Abstract
Background:
B7-H3 is a new member of the B7 ligand family and regulates T-cell responses in various conditions. However, the role of B7-H3 in tumour immunity is largely unknown. The purpose of this study was to evaluate the clinical significance of B7-H3 expression in human pancreatic cancer and the therapeutic potential for cancer immunotherapy.
Methods:
We investigated B7-H3 expression in 59 patients with pancreatic cancer by immunohistochemistry and real-time PCR. Furthermore, we examined the anti-tumour effect of B7-H3-blocking monoclonal antibody in vivo in a murine pancreatic cancer model.
Results:
Tumour-related B7-H3 expression was abundant in most human pancreatic cancer tissues and was significantly higher compared with that in non-cancer tissue or normal pancreas. Moreover, its expression was significantly more intense in cases with lymph node metastasis and advanced pathological stage. B7-H3 blockade promoted CD8+ T-cell infiltration into the tumour and induced a substantial anti-tumour effect on murine pancreatic cancer. In addition, the combination of gemcitabine with B7-H3 blockade showed a synergistic anti-tumour effect without overt toxicity.
Conclusion:
Our data show for the first time that B7-H3 may have a critical role in pancreatic cancer and provide the rationale for developing a novel cancer immunotherapy against this fatal disease.
Keywords: B7-H3, pancreas, immunotherapy, T-cell, antibody
Pancreatic cancer is one of most aggressive and intractable human malignant tumours and a leading cause of cancer-related deaths worldwide (Li et al, 2004; Laheru and Jaffee, 2005). Owing to its extremely high malignant potential, it is usually diagnosed at an advanced stage and often recurs even after curative surgery (Traverso, 2006). Although recent significant advances in cancer therapy, including the introduction of new chemotherapeutic agents, have made a significant impact on the overall survival of pancreatic cancer patients, complete cure is very rare and the incidence rates are almost equal to mortality rates (Burris et al, 1997; Li et al, 2004; Laheru and Jaffee, 2005). Therefore, novel approaches against pancreatic cancer need to be developed and established to improve patient prognosis.
B7-H3 (CD276) is a new member of the B7 family and shares 20–27% identical amino acids with other members (Chapoval et al, 2001). B7-H3 is not expressed on quiescent lymphocytes and can be induced in activated dendritic cells, monocytes, T cells, and in some tumour cell lines (Chapoval et al, 2001; Sun et al, 2002; Steinberger et al, 2004; Zhang et al, 2005). B7-H3 is thought to serve as an accessory co-regulator of T-cell responses after initial antigen priming. At present, there is no consensus with regard to the physiological and pathological roles of B7-H3, as both immunological stimulatory and inhibitory functions have been described (Chapoval et al, 2001; Sun et al, 2002; Suh et al, 2003; Castriconi et al, 2004; Prasad et al, 2004; Steinberger et al, 2004; Wang et al, 2005; Zhang et al, 2005). It was originally identified as a co-stimulatory molecule that promotes T-cell proliferation and IFN-γ production (Chapoval et al, 2001). The experimental evidence that acute and chronic cardiac allograft rejection can be reduced in B7-H3 knockout mice further supports a stimulatory role for B7-H3 on T cells (Wang et al, 2005). By contrast, B7-H3 has been shown to impair type 1 T-helper cell responses and inhibit cytokine production (Suh et al, 2003). In vivo antibody-mediated blockade of B7-H3 in mice has been reported to enhance T-cell activation and to lead to more severe forms of experimental autoimmune encephalitis (Suh et al, 2003; Prasad et al, 2004). In addition, B7-H3 has been implicated to confer protection from natural killer (NK) cell-mediated cytolysis (Castriconi et al, 2004). Therefore, it is likely that B7-H3 exerts dual functions under certain circumstances.
In tumour immunity, the precise role of B7-H3 also remains unclear. B7-H3 expression has been identified in several tumour cell lines and in actual human tumour specimens, including gastric cancer, non-small-cell lung cancer, and prostate cancer (Sun et al, 2006; Wu et al, 2006; Roth et al, 2007; Zang et al, 2007). Several murine studies have shown that introduction of B7-H3 in tumour cells and tissues by various methods activates tumour-specific immunocompetent cells and promotes anti-tumour response, thereby leading to rapid tumour regression, reduction of metastasis, and prolongation of animal survival (Sun et al, 2003; Luo et al, 2004, 2006; Lupu et al, 2006, 2007). In addition, high intra-tumour B7-H3 expression was shown to correlate with better prognosis in gastric cancer (Wu et al, 2006). These data suggested that tumour-associated B7-H3 expression might promote immune response as a positive regulator. By sharp contrast, the negative role of B7-H3 expression has been reported in lung and prostate cancers (Sun et al, 2006; Roth et al, 2007; Zang et al, 2007). In non-small-cell lung cancer, B7-H3 expression was inversely correlated with the number of tumour-infiltrating lymphocytes, and a high B7-H3 expression was more common in patients with lymph node metastasis (Sun et al, 2006). Furthermore, a strong intensity for B7-H3 was significantly correlated with disease spread at the time of surgery, as well as with increased risk of recurrence and poor prognosis in prostate cancer (Roth et al, 2007; Zang et al, 2007). In addition, 4Ig-B7-H3 (B7-H3 protein with four-Ig-like domains) was identified in advanced stage neuroblastoma and has been shown to exert a protective effect on neuroblastoma cells from NK-mediated lysis (Castriconi et al, 2004). Thus, experimental and clinical data are conflicting and the precise role of B7-H3 in tumour immunity has not been fully elucidated yet.
In this study, we investigated the clinical significance of B7-H3 expression in human pancreatic cancer and the therapeutic efficacy of targeting the B7-H3 pathway towards future clinical application for the treatment of pancreatic cancer.
Materials and methods
Patients
We examined 59 patients with pancreatic cancer who underwent surgery at the Department of Surgery, Nara Medical University, between 1996 and 2004 (Table 1). The median age of the patients was 66 years, with a range of 42–80 years. Tissues, both cancerous and non-cancerous, were obtained from resected specimens and were then rapidly frozen at −80°C for storage until use. The remainder of each specimen was fixed in 10% phosphate-buffered formalin and embedded in paraffin. Pancreatic cancers were classified according to the TNM staging system. Post-operative pathological examinations have indicated metastasis in distant lymph nodes (M1, Stage IV) in seven patients. Primary tumour tissues from these patients were included for histological analysis in this study. Follow-up was until death or December 2007. The median follow-up for all patients was 14 months, with a range of 3–157 months. Most of the patients received systemic adjuvant chemotherapy after surgery. Normal pancreatic tissues were obtained from surgical specimens other than pancreatic cancer. Written informed consent was obtained from all patients before treatment, according to our institutional guidelines.
Table 1. Clinicopathological characteristics according to tumor B7-H3 expression.
|
B7-H3 intensity
|
||||
|---|---|---|---|---|
| Variable | n | None or weak (%) | Strong (%) | P-value |
| Tumour status | ||||
| T1 | 3 | 1 (5.0) | 2 (5.1) | 0.646 |
| T2 | 12 | 5 (25.0) | 7 (18.0) | |
| T3 | 26 | 10 (50.0) | 16 (41.0) | |
| T4 | 18 | 4 (20.0) | 14 (35.9) | |
| Nodal status | ||||
| N0 | 26 | 13 (65.0) | 13 (33.3) | 0.020 |
| N1 | 33 | 7 (35.0) | 26 (66.7) | |
| Metastatic status | ||||
| M0 | 52 | 18 (90.0) | 34 (87.2) | 0.751 |
| M1 | 7 | 2 (10.0) | 5 (12.8) | |
| Pathological stage | ||||
| IA, IB | 7 | 4 (20.0) | 3 (7.7) | 0.040 |
| IIA | 13 | 8 (40.0) | 5 (12.8) | |
| IIB | 16 | 4 (20.0) | 12 (30.8) | |
| III | 16 | 2 (10.0) | 14 (35.9) | |
| IV | 7 | 2 (10.0) | 5 (12.8) | |
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues of primary tumour were cut into 5-μm sections, deparaffinised, and rehydrated in a graded series of ethanol. Antigen retrieval was carried out by heating tissue sections using a Target Retrieval Solution, pH 9.0 (DAKO, Tokyo, Japan). To block endogenous peroxidase, sections were immersed in 3% solution of hydrogen peroxide in absolute methanol for 10 min at room temperature and washed thrice in fresh PBS, each of 5 min duration. Purified goat anti-human B7-H3 antibody (100 μg ml−1; R&D Systems, Minneapolis, MN, USA) diluted 1 : 10 with Antibody Diluent (DAKO) was added and incubated overnight at 4°C. Sections were washed thrice in PBS, each of 5 min duration, and then Histofine Simple Stain MAX PO (G) (NICHIREI, Tokyo, Japan) was added and incubated at room temperature for 30 min. After washing thrice, the Histofine DAB substrate kit (NICHIREI) was added and incubated at room temperature for 20 min. Sections were rinsed thrice in distilled water, counterstained with haematoxylin, dehydrated in ethanol, cleared in xylene, and coverslipped. For the staining of mouse CD8+ T cells, purified rabbit anti-CD8 antibody (Abcam, Tokyo, Japan) diluted 1 : 250 with Antibody Diluent (DAKO) was used and incubated overnight at 4°C. Sections were washed thrice in PBS, followed by incubation with the EnVision detection system (DAKO), according to the instructions of the manufacturer.
Evaluation of immunostaining
Immunohistochemistry for B7-H3 was evaluated by authorised pathologists who had no knowledge of the patients’ clinical status and outcome. Each sample was thoroughly evaluated at a magnification of × 100. To count CD8+ T cells in murine pancreatic cancer tissue, three randomly selected areas were counted at × 200 magnification, and the average count was calculated.
Extraction of total RNAs and real-time reverse-transcriptase PCR analysis
Total RNA was isolated using RNAspin Mini (GE Healthcare, Tokyo, Japan) and the first-strand cDNA was synthesised from 1 μg RNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), according to the instructions of the manufacturer. Real-time quantitative PCR analysis was carried out using an ABI Prism 7700 sequence detector system (Applied Biosystems). All primer/probe sets were purchased from Applied Biosystems. PCR was carried out using the TaqMan Universal PCR Master Mix (Applied Biosystems) using 1 μl of cDNA in a 20 μl final reaction volume. The PCR thermal cycle conditions were as follows: initial step at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The expression level of the housekeeping gene β2-microglobulin was measured as an internal reference with a standard curve to determine the integrity of template RNA for all specimens. The ratio of the mRNA level of each gene was calculated as follows: (absolute copy number of each gene)/(absolute copy number of β2-microglobulin).
Animal and cell line
Female C57BL/6 mice (8–12-weeks old) were obtained from CLEA JAPAN (Tokyo, Japan). All mice were maintained under specific pathogen-free conditions in the animal facility at Nara Medical University. All experiments were conducted under a protocol approved by our institutional review board. A murine pancreatic adenocarcinoma, PAN02, was obtained from the DCTD Tumor Repository, National Cancer Institute (Frederick, MD, USA). Cells were grown in RPMI 1640 supplemented with 10% heat-inactivated foetal bovine serum.
Animal experimental protocol
PAN02 was orthotopically implanted in the pancreas of syngeneic C57BL/6 mice. The anti-mouse B7-H3-blocking monoclonal Ab (MJ18, rat IgG1) was generated as previously described (Nagashima et al, 2008). The day after tumour implantation, mAb treatment was started. In the antibody treatment arm, mice were intraperitoneally injected with 0.3 mg of MJ18 every other day for 3 weeks. Control mice received control rat IgG. For in vivo depletion of CD8+ T cells, mice received an anti-CD8-depleting monoclonal Ab (2.43, rat IgG, 200 μg), which was intraperitoneally injected 6, 3, and 1 day before tumour implantation and weekly thereafter as previously described (Sho et al, 2002). Gemcitabine was intraperitoneally given at a dose of 0.8 mg per body, every 3 days, 5 times in total. The doses were determined on the basis of our preliminary experiments. Tumour volume was calculated according to the formula V=A × B2/2 (mm3), where A is the largest diameter (mm) and B is the smallest diameter (mm). At 4 weeks after tumour establishment, the mice were killed and tumours were removed for further analysis.
Cell viability analysis
Cell viability was determined using the Cell-titer 96 aqueous one solution cell proliferation assay kit, according to the instruction manual (Promega Corporation, Madison, WI, USA). Briefly, aliquots of 1 × 103 cells per well were cultured in 96-well plates with MJ18 or control IgG for 72 h. Antibody was used at a concentration of 1 or 10 μg ml−1. Cell-titer 96 aqueous one solution was added to each well and incubated for an additional 1 h. The absorbance at 490 nm was recorded with a 96-well plate reader. Each experiment was performed in triplicate and repeated at least thrice.
Statistical analysis
Results were expressed as mean values±standard error, and Student's t-test was used for evaluating statistical significance. A value less than 0.05 (P<0.05) was considered for statistical significance.
Results
B7-H3 expression in human pancreatic cancer
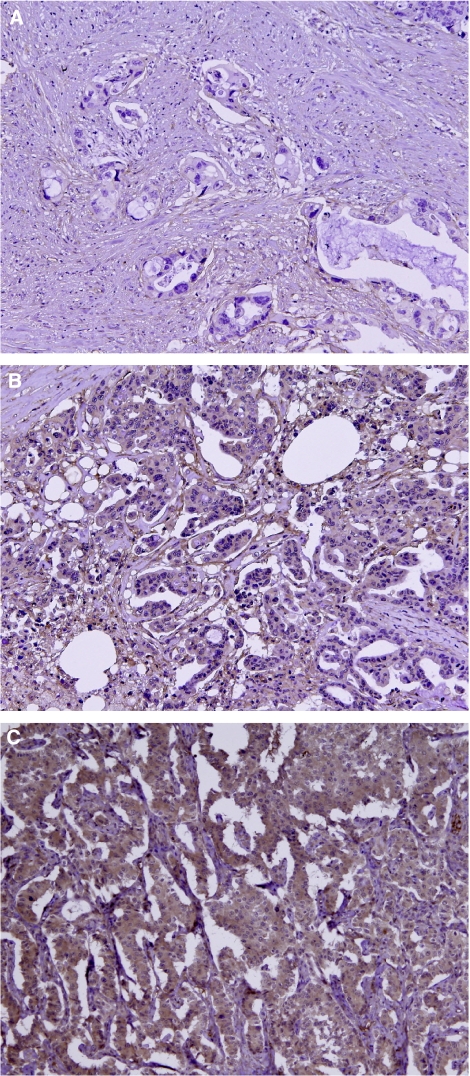
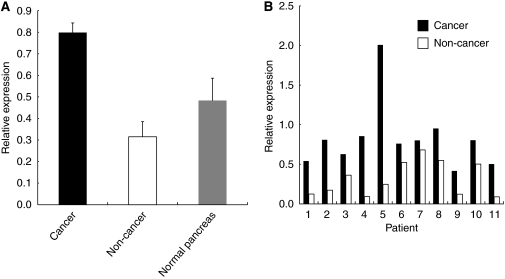
First, we examined the expression of B7-H3 on 59 pancreatic cancer tissues by immunohistochemistry. Positive staining was seen both on the cell membrane and in the cytoplasm of cancer cells in 55 patients (93.2%), whereas only 4 patients (6.8%) had no staining for B7-H3 (Figure 1). In non-cancer tissues, some islet cells were also positive for B7-H3. We then compared the relative expression of B7-H3 between pancreatic cancer and non-cancer tissues using available frozen tissues by quantitative real-time PCR. The B7-H3 expression in cancerous pancreatic tissues was significantly higher than in non-cancerous or normal pancreatic tissues (cancer vs non-cancer, P<0.001; cancer vs normal pancreas, P=0.008; non-cancer vs normal pancreas, N.S.) (Figure 2A). Furthermore, the B7-H3 expression of cancer tissue was consistently higher than that of non-cancer tissue in each individual pancreatic cancer patient (Figure 2B). These data suggested that B7-H3 might have some effect and may be a potential target for immunotherapy in pancreatic cancer.
Figure 1.
Immunohistochemical staining of human pancreatic cancer tissue for B7-H3. Positive staining was seen on the cell membrane and in the cytoplasm of cancer cells in almost all patients. Representative tissue of no staining (A), weak intensity (B), and strong intensity (C) for B7-H3 expression in pancreatic cancer was shown. Original magnification, × 100.
Figure 2.
Comparison of B7-H3 expression between cancer and non-cancer tissues of the pancreas. (A) The cumulative B7-H3 expression in cancer tissue (n=30) was significantly higher compared with that in non-cancer tissue (n=11) and normal pancreatic tissue (n=5) (cancer vs non-cancer, P<0.0001; cancer vs normal pancreas, P=0.0078; non-cancer vs normal pancreas, N.S.). (B) The expression in cancer tissue is consistently higher than that in non-cancer tissue of individual pancreatic cancer patients.
Correlation between B7-H3 expression and pathological findings
B7-H3 was positively stained in over 90% of pancreatic cancer patients. In these 55 positive tissues, it was homogeneously expressed in almost all pancreatic cancer cells in each examined tumour section. Therefore, all specimens were classified into two groups according to staining intensity as follows: 39 tumours with strong staining and 20 tumours with weak or no staining (Figure 1). We evaluated the correlation of the B7-H3 expression with various clinicopathological data. We found that tumours with a strong intensity of B7-H3 expression had more common lymph node metastasis (P=0.020) and advanced pathological stage (P=0.040) (Table 1). These data suggested that B7-H3 expression might be functionally important in tumour progression and metastasis in pancreatic cancer. In this study, there was no significant difference in the post-operative prognosis between strong and weak intensity of tumour B7-H3 expression.
Therapeutic efficacy of anti-B7-H3 mAb in pancreatic cancer
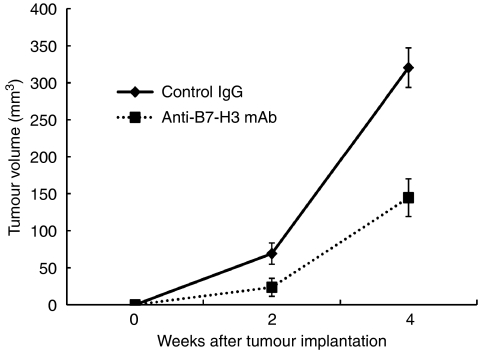
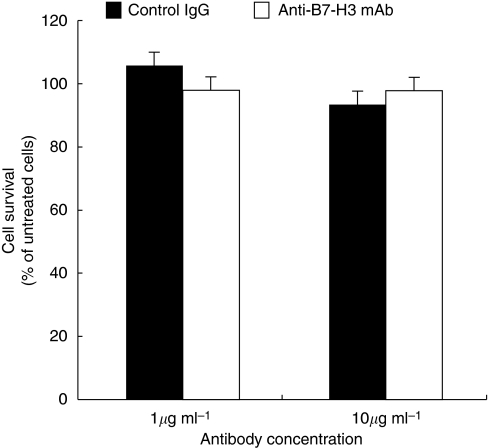
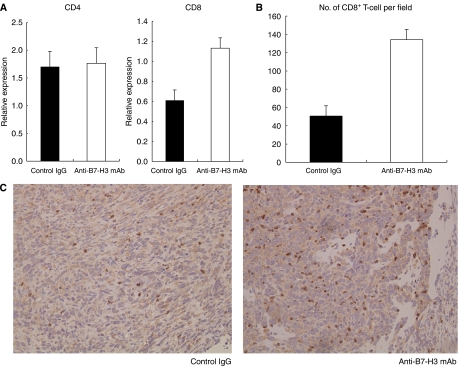
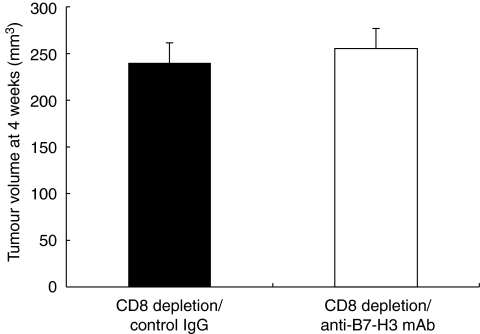
Towards a clinical application of targeting the B7-H3 pathway, we examined the therapeutic efficacy of anti-B7-H3-blocking mAb in pancreatic cancer. To this end, we used a murine pancreatic cancer model using PAN02, a murine pancreatic adenocarcinoma. We confirmed B7-H3 expression in PAN02 by RT-PCR and flow cytometry (data not shown). The anti-B7-H3 mAb, MJ18, treatment induced substantial anti-tumour effect in vivo and significantly inhibited tumour growth (tumour volume at 2 weeks: anti-B7-H3 mAb, n=7, 23.6±12.2 mm3; control, n=5, 69.1±14.4 mm3; P=0.036; tumour volume at 4 weeks: anti-B7-H3 mAb, n=10, 144.6±13.3 mm3; control, n=9, 320.4±36.1 mm3; P=0.0002) (Figure 3). To analyse the underlying mechanisms, we first tested in vitro effect of MJ18 on PAN02. A total of 1000 PAN02s were co-cultured with MJ18. Control rat IgG was used as a control. The survival rate of PAN02 was determined by MTS assay. As a result, B7-H3 blockade did not have any direct effect on cancer cell growth in vitro (Figure 4). We then evaluated tumour-infiltrating T cells after mAb treatment. At 2 weeks after tumour implantation, CD8+, but not CD4+, T cells in tumours treated with MJ18 were significantly more than that in controls, as indicated by real-time PCR (CD8+ T cells, n=3, P=0.024; CD4+ T cells, n=3, N.S.; controls, n=4) (Figure 5A). This was confirmed by immunohistochemical analysis on pancreatic cancer tissues at 4 weeks after tumour implantation. There was a more profound infiltration of CD8+ T cells into the tumour treated with B7-H3 blockade compared with controls (anti-B7-H3 mAb, n=5, 134.2±11.2; control, n=5, 50.5±11.2, P<0.001) (Figure 5B and C). Data indicated that B7-H3 blockade promoted the infiltrations of CD8+ T cells into tumours, thereby resulting in tumour growth inhibition. Furthermore, to confirm the requirement of CD8+ T cells for the anti-tumour effect of B7-H3 blockade, we used an in vivo depletion experiment. As a result, the depletion of CD8+ T cells completely abolished the effect of anti-B7-H3 mAb on murine pancreatic cancer (tumour volume at 4 weeks: anti-B7-H3 mAb, n=5, 255.4±21.4 mm3; control, n=5, 240.1±21.4 mm3; N.S.) (Figure 6). Therefore, data clearly indicated that CD8 T cells are effectors and are also essential for the anti-tumour effect of B7-H3 blockade.
Figure 3.
Therapeutic efficacy of B7-H3 blockade in murine pancreatic cancer. The effect of B7-H3 blockade on pancreatic cancer was examined in vivo. PAN02 was orthotopically implanted in the pancreas of syngeneic C57BL/6 mice. B7-H3 blockade significantly inhibited tumour growth compared with controls. (P=0.036 and 0.0002 at 2 and 4 weeks, respectively).
Figure 4.
Effect of anti-B7-H3 mAb on pancreatic cancer in vitro. A total of 1000 murine pancreatic cancer cells, PAN02s, were co-cultured with anti-B7-H3-blocking mAb, MJ18, or control IgG. B7-H3 blockade did not have any direct effect on cancer cell growth in vitro, as determined by MTS assay.
Figure 5.
Tumour-infiltrating T cells. (A) At 2 weeks after tumour implantation, CD8+, but not CD4+, T cells in tumours treated with anti-B7-H3 mAb were more abundant than in controls (P=0.024), as shown by quantitative real-time PCR. (B) Immunohistochemical analysis of CD8+ T cells in tumours treated with anti-B7-H3 mAb or control IgG (P<0.001). (C) Representative immunohistochemical staining of tumour-infiltrating CD8+ T cells. Original magnification, × 200. A more profound infiltration of CD8+ T cells was observed in the tumour treated with anti-B7-H3 mAb compared with controls.
Figure 6.
Requirement of CD8+ T cells for therapeutic efficacy of B7-H3 blockade. The tumour size between the control group and the treatment group did not have any statistical difference at 4 weeks after tumour implantation in mice depleted of CD8+ T cells.
Synergy between B7-H3 blockade and conventional chemotherapy
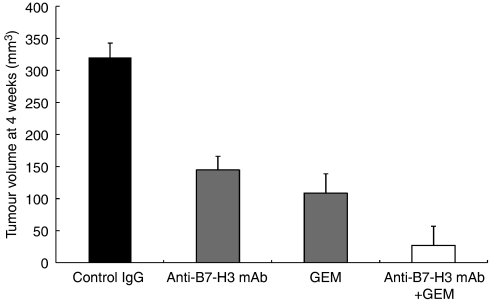
Finally, we evaluated the combination of conventional chemotherapy with B7-H3 blockade in pancreatic cancer. We used gemcitabine, which is currently the standard chemotherapeutic agent for pancreatic cancer. The treatment with gemcitabine alone resulted in significant inhibition of tumour growth (n=5, 108.6±9.3 mm3, P=0.0011 compared with controls). The combined treatment of gemcitabine and B7-H3 blockade showed a substantial synergistic anti-tumour effect on pancreatic cancer (n=5, 27.1±4.9 mm3, P<0.0001 compared with gemcitabine alone or B7-H3 blockade alone) (Figure 7). There were no overt toxicity and death in mice during and after treatment.
Figure 7.
Combination of B7-H3 blockade with gemcitabine. Treatment with gemcitabine (GEM) alone resulted in a significant inhibition of tumour growth (P=0.0011). Furthermore, the combined treatment with gemcitabine and B7-H3 blockade showed a substantial synergistic anti-tumour effect (P<0.0001 compared with gemcitabine alone or B7-H3 blockade alone).
Discussion
Although the cancer-related death rates of various malignancies have been declining because of improvements in early detection and treatment, the overall pancreatic cancer mortality rate is still extremely high (Jemal et al, 2007). To improve patient survival, novel strategies need to be developed. Immunotherapy is an attractive strategy and may provide a breakthrough in the treatment of pancreatic cancer. However, the clinical response rates of current immunotherapies are limited, presumably because of tumour immune escape mechanisms (Laheru and Jaffee, 2005; Plate, 2007). Thus, to elicit sufficient tumour-specific T-cell responses, it is necessary to overcome tumour immune evasion. One of the strategies may be targeting T-cell-inhibitory pathways, including B7/CTLA-4 and PD-L/PD-1 (Dong et al, 2002; Curiel et al, 2003; Ohigashi et al, 2005; Nomi et al, 2007). However, the effect of human CTLA-4-blocking antibody is likely to be limited in current clinical trials (Downey et al, 2007; Hodi et al, 2008). Therefore, further attempts to identify specific negative regulatory molecules for cancer immunotherapy may be needed. We were intrigued with a new member of the B7 family, B7-H3, as a potential negative molecule for T-cell activation in tumour immunity. We first examined the tumour B7-H3 expression in human pancreatic cancer, and found that tumour B7-H3 expression was consistently high in comparison with non-cancer tissue and significantly correlated with lymph node metastasis and advanced pathological stage. These clinical data suggested that tumour B7-H3 might function as a negative regulator in pancreatic cancer. This might be consistent with several previous reports in other malignancies, including lung and prostate cancers (Castriconi et al, 2004; Sun et al, 2006; Roth et al, 2007; Zang et al, 2007). However, there was no significant difference in post-operative prognosis as a result of tumour B7-H3 expression. The reason for this discrepancy may lie, in part, in the development of adjuvant chemotherapy. In fact, the post-operative prognosis in our department is much better because of aggressive chemotherapy for post-operative recurrence. On the other hand, conflicting observations were shown in a clinical study of gastric cancer and in a study of a murine colon cancer model. These indicate that high intra-tumour B7-H3 expression correlated with better prognosis in gastric cancer (Wu et al, 2006), and the introduction of B7-H3 into colon cancer cells leads to a reduction in tumour metastasis and tumour regression (Lupu et al, 2006, 2007). To clarify these discrepancies in tumour B7-H3 expression, a key issue may be to identify unknown receptors for B7-H3. It has been reported that soluble B7-H3 protein binds a putative counter-receptor on activated T cells that is distinct from CD28 and CTLA-4 (Chapoval et al, 2001; Sun et al, 2002). Furthermore, two distinct receptors have been assumed to explain the duality of the stimulatory and inhibitory functions imparted by B7-H3 (Roth et al, 2007). Hashiguchi et al, 2008 have recently reported that the triggering receptor expressed on myeloid cells, (TREM)-like Transcript 2 (TLT-2, TREML2), is a receptor for B7-H3. They showed that the interaction of B7-H3 with TLT-2 on T cells enhanced T-cell activation. B7-H3 may use another inhibitory receptor besides TLT-2. Therefore, depending on the affinity of differential receptors, tumour-associated B7-H3 may have distinct functional effects in vivo. A high-level expression of B7-H3 by transfection may engage TLT-2 to enhance T-cell responses. By contrast, other cancers with relatively low or physiological levels of B7-H3 expression may engage an inhibitory receptor (Lupu et al, 2006, 2007; Yi and Chen, 2009). Further investigations for the identification of receptors are clearly warranted to elucidate the physiological and pathological functions of B7-H3 in various conditions.
On the basis of our clinical data, we then investigated the therapeutic efficacy of blocking the B7-H3 pathway in pancreatic cancer towards future application. We used a murine orthotopic pancreatic cancer model. We found several important observations. First, anti-B7-H3-blocking mAb had a significant anti-tumour effect on tumour growth in vivo. This anti-tumour effect was not observed in vitro, suggesting that mAb had no direct effect on cancer cell growth. Furthermore, we observed that CD8+ T-cell infiltration into tumours was promoted by B7-H3 blockade. Furthermore, the in vivo depletion indicated that CD8+ T cells are required for the anti-tumour effect of B7-H3 blockade. Taken together, these data suggested that the B7-H3 pathway might critically regulate the growth of pancreatic cancer in vivo through the negative interaction between tumours and tumour-reactive CD8+ T cells. Although some islet cells were also positive for B7-H3, blood sugar levels were consistently normal and mice were healthy after mAb treatment. Second, more importantly, our data indicated that the combination of gemcitabine with B7-H3 blockade exerted a synergistic anti-tumour effect on pancreatic cancer. In clinical settings, gemcitabine is still currently the best treatment available for pancreatic cancer (Burris et al, 1997; Li et al, 2004; Hochster et al, 2006; Oettle et al, 2007). However, the effect of gemcitabine alone is limited and most patients develop resistance to the therapy. Therefore, gemcitabine, in combination with other approaches, is currently under investigation (Hochster et al, 2006). Chemotherapy and immunotherapy have usually been regarded as unrelated or potentially antagonistic forms of therapy (Lake and Robinson, 2005). This is because most chemotherapies kill target cells by apoptosis and similarly induce cell death of activated T cells recognising tumour antigen. In addition, lymphopenia is a common side effect of many anti-cancer drugs and this has been assumed to be detrimental to sufficient anti-tumour immune response. On the other hand, a number of studies have showed that chemotherapy could synergise with immunotherapy under some circumstances. In fact, it has been reported that gemcitabine reduces myeloid-derived suppressor cells, accompanied by an increase in the anti-tumour activity of CD8+ T cells and activated NK cells (Suzuki et al, 2005). In addition, several previous animal studies have shown a synergistic anti-tumour effect of gemcitabine with certain immunotherapies, including cytokines, monoclonal antibodies, and vaccines (Nomi et al, 2007). Thus, gemcitabine is a candidate chemotherapeutic agent for the combination therapy with immunotherapy. Although further studies are required to determine the underlying mechanisms in a synergistic effect between gemcitabine and B7-H3 blockade, our data may be clinically important and support future application of B7-H3 blockade for the treatment of pancreatic cancer.
In conclusion, we showed for the first time that B7-H3 is abundantly expressed in human pancreatic cancer and it has clinical significance. Furthermore, our data also indicated that B7-H3 is a potential target for immunotherapy against pancreatic cancer. This study may provide the rationale for developing a novel cancer therapy for this fatal malignant disease.
Acknowledgments
This work was supported by the following grants: Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, No. 19591491, 21591648; Research Grant from the Pancreas Research Foundation of Japan; Research Grant from the Foundation for Promotion of Cancer Research in Japan; Research Grant from Daiwa Securities Health Foundation; Research Grant from The Japanese Society of Gastroenterology; and Research Grant from Nakayama Cancer Research Institute (M Sho).
References
- Burris III HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6): 2403–2413 [DOI] [PubMed] [Google Scholar]
- Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, Negri F, Conte R, Corrias MV, Moretta L, Moretta A, Bottino C (2004) Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci USA 101(34): 12640–12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L (2001) B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol 2(3): 269–274 [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W (2003) Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 9(5): 562–567 [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8(8): 793–800 [DOI] [PubMed] [Google Scholar]
- Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA (2007) Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res 13(22 Pt 1): 6681–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M (2008) Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci USA 105(30): 10495–10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochster HS, Haller DG, de Gramont A, Berlin JD, Philip PA, Moore MJ, Ajani JA (2006) Consensus report of the international society of gastrointestinal oncology on therapeutic progress in advanced pancreatic cancer. Cancer 107(4): 676–685 [DOI] [PubMed] [Google Scholar]
- Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G (2008) Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA 105(8): 3005–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57(1): 43–66 [DOI] [PubMed] [Google Scholar]
- Laheru D, Jaffee EM (2005) Immunotherapy for pancreatic cancer – science driving clinical progress. Nat Rev Cancer 5(6): 459–467 [DOI] [PubMed] [Google Scholar]
- Lake RA, Robinson BW (2005) Immunotherapy and chemotherapy – a practical partnership. Nat Rev Cancer 5(5): 397–405 [DOI] [PubMed] [Google Scholar]
- Li D, Xie K, Wolff R, Abbruzzese JL (2004) Pancreatic cancer. Lancet 363(9414): 1049–1057 [DOI] [PubMed] [Google Scholar]
- Luo L, Chapoval AI, Flies DB, Zhu G, Hirano F, Wang S, Lau JS, Dong H, Tamada K, Flies AS, Liu Y, Chen L (2004) B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol 173(9): 5445–5450 [DOI] [PubMed] [Google Scholar]
- Luo L, Qiao H, Meng F, Dong X, Zhou B, Jiang H, Kanwar JR, Krissansen GW, Sun X (2006) Arsenic trioxide synergizes with B7H3-mediated immunotherapy to eradicate hepatocellular carcinomas. Int J Cancer 118(7): 1823–1830 [DOI] [PubMed] [Google Scholar]
- Lupu CM, Eisenbach C, Kuefner MA, Schmidt J, Lupu AD, Stremmel W, Encke J (2006) An orthotopic colon cancer model for studying the B7-H3 antitumor effect in vivo. J Gastrointest Surg 10(5): 635–645 [DOI] [PubMed] [Google Scholar]
- Lupu CM, Eisenbach C, Lupu AD, Kuefner MA, Hoyler B, Stremmel W, Encke J (2007) Adenoviral B7-H3 therapy induces tumor specific immune responses and reduces secondary metastasis in a murine model of colon cancer. Oncol Rep 18(3): 745–748 [PubMed] [Google Scholar]
- Nagashima O, Harada N, Usui Y, Yamazaki T, Yagita H, Okumura K, Takahashi K, Akiba H (2008) B7-h3 contributes to the development of pathogenic th2 cells in a murine model of asthma. J Immunol 181(6): 4062–4071 [DOI] [PubMed] [Google Scholar]
- Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y (2007) Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 13(7): 2151–2157 [DOI] [PubMed] [Google Scholar]
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297(3): 267–277 [DOI] [PubMed] [Google Scholar]
- Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y (2005) Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 11(8): 2947–2953 [DOI] [PubMed] [Google Scholar]
- Plate JM (2007) Current immunotherapeutic strategies in pancreatic cancer. Surg Oncol Clin N Am 16(4): 919–943, xi [DOI] [PubMed] [Google Scholar]
- Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, Dong C (2004) Murine B7-H3 is a negative regulator of T cells. J Immunol 173(4): 2500–2506 [DOI] [PubMed] [Google Scholar]
- Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML, Cheville JC, Sebo TJ, Kwon ED (2007) B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res 67(16): 7893–7900 [DOI] [PubMed] [Google Scholar]
- Sho M, Yamada A, Najafian N, Salama AD, Harada H, Sandner SE, Sanchez-Fueyo A, Zheng XX, Strom TB, Sayegh MH (2002) Physiological mechanisms of regulating alloimmunity: cytokines, CTLA-4, CD25+ cells, and the alloreactive T cell clone size. J Immunol 169(7): 3744–3751 [DOI] [PubMed] [Google Scholar]
- Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF, Stockl J, Knapp W (2004) Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol 172(4): 2352–2359 [DOI] [PubMed] [Google Scholar]
- Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A, Itie A, Chung S, Da Costa J, Arya S, Horan T, Campbell P, Gaida K, Ohashi PS, Watts TH, Yoshinaga SK, Bray MR, Jordana M, Mak TW (2003) The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol 4(9): 899–906 [DOI] [PubMed] [Google Scholar]
- Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C (2002) Characterization of mouse and human B7-H3 genes. J Immunol 168(12): 6294–6297 [DOI] [PubMed] [Google Scholar]
- Sun X, Vale M, Leung E, Kanwar JR, Gupta R, Krissansen GW (2003) Mouse B7-H3 induces antitumor immunity. Gene Ther 10(20): 1728–1734 [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X (2006) B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer 53(2): 143–151 [DOI] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM (2005) Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 11(18): 6713–6721 [DOI] [PubMed] [Google Scholar]
- Traverso LW (2006) Pancreatic cancer: surgery alone is not sufficient. Surg Endosc 20(Suppl 2): S446–S449 [DOI] [PubMed] [Google Scholar]
- Wang L, Fraser CC, Kikly K, Wells AD, Han R, Coyle AJ, Chen L, Hancock WW (2005) B7-H3 promotes acute and chronic allograft rejection. Eur J Immunol 35(2): 428–438 [DOI] [PubMed] [Google Scholar]
- Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, Zhao JM, Zhang GB, Zhang XG (2006) Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol 12(3): 457–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KH, Chen L (2009) Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev 229(1): 145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP (2007) B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA 104(49): 19458–19463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GB, Zhou H, Chen YJ, Ge Y, Xie F, Shi Q, Ma HB, Fei M, Zhang XG (2005) Characterization and application of two novel monoclonal antibodies against 2IgB7-H3: expression analysis of 2IgB7-H3 on dendritic cells and tumor cells. Tissue Antigens 66(2): 83–92 [DOI] [PubMed] [Google Scholar]