Dr Alain Mareck and Dr Odile Soret for helpful discussions, and Dr Laurent Coquet and Dr Christophe Rihouey for access to the Proteomics platform.
LITERATURE CITED
- Alexandre F, Morvan O, Gaffé J, et al. Pectin methylesterases pattern in the flax seedlings during their development. Plant Physiology and Biochemistry. 1997;35:427–436. [Google Scholar]
- Al-Qsous S, Carpentier E, Klein-Eude D, et al. Identification and isolation of a pectin methylesterase isoform that could be involved in flax cell-wall stiffening. Planta. 2004;219:369–378. doi: 10.1007/s00425-004-1246-1. [DOI] [PubMed] [Google Scholar]
- Andeme-Onzighi C, Girault R, His I, Morvan C, Driouich A. Immunocytochemical characterization of early-developing flax fibre cell walls. Protoplasma. 2000;213:235–245. [Google Scholar]
- Barceló J, Vasquez MD, Poschenrieder C. Cadmium induced structural and ultrastructural changes in the vascular system of bush bean stems. Botanica Acta. 1988;101:254–261. [Google Scholar]
- Bosh M, Hepler PK. Pectin methylesterases and pectin dynamics in pollen tubes. The Plant Cell. 2005;17:3219–3226. doi: 10.1105/tpc.105.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruyant P, Schauman A, Djiana R, Morvan C, Balangé PA. Effect of light on total proteins and peroxidase activities in the medium and in the cell-wall fraction of suspension-cultured cells of flax. Plant Physiology and Biochemistry. 1996;34:417–423. [Google Scholar]
- Burel C, Berthe T, Mery JC, Morvan C, Balangé AP. Isoelectric focusing analysis of peroxydases in flax seedling hypocotyls grown in different light conditions. Plant Physiology and Biochemistry. 1994;32:853–860. [Google Scholar]
- Carpentier E. EP1 and PME1: deux proteines pariétales à motif RGD, quels rôles chez le lin? France: University of Rouen; 2005. PhD Thesis. [Google Scholar]
- Carpin S, Crèvecoeur M, de Meyer M, Simon P, Greppin H, Penel C. Identification of a Ca2+-pectate binding site on an apoplastic peroxidase. The Plant Cell. 2001;13:511–520. doi: 10.1105/tpc.13.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaoui A, Ferjani EE. Impact of cadmium and copper excess on cell wall peroxidases in pea stems. Pakistan Journal of. Biological Sciences. 2004;7:902–904. [Google Scholar]
- Collet A, Cosette P, Beloin C, et al. Impact of rpoS deletion on the proteome of Escherichia coli grown as biofilm. Journal of Proteome Research. 2008;7:4659–4669. doi: 10.1021/pr8001723. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Assembly and enlargement of the primary cell wall in plants. Annual Review of Cell Developmental Biology. 1997;13:171–201. doi: 10.1146/annurev.cellbio.13.1.171. [DOI] [PubMed] [Google Scholar]
- Cosio C, Dunand C. Specific functions of individual class III peroxidase genes. Journal of Experimental Botany. 2009;60:391–408. doi: 10.1093/jxb/ern318. [DOI] [PubMed] [Google Scholar]
- Demarty M, Morvan C, Thellier M. Calcium and the cell wall. Plant, Cell and Environment. 1984;7:441–448. [Google Scholar]
- Derbyshire P, McCann M, Roberts K. Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biology. 2007;7:31–42. doi: 10.1186/1471-2229-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douchiche O. France: University of Rouen; 2006. Implication de la paroi de l'hypocotyle de lin (Linum usitatissimum) dans la réponse au cadmium. Rôle des pectines dans la restructuration des parois. PhD thesis. [Google Scholar]
- Douchiche O, Rihouey C, Schaumann A, Driouich A, Morvan C. Cadmium-induced alterations of the structural features pectins in flax hypocotyl. Planta. 2007;225:1301–1312. doi: 10.1007/s00425-006-0425-7. [DOI] [PubMed] [Google Scholar]
- Dunand C, Tognolli M, Overney S, et al. Identification and characterization of Ca2+-pectate binding peroxidases in Arabidopsis thaliana. Journal of Plant Physiology. 2002;159:1165–1171. [Google Scholar]
- Eticha D, Stass A, Horst WJ. Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant, Cell and Environment. 2005;28:1410–1420. [Google Scholar]
- Femenia A, Garosi P, Roberts K, Waldron KW, Selvendran RR, Robertson JA. Tissue-related changes in methyl-esterification of pectic polysaccharides in cauliflower (Brassica oleracea L. var. botrytis) stems. Planta. 1998;205:438–444. doi: 10.1007/s004250050341. [DOI] [PubMed] [Google Scholar]
- Gaffé J, Morvan C, Jauneau A, Demarty M. Partial purification of flax cell-wall pectin methylesterase. Phytochemistry. 1992;31:761–765. [Google Scholar]
- Gaspar T, Penel C, Castillo FJ, Greppin H. A two step control of basic and acidic peroxidases and its significance for growth and development. Physiologia Plantarum. 1985;64:418–423. [Google Scholar]
- Gerhardt KE, Fieldes MA. Charge and molecular weight differences for fourteen guaïacol peroxidase isozymes in flax indicate that most are encoded by different structural genes. Electrophoresis. 1999;20:1939–1945. doi: 10.1002/(SICI)1522-2683(19990701)20:10<1939::AID-ELPS1939>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Goldberg R, Imberty A, Chu-Ba J. Development of isoperoxidases along the growth gradient in the mung bean hypocotyl. Phytochemistry. 1986;25:1271–1274. [Google Scholar]
- Goldberg R, Morvan C, Jauneau A, Jarvis MC. Methyl-esterification, de-esterificiation and gelation of pectins in the primary cell wall. Review. Progress in Biotechnology. 1996;14:151–172. [Google Scholar]
- Hassinen VH, Tervahauta AI, Halimaa P, et al. Isolation of Zn-responsive genes from two accessions of the hyperaccumulator plant Thlaspi caerulescens. Planta. 2007;225:977–989. doi: 10.1007/s00425-006-0403-0. [DOI] [PubMed] [Google Scholar]
- Heidenreich B, Mayer K, Jr, Sandermann H, Ernst D. Mercury-induced genes in Arabidopsis thaliana: identification of induced genes upon long-term mercuric ion exposure. Plant, Cell and Environment. 2001;24:1227–1234. [Google Scholar]
- His I, Driouich A, Jauneau A. Distribution of cell wall matrix polysaccharides in the epidermis of flax hypocotyl seedlings: calcium-induced acidification of pectins. Plant Physiology and Biochemistry. 1997;35:631–644. [Google Scholar]
- Irshad M, Canut H, Borderies G, Pont-Lezica R, Jamet E. A new picture of cell wall protein dynamics in elongating cells of Arabidopsis thaliana: confirmed actors and newcomers. BMC Plant Biology. 2008;8:94. doi: 10.1186/1471-2229-8-94. http://www.biomedcentral.com/1471-2229/8/94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MC, McCann MC. Macromolecular biophysics of the plant cell wall: concepts and methodology. Plant Physiology and Biochemistry. 2000;38:1–13. [Google Scholar]
- Jauneau A, Cabin-Flaman A, Verdus MC, Ripoll C, Thellier M. Involvement of calcium in the inhibition of endopolygalacturonase activity in epidermis cell wall of Linum usitatissimum. Plant Physiology and Biochemistry. 1994;32:839–846. [Google Scholar]
- Jauneau A, Quentin M, Driouich A. Micro-heterogeneity of pectins and calcium distribution in the epidermal and cortical parenchyma of flax. Protoplasma. 1997;198:9–19. [Google Scholar]
- Jouili H, Bouazizi H, Rossignol M, Borderies G, Jamet E, Ferjani EE. Partial purification and characterization of a copper-induced anionic peroxidase of sunflower roots. Plant Physiology and Biochemistry. 2008;46:760–767. doi: 10.1016/j.plaphy.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Kim JB, Carpita N. Changes in esterification of the uronic acid groups of cell wall polysaccharides during elongation of maize coleoptiles. Plant Physiology. 1992;98:646–653. doi: 10.1104/pp.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavons JA, Bennet RD. Determination of methanol using alcohol oxydase and its application to methyl ester content of pectins. Journal of Agricultural and Food Chemistry. 1986;34:597–599. [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C, Roberts K. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta. 1990;181:512–521. doi: 10.1007/BF00193004. [DOI] [PubMed] [Google Scholar]
- Konno H, Nakato T, Nakashima S, Katoh K. Lygodium japonicum fern accumulates copper in the cell wall pectin. Journal of Experimental Botany. 2005;56:1923–1931. doi: 10.1093/jxb/eri187. [DOI] [PubMed] [Google Scholar]
- Kumari M, Taylor GJ, Deyholos MK. Transcriptomic responses to aluminium stress in roots of Arabidopsis thaliana. Molecular Genetics and Genomics. 2008;279:339–357. doi: 10.1007/s00438-007-0316-z. [DOI] [PubMed] [Google Scholar]
- Lacoux J, Klein D, Domon JM, et al. Antisense transgenesis of Linum usitatissimum with a pectin methylesterase cDNA. Plant Physiology and Biochemistry. 2003;41:241–249. [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee RB, Kim KY, Jung WJ, Avice JC, Ourry A, Kim TH. Peroxidase and lignification in relation to the intensity of water-deficit stress in white clover (Trifolium repens L.) Journal of Experimental Botany. 2007;58:1271–1279. doi: 10.1093/jxb/erl280. [DOI] [PubMed] [Google Scholar]
- Lerouge P, Cabanes-Macheteau M, Rayon C, Fischette-Lainé AC, Gomord V, Faye L. N-Glycoprotein biosynthesis in plant: recent developments and future trends. Plant Molecular Biology. 1998;38:31–48. [PubMed] [Google Scholar]
- Lin CC, Kao HC. NaCl induced changes in ionically bound peroxidase activity in roots of rice seedlings. Plant and Soil. 1999;216:147–153. [Google Scholar]
- McDougall GJ. Accumulation of wall-associated peroxidases during wound-induced suberization of flax. Journal of Plant Physiology. 1993;142:651–656. [Google Scholar]
- Mareck A, Gaffé J, Morvan O, Alexandre C, Morvan C. Characterization of isoforms of pectin methylesterase using polyclonal antibodies. Plant and Cell Physiology. 1995;36:409–417. [Google Scholar]
- Markovic O, Janecek S. Pectin methylesterases: sequence-structural features and phylogenetic relationships. Carbohydrate Research. 2004;339:2281–2295. doi: 10.1016/j.carres.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Micheli F, Holliger C, Goldberg R, Richard L. Characterization of the pectin methylesterase-like gene AtPME3: a new member of a gene family comprising at least 12 genes in Arabidopsis thaliana. Gene. 1998;220:13–20. doi: 10.1016/s0378-1119(98)00431-4. [DOI] [PubMed] [Google Scholar]
- Morvan C, Abdul-Hafez A, Jauneau A, Demarty M. Les composés pectiques, marqueurs de la croissance du lin. Bulletin de la Société de Botanique. 1991;a 138:339–350. [Google Scholar]
- Morvan C, Abdul-Hafez A, Jauneau A, Thoiron B, Demarty M. Incorporation of D-[U-14C]glucose in the cell wall of Linum plantlets during the first steps of growth. Plant and Cell Physiology. 1991;b 32:609–621. [Google Scholar]
- Omann F, Tyson H. An anionic stem-specific flax peroxidase cDNA with C-terminal motifs also found in a blue copper-type pea protein correlated with lignin deposition. Australian Journal of Plant Physiology. 1996;23:773–789. [Google Scholar]
- Omann F, Beaulieu N, Tyson H. cDNA sequence and tissue-specific expression of anionic flax peroxidase. Genome. 1994;37:137–147. doi: 10.1139/g94-018. [DOI] [PubMed] [Google Scholar]
- Pan JW, Ye D, Wang LL, et al. Root border cell development is a temperature-insensitive and Al-sensitive process in barley. Plant and Cell Physiology. 2004;45:751–760. doi: 10.1093/pcp/pch090. [DOI] [PubMed] [Google Scholar]
- Passardi E, Cosio C, Penel C, Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Reports. 2005;24:255–265. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- Passardi F, Penel C, Dunand C. Performing the paradoxical: how plant peroxidases modifiy the cell wall. Trends in Plant Sciences. 2004;9:534–540. doi: 10.1016/j.tplants.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Penel C, Greppin H. Pectin binding proteins: caracterization of the binding and comparison with heparin. Plant Physiology and Biochemistry. 1996;34:479–488. [Google Scholar]
- Rihouey C, Jauneau A, Cabin-Flaman A, Demarty M, Lefèbvre F, Morvan C. Calcium and acidic pectin distribution in flax cell walls: evidence for different kinds of linkage in the cell junction and middle lamella of the cortical parenchyma of flax hypocotyl. Plant Physiology and Biochemistry. 1995;a 33:497–508. [Google Scholar]
- Rihouey C, Morvan C, Borissova I, Jauneau A, Demarty M, Jarvis M. Structural features of cdta-soluble pectins from flax hypocotyls. Carbohydrate Polymers. 1995;b 28:159–166. [Google Scholar]
- Roach MJ, Deyholos MK. Microarray analysis of developing flax hypocotyls identifies novel transcripts correlated with specific stages of phloem fibre differentiation. Annals of Botany. 2008;102:317–330. doi: 10.1093/aob/mcn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger D, Lacoux J, Lamblin F, et al. Isolation of a flax pectin methylesterase promoter and its expression in transgenic tobacco. Plant Science. 2001;160:713–721. doi: 10.1016/s0168-9452(00)00456-8. [DOI] [PubMed] [Google Scholar]
- Roth U, von Roepenack-Lahaye E, Clemens S. Proteome changes in Arabidopsis thaliana roots upon exposure to Cd2+ Journal of Experimental Botany. 2006;57:4003–4013. doi: 10.1093/jxb/erl170. [DOI] [PubMed] [Google Scholar]
- Schmohl N, Horst WJ. Cell wall pectin content modulates aluminium sensitivity of Zea mays L. cells grown in suspension culture. Plant, Cell and Environment. 2000;23:735–742. [Google Scholar]
- Schmohl N, Pilling J, Fisahn J, Horst WJ. Pectin methylestease modulates aluminium sensitivity in Zea mays and Solanum tuberosum. Physiologia Plantarum. 2000;109:419–427. [Google Scholar]
- Shah K, Penel C, Gagnon J, Dunand C. Purification and identification of a Ca2+-pectate binding peroxidase from Arabidopsis leaves. Phytochemistry. 2004;65:307–312. doi: 10.1016/j.phytochem.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Siedlecka A, Wicklund S, Péronne MA, et al. Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cell of Populus. Plant Physiology. 2008;146:554–565. doi: 10.1104/pp.107.111963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandenBosch KA, Bradley DJ, Knox JP, Perotto S, Butcher GW, Brewin N. Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO Journal. 1989;8:335–342. doi: 10.1002/j.1460-2075.1989.tb03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma K, Shekhawat GS, Sharma A, Mehta SK, Sharma V. Cadmium induced oxidative stress and changes in soluble and ionically bound cell wall peroxidase activities in roots of seedling and 3–4 leaf stage plants of Brassica juncea (L.) Czern. Plant Cell Reports. 2008;27:1261–1269. doi: 10.1007/s00299-008-0552-7. [DOI] [PubMed] [Google Scholar]
- Vilain S, Cosette P, Hubert M, Lange C, Junter GA, Jouenne T. Comparative proteomic analysis of planktonic and immobilized Pseudomonas aeruginosa cells: a multivariate statistical approach. Annals of Biochemistry. 2004;329:120–130. doi: 10.1016/j.ab.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Wolf S, Rausch T, Greiner S. The N-terminal pro region mediates retention of unprocessed type-I PME in the Golgi apparatus. The Plant Journal. 2009;58:361–375. doi: 10.1111/j.1365-313X.2009.03784.x. [DOI] [PubMed] [Google Scholar]
- Yamaoka T, Chiba N. Changes in the coagulating ability of pectin during the growth of soybean hypocotyls. Plant and Cell Physiology. 1983;24:1281–1290. [Google Scholar]
- Yang JL, Ying Li Y, Zhang YJ, et al. Cell wall polysaccharides are specifically involved in the exclusion of aluminium from the rice root apex. Plant Physiology. 2008;146:602–611. doi: 10.1104/pp.107.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.