Abstract
The combined oral contraceptive pill (COC) consisting of drospirenone 3 mg/ethinyl estradiol 20 μg (3 mg DRSP/20 μg EE-24/4) supplies 24 days of pills with hormones followed by 4 days of hormone-free pills. This regimen is called the 24/4 regimen. The progesterone component of this oral contraceptive pill (OCP), drospirenone (DRSP), is a fourth-generation progestin that has potent progestogenic, antimineralocorticoid, and antiandrogenic activity, which are unique characteristics compared with the other progestogens contained in most of the other OCPs currently marketed. This formulation, in addition to being an effective long-term OCP, has the additional medical benefit of providing a good parallel treatment for premenstrual dysphoric disorder and moderate acne. The effectiveness of 3 mg DRSP/20 μg EE-24/4, its tolerability and safety, and its additional non-contraceptive benefits are discussed.
Keywords: drospirenone, premenstrual dysphoric disorder, acne vulgaris, contraception, antimineralocorticoid activity, antiandrogenic activity
Introduction to the 3 mg DRSP/20 μg EE (24/4) oral contraceptive pill
Between 1973 and 1974 the US Food and Drug Administration first approved several low dose pills. By 1975, no pill recommended for contraception had more than 50 μg of estrogen, with most containing estrogen levels in the range of 20 to 30 μg. A decrease in the progesterone component to 1 mg or less as well as different progestins were also introduced into the oral contraceptive pill (OCP) formulations. Today, newer OCPs have not only altered estrogen and progestin levels, but also have varied the number of active pills. These changes in OCP have had the goal of acceptable contraceptive efficacy with minimal side effects and risks. In more recent years, certain OCP formulations have been prescribed to treat various medical conditions, such as premenstrual dysphoric disorder (PMDD), moderate acne vulgaris, polycystic ovarian syndrome and hirsuitism.
There are 60 million women of reproductive age in the US, approximately 64% of whom use some method of contraception.1 Oral contraceptives are the most popular reversible method of contraception in America (26.9%) and second only in popularity to the combined permanent sterilization of women (27.7%) and vasectomies (10.9%). Fifty-three percent of US contraception users aged 15 to 24 use OCPs for their high efficacy, ease of use, minimal side effects and good cycle control.2
However, effective hormonal contraception may be discontinued by sexually active, reproductive-age women despite their continued need due to the intolerability of side effects. Bleeding irregularities, nausea, headaches and fluid retention related symptoms such as breast tenderness and bloating are frequently the side effects that cause discontinuance of OCP use.3
The fourth-generation progestins used in OCPs were introduced to the market in an attempt to minimize the negative side effects of progestins that were used earlier in OCPs and to address the other medical conditions that they could be effective in treating.
PMDD is included in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).4 Women with PMDD report mood symptoms such as lability and behavioral symptoms such as changes in eating patterns. PMDD also involves physical symptoms such as breast tenderness, edema and/or headaches. PMDD symptoms occur during the last week of the luteal phase of the menstrual cycle and resolve in the week after menses.4 Using the formulation of drospirenone 3 mg/ethinyl estradiol 20 μg (3 mg DRSP/20 μg EE-24/4) in women with PMDD is desirable as the progestin may ameliorate physical symptoms and the shortened hormone-free interval (4 hormone-free days) will provide greater suppression of follicle development and a more stable level of exogenous hormones throughout the cycle.3 A multicenter, double-blind, placebo-controlled crossover study showed that this OCP formulation decreased the mood, behavioral and physical symptoms of PMDD.5 Another study looking at the efficacy of this combination OCP in the treatment of PMDD drew similar conclusions.6
Acne vulgaris is caused by an overproduction of androgen by the sebaceous glands, which can lead to increased sebum production and acne. Moderate acne vulgaris is the most common form of acne and patients present with various types of lesions with whiteheads, papules and pustules. Severe acne on the other hand is characterized by the presence of nodules and cysts on the dermatologic surfaces. The benefit of treating moderate acne vulgaris is important, since the condition is often found in young adults and the lesions that are obvious, particularly on the face and neck, can lead to negative psychosocial consequences.7 The main hormonal mechanism by which acne may be treated by OCP is through the effects of the estrogen component. That is, the estrogen in the OCP augments the levels of the sex hormone binding globulin (SHBG), which in turn increases the binding of free testosterone. Therefore, there is less free androgen in the circulation, to exert its action on the sebaceous glands. Drospirenone has another hormonal mechanism: that of an antiandrogenic hormone, which exerts its progestational and antimineralocorticoid effects by competitively inhibiting and binding androgens to its receptors. This mechanism would explain the reduction in sebum excretion rates and lesion counts.7
There have been no clinical trials exploring the role of 3 mg DRSP/20 μg EE-24/4 in PCOS, patients. It can be hypothesized that this combination of hormones would be effective in reducing adrenal androgens to the point of clinical significance. Although this formulation has not been studied in this condition, there are some data on a similar formulation that suggest that it may be effective. This formulation may be effective in the treatment of hyperandrogenism, not only causing blockade of ovarian steroid production but also by acting on the adrenals to reduce adrenal androgen synthesis.8 In one prospective study, a 30 μg EE with 3 mg DRSP (21/7) OCP was shown to affect steroid genesis by reducing synthesis and release of androgens in response to adrenocorticotropic hormone (ACTH).
Since most oral contraceptives, such as levonorgestrel, norgestrel, norethindrone, and desogestrel, use synthetic progestins derived from 19 nortestosterone or 17 alpha hydroxyprogesterone derivatives, they are not structurally similar to aldosterone and therefore do not possess antimineralocorticoid effects. These progestins do not antagonize the salt-retaining effect of estrogen, which may contribute to weight gain and increased blood pressure seen in some women using OCPs. On the other hand, drospirenone may prevent the sodium and water retention via the reninangiotensin-aldosterone system.9
DRSP is similar to progesterone in its ability to have potent antiandrogenic and antimineralocorticoid activities.8 The only other DRSP containing OCP that is currently marketed is the OCP with DRSP 3 mg and EE 30 μg. The difference between 3 mg DRSP/20 μg EE-24/4 and 3 mg DRSP/30 μg EE-21/7 is that each tablet in the prior formulation contains a lower dose of EE (20 vs 30 μg) and has a shorter hormone-free interval (4 days vs 7 days). The shorter hormone-free interval leads to a greater suppression of follicle development and a more stable hormone time frame than the traditional regimens of 21-day hormone-containing pill and 7 days placebo. As expected, greater pituitary and ovarian suppression are seen with the shorter hormone-free interval.9 Because of this effect, the 24/4 regimen should better alleviate symptoms such as pelvic pain, headaches, bloating and breast tenderness that are commonly reported during the 7 days of placebo pills by women taking a regimen of 21 days of active pills followed by seven hormone free pill days (21/7 regimen). Extending the days on active treatment reduces hormone withdrawal effects in most women since the fluctuations in hormone levels is minimal.10
Biochemical properties/chemistry
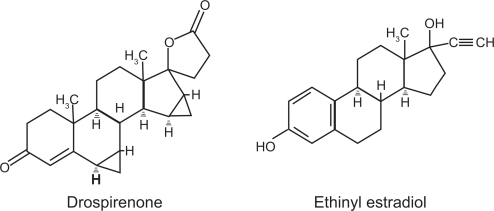
Drospirenone is a synthetic progestin with the molecular weight of 366.5 and the molecular formula of C24H30O3. Ethinyl estradiol is a synthetic estrogen with the molecular weight of 296.4 and the molecular formula of C20H24O2 (Bayer Pharmaceutical Company; http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7949). The structural formulas are given in Figure 1.
Figure 1.
Structure of drospirenone and ethinyl estradiol.
Pharmacodynamics
The (24/7) oral contraceptive regimen has 24 active film-coated tablets that contain 3 mg of drospirenone and 0.02 mg of EE. These hormones are stabilized in the tablet by betadex as a clathrate. The rest of the regimen consists of 4 inert film-coated tablets.11 The drospirenone structure is an analogue of 17 alpha spironolactone.
The mechanism of action is through the suppression of gonadotropin release from the hypothalamus. With suppression of gonadotropin release, ovarian stimulation and follicular maturation do not occur, and the estradiol levels are low due to diminished follicular production. The follicles are the major producers of estradiol and with a sparsity of follicular maturation, estradiol production therefore plummets.
In the typical 21/7 OCP regimen, there is less gonadotropin suppression and follicular maturation may occur. Since the pill-free interval is only 7 days, in most women the follicle does not mature to the point of ovulation, and therefore most OCP cycles are anovulatory. Since the OCP also has a progestin for at least 21 day, the cervical mucus is also altered. With estrogen alone, the cervical mucus is thin, clear, and copious with Spinnbarkeit noted. Once a progestin is introduced, the cervical mucus becomes thick, sticky, pasty and scant. It is this progestin-affected cervical mucus in the combination OCP that produces an environment that makes it difficult for sperm to enter the uterus. The progestin also alters motility of the uterus and oviduct as the normal transport of both the ovulated ova traveling down the fallopian tube and the sperm that are traveling up the fallopian tube to fertilize this ova is interrupted. Progestins also alter the endometrium, such that the glandular production of glycogen is diminished.12 In addition to these progestin effects, the drospirenone, which is similar to endogenous progesterone, contains antimineralocorticoid, progestogenic and antiandrogenic properties. Drospirenone increases urinary sodium and aldosterone excretion and causes reversible increase in plasma aldosterone levels.2
Pharmacokinetics and metabolism
The pharmacokinetic profile of 3 mg DRSP/20 μg EE-24/4 has been studied preclinically and clinically.11 When evaluating a single tablet with DRSP only, the absolute bioavailability of DRSP is about 76%, a percentage measured after it undergoes first pass metabolism through the liver. The absolute bioavailability of EE (without the DRSP component) is approximately 40%, measured after the first pass, taking into consideration the presystemic conjugation. The absolute bioavailability of the combination tablet of 3 mg DRSP/20 μg EE tablet as ultilized in the currently marketed OCP has not been evaluated.11
These concentrations are reduced by 40% when the DRSP tablet or the EE tablet is taken with food. Food consumption affects the rate but not the extent of absorption and is not considered to be clinically important.
EE, a synthetic hormone that is highly protein bound to albumin in the circulation, is metabolized in the liver as well as in the small bowel mucosa. EE metabolism is mainly by aromatic hydroxylation, which forms a wide variety of free and glucoronide or sulfate-conjugated metabolites. With 2 hydroxylation by cytochrome P450 (CYP) 3A4 enzymes, this synthetic estrogen metabolite undergoes methylation and glucoronidation before it is excreted in the urine and feces.2,11
DRSP is highly bound to serum metabolites (approximately 97%) but does not bind to sex hormone binding globulin (SHBG). It is metabolized mostly independently of P450 enzymes, with a minor degree of metabolism by CYP3A4. The acid form of drospirenone and 4,5 dihydrodrospirenone-3-sulfate are the two main metabolites, neither of which are pharmacologically active.2,11 In women with liver impairment the DRSP levels are approximately 3 times higher. In women with renal dysfunction, the DRSP levels are approximately 37% higher.
Clinical efficacy, safety and tolerability
The primary efficacy clinical trial was a 1-year study that looked at the number of pregnancies that occurred in reproductive aged women using 3 mg DRSP/20 μg EE-24/4. This 1-year study recruited 1027 subjects who completed 11,480 of 28-day cycles of use. The subjects, who were racially diverse, ranged in age from 17 from 36 years. The racial demographics of the cohort was: 87.8% Caucasian, 4.6% Hispanic, 4.3% Black, 1.2% Asian and 2.1% other. Data at completion of the study reported the Pearl index as 1.41 per 100 women-years of use. Twelve pregnancies occurred after the onset of treatment and within 14 days after the last dose of 3 mg DRSP/20 μg EE-24/4.13 Another multicenter trial primarily examined not only contraceptive efficacy but also evaluated tolerability and patient satisfaction.3 These data showed a 99% contraception protection rate which is comparable to other low dose combined oral contraceptive pills (COCs). Of note, only 77 (7.5%) of the subjects discontinued the OCP due to adverse events. This number is favorable when compared to other OCP studies where the withdrawal rates due to adverse events are usually closer to 10% to 17%. Of the women who rated their overall satisfaction, 86% stated that they were satisfied or very satisfied with the OCP and 73% stated that they would continue this OCP if it were available.
Two multicenter, double-blind, randomized, placebo-controlled studies on PMDD treatment efficacy of 3 mg DRSP/20 μg EE-24/4 were performed on women who met full criteria for PMDD, compared with the milder PMS. One study recruited n = 449 in a parallel group study.6 In this study, there were two run in/qualification and three treatment cycles. Another PMDD study was done in which n = 64 women with PMDD were recruited. In these studies, the subjects’ symptoms were rated using the Daily Record of Severity of Problems Scale. With this scale, a measurement is collected on the severity of 21 PMDD symptoms, such as feeling depressed, mood swings, diminished interest, breast swelling and headache. Women in these studies were between 18 and 40 years with a diagnosis of PMDD and lacking other recognized psychiatric disorders. They also had to have regular menstrual cycles and have no contraindications to OCP use. The outcome of both studies showed a significant difference in mean luteal phase, total Daily Record of Severity of Problems between qualification cycles and treatment cycles with a greater than 50% decrease in premenstrual symptoms and improvement in quality of life in these women suffering from PMDD.14
The therapeutic efficacy of 3 mg DRSP/20 μg EE-24/4 in women with moderate acne vulgaris was also assessed. Two double-blind, randomized, placebo-controlled multicenter trials using this OCP were conducted.2 In these trials subjects with moderate acne vulgaris (minimal of 40 facial lesions) received 3 mg DRSP/20 μg EE-24/4 or placebo for six cycles. In addition to the moderate acne vulgaris, subjects had to be able to take the OCP, have had a menstrual cycle in the last 3 months and a normal pap smear. The primary outcome confirmed the hypothesis that this formulation was more effective than placebo in reducing the severity of moderate acne.7
Drug interactions
As with other formulations, the effectiveness of this formulation may be decreased with rifampin use because rifampin increases the metabolism of EE. Contraceptive efficacy may also be decreased by some anticonvulsants such as phenobarbital, phenytoin and carbamazepine, as these drugs increase the metabolism of EE and some progestins. Some herbal medicines and vitamins also affect OCP effectiveness. St. John’s Wort (Hypericum perforatum), for example, may reduce OCP efficacy by inducing cytochrome P450 and p-glycoprotein transporter. Of interest, ascorbic acid in some studies has been shown to increase the plasma concentrations of some synthetic estrogens. Increase in the plasma concentrations of cyclosporine, prednisolone and theophylline have been reported with concomitant administration of OCPs.11
Because of the DRSP structure, serum potassium levels may be slightly elevated in women taking 3 mg DRSP/20 μg EE-24/4, but this increase is usually not significant.
Other metabolic interactions with DRSP were also studied. In vitro and in vivo studies evaluating the cytochrome P450 enzymes were investigated. Also, the potential effect of DRSP on CYPC219 activity was investigated in a clinical pharmacokinetic study using omeprazole as a marker substrate. These studies showed that DRSP did not inhibit CYP2C19 and CYP3A4 in vivo. Two additional clinical drug–drug interaction studies were performed using simvastatin and midazolam, which had similar results.
Studies on carbohydrate metabolism, lipid and hemostatic parameters showed that high density lipoprotein increases, and low density lipoprotein decreases, thus suggesting cardioprotective benefit. Blood glucose levels only slightly elevated, but were still within normal range. Hemostatic parameters showed an increase in the activation markers of thrombin and fibrin turnover, findings comparable to those observed with other COCs.10
Adverse events
While using 3 mg DRSP/20 μg EE-24/4 some side affects such as spotting, dysmenorrhea, vomiting, breast tenderness, clumsiness, nervousness and increased irritability were reported by subjects that led to study discontinuation.6 Other side effects reported include upper respiratory infection, metrorrhagia, headache, nausea, sinusitis, suspicious Pap smears and vaginal moniliasis.7 These adverse events reported with 3 mg DRSP/20 μg EE-24/4 were typical of hormonal contraceptive use and were generally mild to moderate in intensity.15
Conclusion
In summary, the regimen 3 mg DRSP/20 μg EE-24/4 is safe and efficacious as a contraceptive. It is well tolerated and provides additional, non-birth control/non-contraceptive benefits. This OCP significantly improved the physical and emotional symptoms in women who suffer from PMDD. It was also beneficial in the treatment of women with moderate acne vulgaris. In addition, this OCP has the potential for clinical use in women with PCOS and hirsutism.
Expert opinion
Why should fourth-generation progestins be used?
The goal of newer progestin formulations are to decrease side effects. These newer progestins are used to better address medical conditions such as PMDD that affect women’s health and psychosocial well-being and to provide efficacious contraception with the lowest doses of hormones. Clinically, DRSP appears to prevent sodium and water retention via the reninangiotensin-aldosterone system (RAAS). This antimineralocorticoid effect may decrease bloating, weight gain and blood pressure.
Together with the safety and efficacy evidence from clinical trials, it is evident that 3 mg DRSP/20 μg EE-24/4 provides therapeutic efficacy that corresponded to 99% contraceptive protection.
Improving the tolerability of OCPs without affecting their efficacy or safety has been a big challenge over the years. Reduction of hormone-free days in 3 mg DRSP/20 μg EE-24/4 reduces the incidence of hormone withdrawal adverse effects and decreases the incidence of ovulation. It has a high overall user satisfaction rating, and provides a close to physiological bleeding pattern. Because it is well tolerated with a lower incidence of adverse effects, we can extrapolate increased compliance.
Additional medical benefits were seen with PMDD and moderate acne vulgaris (the most common form of acne which is considered to decrease the quality of life in many young women). DRSP in this particular regimen offers extended days of acne treatment because of its long half-life compared to that of traditional OCPs. The anti-androgenic effects are stronger because DRSP is a fourth-generation progestin. It has more direct antiandrogenic effects compared to other progestins, contributing to an overall decrease in androgen synthesis and therefore a decrease in acne formation.
The symptoms of PMDD are statistically significantly improved with the use of 3 mg DRSP/20 μg EE-24/4.
Further studies on the 3 mg DRSP/20 μg EE-24/4 regimen will probably show additional benefits for PCOS patients since studies already conducted on other DRSP-containing COCs have already proven beneficial.
In the near future DRSP may prove to be of some benefit for postmenopausal women in that it may be cardio-protective. Menopausal hormone therapy containing DRSP may cause blood pressure reduction in hypertensive post-menopausal women through rapid activation of endothelial nitric oxide synthase. These effects are mediated by action on both progesterone and mineralocorticoid receptors.13 The combination of DRSP and estrogen as menopausal hormone therapy for hypertensive postmenopausal women sounds promising, as it would have the potential to treat hot flashes and reduce blood pressure.16
Therefore in the near future DRSP with a low dose estrogen may have its use expanded beyond that of contraception, to medical conditions that affect quality of life.
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Mosher WD, Martinex GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004;350:1–36. [PubMed] [Google Scholar]
- 2.Fenton C, Wellington K, Moen MD, Robinson DM. Drospirenone/ethinylestradiol 3 mg/20 mcg (24/4 day regimen): a review of its use in contraception, premenstrual dysphoric disorder and moderate acne vulgaris. Drugs. 2007;67(12):1748–1765. doi: 10.2165/00003495-200767120-00007. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann G, Sulak PJ, Sampson-Landers C, et al. Efficacy and safety of a low dose 24 day combined oral contraceptive containing 20 mcg ethinylestradiol and 3 mg drospirenone. Contraception. 2004;70(3):191–198. doi: 10.1016/j.contraception.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 4.First MB, editor. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington (DC): American Psychiatric Press; 2000. Premenstrual dysphoric disorder; pp. 771–774. [Google Scholar]
- 5.Steiner M, Born L. Diagnosis and treatment of premenstrual dysphoric disorder: An update. Int Clin Psychopharmacol. 2000;15(Suppl 3):S5–S17. [PubMed] [Google Scholar]
- 6.Yonkers KA, Brown C, Pearlstein TB, Foegh M, Sampson-Landers C, Rapkin A. Efficacy of a new low dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol. 2005;106:492–501. doi: 10.1097/01.AOG.0000175834.77215.2e. [DOI] [PubMed] [Google Scholar]
- 7.Koltun W, Lucky A, Thiboutot D, et al. Efficacy and safety of 3 mg drospirenone/20 mcg ethinylestradiol oral contraceptive administered in 24/4 regimen in the treatment of acne vulgaris:a randomized, double-blind, placebo-controlled trial. Contraception. 2008;77:249–256. doi: 10.1016/j.contraception.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 8.De Leo V, Morgante G, Piomboni P, et al. Evaluation of effects of an oral contraceptive containing ethinylestradiol combined with drospirenone on adrenal steroidogenesis in hyper-androgenic women with polycystic ovary syndrome. Fertil Steril. 2007;88(1):113–117. doi: 10.1016/j.fertnstert.2006.11.137. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Lopéz FR. Clinical experiences with drospirenone: from reproductive to post-menopausal years. Maturitas. 2008;60(2):78–91. doi: 10.1016/j.maturitas.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Klipping C, Marr J. Effects of two combined oral contraceptives containing ethinyl estradiol 20 mcg combined with either drospirenone or desogestrel on lipids, hemostatic parameters and carbohydrate metabolism. Contraception. 2005;71:409–416. doi: 10.1016/j.contraception.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Koltun W, Lucky AW, Thiboutot D, et al. Efficacy and safety of 3 mg drospirenone/20 mcg ethinylestradiol oral contraceptive administered in 24/4 regimen in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled trial. Contraception. 2008;77(4):249–256. doi: 10.1016/j.contraception.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Droegemuller W. Endometriosis and adenomyosis: etiology, pathology, diagnosis and management. Stenchever, Droegemuller, Herbst, Michell, editors. Comprehensive Gynecology. 2001:531–564. [Google Scholar]
- 13.Hernadi L, Marr J, Petralgia F. Kuala Lumpur: 2006. Nov 5–10, Efficacy of a new low-dose 24-day combined oral contraceptive containing drospirenone 3 mg and ethinyl estradiol 20 μg [oral presentation] XVIII World Congress of Gynecology and Obstetrics (FIGO) [Google Scholar]
- 14.Pearlstein TB, Bachmann GA, Zacur HA, et al. Treatment of premenstrual dysphoric disorder with a new drospirenone containing oral contraceptive formulation. Contraception. 2005;72(6):414–421. doi: 10.1016/j.contraception.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Cibula D, Karck U, Weidenhammer HG, Kunz J, Alinicic S, Marr J. Efficacy and safety of a low-dose 21-day combined oral contraceptive containing ethinylestradiol 20 mcg and drospirenone 3 mg. Clinical Drug Investig. 2006;26(3):143–150. doi: 10.2165/00044011-200626030-00004. [DOI] [PubMed] [Google Scholar]
- 16.White WB, Hanes V, Korner P. Drospirenone and 17-B estradiol reduces 24 h BP in postmenopausal women with hypertension. Obstet Gynecol. 2006;107:23S–24S. [Google Scholar]