Abstract
Background
In Cancer and Leukemia Group B 39801, we evaluated whether induction chemotherapy before concurrent chemoradiotherapy would result in improved survival, and demonstrated no significant benefit from the addition of induction chemotherapy. The primary objective of this analysis was to dichotomize patients into prognostic groups using factors predictive of survival, and to investigate if induction chemotherapy was beneficial in either prognostic group.
Patients and Methods
A Cox proportional hazard model was used to assess the impact on survival of the following factors: (≥ 70 vs. < 70 years), gender, race, stage (IIIB vs. IIIA), hemoglobin (hgb) (< 13 vs. ≥13 g/dl), performance status (PS) (1 vs.0), weight loss (≥5% vs. < 5%), treatment arm, and the interaction between weight loss and hgb.
Results
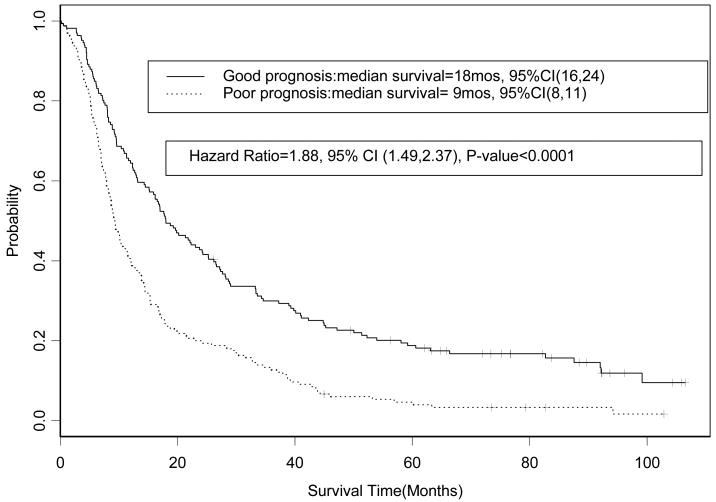
Factors predictive of decreased survival were weight loss ≥ 5%, age ≥ 70 years, PS of 1, and hgb < 13 g/dl (p<0.05). Patients were classified as having ≥2 poor prognostic factors (n=165) or ≤ 1 factor (n=166). The hazard ratio (HR) for overall survival for the patients with ≥ 2 versus patients with ≤ 1 was 1.88 (95% CI, 1.49 to 2.37; p= < 0.0001); median survival times observed were 9 (95% CI, 8 to 11) and 18 (95% CI, 16 to 24) months, respectively. There was no significant difference in survival between treatment arms in patients with ≥ 2 factors (HR=0.86, 95% CI, 0.63 to 1.17; p=0.34) or ≤1 factor (HR=0.97, 95% CI, 0.70 to 1.35; p=0.87)
Conclusions
There is no evidence that induction chemotherapy is beneficial in either prognostic group.
Keywords: locally advanced non-small cell lung cancer, combined modality therapy, chemoradiation, CALGB, prognostic factors, induction chemotherapy
Lung cancer is the leading cause of cancer-related mortality in the United States, and it is estimated that in 2008 more patients died from lung cancer than prostate, breast, and colorectal cancer combined.1 Eighty-seven percent of patients with lung cancer have non-small cell lung cancer (NSCLC), and approximately one third of patients with NSCLC will have stage III disease at the time of diagnosis.2,3 Phase III trials have demonstrated an improvement in survival with the combination of chemotherapy and radiotherapy over radiation alone for patients with stage III NSCLC, and for appropriate patients with a good performance status combined modality therapy is the standard of care in the United States.4 However, there has been significant variability in the overall survival observed on combined modality therapy trials. The median survival time and 5-year survival rate observed on cooperative group phase II or III trials is 15-18 months and 25%, respectively.5 Currently a variety of factors such as single versus multilevel nodal involvement, the presence of co-morbidities, variable definitions of resectability and physician preference contribute to the heterogeneity of patients selected for combined modality therapy. Differences in the patient population enrolled on the clinical trials could have contributed to the observed differences in survival, and makes assessment of the efficacy of treatment paradigms difficult
Cancer and Leukemia Group B (CALGB) trial 39801 was designed to investigate the impact of induction chemotherapy in addition to standard chemoradiotherapy on overall survival.6 The difference in survival was not statistically significant (p=0.3), and the median survival observed on the induction chemotherapy followed by chemoradiotherapy and the chemoradiotherapy arm alone arms was 14 and 12 months, respectively. The median survival time on both treatment arms was lower than the survival observed on previous CALGB combined modality trials.7,8 Previous CALGB trials had excluded patients with performance status (PS) 2 and > 5% weight loss in the three months before diagnosis, but the latter criterion was abandoned since it was felt to be redundant with PS. The lack of weight loss criteria, the increasing acceptance of combined modality therapy, and the perception that carboplatin and paclitaxel based chemoradiotherapy is well tolerated could have led to a broader patient population being enrolled than on previous trials.
When eligible patients with weight loss < 5% were analyzed (n=244) the median survival was numerically higher among patients who received chemoradiotherapy alone than among patients who received induction chemotherapy followed by chemoradiotherapy, 16 and 14 months, respectively.6 In contrast when the eligible patients with weight loss of ≥ 5% (n=87) were analyzed the median survival was numerically lower among patients who received chemoradiotherapy alone than among patients who received induction chemotherapy followed by chemoradiotherapy than chemoradiotherapy alone, 8 and 10 months, respectively.6 This raised a question if different prognostic groups may benefit greater from one of the treatment paradigms. In order to investigate this hypothesis we sought to identify good and poor prognostic groups for survival and to determine if induction chemotherapy was beneficial in either prognostic group.
Patients and Methods
Patients were enrolled on CALGB 39801 from July 1998 to May 2002. Patients were required to have histological or cytological documentation of NSCLC, and previously untreated unresectable or inoperable stage III disease.9 The eligibility criteria have been published previously,6 and the forced expiratory volume in 1 second (FEV-1) was required to be > 800 ml. Each participant signed an institutional review board (IRB) approved, protocol specific informed consent in accordance with federal and institutional guidelines. Patient random assignment and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson, following standard CALGB policies. All statistical analyses were performed by CALGB statisticians.
As part of the quality assurance program of the CALGB, members of the Audit Committee visit all participating institutions at least once every three years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 214 patients (58%) of the 366 patients under this study.
Treatment Plan
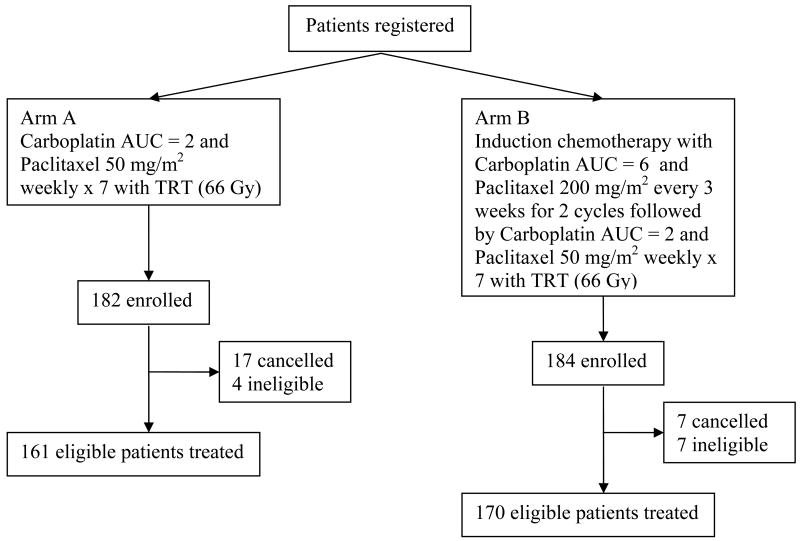
Following registration, patients were randomly assigned to one of the two treatment arms (Figure 1). Patients on arm A received concurrent chemoradiotherapy with paclitaxel 50 mg/m2 and carboplatin dose of area under the curve (AUC) of 2 using the Calvert equation10 weekly for seven consecutive weeks concurrent with thoracic radiation therapy (TRT). Patients on arm B received two cycles of induction chemotherapy with carboplatin (AUC=6) and paclitaxel 200 mg/m2 every 21 days followed by the identical concurrent chemoradiotherapy beginning on day 43. The details of the chemotherapy administration, dose modifications, and management of treatment related toxicity have been published previously.6
Figure 1. CALGB 390801: Study Design.
Abbreviations: AUC = Area Under the Curve; TRT = Thoracic Radiation Therapy
Radiation Therapy
TRT began on day 1 for patients on arm A and on day 43 for patients on arm B. Patients on arm B who experienced extra-thoracic disease progression during induction therapy were removed from protocol therapy, whereas patients whose disease progression was within the planned radiation field continued their planned TRT. Concurrent radiotherapy to 66 Gy was administered at five fractions per week for seven consecutive weeks at 2 Gy/fraction. A central review of the radiation therapy for each case, including review of the diagnostic imaging, was performed at the Quality Assurance Review Center (QARC) by the radiation oncology co-chair, a thoracic radiologist, and QARC staff. The details of the radiation treatment planning have been published previously.6
Statistical Considerations
A Cox proportional hazards model was used to assess the impact on survival of the following factors previously reported to be prognostic among patients who received combined modality therapy with stage III disease11-14 : age (≥70 vs. <70 years), gender (female vs. male), race/ethnicity (non-white vs. white), hemoglobin (hgb) < 13 vs. ≥13 g/dl, PS (1 vs.0), pretreatment weight loss (≥5% vs. <5%), stage (IIIB vs. IIIA), treatment arm, and the interaction between weight loss and hgb. The median hgb rounded to the nearest g/dl was 13; therefore, patients were divided into hgb of < 13 or ≥ 13 g/dl. Patients without documented weight loss (n=12) were classified in the weight loss <5% category. Factors shown to be jointly predictive of survival (p<0.05) were used to define two prognostic groups. The poor prognosis group was defined by the presence of 2 or more poor prognostic factors; whereas the good prognosis group was defined by the presence of 1 or fewer poor prognostic factors. The Kaplan-Meier product limit estimator was used to describe the survival experience in the two prognostic groups; a log-rank test was conducted.
Results
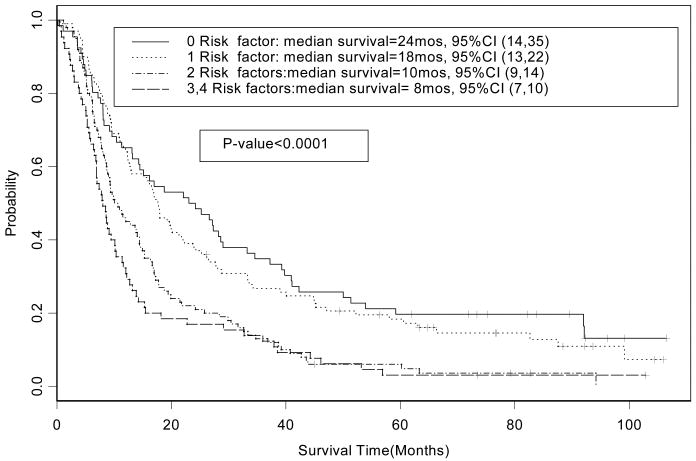
The patient characteristics are summarized in Table 1. The median follow-up time for survivors is 88 months (range 0.2 to 106 months). The Cox proportional hazards regression model identified four factors that were significant predictors of poor survival: age ≥ 70 years, hgb < 13 g/dl, weight loss ≥ 5%, and PS of 1 (p< 0.05) (Table 2). A significant interaction effect on overall survival between weight loss and hemoglobin was detected. The number of patients with 0, 1, 2, or ≥ 3 poor prognostic factors and the overall survival by risk factors are summarized in Table 3 and Figure 2. The overall survival observed for the poor prognosis group (n=166) in comparison to good prognosis group (n=165) was significantly lower (hazard ratio (HR) for overall survival = 1.88, 95 % CI 1.49 to 2.37; p-value <0.0001); the median survival times observed in the poor and good prognosis groups were 9 (95% CI, 8 to 11) and 18 (95% CI, 16 to 24) months, respectively (Figure 3). The five-year overall survival rate observed of all patients was 11.8% (95% CI, 8.7 to 15.8%), and the five-year overall survival rate observed of the poor and good prognosis groups was 4.7% (95% CI, 2.3 to 9.4%) and 18.8% (95% CI, 13.7 to 25.9%), respectively. The reasons for discontinuation of treatment (Table 4) and the rates of hematological toxicities and non-hematological adverse events (Table 5) were similar between the two prognostic groups. There is no significant difference between the good and poor prognosis groups in the maximum grade of adverse event (p=0.12), hematological adverse events (p=0.09) and non-hematological adverse events (p=0.09).
Table 1. Patient Characteristics.
| Characteristic | Overall (%) N=331 |
Poor Prognosis (%) N=165 |
Good Prognosis (%) N=166 |
|---|---|---|---|
| Median Age, years (Range) | 63 (37-85) | 68 (37-85) | 60(38-81) |
| <70 | 247 (75) | 89 (54) | 158 (95) |
| ≥70 | 84 (25) | 76 (46) | 8 (5) |
| Gender | |||
| Male | 218 (66) | 105 (64) | 113 (68) |
| Female | 113 (34) | 60 (36) | 53 (32) |
| Ethnicity | |||
| White | 282 (85) | 132 (80) | 150 (90) |
| African American | 40 (12) | 27 (16) | 13 (8) |
| Hispanic American | 5 (2) | 3 (2) | 2 (1) |
| Other | 4 (2) | 3 (2) | 1 (1) |
| Performance Status | |||
| 0 | 148 (45) | 34 (21) | 114 (69) |
| 1 | 183 (55) | 131(79) | 52 (31) |
| Weight loss | |||
| <5%* | 244 (74) | 90 (55) | 154 (93) |
| ≥5% | 87 (26) | 75 (45) | 12 (7) |
| Stage | |||
| IIIA | 168 (51) | 77 (47) | 91 (55) |
| IIIB | 163 (49) | 88 (53) | 75 (45) |
| Hemoglobin | |||
| ≥13g/d1 | 181 (55) | 43 (26) | 138 (83) |
| <13g/d1 | 150 (45) | 122 (74) | 28 (17) |
| Treatment arms | |||
| Arm A | 161 (49) | 82 (50) | 79 (48) |
| Arm B | 170 (51) | 83 (50) | 87 (52) |
The 12 patients with no documentation of weight loss were included in the <5% weight loss category; 7 patients were treated on arm A and 5 patients were treated on arm B
Table 2. Cox Proportional Hazard Model.
| Variable | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Weight loss (≥5% vs <5%) | 1.92 | 1.33 to 2.76 | <0.01 |
| Hgb (<13 vs ≥ 13) | 1.65 | 1.24 to 2.20 | <0.01 |
| Age (≥70 vs <70) | 1.45 | 1.11 to 1.90 | 0.01 |
| PS (1 vs 0) | 1.35 | 1.06 to 1.71 | 0.01 |
| Race (Non-White vs White) | 1.28 | 0.91 to 1.79 | 0.15 |
| Stage (IIIB vs IIIA) | 1.11 | 0.87 to 1.40 | 0.40 |
| Arm (Arm A vs Arm B) | 0.94 | 0.75 to 1.18 | 0.60 |
| Gender (Female vs Male) | 0.89 | 0.70 to 1.14 | 0.34 |
| Weight loss * Hgb | 0.49 | 0.29 to 0.81 | 0.01 |
Abbreviations: CI=confidence interval, hgb=hemoglobin, PS=performance status, arm A=concurrent chemoradiotherapy, arm B=induction chemotherapy and concurrent chemoradiotherapy Weight loss
hgb: the interaction between weight loss and hemoglobin
Table 3. Median Survival by Risk factors.
| Number of factors | Number of patients | Median survival (95% CI) |
|---|---|---|
| 0 | 66 | 24 months (14 to 25) |
| 1 | 100 | 18 months (13 to 22) |
| 2 | 100 | 10 months (9 to 14) |
| ≥ 3 | 65 | 8 months (7 to 10) |
Abbreviations: CI=confidence interval
Figure 2. CALGB 39801: Overall Survival by Risk Factors (n=331).
Figure 3. CALGB 39801: Overall survival by Good Prognosis vs. Poor Prognosis (n=331).
Table 4. Reasons for discontinuing protocol therapy.
| Poor Prognosis (%) N=165 |
Good Prognosis (%) N=166 |
|
|---|---|---|
| Treatment Completed | 119 (72) | 118 (71) |
| Adverse Events | 17 (10) | 25 (15) |
| Disease Progression | 9 (5) | 7 (4) |
| Died | 9 (5) | 4 (2) |
| Patient Refusal | 4 (2) | 3 (2) |
| Taken off protocol treatment | 2 (1) | 2 (1) |
| Other | 3 (2) | 3 (2) |
| Missing | 2 (1) | 4 (2) |
Table 5. Select Adverse events in the Good and Poor Prognosis groups.
| Variable | Poor Prognosis (n=165) | Good Prognosis (n=166) | ||
|---|---|---|---|---|
| Grade 3 (%) | Grade 4 (%) | Grade 3 (%) | Grade 4 (%) | |
| Hematologic | ||||
| Febrile Neutropenia | 4 (3) | 1 (1) | 4 (2) | 1 (1) |
| Hemoglobin | 11 (7) | 0 (0) | 15 (9) | 0 (0) |
| Neutropenia | 39 (24) | 25 (15) | 21 (13) | 25 (15) |
| Red blood cell transfusion | 9 (5) | 0 (0) | 9 (5) | 0 (0) |
| Thrombocytopenia | 3 (2) | 0 (0) | 10 (6) | 0 (0) |
| Maximum toxicity | 80 (48) | 40 (24) | 83 (50) | 33 (20) |
| Non Hematologic | ||||
| Anorexia | 19 (12) | 8 (5) | 21 (13) | 9 (5) |
| Dysphagia-esophageal | 39 (24) | 6 (4) | 48 (29) | 7 (4) |
| Dyspnea | 20 (12) | 8 (5) | 18 (11) | 3 (2) |
| Fatigue | 21 (13) | 6 (4) | 30 (18) | 1 (1) |
| Nausea | 10 (6) | 0 (0) | 14 (8) | 0 (0) |
| Pneumonitis | 7 (4) | 4 (2) | 7 (4) | 1 (1) |
| Vomiting | 8 (5) | 0 (0) | 10 (6) | 0 (0) |
| Weight loss | 8 (5) | 0 (0) | 4 (2) | 0 (0) |
| Maximum toxicity | 78 (47) | 26 (16) | 82 (50) | 26 (16) |
There were 3 grade 5 non-hematological adverse events; 2 (1%) in the poor prognosis group and 1 (1%) in the good prognosis group
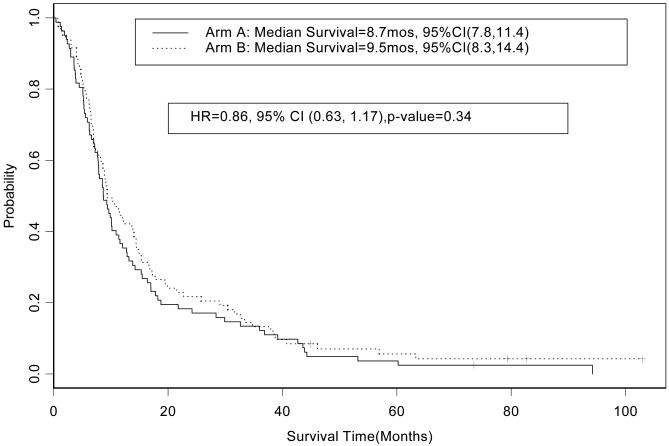
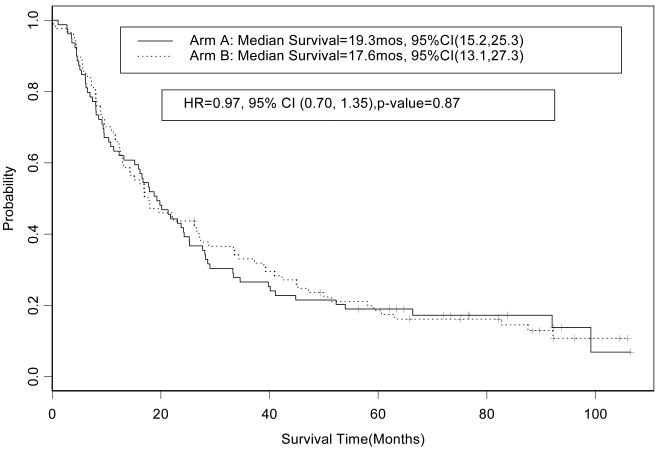
An exploratory analysis was performed to test if there was a difference in survival related to treatment arm within the good and poor prognosis subgroups. In the poor prognosis group the HR for overall survival for patients who received treatment on arm A (concurrent chemoradiotherapy alone, n=82) in comparison to patients who received treatment arm B (induction chemotherapy followed by chemoradiotherapy, n=83) was 0.86 (95% CI, 0.63 to 1.17, p=0.34); the median survival times observed on arms A and B were 8.7 (95% CI, 7.8 to 10.9) and 9.5 (95% CI, 8.2 to 14.1) months, respectively (Figure 4). In the good prognosis group the HR for overall survival for patients on arm A (n=79) in comparison to patients on arm B (n=87) was 0.97 (95% CI, 0.70 to 1.35; p=0.87); the median survival times observed on arms A and B were 19.3 (95% CI, 13.2 to 25.2) and 17.6 (95% CI, 13.0 to 27.2) months, respectively (Figure 5). In the good prognosis group the five-year overall survival rate observed among patients on arm A and B was 19% (95% CI, 12.0 to 29.9%) and 18.6% (95% CI, 11.9 to 29.1%), respectively. In the poor prognosis group the five-year overall survival rate among patients on arm A and B was 3.7% (85% CI, 1.2 to 11.1%) and 5.6% (95% CI, 2.3 to 13.9%), respectively.
Figure 4. CALGB 39801: Overall survival by treatment arm in the poor prognosis group (n=165).
Figure 5. CALGB 39801: Overall Survival by treatment arm in the good prognosis group (n=166).
Discussion
Treatment with chemoradiotherapy is the standard of care for unresectable stage III NSCLC, but the majority of patients will still experience local and/or distant disease progression and die from progressive disease. The patient population enrolled on this trial may be more reflective of the patient population who receive chemoradiotherapy outside the context of clinical trial, and the treatment paradigms investigated continue to be frequently used. In order to divide this homogenous patient population with a similar prognosis into two different prognostic groups we divided patients based on the presence of ≥ 2 or ≤ 1 prognostic factor. This division is arbitrary and an oversimplification of the multiple factors and complex interactions that probably contributed to the differences in survival observed.
In the poor prognosis group, the median survival time of 9 months observed is less than the median survival observed with chemotherapy alone in patients with stage IIIB disease due to pleural or pericardial effusion or metastatic disease.15-17 Median survival is arguably a sub-optimal parameter to evaluate chemoradiotherapy treatment when the intent of the therapy is to cure a minority of the patients. However, the long term survival of the poor prognosis patients is also poor with a 5-year survival rate of 4.7%. This raises a concern that the majority of patients in the poor prognosis patient population derives no long term benefit from concurrent chemoradiotherapy and may be exposed to a therapy associated a higher rate of esophagitis and/or myelosuppression in comparison to sequential chemotherapy and TRT or TRT alone.18-23
The rates of hematological and non-hematologic adverse events, and maximum toxicity were similar between the two prognostic groups (Table 4), and the percentage of patients who have discontinued therapy related to toxicity was similar between the two prognostic groups (Table 3). These data suggest that the difference in survival between the prognostic groups did not result from excessive toxicity or the inability to complete the protocol therapy. Another potential confounding factor is that the patients in the poor prognosis group may have experienced extra-thoracic disease progression at a higher rate due to the fact that constitutional symptoms such as weight loss are frequently associated with occult metastatic disease. However, the percentage of patients who discontinued therapy due to disease progression (4% and 5%, respectively) and due to death (2% and 5%, respectively) was similar in the poor and good prognostic groups. Thus, disease progression while on therapy does not appear to be responsible for the differences in survival.
In the original analysis among eligible patients with weight loss ≥ 5% (n=87) a numerically longer median survival was observed on arm B, and in contrast among the eligible patients with weight loss < 5% (n=244) a numerically longer survival was observed on arm A. These numerical differences observed in survival led us to perform an exploratory analysis of effect of the treatment arm in the poor (n=165) and good prognosis groups (n=166). These data provide no evidence that patients in each prognostic subgroup will benefit from induction chemotherapy in addition to concurrent chemoradiotherapy over concurrent chemoradiotherapy alone. Importantly, this retrospective analysis lacks statistical power to definitively determine a lack of benefit of induction chemotherapy and should be interpreted cautiously.
Only 66 patients enrolled on this trial (20% of the entire patient population) had no factors predictive of poor survival. Of the remaining patients in the good prognosis group only 5% were age ≥ 70 and 7% had weight loss ≥ 5%. Patients with an hgb < 13 g/dl (17%) or a PS of 1 (31%) were more common. The median survival time of 18 months observed in this good prognosis patient population is similar to a previous CALGB trial for unresectable stage III disease.7 A direct comparison of the prevalence of these adverse factors in this trial and other trials is difficult due to the lack of reporting of all the risk factors we used and differences in eligibility criteria.7,8,24-26
Outside the context of a clinical trial the percentage of patients who receive chemoradiotherapy who fit the criteria for the good prognosis category may be different due to patient and physician selection related to trial participation and the eligibility requirements of clinical trials. A recent review of a population-based cancer registry of patients with locally advanced lung cancer revealed that only a minority of patients would be eligible for concurrent chemoradiotherapy trials.27 The majority of the patients had NSCLC (81%), and patients were analyzed for trial eligibility according to the most lenient inclusion parameters from recent and ongoing concurrent chemoradiation trials: age ≤ 74 years, PS ≤ 2, weight loss <10% in last three months, and FEV-1 at least 40% age-predicted value. Of the 686 patients analyzed 408 patients (59%) were considered ineligible for trial participation. A significant percentage (23.3%) of the patients were excluded based on age ≥ 75 years, but in the age group of 60 to 69 only 50% of patients were eligible, and in the age group of 70 to 74 less than half were eligible. Thus, the patients with stage III disease who participate in clinical trials may represent a select group of patients, and the patients in the good prognosis category may represent an even more select group of patients.
This trial was performed before positron emission tomography (PET) was routinely performed in the staging of patients with NSCLC. Approximately 25% of patients with clinical stage III disease will have metastatic disease detected by PET scans.28 PET scan staging has led to a statistically significant increase in the proportion of patients being determined to have stage IV disease, and patients who have stage III or IV disease using PET scan staging have an improved survival compared to patients who have stage III or IV determined by conventional staging.29 The proportion of patients with metastatic disease detected with PET scan staging may be higher in patients with the clinical characteristics associated with the poor prognosis group. Thus, PET scan staging may reduce the percentage of stage III patients with characteristics of the poor prognosis group.
The use of weekly low dose carboplatin and paclitaxel with concurrent radiotherapy may have compromised the efficacy of both treatment paradigms investigated in this trial. The trials that have demonstrated a benefit of concurrent chemoradiotherapy over sequential chemotherapy and radiotherapy have used systemic dose cisplatin-based therapy concurrent with the radiotherapy.18,30 However, when newer agents (e.g. taxanes and gemcitabine) are used the chemotherapy dose must be reduced to avoid excessive toxicity. A phase III trial that compared weekly paclitaxel with concurrent TRT versus TRT alone after induction chemotherapy with carboplatin and paclitaxel demonstrated a significant improvement in time to tumor progression (p<0.001) but not overall survival in comparison to TRT alone (p=0.091).31 Carboplatin as a single agent in combination with radiation TRT appears to have limited activity based on a previous CALGB trial,32 as well as trials by other groups.33,34
The subsequent CALGB trial, 30407, was a randomized phase II trial that investigated systemic doses of carboplatin (AUC=5) and pemetrexed (500 mg/m2) in combination with thoracic radiation therapy (70 Gy) with and without cetuximab. The investigational arm of carboplatin and pemetrexed was based on the results of a promising phase I trial35, and the investigational including cetuximab was included based on the successful results observed in the treatment of cetuximab in combination radiation therapy in head and neck cancer.36 On preliminary analyses the median survival time observed among patients on the carboplatin and pemetrexed alone and with cetuximab treatment arms was 22.3 and 18.7 months, respectively.37
Other groups have retrospectively examined prognostic factors of patients who received chemoradiotherapy for stage III disease as well. The recent Hoosier Oncology Group phase III trial comparing cisplatin and etoposide with concurrent TRT with and without docetaxel revealed a median survival time of 21.7 months for all patients (n=203), and no significant difference in survival between treatment arms was observed.38 Patients with weight loss of > 5% were excluded from this trial. An analysis of the prognostic factors including age (< 70 versus ≥ 70 years) sex, race/ethnicity, body mass index, PS (0 versus 1), FEV-1 (>2 liters (L) versus 1-2 L), smoking status (current versus never/former), hgb, stage (IIIA versus IIIB) and the use of PET scan staging.14 A total of 203 patients were enrolled, and 26% were age ≥ 70 years, 42% had a PS of 1, and 14% had a baseline hgb < 12. On Cox regression analysis baseline hgb level as a continuous covariate (HR=0.84; 95% CI, 0.73 to 0.96, p-value=0.012) and FEV-1 > 2 L (HR=0.57; 95% CI, 0.35 to 0.92, p-value= 0.021) were independent prognostic factors for survival. Unfortunately we do not have baseline FEV-1 values to investigate FEV-1 as a prognostic factor for patients treated on CALGB 39801. The previously performed Cox proportional hazards regression model for survival perform for patients who received treatment on CALGB 39801 investigate anemia, defined as hgb < 12 g/dl, and did not find anemia predictive of survival.6
A retrospective analysis of prognostic factors of patients who were treated on Radiation Therapy Oncology Group (RTOG) trials 980139 and 941019 was recently performed (n=824).13 The eligibility criteria were inoperable stage II or unresectable stage III NSCLC, a Karnofsky Performance Status (KPS) ≥ 70, and weight loss ≤ 5%. The median baseline hgb was 13.4 g/dl and 17% had hgb < 12 g/dl, 75% of patients had a KPS of 90-100, and 18% were age ≥ 70 years. In Cox proportional univariate hazard models KPS (90-100 vs. 70-80); nodal status (0 and 1 vs. 2 and 3), female gender, and lobar location were statistically significant. Age, stage, gender, nodal status, KPS, tumor location and hgb were dichotomized into two levels and patients were grouped into three cohorts of combined prognostic factors (0 or 1 vs. 2 or 3 vs. >3). The HR for survival observed for the patient cohort of 2 or 3 and > 3 poor prognostic factors in comparison to the cohort of 0 or 1 poor prognostic risk factors was 1.31 (95% CI, 0.99 to 1.79; p-value 0.0585) and 1.74 (95% CI, 1.28 to 2.35; p=0.0003), respectively. The median survival observed in the three groups was 22.7, 17.3, and 13.5 months, respectively; and the 5-year survival rates observed were 18.5, 14.7, and 9.8%, respectively.
Gross tumor volume (GTV) has been identified as a prognostic factor in patients with NSCLC who receive radiation therapy,40-44 and the post-chemotherapy GTV after induction therapy for patients with stage III disease may be a prognostic factor.45 Several studies have found a poor correlation between stage and tumor volume probably due to the fact that there can be significant variability of the tumor volume within a stage.46-48 The percentage of the total lung volume receiving ≥ 20 Gy (V20) is a risk factor for the development of radiation pneumonitis, and has been identified on multivariate analysis to be an independent predictor of survival.49 We did not investigate GTV or V20 as prognostic factors in this trial.
In summary, our analysis indicated that four clinical variables (age, baseline hgb, the presence of weight loss, and performance status) were predictive of survival and if patients are divided into prognostic subgroups there is no evidence that induction chemotherapy is beneficial in either prognostic group.
Acknowledgments
The research for CALGB 39801 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
Statistical Center, Duke University Medical Center, Durham, NC–Stephen George, Ph.D., supported by CA33601
Baptist Cancer Institute CCOP, Memphis, TN–Lee S. Schwartzberg, M.D., supported by CA71323
Cancer Centers of the Carolinas, Greenville, SC–Jeffrey K. Giguere, M.D., supported by CA29165
Dana-Farber Cancer Institute, Boston, MA–Eric P. Winer, M.D., supported by CA32291
Dartmouth Medical School - Norris Cotton Cancer Center, Lebanon, NH–Marc S. Ernstoff, M.D., supported by CA04326
Duke University Medical Center, Durham, NC–Jeffrey Crawford, M.D., supported by CA47577
Georgetown University Medical Center, Washington, DC–Minetta C. Liu, M.D., supported by CA77597
Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY–Jeffrey Kirshner, M.D., supported by CA45389
Massachusetts General Hospital, Boston, MA–Jeffrey W. Clark, M.D., supported by CA12449
Medical University of South Carolina, Charleston, SC–Mark Green, M.D., supported by CA03927
Mount Sinai Medical Center, Miami, FL–Rogerio Lilenbaum, M.D., supported by CA45564
Mount Sinai School of Medicine, New York, NY–Lewis R. Silverman, M.D., supported by CA04457
Northern Indiana Cancer Research Consortium CCOP, South Bend, IN–Rafat Ansari, M.D., supported by CA86726
North Shore-Long Island Jewish Health System, New Hyde Park, NY - Daniel Budman, M.D., supported by CA11028
Rhode Island Hospital, Providence, RI–William Sikov, M.D., supported by CA08025
Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, M.D., supported by CA02599
Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC–James N. Atkins, M.D., supported by CA45808
Southern Nevada Cancer Research Foundation CCOP, Las Vegas, NV–John Ellerton, M.D., supported by CA35421
State University of New York Upstate Medical University, Syracuse, NY–Stephen L. Graziano, M.D., supported by CA21060
The Ohio State University Medical Center, Columbus, OH–Clara D Bloomfield, M.D., supported by CA77658
University of California at San Diego, San Diego, CA–Barbara A. Parker, M.D., supported by CA11789
University of Chicago, Chicago, IL–Gini Fleming, M.D., supported by CA41287
University of Illinois MBCCOP, Chicago, IL–Lawrence E. Feldman, M.D., supported by CA74811
University of Iowa, Iowa City, IA–Daniel A. Vaena, M.D., supported by CA47642
University of Maryland Greenebaum Cancer Center, Baltimore, MD–Martin Edelman, M.D., supported by CA31983
University of Massachusetts Medical School, Worcester, MA–William V. Walsh, M.D., supported by CA37135
University of Minnesota, Minneapolis, MN–Bruce A Peterson, M.D., supported by CA16450
University of Missouri/Ellis Fischel Cancer Center, Columbia, MO–Michael C Perry, M.D., supported by CA12046
University of Nebraska Medical Center, Omaha, NE–Anne Kessinger, M.D., supported by CA77298
University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, M.D., supported by CA47559
University of Tennessee Memphis, Memphis, TN–Harvey B. Niell, M.D., supported by CA47555
University of Texas Southwestern Medical Center, Dallas, TX–Debasish Tripathy, M.D.
Wake Forest University School of Medicine, Winston-Salem, NC–David D Hurd, M.D., supported by CA03927
Walter Reed Army Medical Center, Washington, DC–Thomas Reid, M.D., supported by CA26806
Washington University School of Medicine, St. Louis, MO–Nancy Bartlett, M.D., supported by CA77440
Weill Medical College of Cornell University, New York, NY–John Leonard, M.D., supported by CA07968
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Yang P, Allen MS, Aubry MC, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–62. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 3.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 4.Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology Treatment of Unresectable Non-Small Cell Lung Cancer Guideline: Update 2003. J Clin Oncol. 2004;22:330–353. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Blackstock AW, Govindan R. Definitive chemoradiation for the treatment of locally advanced non small-cell lung cancer. J Clin Oncol. 2007;25:4146–52. doi: 10.1200/JCO.2007.12.6581. [DOI] [PubMed] [Google Scholar]
- 6.Vokes EE, Herndon JE, 2nd, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25:1698–704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 7.Vokes EE, Herndon JE, 2nd, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol. 2002;20:4191–8. doi: 10.1200/JCO.2002.03.054. [DOI] [PubMed] [Google Scholar]
- 8.Akerley W, Herndon JE, Jr, Lyss AP, et al. Induction paclitaxel/carboplatin followed by concurrent chemoradiation therapy for unresectable stage III non-small-cell lung cancer: a limited-access study--CALGB 9534. Clin Lung Cancer. 2005;7:47–53. doi: 10.3816/CLC.2005.n.021. [DOI] [PubMed] [Google Scholar]
- 9.Ginsburg R, Vokes EE, Rosenzweig KE. Non-small cell lung cancer. In: DeVita J, Hellman S, Rosenberg S, editors. Cancer:Principles and Practice of Oncology. 7. Philadelphia, PA, Lippencott: Williams, and Wilkins; 2004. [Google Scholar]
- 10.Calvert A, Newell D, Gumbrell L, et al. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 11.Socinski MA, Zhang C, Herndon JE, 2nd, et al. Combined modality trials of the Cancer and Leukemia Group B in stage III non-small-cell lung cancer: analysis of factors influencing survival and toxicity. Ann Oncol. 2004;15:1033–41. doi: 10.1093/annonc/mdh282. [DOI] [PubMed] [Google Scholar]
- 12.Langer C, Hsu C, Curran WJ, et al. Proc Am Soc Clin Oncol. Orlando, FL: 2002. Elderly patients (pts) with locally advanced non-small cell lung cancer (LA-NSCLC) benefit from combined modality therapy: secondary analysis of Radiation Therapy Oncology Group (RTOG) 94-10; p. 299a. [Google Scholar]
- 13.Langer CJ, Paulus R, Curran W, et al. Rethinking prognostic factors in the era of combined modality therapy for Locally Advanced Non-small Cell Lung Cancer (LANSCLC): a retrospective analysis of RTOG Protocols 9410 and 9801. Journal of Thoracic Oncology. 2007;2:s309. abstract A1-03. [Google Scholar]
- 14.Ademuyiwa FO, Johnson CS, White AS, et al. Prognostic factors in stage III non-small-cell lung cancer. Clin Lung Cancer. 2007;8:478–82. doi: 10.3816/CLC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 15.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III Study Comparing Cisplatin Plus Gemcitabine With Cisplatin Plus Pemetrexed in Chemotherapy-Naive Patients With Advanced-Stage Non-Small-Cell Lung Cancer. J Clin Oncol. 2008 doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 16.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 17.Pirker R, Szczesna A, von Pawel J, et al. FLEX: A randomized, multicenter, phase III study of cetuximab in combination with cisplatin/vinorelbine (CV) versus CV alone in the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2008;26 abstract 3. [Google Scholar]
- 18.Furuse K, West Japan Lung Cancer Group Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 19.Curran D, Scott C, Langer C, et al. Proc Am Soc Clin Oncol. Chicago, Illinois: 2003. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresected stage III NSCLC: RTOG 9410; p. 621. [Google Scholar]
- 20.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol. 2005;23:5910–7. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 21.Dillman R, Seagren S, Propert K, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small cell lung cancer. N Engl J Med. 1990;323:940–945. doi: 10.1056/NEJM199010043231403. [DOI] [PubMed] [Google Scholar]
- 22.Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–91. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 23.Zatloukal P, Petruzelka L, Zemanova M, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004;46:87–98. doi: 10.1016/j.lungcan.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–63. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 25.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–6. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 26.Hanna NH, Neubauer M, Ansari R, et al. Phase III trial of cisplatin (P) plus etoposide (E) plus concurrent chest radiation (XRT) with or without consolidation docetaxel (D) in patients (pts) with inoperable stage III non-small cell lung cancer (NSCLC): HOG LUN 01-24/USO-023. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. 2007;25 abstract 7512. [Google Scholar]
- 27.De Ruysscher D, Botterweck A, Dirx M, et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study. Ann Oncol. 2009;20:98–102. doi: 10.1093/annonc/mdn559. [DOI] [PubMed] [Google Scholar]
- 28.MacManus MP, Hicks RJ, Matthews JP, et al. High rate of detection of unsuspected distant metastases by pet in apparent stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:287–93. doi: 10.1016/s0360-3016(01)01477-8. [DOI] [PubMed] [Google Scholar]
- 29.Morgensztern D, Goodgame B, Baggstrom MQ, et al. The effect of FDG-PET on the stage distribution of non-small cell lung cancer. J Thorac Oncol. 2008;3:135–9. doi: 10.1097/JTO.0b013e3181622c2c. [DOI] [PubMed] [Google Scholar]
- 30.Curran WJ, Scott C, Langer C. Phase III comparison of sequential vs concurrent chemoradiation for PTS with unresected stage III non-small cell lung cancer (NSCLC): initial report of Radiation Therapy Oncology Group (RTOG) 9410. Proc Am Soc Clin Oncol. 2000;19:484a. [Google Scholar]
- 31.Huber RM, Flentje M, Schmidt M, et al. Simultaneous chemoradiotherapy compared with radiotherapy alone after induction chemotherapy in inoperable stage IIIA or IIIB non-small-cell lung cancer: study CTRT99/97 by the Bronchial Carcinoma Therapy Group. J Clin Oncol. 2006;24:4397–404. doi: 10.1200/JCO.2005.05.4163. [DOI] [PubMed] [Google Scholar]
- 32.Clamon G, Herndon J, Cooper R, et al. Radiosensitization with carboplatin for patients with unresectable stage III non-small-cell lung cancer: a phase III trial of the Cancer and Leukemia Group B and the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:4–11. doi: 10.1200/JCO.1999.17.1.4. [DOI] [PubMed] [Google Scholar]
- 33.Gervais R, Ducolone A, Le Chevalier T, et al. Conventional radiation (RT) with daily carboplatin compared to RT alone after induction chemotherapy (ICT) : Final results of a randomized phase III trial in stage III unresectable non-small cell lung cancer (NSCLC). Study CRG/BMS/NPC/96 of the French Lung Cancer Study Group (FNCLCC) and IFCT. Journal of Clinical Oncology. 2005;23 abstract 7016. [Google Scholar]
- 34.Ball D, Bishop J, Smith J, et al. A randomised phase III study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable non-small cell lung cancer: final report of an Australian multi-centre trial. Radiother Oncol. 1999;52:129–36. doi: 10.1016/s0167-8140(99)00093-6. [DOI] [PubMed] [Google Scholar]
- 35.Seiwert T, Connell P, Mauer A, et al. Pemetrexed-based concurrent chemoradiotherapy (CRT) for locally advanced or metastatic non-small cell lung cancer : A phase I dose escalating study. Lung Cancer. 2005;49:S80. abstract PD-048. [Google Scholar]
- 36.Bonner JA, Giralt J, Harai PM, Cohen R, Jones C, Sur RK, Rabin D, Azarnia N, Needle MN, Ang KK. Cetuximab prolongs survival in patients with locoregionally adanced squamous cell carcinoma of head and neck: A phase III study of high doe radiation thereapy with and without cetuximab. Journal of Clinical Oncology. 2004;22 abstract 5507. [Google Scholar]
- 37.Govindan R, Bogart J, Wang X, et al. Phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small cell lung cancer: CALGB 30407. American Society of Clinical Oncology Annual Meeting. 2009 doi: 10.1200/JCO.2010.33.4979. Abst 7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III Study of Cisplatin, Etoposide, and Concurrent Chest Radiation With or Without Consolidation Docetaxel in Patients With Inoperable Stage III Non-Small-Cell Lung Cancer: The Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 39.Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98-01. J Clin Oncol. 2005;23:2145–54. doi: 10.1200/JCO.2005.07.167. [DOI] [PubMed] [Google Scholar]
- 40.Bradley JD, Leumwananonthachai N, Purdy JA, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2002;52:49–57. doi: 10.1016/s0360-3016(01)01772-2. [DOI] [PubMed] [Google Scholar]
- 41.Etiz D, Marks LB, Zhou SM, et al. Influence of tumor volume on survival in patients irradiated for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;53:835–46. doi: 10.1016/s0360-3016(02)02814-6. [DOI] [PubMed] [Google Scholar]
- 42.Werner-Wasik M, Xiao Y, Pequignot E, et al. Assessment of lung cancer response after nonoperative therapy: tumor diameter, bidimensional product, and volume. A serial CT scan-based study. Int J Radiat Oncol Biol Phys. 2001;51:56–61. doi: 10.1016/s0360-3016(01)01615-7. [DOI] [PubMed] [Google Scholar]
- 43.Basaki K, Abe Y, Aoki M, et al. Prognostic factors for survival in stage III non-small-cell lung cancer treated with definitive radiation therapy: impact of tumor volume. Int J Radiat Oncol Biol Phys. 2006;64:449–54. doi: 10.1016/j.ijrobp.2005.07.967. [DOI] [PubMed] [Google Scholar]
- 44.Werner-Wasik M, Swann RS, Bradley J, et al. Increasing tumor volume is predictive of poor overall and progression-free survival: secondary analysis of the Radiation Therapy Oncology Group 93-11 phase I-II radiation dose-escalation study in patients with inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70:385–90. doi: 10.1016/j.ijrobp.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 45.Stinchcombe TE, Morris DE, Moore DT, et al. Post-chemotherapy gross tumor volume is predictive of survival in patients with stage III non-small cell lung cancer treated with combined modality therapy. Lung Cancer. 2006;52:67–74. doi: 10.1016/j.lungcan.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Ball DL, Fisher R, Burmeister B, et al. Stage is not a reliable indicator of tumor volume in non-small cell lung cancer: a preliminary analysis of the Trans-Tasman Radiation Oncology Group 99-05 database. J Thorac Oncol. 2006;1:667–72. [PubMed] [Google Scholar]
- 47.Martel MK, Strawderman M, Hazuka MB, et al. Volume and dose parameters for survival of non-small cell lung cancer patients. Radiother Oncol. 1997;44:23–9. doi: 10.1016/s0167-8140(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 48.Kupelian PA, Komaki R, Allen P. Prognostic factors in the treatment of node-negative nonsmall cell lung carcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys. 1996;36:607–13. doi: 10.1016/s0360-3016(96)00364-1. [DOI] [PubMed] [Google Scholar]
- 49.Gaspar LE, Redman MW, Chansky K, et al. Analysis of V20 and radiation pneumonits on SWOG 0023: A phase III trial of concurrent chemoradiation and docetaxel consolidation followed by gefitinib or placebo in stage III non-small cell lung cancer. Journal of Thoracic Oncology. 2008;3:s264. [Google Scholar]