Abstract
CD8α+ dendritic cells (DCs) have been shown to be the principal DC subset involved in priming MHC class I-restricted CTL immunity to a variety of cytolytic viruses, including HSV type 1, influenza, and vaccinia virus. Whether priming of CTLs by CD8α+ DCs is limited to cytolytic viruses, which may provide dead cellular material for this DC subset, or whether these DCs selectively present intracellular Ags, is unknown. To address this question, we examined Ag presentation to a noncytolytic virus, lymphocytic choriomeningitis virus, and to an intracellular bacterium, Listeria monocytogenes. We show that regardless of the type of intracellular infection, CD8α+ DCs are the principal DC subset that initiate CD8+ T cell immunity.
The CD8+ T cell recognizes pathogen-derived peptides presented by MHC-encoded class I (MHC class I) molecules. Although peptide/MHC class I complexes are expressed on the surface of most cells, their expression by dendritic cells (DCs)3 is essential for priming of pathogen-specific CD8+ T cell responses (1). DCs are a heterogenous population of cells. In mice, they are composed of at least six phenotypically distinguishable subsets, some of which have been shown to perform discrete functions during the immune response to pathogens (2–11). Induction of naive CD8+ T cell responses to cytolytic viruses appears to depend largely on Ag presentation by a single subset of DCs, the CD8α+CD11c+ conventional DCs (CD8 DCs), although other DC subsets may be used in the priming process (6 – 8, 12). In addition, Ag carried in apoptotic cells is also selectively presented to naive CD8+ T cells by this same subset of DCs (13, 14). The death of cells as a result of viral infection might therefore provide a source of viral Ags particularly suitable for uptake by CD8 DCs. It was not clear whether immunity to noncytolytic pathogens, such as lymphocytic choriomeningitis virus (LCMV), or intracellular bacteria such as Listeria monocytogenes (LM) would also rely heavily on the CD8+ subset of DCs for T cell priming.
LCMV is a negative-stranded RNA virus that can infect cells of the immune system and establish either acute or persistent infection in its natural host, the mouse. The outcome of infection depends on the viral variant, the route of infection, and the infectious dose. Viral infection involves receptor-mediated uptake of virions into the cell in endoplasmic vesicles. Endosomal acidification allows release of viral nucleocapsids into the cell cytoplasm, where replication of virus is initiated. After transcription, translation, and assembly of viral particles, noncytolytic shedding leads to dissemination of virus by infected cells. The LCMV Armstrong (ARM) 53b strain of virus (and most viral variants isolated from the CNS) causes an acute fulminant systemic infection that is efficiently controlled within 7–10 days by LCMV-specific CD8+ T cells. In contrast, clone 13 LCMV differs from ARM by only 2 aa, but shows preferential replication in CD11c+ cells. It is associated with immunosuppression and establishes persistent infection (15–19).
LM is a Gram-positive facultative intracellular bacterium with mechanisms that enable it to invade and colonize most cell types. LM infects by inducing phagocytosis by cells through the interaction of internalin A expressed by LM and E-cadherin at the cell’s surface (20). Listeriolysin O, a virulence factor that disrupts the phagosomal membrane, allows release of LM from the phagocytic vacuole into the cytoplasm (21). The bacteria then replicate in the cytoplasm and move directly into adjacent cells by propulsion along polymerized host cell actin filaments that extend into pseudopodia (22, 23). Once the bacteria has invaded the neighboring cell, escape from the secondary vacuole into the cytoplasm now requires LM-derived phosphatidylcholine-specific lipase and zinc metalloproteinase, in addition to listeriolysin O. Infection can extend in this manner through many cells without cellular destruction.
DCs are crucial in mounting an effective cytotoxic T cell response to both LCMV and LM (1, 24, 25). This most likely involves a complex interplay of different DC populations encompassing not only conventional DCs, but also plasmacytoid DCs and novel subsets such as Tip DCs (9, 11, 24). In the case of LCMV, the influx of IFN-α-producing plasmacytoid DCs limits viral replication (26). In LM infection, Tip DCs (CD11c+CD11b+ DCs), so named for their production of TNF-α and inducible NO synthase (iNOS), provide cytokine-directed innate control of infection (9). Although both of these DC types can present pathogen-derived Ags, presentation is very inefficient when compared with conventional DC and was not required for generation of Ag-specific immunity (9). Which DCs are important for priming the CD8+ T cell immune response to these noncytolytic pathogens and whether they are the same DC subset used by cytolytic pathogens is unclear.
The objective of this study was to determine the DC subsets responsible for the Ag presentation that initiates CD8+ T cell responses to the noncytolytic virus LCMV, and to the intracellular bacterium LM. We show that, as for lytic viruses, CD8 DCs are the dominant presenting population, implicating these DCs as central to immunity to intracellular pathogens.
Materials and Methods
Mice, virus, and infections
C57BL/6 (B6) mice were purchased from Taconic Farms, and P14 (transgenic mice that express a CD8+ TCR specific for the immunodominant MHC class I-restricted epitope of LCMV glycoprotein) TCR transgenic mice were maintained under standard conditions at the animal facility at the University of Washington. Experiments with all mice began when they were between 6 and 10 wk of age. P14-specific TCR transgenic mice express a TCR (Vα2) that recognizes the immunodominant MHC class I-restricted epitope of LCMV gp33– 41 (27). Mice were infected either i.v. or i.p. with 2 × 105 PFU of LCMV ARM 53b diluted in 200 μl of sterile PBS, or i.v. infected with 1 × 103 PFU of recombinant isogenic strains of LMs (rLM). rLM encoded either a secreted or nonsecreted fusion protein comprising the LCMV glycoprotein peptide linked to the LCMV nucleoprotein (NP118 –126), which will be referred to as rLM expressing secreted glycoprotein and rLM expressing nonsecreted glycoprotein, respectively (28 –30). Frozen stocks of rLM were grown in brain-heart infusion broth supplemented with 5 mg/ml erythromycin. At mid-log growth phase, culture samples were measured by OD and diluted in PBS for tail vein injection into animals. Bacterial counts were further verified by plating culture dilutions on brain-heart infusion agar plates and incubating overnight. All experiments were undertaken in accordance with ethical guidelines of the University of Washington.
DC isolation from spleen
DCs were isolated essentially as previously described (7, 8, 31). Briefly, spleen fragments were digested for 20 min at room temperature with collagenase/DNase, and then treated for 5 min with EDTA to disrupt T cell-DC complexes. Cells not of the DC lineage were depleted by incubating in predetermined optimal concentrations of purified Abs: anti-CD3 (KT3), anti-Thy-1 (T24/31.7), anti-CD19 (ID3), anti-GR-1 (RB6-8C5), and anti-erythrocyte (TER-119), and then removing the Ab-binding cells with anti-rat Ig-coupled magnetic beads (Dynabeads; Dynal Biotech). Note that, in our hands, plasmacytoid DCs (pDCs) are not depleted using anti-GR-1 mAb (4, 32). The DCs in the enriched populations were gated as CD11c+ cells before sorting into specific subsets.
CFSE labeling of transgenic T cells
Lymph nodes (inguinal, axillary, sacral, cervical, and mesenteric) were obtained from P14 TCR transgenic mice and CD8+ T cells purified using a mixture of optimally titered Abs to deplete cells expressing Mac-1 (M1/70), Mac-3 (F4/80), TER-119, GR1 (RB6-8C5), MHC class II (M5/114), and CD4 (GK1.5), followed by sheep anti-mouse and anti-rat Dynabeads (Dynal Biotech). Enriched cells contained 84 –96% specific TCR transgenic CD8+ T cells. These were labeled with CFSE (Molecular Probes) by incubating 107 purified cells/ml with 5 μM CFSE for 10 min at 37°C. Cells were then washed three times in HEPES modified Eagle’s medium containing 2.5% FCS.
Analysis of in vitro activation of naive T cells by DCs
A total of 5 × 104 enriched CFSE-labeled P14 CD8+ TCR transgenic cells was added to graded numbers of flow cytometrically sorted DCs in 200 μl of RPMI 1640 containing 10% FCS, 50 μM 2-ME, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin in 96-well U-bottom plates (Falcon; BD Biosciences). Each culture was performed in duplicate. Cultures were analyzed for proliferation after 60 h. Cells were stained with anti-CD8α (53-6.7; BD Pharmingen) and anti-CD11c (HL3; BD Pharmingen). Cells were gated on CD11c− propidium iodide− cells.
Results
Presentation of Ag from a noncytolytic virus
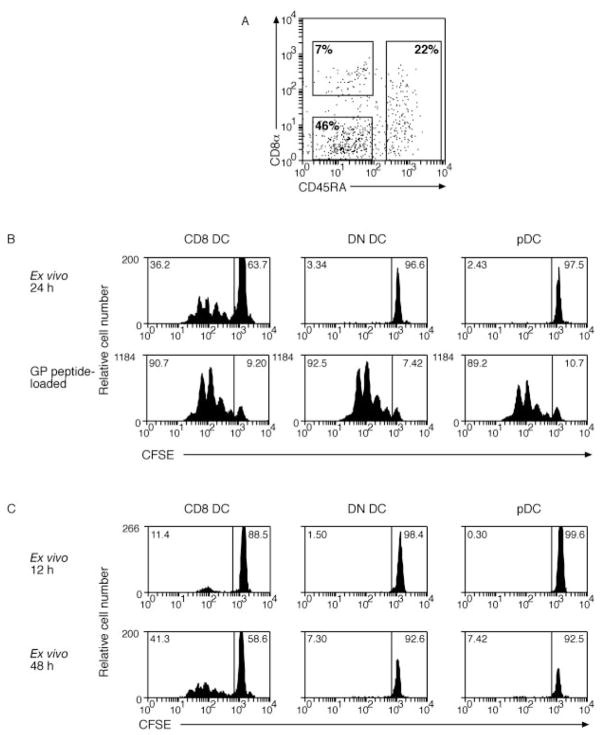
For i.v. and s.c. routes of infection with cytolytic viruses such as HSV, vaccinia, and influenza virus, Ag presentation to CD8+ T cells is largely restricted to the CD8 DC subset (6, 7, 12). By limiting our studies to these cytolytic viruses, it remained possible that the dominant role of CD8 DC was due to their specialized ability to capture Ag from apoptotic or dying cells (14, 33). To determine whether additional or alternative DC subsets were involved in presenting viral Ags from nonlytic viruses, Ag presentation was examined following i.v. infection with LCMV. The use of LCMV also had the advantage of using an authentic mouse pathogen, as earlier studies used virus from other species. One day after i.v. infection with LCMV, DCs were purified from the spleen and separated into subsets based on expression of CD8α and CD45RA. This divided the DCs into conventional (CD45RA−) DCs of CD8α− (double-negative (DN DCs)) or CD8α+ (CD8 DCs) phenotypes and the pDCs (CD45RA+) (Fig. 1A). In this case, the DN DCs contained more than one DC subtype. Each subset from infected mice was examined for the ability to induce proliferation of naive CFSE-labeled P14 CD8+ T cells specific for the immunodominant MHC class I-restricted epitope of glycoprotein encoded by aa 33– 41 (Fig. 1B, top panel). Similar to our previous finding with cytolytic viruses (7), CD8 DCs were the only subset capable of stimulating naive P14 CD8+ T cells following i.v. infection with noncytolytic LCMV. Failure of other DC subsets to present LCMV Ags was not due to any form of nonspecific inhibition or toxicity to the DCs or the cultures, as P14 T cells were efficiently stimulated by each DC population when the DCs were directly coated with the glycoprotein peptide (Fig. 1B, lower panel).
FIGURE 1.
CD8 DCs are the only subset that activates naive CD8+ T cells after i.v. infection with LCMV virus. A, Sorting gates for DCs enriched from spleen at various time points after infection. Cells were enriched for DCs by magnetic depletion of cells staining for CD3, Thy-1, CD19, anti-Gr-1, and erythrocytes. Enriched cells were then stained with anti-CD11c, anti-CD8α, and anti-CD45RA. Rectangles show sorting gates for CD8α+CD45RA− (CD8), CD45RA+ (pDC), and CD8α−CD45RA− DC (DN DCs). B, Mice were infected with LCMV by i.v. inoculation 24 h after infection; DCs were enriched from spleen and sorted by flow cytometry into CD8 DC, DN DC, or pDC before culturing 2.5 × 104 cells with 5 × 104 CFSE-labeled glycoprotein-specific CD8+ T cells. Some DCs were coated with 0.1 μM glycoprotein peptide for 1 h at 37°C and washed three times before coculture with CFSE-labeled glycoprotein-specific CD8+ T cells (lower panel). Proliferation was analyzed at 60 h of culture. The percentage of proliferating cells for each culture is indicated in the top left corner of each histogram (C). At 12 and 48 h after infection, DCs were enriched from spleen of infected mice and responses were analyzed as in B. Each time point was performed twice with similar results. A 6-h time point was also examined in a single experiment.
Previous studies of Ag presentation during i.v. infection with cytolytic viruses such as HSV focused on the 24-h time point because presentation peaked at this time point (7), possibly due to limited virus replication. In the case of LCMV, however, extensive virus replication occurs in the spleen, raising the possibility that optimal Ag presentation, by either CD8 DC or other subsets, may occur at earlier or later time points. To address this possibility, mice were infected i.v. with LCMV at 6, 12, and 48 h before sampling (Fig. 1C, and data not shown). At any time point, the CD8 DCs were the only subset presenting LCMV glycoprotein Ag to P14 CD8+ T cells, although responses were very poor at 12 h and undetectable at 6 h after infection.
Presentation of LCMV Ag does not change with the i.p. route of infection
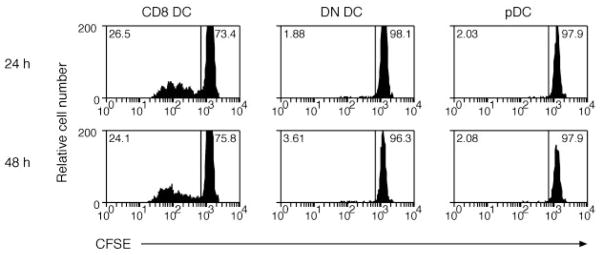
In previous analyses, we showed that MHC class I viral Ag presentation was restricted to the CD8 DC subset following i.v. infections, but presentation of inhaled Ags involved the conventional CD8 DC and, in addition, a CD8-negative DC subset (7, 8). Therefore, we examined a second route of infection, namely i.p. infection, to determine whether other DC subsets might contribute to Ag presentation when the virus gained access to DCs from a different inoculation site. LCMV was given via i.p. inoculation, and DCs were purified from the spleen 24 and 48 h after infection. DC subsets were cocultured with CFSE-labeled P14 CD8+ T cells, and the number of proliferated cells enumerated after 60 h of culture (Fig. 2). Although administration of Ags via the i.p. route provides ready access to peritoneal macrophages that could also potentially contribute to the presentation of viral Ags, the CD8 DC remained the predominant subset of DC priming the immune response in the spleen. No response was detected in a single analysis performed 6 and 12 h after i.p. LCMV infection (data not shown).
FIGURE 2.
CD8 DCs, but not other DCs, activate naive LCMV-specific CD8+ T cells after i.p. infection with virus. Mice were infected with LCMV by i.p. injection. One or 2 days after infection, DCs were enriched from spleen and then sorted into CD8 DC, DN DC, and pDC before culturing 2.5 × 104 cells with 5 × 104 CFSE-labeled glycoprotein-specific CD8+ T cells. Proliferation was analyzed at 60 h of culture. The percentage of proliferating cells for each culture is indicated in the top left corner of each histogram. This experiment was performed twice with similar results.
Intracellular bacterial pathogens also selectively rely on CD8 DC for Ag presentation
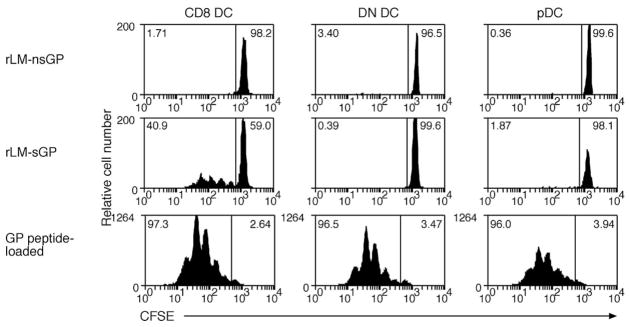
Bacterial pathogens use a number of strategies to allow them to colonize a broad range of cells in the body. Such wide infection implies that infected APCs could be involved in activating naive T cells during the immune response. In addition to conventional DCs, Tip DCs, a novel DC subset that produces TNF-α/iNOS, are recruited into the immune response against Listeria. Tip DCs have limited Ag presentation capacity and are not required for priming of T cells in the immune response, suggesting that other subsets of APCs perform this function. To determine whether intracellular pathogens, such as LM, use a much broader repertoire of DC subsets to prime virus-specific CD8+ T cells, DCs were isolated from mice infected i.v. with a recombinant form of LM that expressed the LCMV viral peptide determinant, glycoprotein, as a surrogate Ag (28, 30). This allowed us to use the same CD8+ TCR transgenic mouse line as in previous studies. The recombinant bacterium expresses the glycoprotein Ag as either a secreted or nonsecreted protein, permitting investigation of whether such differences affect the DC subset(s) driving T cell responses. DCs were isolated from the spleen of mice 24 or 48 h after infection with rLM and were then cocultured with CFSE-labeled P14 CD8+ T cells. The number of proliferated cells was enumerated after 60 h of culture (Fig. 3, upper panels). As with viral infections, secreted Ag from LM was only presented by the CD8 DC. Presentation of nonsecreted Ag was not detected. This was not due to inhibitory effects of the bacteria, as all DC populations presented exogenously loaded peptide to naive CD8+ T cells (Fig. 3, lower panel).
FIGURE 3.
Only CD8 DCs present rLM expressing the glycoprotein Ag. Mice were infected with nonsecreted (upper panel) or secreted (middle panel) forms of the rLM glycoprotein (29) by i.v. injection. DCs were enriched from the spleen 24 h after infection and sorted by flow cytomety into CD8 DC, DN DC, or pDC. Purified DC subsets (2.5 × 104 DC) were then cocultured with 5 × 104 CFSE-labeled glycoprotein-specific CD8+ T cells for 60 h. Twenty-four hour infected DCs from each subset were coated with 0.1 μM glycoprotein peptide for 1 h at 37°C and washed three times before coculture with CFSE-labeled glycoprotein-specific CD8+ T cells (lower panel). The percentage of proliferating cells for each culture is indicated in the top left corner of each histogram. Each experiment was performed twice with similar results. Ag presentation was also examined 12 and 18 h after infection with secreted and nonsecreted forms of rLM glycoprotein (data not shown; each experiment was performed once). No proliferation of naive glycoprotein-specific CD8+ T cells was detected at either of these time points.
Discussion
DCs express specialized Ag-presenting machinery, costimulatory molecules, and cytokines that allow them to initiate immunity to Ags. Although it has been clear for some years that bone marrow-derived cells are necessary to elicit pathogen-specific responses, it has been more difficult to definitively identify which APC was responsible for this function (34, 35). More recently, this was resolved by Jung et al. (1), who used an elegant transgenic mouse model to eliminate CD11c+ cells by inducible short-term ablation in vivo. Mice that lacked DCs failed to develop T cell responses following either malaria or Listeria infection, indicating the crucial importance of DCs to the immune response to these pathogens.
In the mouse, at least six different DC subsets have been described. Although most DC express a common set of pattern recognition receptors, such as TLR2 and 4, some populations have a distinct pattern of TLR expression, raising the idea that different DC populations may be able to discriminate different pathogens and therefore be specialized in initiating the adaptive immune response to those pathogens. Previously, we have determined which DC subsets were responsible for initiation of the immune response to cytolytic viruses. Only a limited number of DCs, and principally (but not exclusively) the CD8 DC, was shown as a key player for priming CD8+ T cells (6 – 8, 12). Intriguingly, this subset also expresses the TLR3 that recognizes viral products (36). Whether the CD8 DC subset, or another subset, plays a broader role in handling different types of pathogen-derived Ags remained to be elucidated. Therefore, we extended our earlier studies to examine infections with either a noncytolytic virus, LCMV, or an intracellular bacteria, LM. This revealed that the CD8 DC was also the principal APC recruited to prime naive CD8+ T cells in these very different types of infections.
The critical importance of disruption of DC function following LCMV infection was first alluded to by Zinkernagel and colleagues (25), who observed that mice infected with LCMV variants (WE or DOCILE strains) developed immune suppression. Similarly, infection with the LCMV clone 13 strain, but not the parental strain LCMV ARM, resulted in generalized suppression of the host immune response (19, 24). Further analysis of the differences between these latter two strains revealed mutations in LCMV clone 13, enabling it to directly target DCs by binding to the laminin receptor, α-dystoglycan. This receptor is predominantly found on DCs that express the C-type lectin, CD205. As CD8 DCs isolated from spleen are the sole subset that expresses CD205, this implicates the CD8 DCs as direct targets for the action of LCMV that leads to crippling of their ability to activate naive CD8+ T cells. In contrast, LCMV ARM only poorly infects DCs, and mice rapidly resolve the infection by mounting an effective virus-specific cytotoxic T cell response (19). These data suggest that activation of naive T cells in LCMV infection may involve transfer of Ag from infected cells to the CD8 DC, and that direct infection of these DCs, as occurs for LCMV clone 13, destroys their capacity to initiate the cytotoxic T cell response.
Clearance of LM infection is dependent on generation of a T cell response, but early containment and bacterial clearance rely on the induction of iNOS (37). Of the different splenic DC populations that could participate in the immune response to LM, a novel subset, Tip DCs have recently been identified as a dominant source of TNF-α and iNOS (9). This subset is the major producer of TNF-α and iNOS during the first 48 h of infection. They are critical to the innate immune defense, and mice lacking these cells ultimately die. Despite this, the apparent absence of this subset from CCR2-deficient mice does not interfere with the normal development of T cell responses implicating another DC subset as responsible for presenting bacterial Ag and priming naive T cells (9). In this report, we investigated which DC subset was responsible for presenting LM Ag. Similar to viral infections, only the CD8 DC subset was able to activate naive CD8+ T cells to LM.
Intravenous infection of mice with rLM expressing either a secreted or a nonsecreted form of LCMV-derived Ag (NP alone, or an NP-glycoprotein fusion protein) (29) elicits CD8+ T cell expansion and contraction that are similar for both pathogens, although the magnitude of the response is diminished for the nonsecreted Ag (28, 29). Despite this, mice previously infected with LCMV were protected against rLM challenge only when the Ag was secreted into the cytoplasm of the cell. As an indirect measure of Ag presentation, we analyzed presentation by DC subsets isolated from either rLM expressing secreted glycoprotein- or rLM expressing nonsecreted glycoprotein-infected mice. The amount of Ag presented by the nonsecreted form of the rLM was insufficient to induce proliferation of our transgenic T cells, while Ag presentation from the secreted form was detected on the CD8 DC subset. Our failure to detect presentation by the nonsecreted variant may reflect some loss of function of the DCs in the preparation procedure, or alternately, the potentially lower levels of Ag obtained by cross-presentation of a nonsecreted Ag are more difficult for us to analyze by this approach. Despite this, the differences in the level of Ag presentation detected via the ex vivo assay suggest that the blunted Ag presentation observed in the nonsecreted form of LM could shorten the developmental phase of effector and memory cells, thereby contributing to the diminished capacity of mice infected with this pathogen to develop potent long-term CD8+ memory T cells (28, 29, 38). These results reinforce our previous observations of the importance of the CD8 DC subset in priming naive CD8+ T cells in response to intracellular pathogens. The importance of the interplay between different functionally specialized DC subsets is underlined by LM infection, in which it is the concert of different DC populations that is essential for control and clearance of the pathogen. Investigating the rules governing the cellular and molecular signals regulating these interactions is essential to understanding the generation of long-lasting protective memory.
Acknowledgments
We thank David Vremec, Walter and Eliza Hall Institute of Medical Research, staff of the University of Washington Flow Cytometry Facility, and members of Prof. M. Bevan’s laboratory for assistance. We are grateful to H. Shen (University of Pennsylvania School of Medicine, Philadelphia, PA) for providing rLM glycoprotein.
Footnotes
Disclosures
The authors have no financial conflict of interest.
Abbreviations used in this paper: DC, dendritic cell; ARM, lymphocytic choriomeningitis virus Armstrong 53b strain; DN, double negative; iNOS, inducible NO synthase; LCMV, lymphocytic choriomeningitis virus; LM, Listeria monocytogenes; NP, nucleoprotein; pDC, plasmacytoid DC.
This work was supported by grants from the National Health and Medical Research Council of Australia (to G.T.B., K.S., and W.R.H.) and by National Institutes of Health Grant AI19335 (to M.J.B.). G.T.B. is a Wellcome Trust Senior Overseas Fellow; W.R.H. is a Howard Hughes Medical Institute International Fellow; and M.J.B. is an investigator of the Howard Hughes Medical Institute.
References
- 1.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220.x. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 3.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM, Wu L, Lahoud MH, Henri S, Scott B, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8+ dendritic cells only after microbial stimulus. J Exp Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 6.Smith CM, Belz GT, Wilson NS, Villadangos JA, Shortman K, Carbone FR, Heath WR. Cutting edge: conventional CD8α+ dendritic cells are preferentially involved in CTL priming after footpad infection with herpes simplex virus-1. J Immunol. 2003;170:4437–4440. doi: 10.4049/jimmunol.170.9.4437. [DOI] [PubMed] [Google Scholar]
- 7.Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Heath WR. Cutting edge: conventional CD8α+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 8.Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci USA. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allan RS, Smith CM, Belz G, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity is induced by CD8α+ dendritic cells but not Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 13.Den Haan JM, Lehar SM, Bevan MJ. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matloubian M, Kolhekar SR, Somasundaram T, Ahmed R. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1993;67:7340–7349. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed R, Simon RS, Matloubian M, Kolhekar SR, Southern PJ, Freedman DM. Genetic analysis of in vivo-selected viral variants causing chronic infection: importance of mutation in the L RNA segment of lymphocytic choriomeningitis virus. J Virol. 1988;62:3301–3308. doi: 10.1128/jvi.62.9.3301-3308.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvato M, Shimomaye E, Southern P, Oldstone MB. Virus-Lymphocyte interactions. IV. Molecular characterization of LCMV Armstrong (CTL+) small genomic segment and that of its variant, clone 13 (CTL−) Virology. 1988;164:517–522. doi: 10.1016/0042-6822(88)90566-1. [DOI] [PubMed] [Google Scholar]
- 18.Salvato M, Borrow P, Shimomaye E, Oldstone MB. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J Virol. 1991;65:1863–1869. doi: 10.1128/jvi.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MB. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaillard JL, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from Gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 21.Bielecki J, Youngman P, Connelly P, Portnoy DA. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 22.Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wachter M, Wuenscher M, Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 24.Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odermatt B, Eppler M, Leist TP, Hengartner H, Zinkernagel RM. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc Natl Acad Sci USA. 1991;88:8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O’Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 27.Kyburz D, Aichele P, Speiser DE, Hengartner H, Zinkernagel RM, Pircher H. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur J Immunol. 1993;23:1956–1962. doi: 10.1002/eji.1830230834. [DOI] [PubMed] [Google Scholar]
- 28.Zenewicz LA, Foulds KE, Jiang J, Fan X, Shen H. Nonsecreted bacterial proteins induce recall CD8 T cell responses but do not serve as protective antigens. J Immunol. 2002;169:5805–5812. doi: 10.4049/jimmunol.169.10.5805. [DOI] [PubMed] [Google Scholar]
- 29.Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 30.Jiang J, Lau LL, Shen H. Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J Immunol. 2003;171:4352–4358. doi: 10.4049/jimmunol.171.8.4352. [DOI] [PubMed] [Google Scholar]
- 31.Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8α+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Keeffe M, Hochrein H, Vremec D, Scott B, Hertzog P, Tatarczuch L, Shortman K. Dendritic cell precursor populations of mouse blood: identification of the murine homologues of human blood plasmacytoid pre-DC2 and CD11c+ DC1 precursors. Blood. 2003;101:1453–1459. doi: 10.1182/blood-2002-03-0974. [DOI] [PubMed] [Google Scholar]
- 33.Schulz O, Reis e Sousa C. Cross-presentation of cell-associated antigens by CD8α+ dendritic cells is attributable to their ability to internalize dead cells. Immunology. 2002;107:183–189. doi: 10.1046/j.1365-2567.2002.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lenz LL, Butz EA, Bevan MJ. Requirements for bone marrow-derived antigen-presenting cells in priming cytotoxic T cell responses to intra-cellular pathogens. J Exp Med. 2000;192:1135–1142. doi: 10.1084/jem.192.8.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 36.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8α+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 37.Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 38.Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]