Abstract
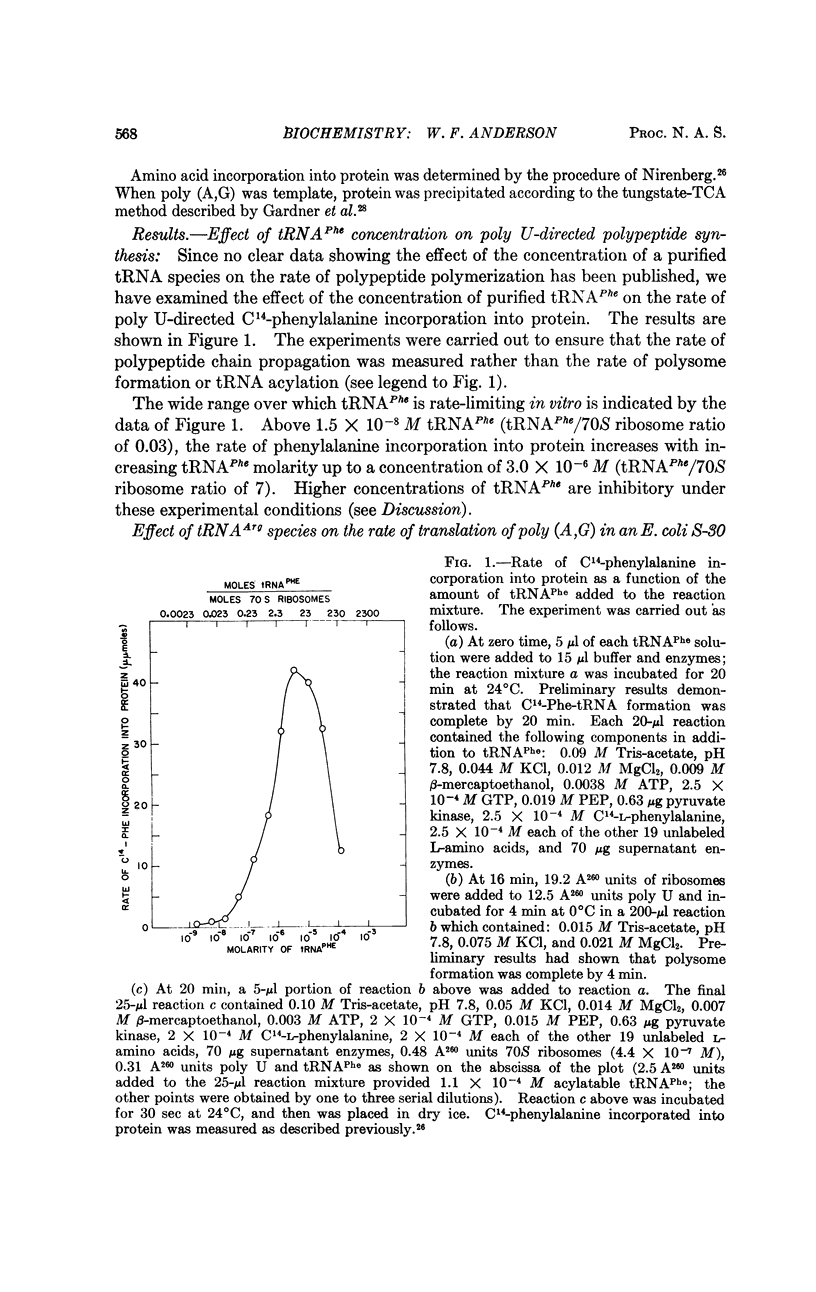
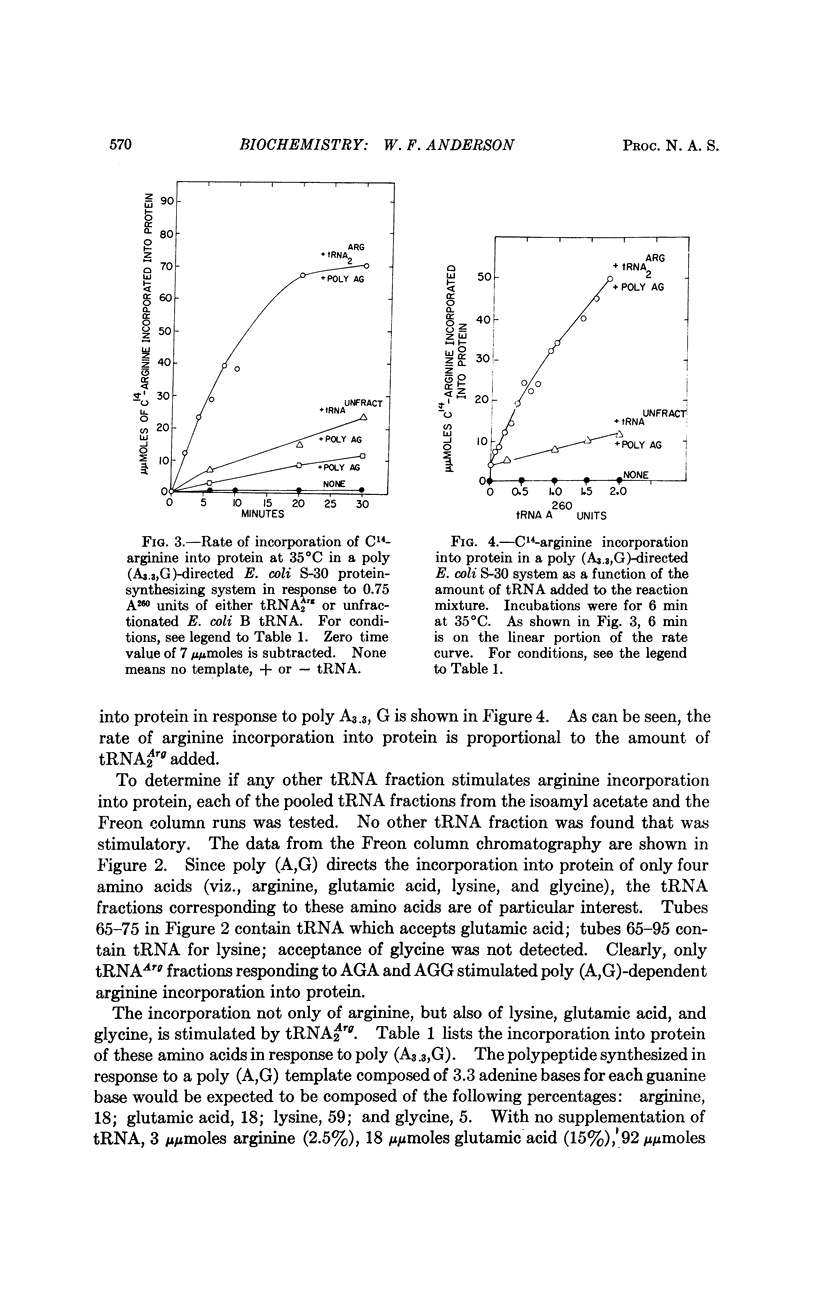
Two in vitro protein-synthesizing systems derived from E. coli have been utilized to demonstrate that the concentration of a tRNA species can regulate the rate of translation of a messenger RNA. (a) The rate of poly-U-directed C14-phenylalanine incorporation into protein is stimulated by concentrations of tRNAPhe from 1.5 × 10-8 M to 3.0 × 10-6 M, the latter representing a tRNAPhe/70S ribosome ratio of 7. (b) The rate of translation of poly A,G in a S-30 protein-synthesizing system derived from E. coli is limited by the amount of tRNAArg recognizing the codewords AGA and AGG present in the extract. Polypeptide synthesis can be stimulated in direct proportion to the amount of this tRNAArg species added to the reaction mixture. A mechanism for regulating the rate of protein synthesis at the translational level may be the slowing of polypeptide chain propagation at certain codons due to the presence of rate-limiting tRNA species.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Weinstein B., Farber E. Patterns of transfer RNA in normal rat liver and during hepatic carcinogenesis. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1255–1260. doi: 10.1073/pnas.58.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R., Trupin J., Nirenberg M., Leder P., Bernfield M., Jaouni T. RNA codewords and protein synthesis, 8. Nucleotide sequences of synonym codons for arginine, valine, cysteine, and alanine. Proc Natl Acad Sci U S A. 1965 Sep;54(3):954–960. doi: 10.1073/pnas.54.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. T., Beaudet A., Nirenberg M. RNA codons and protein synthesis. 15. Dissimilar responses of mammalian and bacterial transfer RNA fractions to messenger RNA codons. J Mol Biol. 1968 Oct 14;37(1):99–118. doi: 10.1016/0022-2836(68)90076-4. [DOI] [PubMed] [Google Scholar]
- Doi R. H., Kaneko I., Igarashi R. T. Pattern of valine transfer ribonucleic acid of Bacillus subtilis under different growth conditions. J Biol Chem. 1968 Mar 10;243(5):945–951. [PubMed] [Google Scholar]
- GARDNER R. S., WAHBA A. J., BASILIO C., MILLER R. S., LENGYEL P., SPEYER J. F. Synthetic polynucleotides and the amino acid code. VII. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2087–2094. doi: 10.1073/pnas.48.12.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. II. The polypeptide chain and S-RNA. J Mol Biol. 1963 May;6:389–403. doi: 10.1016/s0022-2836(63)80051-0. [DOI] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Kellogg D. A., Doctor B. P., Loebel J. E., Nirenberg M. W. RNA codons and protein synthesis. IX. Synonym codon recognition by multiple species of valine-, alanine-, and methionine-sRNA. Proc Natl Acad Sci U S A. 1966 Apr;55(4):912–919. doi: 10.1073/pnas.55.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelmers A. D., Novelli G. D., Stulberg M. P. Separation of transfer ribonucleic acids by reverse phase chromatography. J Biol Chem. 1965 Oct;240(10):3979–3983. [PubMed] [Google Scholar]
- LAZZARINI R. A., PETERKOFSKY A. THE CHARACTERIZATION OF A NEW SPECIES OF LEUCYL-SRNA FORMED DURING METHIONINE DEPRIVATION OF ESCHERICHIA COLI WITH RELAXED CONTROL. Proc Natl Acad Sci U S A. 1965 Mar;53:549–556. doi: 10.1073/pnas.53.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Ingram V. M. Erythrocyte transfer RNA: change during chick development. Science. 1967 Dec 8;158(3806):1330–1332. doi: 10.1126/science.158.3806.1330. [DOI] [PubMed] [Google Scholar]
- Muench K. H., Safille P. A. Transfer ribonucleic acids in Escherichia coli. Multiplicity and variation. Biochemistry. 1968 Aug;7(8):2799–2808. doi: 10.1021/bi00848a015. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Nirenberg M., Caskey T., Marshall R., Brimacombe R., Kellogg D., Doctor B., Hatfield D., Levin J., Rottman F., Pestka S. The RNA code and protein synthesis. Cold Spring Harb Symp Quant Biol. 1966;31:11–24. doi: 10.1101/sqb.1966.031.01.008. [DOI] [PubMed] [Google Scholar]
- Rosset R., Julien J., Monier R. Ribonucleic acid composition of bacteria as a function of growth rate. J Mol Biol. 1966 Jul;18(2):308–320. doi: 10.1016/s0022-2836(66)80248-6. [DOI] [PubMed] [Google Scholar]
- SINGER M. F., GUSS J. K. The dependence of reactions catalyzed by polynucleotide phosphorylase on oligonucleotides. J Biol Chem. 1962 Jan;237:182–189. [PubMed] [Google Scholar]
- STENT G. S. THE OPERON: ON ITS THIRD ANNIVERSARY. MODULATION OF TRANSFER RNA SPECIES CAN PROVIDE A WORKABLE MODEL OF AN OPERATOR-LESS OPERON. Science. 1964 May 15;144(3620):816–820. doi: 10.1126/science.144.3620.816. [DOI] [PubMed] [Google Scholar]
- SUEOKA N., KANO-SUEOKA T. A SPECIFIC MODIFICATION OF LEUCYL-SRNA OF ESCHERICHIA COLI AFTER PHAGE T2 INFECTION. Proc Natl Acad Sci U S A. 1964 Dec;52:1535–1540. doi: 10.1073/pnas.52.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M., Smith M. A., Stanley W. M., Jr, Wahba A. J., Ochoa S. Direction of reading of the genetic message. J Biol Chem. 1965 Oct;240(10):3988–3995. [PubMed] [Google Scholar]
- Söll D., Jones D. S., Ohtsuka E., Faulkner R. D., Lohrmann R., Hayatsu H., Khorana H. G. Specificity of sRNA for recognition of codons as studied by the ribosomal binding technique. J Mol Biol. 1966 Aug;19(2):556–573. doi: 10.1016/s0022-2836(66)80023-2. [DOI] [PubMed] [Google Scholar]
- Taylor M. W., Granger G. A., Buck C. A., Holland J. J. Similarities and differences among specific tRNA's in mammalian tissues. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1712–1719. doi: 10.1073/pnas.57.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S., Sypherd P. S. Modification in transfer RNA during the differentiation of wheat seedlings. Proc Natl Acad Sci U S A. 1968 Feb;59(2):453–458. doi: 10.1073/pnas.59.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. F., Kelmers A. D. A new chromatographic system for increased resolution of transfer ribonucleic acids. Biochemistry. 1967 Aug;6(8):2507–2513. doi: 10.1021/bi00860a030. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Novelli G. D. Isoaccepting +RNA's in mouse plasma cell tumors that synthesize different myeloma protein. Biochem Biophys Res Commun. 1968 May 23;31(4):534–539. doi: 10.1016/0006-291x(68)90510-x. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Novelli G. D. Multiple isoaccepting transfer RNA's in a muouse plasma cell tumor. Proc Natl Acad Sci U S A. 1968 Jan;59(1):208–215. doi: 10.1073/pnas.59.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]



