Abstract
Aortic sclerosis is associated with cardiovascular events in patients without coronary heart disease (CHD), but it is unclear whether this association exists in patients with established CHD or is independent of baseline cardiac disease severity. It is also unclear whether statins modify this association. In a prospective cohort study of 814 outpatients with established CHD and no evidence of aortic stenosis, the association of aortic sclerosis with subsequent cardiovascular events was examined using a multivariable Cox proportional hazards model. Of 814 participants, 324 (40%) had aortic sclerosis. During 4 years of follow-up, 10% with aortic sclerosis experienced a myocardial infarction (MI) compared with 5% of those without aortic sclerosis (hazard ratio [HR] 1.8, 95% confidence interval [CI] 1.1 to 3.1, p = 0.02). This association was unchanged after adjustment for potential confounders and mediators (HR 2.4, 95% CI 1.3 to 4.8, p = 0.009). However, the association between aortic sclerosis and MI appeared to differ by statin use (p = 0.15 for interaction). Aortic sclerosis predicted subsequent MI in subjects not administered statins (adjusted HR 4.1, 95% CI 1.1 to 15.7, p = 0.04), but not in those administered statins (adjusted HR 1.7, 95% CI 0.8 to 3.9, p = 0.18). In conclusion, aortic sclerosis was present in 40% of patients with CHD and is independently associated with a 2.4-fold increased rate of subsequent MI. Statins may attenuate the increased risk of future MI in patients with aortic sclerosis.
We sought to determine whether the presence of aortic sclerosis, which has been associated with increased risk of cardiovascular (CV) events,1–9 predicts adverse CV outcomes in patients with established coronary heart disease (CHD); whether this association is independent of baseline CV risk factors and cardiac disease severity; and whether statin therapy attenuates this association. In a cohort of 814 outpatients with established CHD followed for a median of 4 years, we examined the independent association of aortic sclerosis with subsequent myocardial infarction (MI), unstable angina, heart failure (HF), CHD death, and all-cause mortality.
Methods
The Heart and Soul Study is a prospective cohort study of psychosocial factors and health outcomes in patients with coronary disease. Methods and objectives have been previously described.10–12 We used administrative databases to identify outpatients with documented coronary artery disease at 2 Department of Veterans Affairs Medical Centers (San Francisco Veterans Affairs Medical Center and Veterans Affairs Palo Alto Health Care System, California), 1 university medical center (University of California, San Francisco), and 9 public health clinics in the Community Health Network of San Francisco. Patients were eligible to participate if they had ≥1 of the following: a history of MI, angiographic evidence of ≥50% diameter stenosis of ≥1 coronary artery, previous evidence of exercise-induced ischemia using treadmill or nuclear testing, history of coronary revascularization, or diagnosis of CHD listed in the medical record by an internist or cardiologist.
From September 2000 to December 2002, a total of 1,024 participants enrolled, including 549 (54%) with a history of MI, 237 (23%) with a history of coronary revascularization, but no MI, and 238 (23%) with angiographic evidence of ≥50% diameter stenosis of ≥1 coronary artery or evidence of exercise-induced ischemia using treadmill or nuclear testing, but no history of MI or revascularization. Participants completed a daylong baseline study appointment that included a medical history interview, physical examination, exercise treadmill test with a baseline and stress echocardiogram, laboratory testing (including 24-hour urine collection), and comprehensive health status questionnaire. Of 1,024 participants, we excluded 84 participants who had aortic stenosis, defined as a peak aortic valve velocity ≥2.0 m/s. Of these 84 subjects with aortic stenosis, only 30 had a peak velocity >2.5 m/s and only 13 had a peak velocity >3.0 m/s. We excluded an additional 126 participants for whom the presence or absence of aortic sclerosis could not be determined, leaving 814 subjects for this analysis. The study protocol was approved by the institutional review boards at each participating site. All participants provided written informed consent.
A complete resting 2-dimensional echocardiogram and Doppler ultrasound examination, including all standard views and subcostal imaging of the inferior vena cava, were performed using a 3.5-MHz transducer (Acuson Sequoia Ultrasound System, Mountain View, California). A single experienced reader (NBS), blinded to clinical history, physical examination, laboratory data, and outcome variables, interpreted all echocardiograms for aortic valve morphologic characteristics, sclerosis, and stenosis. Similar to other studies,3,4 we defined aortic sclerosis as the presence of focal areas of aortic valve leaflet thickening and/or increased echogenicity with preserved leaflet mobility and a peak Doppler velocity across the aortic valve of <2.0 m/s. We found good intraobserver agreement for aortic valve morphologic characteristics during an intraobserver reliability analysis of 31 participants (κ = 0.62, 95% confidence interval [CI] 0.36 to 0.87).
We conducted annual telephone follow-up interviews with participants (or their proxies) to ask about death or hospitalization for “heart trouble.” For any reported event, medical records, electrocardiograms, death certificates, and coroner’s reports were retrieved and reviewed by 2 independent and blinded adjudicators. If the adjudicators agreed on the outcome classification, their classification was binding. If they disagreed, they conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator as necessary.
Outcomes were defined as follows. MI and unstable angina pectoris were determined using standard diagnostic criteria developed by the American Heart Association.13 Death was considered caused by CHD if (1) the participant died during the same hospitalization in which an acute MI was documented or (2) the participant experienced sudden CHD death, defined as an unexpected otherwise unexplained fatality within 1 hour of the onset of terminal symptoms. HF was defined using Framingham criteria.14
During echocardiography, we obtained standard parasternal short-axis and apical 2- and 4-chamber views during held inspiration. These were planimetered with a computerized digitization system to determine end-diastolic and end-systolic left ventricular volume. We calculated left ventricular ejection fraction as (end-diastolic volume – end-systolic volume)/end-diastolic volume.15 Based on previously validated criteria,14 we defined 3 categories of diastolic dysfunction (impaired relaxation, pseudonormal, and restrictive) by measuring the ratio of early (E) to late (A) diastolic transmitral peak filling velocities and pulmonary venous flow.
To examine whether inducible ischemia mediated the association between aortic sclerosis and CV outcomes, all participants underwent full exercise treadmill testing according to a standard Bruce protocol with continuous 12-lead electrocardiographic monitoring. Echocardiography was performed immediately before and after exercise. Inducible ischemia was defined as the presence of ≥1 new wall motion abnormality at peak exercise that was not present at rest. We also computed a wall motion score index based on number and severity of exercise-induced abnormal myocardial segments.15,16 In addition, we examined whether valvular abnormalities accounted for any association of aortic sclerosis with outcomes. We measured peak aortic transvalvular velocity across the aortic valve and documented the presence or absence of mitral annular calcium in all subjects.
Differences in baseline characteristics in participants with and without aortic sclerosis were determined using t tests (or nonparametric equivalent) for continuous variables and chi-square tests for dichotomous variables. Because aortic sclerosis was highly associated with increasing age (odds ratio 1.8 for each 10-year increase in age, 95% CI 1.6 to 2.1, p <0.0001), for each variable associated with aortic sclerosis on univariate analysis, we first performed logistic regression with aortic sclerosis as the dependent variable and 2 variables (age and each univariate predictor) as the independent variables. This analysis allowed us to determine which variables were associated with aortic sclerosis after age adjustment.
To examine the independent association of aortic sclerosis with CV outcomes, we used a Cox proportional hazards multivariable analysis with aortic sclerosis as the independent variable and CV outcomes as dependent variables. In our initial multivariable models, we first adjusted for age and only variables still associated with aortic sclerosis after age adjustment: statin use, blood pressure, peak aortic transvalvular velocity, mitral annular calcium, and mitral A-wave velocity. In highly adjusted models, we adjusted for all variables from Tables 1 and 2 associated with aortic sclerosis at p <0.1. Cumulative incidence curves were generated to illustrate differences in risk-adjusted rate of MI.
Table 1.
Baseline characteristics of participants with normal aortic valves and aortic valve sclerosis
| Characteristic | Normal Aortic Valve (n = 490) | Aortic Valve Sclerosis (n = 324) | p Value |
|---|---|---|---|
| Age (yrs) | 64 ± 11 | 70 ± 9 | <0.0001 |
| Men | 397 (81%) | 276 (85%) | 0.14 |
| Height (cm) | 172 ± 9 | 171 ± 9 | 0.02 |
| Physically inactive | 168 (34%) | 109 (34%) | 0.82 |
| White | 270 (55%) | 215 (66%) | 0.002 |
| Black | 103 (21%) | 30 (9%) | <0.0001 |
| Other | 116 (24%) | 79 (24%) | 0.83 |
| Hypertension | 326 (67%) | 234 (73%) | 0.08 |
| HF | 75 (15%) | 63 (20%) | 0.13 |
| Diabetes mellitus | 120 (24%) | 77 (24%) | 0.80 |
| MI | 261 (54%) | 180 (56%) | 0.57 |
| Current smoker | 116 (24%) | 48 (15%) | 0.002 |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | 236 (48%) | 176 (54%) | 0.09 |
| Statin | 298 (61%) | 227 (70%) | 0.007 |
| Aspirin | 377 (77%) | 261 (81%) | 0.22 |
| β blocker | 278 (57%) | 196 (60%) | 0.29 |
| Warfarin | 37 (8%) | 20 (6%) | 0.46 |
| Body mass index (kg/m2) | 28.5 ± 5.4 | 27.8 ± 4.5 | 0.06 |
| Total cholesterol (mg/dl) | 179 ± 43 | 174 ± 40 | 0.12 |
| Low-density lipoprotein cholesterol (mg/dl) | 106 ± 35 | 101 ± 31 | 0.04 |
| High-density lipoprotein cholesterol (mg/dl) | 45 ± 13 | 46 ± 14 | 0.17 |
| Triglycerides (mg/dl) | 144 ± 133 | 137 ± 131 | 0.51 |
| C-reactive protein (mg/dl) | 4.4 ± 8.2 | 3.9 ± 6.3 | 0.43 |
| N-terminal pro-B-type natriuretic peptide (pg/ml) | 357.5 ± 793.1 | 459.9 ± 907.3 | 0.10 |
| Systolic blood pressure (mm Hg) | 129 ± 17 | 134 ± 21 | 0.0004 |
| Diastolic blood pressure (mm Hg) | 75 ± 10 | 74 ± 11 | 0.06 |
| Pulse pressure (mm Hg) | 56 ± 14 | 61 ± 17 | <0.0001 |
| Serum creatinine (mg/dl) | 1.1 ± 0.68 | 1.1 ± 0.45 | 0.63 |
| Measured creatinine clearance (ml/min) | 86.4 ± 28.3 | 77.3 ± 27 | <0.0001 |
Data presented as mean ± SD or number (percent).
Table 2.
Echocardiographic characteristics of participants with normal aortic valves and aortic valve sclerosis
| Variable | Normal Aortic Valve (n = 490) | Aortic Valve Sclerosis (n = 324) | p Value |
|---|---|---|---|
| Peak aortic transvalvular velocity (m/s) | 1.3 ± 0.23 | 1.4 ± 0.25 | <0.0001 |
| Mitral annular calcium | 51 (10%) | 82 (25%) | <0.0001 |
| LV mass index (g/m2) | 95.9 ± 24.5 | 97.8 ± 24 | 0.27 |
| LV end-systolic volume index (ml/m2) | 21 ± 15.6 | 21.9 ± 17.9 | 0.47 |
| LV end-diastolic volume index (ml/m2) | 50.8 ± 18.6 | 52.1 ± 16.9 | 0.33 |
| LV ejection fraction (%) | 62 ± 10 | 62 ± 9 | 0.79 |
| LV diastolic function | |||
| Normal | 284 (66%) | 173 (60%) | 0.08 |
| Impaired relaxation | 97 (23%) | 84 (29%) | 0.05 |
| Pseudonormal | 27 (6%) | 20 (7%) | 0.74 |
| Restrictive | 22 (5%) | 13 (4%) | 0.70 |
| Left atrial volume index (ml/m2) | 32 ± 11.4 | 33.4 ± 12.6 | 0.11 |
| Isovolumic relaxation time (ms) | 116.8 ± 25.3 | 116.6 ± 25.2 | 0.91 |
| Early LV diastolic filling velocity (E wave velocity) (m/s) | 0.76 ± 0.22 | 0.76 ± 0.20 | 0.87 |
| Late (atrial) LV diastolic filling velocity (A-wave velocity) (m/s) | 0.74 ± 0.24 | 0.82 ± 0.24 | <0.0001 |
| Early/atrial LV filling ratio (E/A ratio) | 1.1 ± 0.46 | 1 ± 0.48 | 0.002 |
| Early mitral inflow deceleration time (ms) | 237.8 ± 61.1 | 244.4 ± 65 | 0.14 |
| Inducible myocardial ischemia | 93 (19%) | 88 (27%) | 0.006 |
| Post-exercise wall motion score | 1.16 ± 0.36 | 1.19 ± 0.36 | 0.25 |
Data presented as mean ± SD or number (percent).
LV = left ventricular.
Because there appeared to be an interaction between statin use and aortic sclerosis with regard to MI outcome (p = 0.15 for the interaction), we stratified by baseline statin use to explore whether statin use attenuated the risk of MI. Finally, we stratified by history of MI at baseline to determine whether aortic sclerosis predicted incident MI in those with a history of CHD but no previous MI. All analyses were performed using Statistical Analysis Software (version 8, SAS Institute, Inc, Cary, North Carolina) and Stata (version 9, StataCorp LP, College Station, Texas).
Results
Of 814 participants, 324 (40%) had aortic sclerosis. Tables 1 and 2 list differences in clinical, laboratory, and echocardiographic characteristics between patients with and without aortic sclerosis. After simple age adjustment in logistic regression analyses, only 6 variables were still associated with aortic sclerosis (Table 3): statin use, systolic blood pressure, pulse pressure, peak aortic transvalvular velocity, mitral annular calcium, and mitral A-wave velocity.
Table 3.
Factors associated with aortic sclerosis after age adjustment on logistic regression analysis
| Factor | OR (95% CI) | p Value |
|---|---|---|
| Statin use | 1.45 (1.06–1.98) | 0.021 |
| Systolic blood pressure* | 1.11 (1.03–1.20) | 0.009 |
| Pulse pressure† | 1.19 (1.07–1.31) | 0.001 |
| Peak aortic transvalvular velocity‡ | 2.41 (1.76–3.30) | <0.0001 |
| Mitral annular calcium | 1.75 (1.23–2.48) | 0.002 |
| Mitral A-wave velocity§ | 1.07 (1.01–1.14) | 0.033 |
Per 10 mm Hg increase in pressure.
Per 10 mm Hg increase in pressure.
Per 0.5 m/s increase in velocity.
Per 0.1 m/s increase in velocity.
OR = odds ratio; CI = confidence interval.
During a median 4 years of follow-up, we observed an increase in MI, HF, and all-cause mortality, but no difference in angina or CHD mortality between those with and without aortic sclerosis (Table 4). In unadjusted analyses, 10% of participants (32 of 324) with aortic sclerosis were hospitalized for MI compared with 5% of those (26 of 490) without aortic sclerosis (hazard ratio [HR] 1.8, 95% CI 1.1 to 3.1, p = 0.01). Simple adjustment for age alone eliminated the increased risk of HF and all-cause mortality found on univariate analysis.
Table 4.
Number of participants with cardiovascular events by the presence or absence of aortic sclerosis
| Outcome | Normal Aortic Valve (n = 490) | Aortic Valve Sclerosis (n = 324) | p Value |
|---|---|---|---|
| MI | 26 (5%) | 32 (10%) | 0.01 |
| Angina pectoris | 54 (11%) | 47 (15%) | 0.14 |
| HF | 35 (7%) | 45 (14%) | 0.002 |
| CHD death | 13 (3%) | 11 (3%) | 0.54 |
| All-cause mortality | 56 (11%) | 59 (18%) | 0.007 |
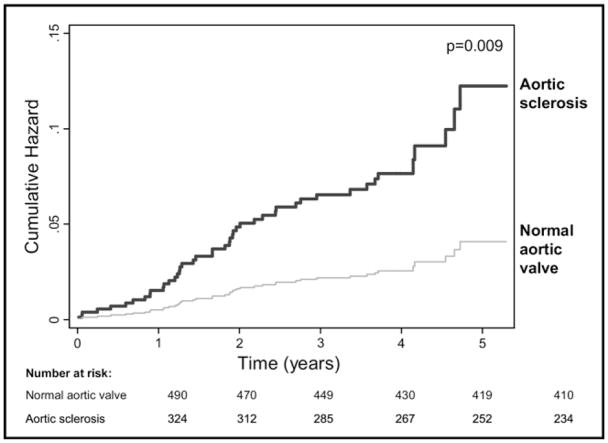
Full results of our multivariable analysis are listed in Table 5. In our initial multivariate analysis, we adjusted for only age and all variables in Table 3. In highly adjusted models, after adjustment for age, height, race, hypertension, history of MI, smoking, medication use, body mass index, low-density lipoprotein cholesterol, N-terminal pro-B-type natriuretic peptide, systolic and diastolic blood pressure, creatinine clearance, and echocardiographic variables (ejection fraction, diastolic function, A-wave velocity, and E/A ratio), the presence of aortic valve sclerosis remained associated with a 2.4-fold increased rate of MI. After further adjustment for potential mediators (inducible ischemia, after-exercise wall motion score index, aortic valve peak transvalvular velocity, and mitral annular calcium), the association between aortic sclerosis and MI persisted (HR 2.4, 95% CI 1.3 to 4.8, p = 0.009). Figure 1 shows adjusted cumulative hazards of MI for subjects with and without aortic sclerosis.
Table 5.
Association of aortic sclerosis with cardiovascular outcomes
| Outcome | Unadjusted |
Adjusted for Age and Variables in Table 3* |
Adjusted for All Potential Confounders† |
Further Adjusted for Potential Mediators‡ |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| MI | 1.8 (1.1–3.1) | 0.02 | 1.9 (1.1–3.3) | 0.03 | 2.4 (1.3–4.7) | 0.007 | 2.4 (1.3–4.8) | 0.009 |
| Angina pectoris | 1.3 (0.9–2.0) | 0.16 | 1.1 (0.7–1.6) | 0.77 | 1.3 (0.8–2.1) | 0.22 | 1.2 (0.7–1.9) | 0.48 |
| HF | 2.0 (1.3–3.1) | 0.003 | 1.6 (1.0–2.7) | 0.08 | 1.5 (0.8–2.7) | 0.20 | 1.6 (0.9–3.0) | 0.13 |
| CHD | 1.2 (0.5–2.7) | 0.63 | 1.3 (0.5–3.2) | 0.62 | 1.0 (0.4–2.9) | 0.98 | 1.2 (0.4–3.9) | 0.31 |
| All-cause mortality | 1.5 (1.1–2.2) | 0.02 | 1.4 (0.9–2.2) | 0.10 | 1.2 (0.8–1.9) | 0.38 | 1.2 (0.7–1.9) | 0.46 |
Adjusted for age, statin medications, systolic blood pressure, pulse pressure, peak aortic transvalvular velocity, mitral annular calcium, and mitral A-wave velocity.
Adjusted for age; height; race; hypertension; history of MI; smoking; use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and statin medications; body mass index; low-density lipoprotein cholesterol; N-terminal pro-B-type natriuretic peptide; systolic and diastolic blood pressure; creatinine clearance; and echocardiographic variables (diastolic function, A-wave velocity, and E/A ratio).
Adjusted for all listed variables plus inducible ischemia, after-exercise wall motion score index, and valvular variables (peak aortic transvalvular velocity and mitral annular calcium).
Figure 1.
Cumulative incidence of MI in 324 subjects with aortic valve sclerosis and 490 subjects with normal aortic valves. Rates were adjusted for age; height; race; hypertension; smoking; use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and statin medications; body mass index; low-density lipoprotein cholesterol; N-terminal pro-B-type natriuretic peptide; systolic and diastolic blood pressure; creatinine clearance; and echocardiographic variables (diastolic function, A-wave velocity, E/A ratio, inducible ischemia, after-exercise wall motion score, aortic valve peak velocity, and mitral annular calcium).
To explore the association between aortic sclerosis, statins, and subsequent MI, we stratified by baseline statin use and found that in patients who were not on statin therapy at baseline (n = 289), the association between aortic sclerosis and MI persisted (adjusted HR 4.1, 95% CI 1.1 to 15.7, p = 0.04). However, in patients who were on statin therapy at baseline (n = 525), the association between aortic sclerosis and MI was no longer present (unadjusted HR 1.7, 95% CI 0.6 to 1.7, p = 0.09; adjusted HR 1.7, 95% CI 0.8 to 3.9, p = 0.18).
Finally, when we stratified by baseline history of MI, we found that in patients with no history of MI (n = 365), aortic sclerosis was an even stronger predictor of MI. In unadjusted analysis, aortic sclerosis was associated with a 3.2-fold increased risk of MI (95% CI 1.4 to 7.5, p = 0.007). After adjustment for all potential confounders and mediators listed, aortic sclerosis was associated with a 3.6-fold increased risk of MI (95% CI 1.2 to 10.8, p = 0.02). In patients with a history of MI (N = 437), aortic sclerosis was no longer associated with MI (unadjusted HR 1.2, 95% CI 0.6 to 2.4, p = 0.53).
Discussion
In this cohort of 814 outpatients with established CHD, we found that the presence of aortic sclerosis predicted a 2.4-fold increased rate of MI during a median of 4 years of follow-up. This association persisted after adjustment for multiple potential confounding variables, including known CV risk factors, baseline left ventricular function, valvular abnormalities, and the presence of inducible myocardial ischemia. These findings suggest that aortic sclerosis is a robust independent risk factor for MI in patients with established CHD.
Aortic sclerosis predicts MI in patients without CHD.3 Our results show that the presence of aortic sclerosis is a powerful predictor of MI events and adds further credence to the hypothesis that aortic sclerosis is a marker of atherosclerotic burden. Because patients with aortic sclerosis have more cardiac calcium and worse left ventricular function than those without aortic sclerosis,4,7,17 it is important to account for echocardiographic differences when examining the association of aortic sclerosis with CV outcomes. We found that aortic sclerosis predicts MI even after adjustment for echocardiographic variables, including mitral annular calcium, exercise-induced ischemia, and left ventricular diastolic function.
Previous studies found that patients with aortic sclerosis were more likely to have an atherogenic risk profile,1,4–6 suggesting that aortic sclerosis may be a marker of greater disease severity rather than an independent risk factor for CV outcomes. However, adjustment for age, medical history, smoking, low-density lipoprotein cholesterol, cardiac medications, body mass index, blood pressure, and echocardiographic variables did not affect the association between aortic sclerosis and MI. Furthermore, it does not appear that the association of aortic sclerosis and MI is due entirely to baseline ischemic burden because adjustment for the presence of inducible ischemia on stress echocardiography did not affect the association between aortic sclerosis and MI.
Because aortic sclerosis shares pathologic similarities with coronary atherosclerosis,2 it seems intuitive that statin use may decrease the risk of MI in patients with aortic sclerosis and CHD. Although virtually all patients with CHD should be administered statins unless contraindicated, it is possible that the presence of aortic sclerosis should signal an even heightened risk of MI and another reason these patients should be on statin therapy. Of note, in a prevalence cohort like the Heart and Soul Study, it is not surprising that patients with aortic sclerosis were more likely to be administered statins and had lower baseline low-density lipoprotein cholesterol, because patients with aortic sclerosis are older and more likely to have worse atherosclerotic disease. Our finding that aortic sclerosis predicts MI in patients without a history of MI also supports the possibility that statins attenuate the association between aortic sclerosis and future MI because those without a history of MI were less likely to be using statins (59% vs 70%, p = 0.001).
Although our study includes comprehensive measurement of clinical and echocardiographic risk factors for CV outcomes and detailed adjudication of outcome events, several limitations must be considered in interpreting our results. First, the diagnosis of aortic sclerosis was based on subjective interpretation of echocardiographic images. However, our definition was similar to those used by others,3,8 and any misclassification of aortic sclerosis would have decreased the association between aortic sclerosis and MI. Second, the number of CV events (including MI) during our study was small, although this finding is consistent with other contemporary studies of patients with CHD. For example, the Treating to New Targets (TNT) study of high-dose atorvastatin in patients with CHD found a 5% incidence of total CV events during a 3-year follow-up period18 compared with a 7% incidence of MI alone in our study during a median 4 years of follow-up. Third, we cannot exclude the possibility that our study was underpowered to detect an increased risk of CV events other than MI in patients with aortic sclerosis. Finally, our study is also limited by a predominantly male urban cohort and thus our results may not apply to women or nonurban populations.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs, Washington, DC, Grant No. R01 HL079235 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland; the American Federation for Aging Research (Paul Beeson Scholars Program), New York, New York; the Robert Wood Johnson Foundation (Faculty Scholars Program), Princeton, New Jersey; and the Nancy Kirwan Heart Research Fund, San Francisco, California.
References
- 1.Chandra HR, Goldstein JA, Choudhary N, O’Neill CS, George PB, Gangasani SR, Cronin L, Marcovitz PA, Hauser AM, O’Neill WW. Adverse outcome in aortic sclerosis is associated with coronary artery disease and inflammation. J Am Coll Cardiol. 2004;43:169–175. doi: 10.1016/j.jacc.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 2.Nightingale AK, Horowitz JD. Aortic sclerosis: not an innocent murmur but a marker of increased cardiovascular risk. Heart. 2005;91:1389–1393. doi: 10.1136/hrt.2004.057117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 4.Agno FS, Chinali M, Bella JN, Liu JE, Arnett DK, Kitzman DW, Oberman A, Hopkins PN, Rao DC, Devereux RB. Aortic valve sclerosis is associated with preclinical cardiovascular disease in hypertensive adults: the Hypertension Genetic Epidemiology Network Study. J Hypertens. 2005;23:867–873. doi: 10.1097/01.hjh.0000163157.14493.c7. [DOI] [PubMed] [Google Scholar]
- 5.Agmon Y, Khandheria BK, Meissner I, Sicks JR, O’Fallon WM, Wiebers DO, Whisnant JP, Seward JB, Tajik AJ. Aortic valve sclerosis and aortic atherosclerosis: different manifestations of the same disease? Insights from a population-based study. J Am Coll Cardiol. 2001;38:827–834. doi: 10.1016/s0735-1097(01)01422-x. [DOI] [PubMed] [Google Scholar]
- 6.Agmon Y, Khandheria BK, Jamil Tajik A, Seward JB, Sicks JD, Fought AJ, O’Fallon WM, Smith TF, Wiebers DO, Meissner I. Inflammation, infection, and aortic valve sclerosis; insights from the Olmsted County (Minnesota) population. Atherosclerosis. 2004;174:337–342. doi: 10.1016/j.atherosclerosis.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Olsen MH, Wachtell K, Bella JN, Gerdts E, Palmieri V, Nieminen MS, Smith G, Ibsen H, Devereux RB. Aortic valve sclerosis relates to cardiovascular events in patients with hypertension (a LIFE substudy) Am J Cardiol. 2005;95:132–136. doi: 10.1016/j.amjcard.2004.08.080. [DOI] [PubMed] [Google Scholar]
- 8.Taylor HA, Jr, Clark BL, Garrison RJ, Andrew ME, Han H, Fox ER, Arnett DK, Samdarshi T, Jones DW. Relation of aortic valve sclerosis to risk of coronary heart disease in African-Americans. Am J Cardiol. 2005;95:401–404. doi: 10.1016/j.amjcard.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 9.Aronow WS, Ahn C, Shirani J, Kronzon I. Comparison of frequency of new coronary events in older subjects with and without valvular aortic sclerosis. Am J Cardiol. 1999;83:599–600. doi: 10.1016/s0002-9149(98)00922-9. [DOI] [PubMed] [Google Scholar]
- 10.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. B-Type natriuretic peptide and ischemia in patients with stable coronary disease: data from the Heart and Soul Study. Circulation. 2003;108:2987–2992. doi: 10.1161/01.CIR.0000103681.04726.9C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beattie MS, Shlipak MG, Liu H, Browner WS, Schiller NB, Whooley MA. C-Reactive protein and ischemia in users and nonusers of beta-blockers and statins: data from the Heart and Soul Study. Circulation. 2003;107:245–250. doi: 10.1161/01.cir.0000044387.23578.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 14.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 15.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 16.Moller JE, Hillis GS, Oh JK, Reeder GS, Gersh BJ, Pellikka PA. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am Heart J. 2006;151:419–425. doi: 10.1016/j.ahj.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 18.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Ruchert J-C, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger N. Intensive lipid-lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]